Abstract
Japanese larch (Larix kaempferi = L. leptolepis) is often characterized by its high growth rate with heterophyllous shoots, but the functional differences of heterophyllous shoots still remain unclear. Recently, abrupt high temperature and drought during spring induced high photosynthetic rate via change in leaf morphology of the deciduous habit. In order to reveal the photosynthetic characteristics of both short and long-shoot needles of sunny canopy of the larch trees using a canopy tower, we calculated the seasonal change of gas exchange characters and leaf mass per area (LMA) and foliar nitrogen content (N) of heterophyllous needles: short and long-shoot needles over 3 years. No marked difference in light-saturated photosynthetic rates (Psat) was observed between short and long shoots after leaf maturation to yellowing, although the difference was obvious in a specific year, which only shows that seasonal change in temperature and soil moisture determines the in situ photosynthetic capacity of needles. The large annual and seasonal variations in Psat in both shoots were found to be mainly determined by climatic variations, while shoot types determined the strategy of their photosynthetic N utilization as well as the stomatal regulation.
1. Introduction
Plants have high morphological and functional plasticity because they do not have the ability to move in order to avoid harsh environments and conditions [1,2]. According to the Fifth Assessment Report of IPCC [3], annual fluctuation in terms of climate condition has been observed to increase in the past years. As a result, we recently have extremely warm weather during spring with little precipitation [4,5]. The photosynthetic rate of some deciduous tree seedlings responds well to warm springs or drought in terms of leaf morphological changes and leaf nitrogen (N) accumulation [6,7,8]. Moreover, under stress conditions (light, water, nutrient, etc.), plants can cope by changing their leaf mass per area (LMA) for efficient use of their resources as well as for allocation of biomass (e.g., [7,9]). The different roles of heterophyllous growth traits have been examined in birch [10,11,12], larch [13,14,15,16], and others [17,18].
Most larch (Larix sp.) species are characterized by its high growth rate with heterophyllous shoots [11,13] in deciduous leaf habit among conifer species [13]. Dahurian larch (Larix gmelinii) can grow on severe environmental conditions in East Siberia and Far East Russia, i.e., permafrost with small amounts of precipitation, extreme sunlight, huge daily temperature differences, etc. [19,20,21]. In addition, larch can survive at harsh environments but with mostly short-shoot needles, except in the initial stage of seedlings. Many studies have been carried out to determine the environmental responses of larch seedlings for long-shoot needles but not many on adult trees for short- and long-shoot needles [22].
We have been fascinated with the high growth rate of larch (Larix sp.) species and aimed to analyze their photosynthetic function [14,23,24,25,26,27]. Irrespective of the former expectation, it was concluded that no special metabolic pathway could be found [28], but the high photosynthetic rate may be realized through a unique arrangement of the different types of needles in a larch canopy (i.e., the heterophyllicity) [7,16,24] and deciduous leaf habit for avoiding cold and dry season and re-accumulation of N to plant body [25,29]. Is there any specific functional difference in heterophyllous shoots, i.e., short and long shoots of the Japanese larch? As deciduous leaf habit [2], we should determine the remobilization capacity of N before leaf shedding for the next growing season.
The roles of heterophyllous leaves were reported in birch, which has similar photosynthetic traits to larch [20,29], namely, early leaves and late leaves. The growth of late birch leaves was substantially suppressed when shading occured for early leaves [12,16]. In Japanese larch, the temporal growth patterns of the apices of short and long shoots were found to differ: the apices of long shoots have continual growth phases, but short shoots do not repeat annually the formation of winter buds [30]. Therefore, the growth of larch is strongly dependent on the photosynthetic activities of short-shoot needles. We expected that short-shoot needles should have high photosynthetic rate under various environmental conditions.
However, there are two contradictory results regarding the photosynthetic rate at light saturation (Psat) and ambient CO2 in Japanese larch, namely, short-shoot needles have lower Psat than long-shoot needles [15]; in contrast, no difference in Psat was found for both shoot types [14]. The defoliation treatment on short-shoot needles at lower canopy of Dahurian larch concluded that there is substantial suppression of both diameter and length growth at the upper canopy, though only the diameter is significantly decreased at the lower canopy [31]. These studies found that the functional role of these two shoot types may be different in crown development.
To assess the functional role of heterophyllous larch needles, in situ measurements should be performed, such as canopy photosynthesis in larches, including its hybrid [14,24,25,26], which is considered essential in understanding the carbon balance of a forest from leaf to stand [19,32,33,34]. However, studies examining the functional difference in heterophyllous larch shoot in situ are still very limited [22], and some of them were only good for one season’s results. There is a big yearly variation in Psat among the four tree saplings in larch forests found in northern Japan, which is caused by the difference in air temperature and precipitation during leaf unfolding period [7,8,19,35]. If spring would bring few precipitation and high temperature, photosynthetic rate was high with high N, and this trend could be induced by manipulated experiments in a greenhouse [7]. Therefore, long-term measurement in situ will be needed to reveal the role and functional difference in heterophyllous shoots of larch species under certain field conditions.
As a typical heterophyllous conifer species, short shoots grow on older branches with bundles of needles, whereas long shoots of the Japanese larch generally develop at the top branches with separated needles directly on current-year branches [14,30]. However, the difference between short and long shoots has not well been discussed [16,20,26], and some ecophysiological questions still remain, e.g., differences in the relations between leaf N and photosynthetic rate (Psat), maximum carboxylation rate (Vcmax) and maximum electron transportation rate (Jmax), and N remobilization rate before needle shedding [9,17,32]. Are there any variations in the relations of Psat–2N, Vcmax–N, and Jmax–N between short- and long-shoot needles?
Photosynthetic capacity is substantially affected by the anatomical structure of leaves [36,37,38,39,40] as well as stomatal and mesophyll resistance in gas diffusion of water and CO2 [39,41,42]. Leaf functional structures, including leaf thickness and water availability, affect gas diffusion from air to chloroplasts and vice versa [39,40]. These former evidences are applicable to heterophyllous shoots in Japanese larch trees.
To address these questions, we monitored the in situ seasonal and annual changes of canopy photosynthetic capacity (Psat and Pmax) in the needle traits of short and long shoots, together with environmental factors, using the canopy tower, from 2001 to 2003. The needle nitrogen remobilization rate (NRMA) and photosynthetic N relations (Psat–N and Pmax–N, Vcmax–N, and Jmax–N) were all examined in relation to the factors affecting photosynthesis of a larch canopy. The goal of this study is to reveal seasonal and yearly variations in the photosynthetic capacity in situ of both short- and long-shoot needles in order to maintain the canopy function in a field.
2. Results
2.1. Seasonal Changes of Air Temperature and Soil Moisture
The peak air temperature was recorded in July and August. We should highlight that the air temperature in July and August 2001 and 2002 was much lower than that in 2003. In 2003, air temperature was higher, and soil moisture was recorded to be lower. From April to May, soil moisture in 2003 has sharply decreased as compared with that in 2001 and 2002. Soil moisture data during late April and May when short-shoot needles flushed indicated about 34% (v/v) in 2001 and 2002 while 27% in 2003. In late June to July, soil moisture was recorded to be at 35%, 31%, and 26% in 2001, 2002, and 2003, respectively (Figure 1).
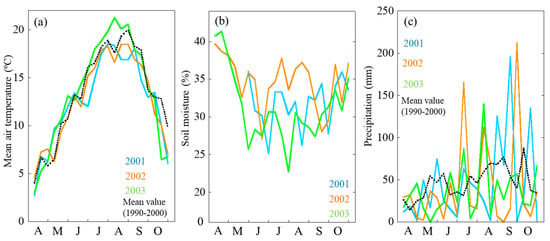
Figure 1.
Seasonal and annual changes in mean daily air temperature (a), soil moisture (b) and precipitation (c). Different colors indicate the different year and mean value: light blue, 2001; orange, 2002; light green, 2003; dashed line, mean value from 1990 to 2000.
2.2. Seasonal and Annual Changes in Psat, LMA, and Nitrogen Content
Short-shoot needles flushed in mid-May. After complete expansion of the short-shoot needles (about 20 days later), long-shoot needles started to develop. In short-shoot needles, Psat gradually increased from mid-May to August and then decreased toward late October; the values ranged from about 2.0 to 8.0 µmol m−2s−1. Long-shoot needles displayed a similar tendency to short-shoot needles; however, Psat of long-shoot needles increased from June to August and then decreased from late September toward October (Figure 2, left). Comparing the seasonal change in Psat, Psat of short and long shoot was similar in 2001 and 2002. In contrast, there was a clear yearly difference in Psat in 2003 (Figure 2, left). The Psat of short shoots was generally higher than that of long shoots in 2001 and 2002, but a reverse trend was observed in 2003. Moreover, the difference between short and long shoots was significantly higher in the early phase of the growing season than in the late growing season (Figure 2, left). In the midst of summer, Psat was found to be 30% higher in 2003 than in 2001 for short shoots and 40% higher for long shoots (Figure 2, left). However, the difference in Psat between short and long shoots was found to insignificant (p > 0.05).
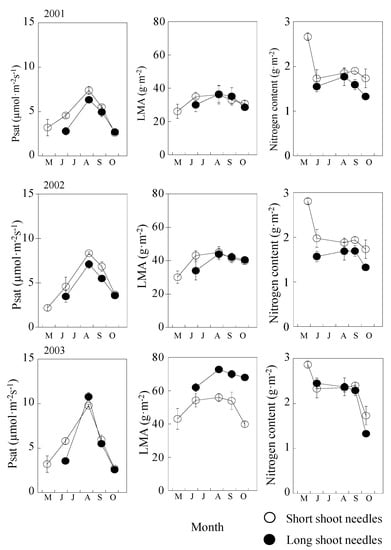
Figure 2.
Seasonal and annual changes of Psat, LMA, and nitrogen content from 2001 to 2003. Open cycles are short shoots, and closed cycles are long shoots.
Except for the year 2003, no difference was found in the pattern of LMA of short- and long-shoot needles (Figure 2, center). LMA of both short- and long-shoot needles in 2003 showed 10–20 g m−2 higher than that in 2001 and 2002. After maturation of long-shoot needles in 2003, LMA of long-shoot needles did not clearly decrease, while LMA of both shoot needles slightly decreased in 2001 and 2002.
Leaf nitrogen (N) content of both types of needles showed similar pattern, in terms of seasonal change in the successive 3 years (Figure 2, right). N was high in May for short-shoot needles within the 3-year period. N showed a stable value with needle maturation in both needle types and then decreased after late September.
2.3. Photosynthesis–Nitrogen Relation
Photosynthetic N use efficiency (PNUE) is expressed by the gradient of the linear part of the Psat–N relation. A steeper slope means more efficient use of N in photosynthesis. For both short and long shoots, accurate linear relations between leaf nitrogen and Psat were observed. However, the difference between their gradients was found to be significant (p < 0.05). The gradient was steeper for short shoots (PNUE = 11.1) than for long shoots (PNUE = 5.6) (Figure 3a). PNUE of short-shoot needles was higher than that of the long-shoot needles. Psat at CO2 and light saturation (=Pmax) and Pmax of short and long shoots were equally correlated with their foliar N concentration (PNUE = 9.5) (Figure 3b).
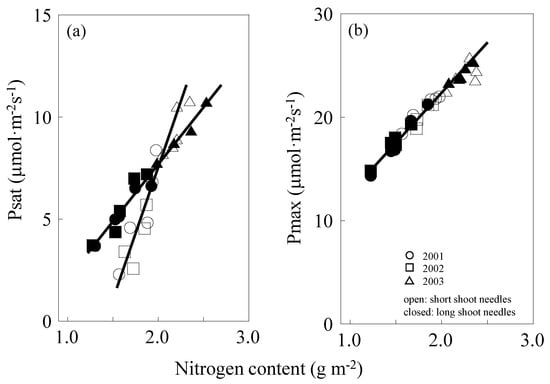
Figure 3.
Psat–N relationship (a) and Pmax–N relationship (b). Open cycles are short shoots, and closed cycles are long shoots. Different shapes of symbols indicate different years, ○, 2001; □, 2002; △, 2003. Data are after leaf maturation till leaf senescence (June to October for short shoots, July to October for long shoots). Best-fitting equations: (a), Psat = −3.90 + 5.62N, r2 = 0.97, p < 0.001 (long shoots); Psat = −15.1 + 11.1N, r2 = 0.91, p < 0.001 (short shoots); (b), Pmax = 2.22 + 9.47N, r2 = 0.90, p < 0.001 (short and long shoots).
2.4. Variation of Vcmax and Jmax
In Vcmax, no significant difference was observed in Vcmax of short- and long-shoot needles (p > 0.05, Figure 4). However, in short-shoot needles, Vcmax of 2003 was significantly recorded to be higher than the other 2 years (p < 0.01). In long-shoot needles, Vcmax in 2003 tended to show marginally higher value than the other 2 years.
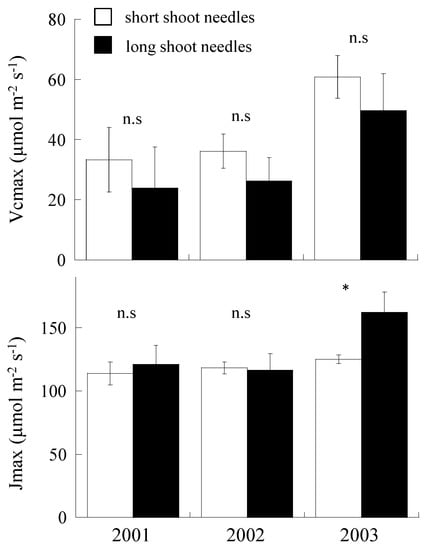
Figure 4.
The maximum rates of RuBP carboxylation (Vcmax) and the maximum rate of electron transport driving the RuBP regeneration (Jmax) of short- and long-shoot needles in August for 3 years. Open bars indicate short-shoot needles, while closed bars indicate long-shoot needles. n.s. means not significant. * indicates the statistical difference between short- and long-shoot needles. Vertical line on each bar indicates standard error (SE).
In Jmax, except 2003, no significant differences were observed in short- and long-shoot needles in 2001 and 2002. In 2003, Jmax in short-shoot needles showed significantly lower value than that in long-shoot needles. In long-shoot needles, Jmax value of 2003 was significantly higher than the other 2 years (p < 0.01).
2.5. Vcmax, Jmax–N Relation
No marked difference was observed between short and long shoots in Vcmax–N relation (Figure 5a). However, a significant difference between short-shoot and long-shoot needles in Jmax–N relation was also found, i.e., when N increased, Jmax of short-shoot needles increased gentler than that of the long ones (Figure 5b).
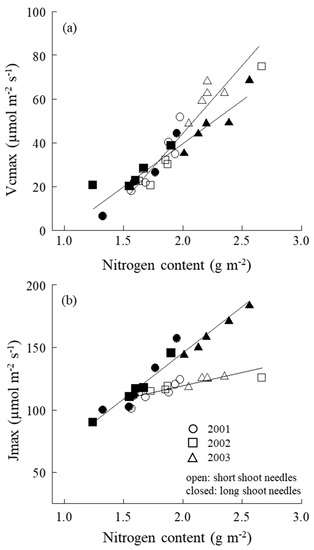
Figure 5.
(a) Relationship between the maximum rates of RuBP carboxylation (Vcmax) and leaf nitrogen content and (b) the maximum rate of electron transport driving the RuBP regeneration (Jmax) and leaf nitrogen content of both short- and long-shoot needles. ○, 2001; □, 2002; △, 2003. Open cycles are short-shoot needles, while closed cycles are long-shoot needles.
2.6. Nitrogen Remobilization Rate (NRMR)
Large annual variations in NRMR were observed for both short and long shoots (Figure 6). For short shoots, the minimum NRMR was recorded to be at 17%, observed in 2002, and the maximum NRMR was at 25% in 2003. For long shoots, the minimum NRMR was determined to be at 26%, and the maximum NRMR was at 43% (Figure 6). In each year, short shoots were able to remobilize less nitrogen during leaf senescence than that of long-shoot needles (p < 0.01) (Figure 6). On average, short shoots could remobilize 20% N, while long shoots could remobilize 33% N.
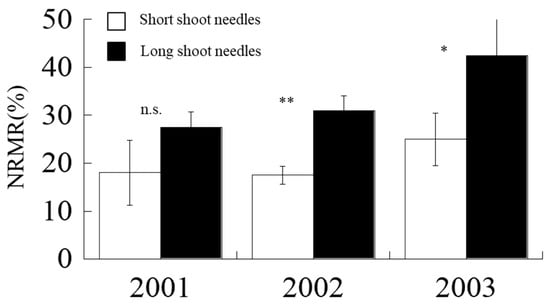
Figure 6.
Nitrogen remobilization rates (NRMR) of short and long shoots from 2001 to 2003. Vertical line on each bar indicates standard error (SE). n.s. means not significant. * and ** imply significant at p < 0.05 and p < 0.01, respectively
3. Discussion
3.1. Seasonal and Yearly Variation in Photosynthetic Rate: Climatic Limitation
Our study found that there were large seasonal and yearly variations in the photosynthetic capacity of the two shoot types (Figure 2). The pattern of seasonal changes in LMA and N was found to be similar in needles of both shoot types. As a result, a clear positive correlation was determined between N and Psat or Pmax (Figure 3). Photosynthetic capacity is determined both by climatic conditions and by status of leaf morphology, CO2 diffusion, N allocation to photosynthetic organs, kinetics of Rubisco, and others. [1,2,8,40,43,44]. In loblolly pine (Pinus taeda) canopy, year-to-year variation in its microenvironment led to large variations in Psat, which was shown as a stomatal limitation [34]. In a mixed deciduous forest, Bassow and Bazzaz [35] found that Psat differs significantly not only between species but also among individuals within a species.
Temperature significantly affects the yearly variation of Psat through optimal temperature for photosynthesis, vapor pressure deficit [1,2,32], and increases of leaf LMA and N [8,45,46]. The air temperature in July and August 2001 was at 3 °C lower than that in 2002 and 2003 and that in 2002 was about 1 °C lower than that in 2003 (Figure 1). There was a clear positive correlation between Psat and N (Figure 3). From manipulated experiments in a greenhouse for four kinds of deciduous species with different leaf developments (determinant vs. indeterminant) [7], their Psat was also found to positively correlate with LAM and N. With increasing temperature, larch needles were adequately developed with higher photosynthetic capacity. As a result, more energy from the photosynthates could be used in absorbing N; thus, more N was detected in needles (Figure 2).
Except in 2003, no significant difference was observed in LMA between short and long shoots (Figure 2) at leaf stable period [36], i.e., from the maturation of leaves to the start of leaf senescence. Significant high LMA in 2003 was observed in short-shoot leaves in June and in long-shoot leaves from July to September (Figure 2). In 2003, the soil moisture was much dryer than in 2001 and 2002 (Figure 1). Our finding of both shoots having higher LMA in the dry spring of 2003 suggests that the structure of needles was affected and changed by the low soil moisture conditions. Similar responses of the three kinds of understory seedlings in a Japanese larch forest to variations in climatic condition were reported in previous studies [1,8,43]. Soil moisture condition during leaf unfolding increased the Psat of deciduous oak, magnolia, and hornbeam (Carpinus sp.) with high LMA and high N [8]. Similar responses were also reported. In the Central USA, maple seedlings had higher leaf mass leaves, responding to severe drought [6].
The evidence may indicate greater investment in leaf construction to endure desiccation in a water-limited environment. In dry and hot summer (2003), larch generally needs higher LMA, which is rewarded by higher photosynthetic rates per unit leaf area in both short and long shoots (Figure 2) and also higher NRMR for storage for the next year’s growth (Figure 6), which accord with the process of leaf economy [44,47]. Their findings [44,47] also agreed with our results. A previous study suggested that the different development stages of shoots and leaves may have important impacts on the seasonal course of photosynthesis [48]. This is one limitation of this study. The evaluation of combination effects of developing stage differences and impacts of climatic factors should be revealed in further studies.
3.2. Differences in the Photosynthetic Nitrogen Relations Between Short and Long Shoots
A positive correlation between leaf N and photosynthesis has been observed across many species [1,2,49] with large variations among species [47], even in yearly variation within the same species [28]. Weak linear relations have also been observed in some canopy species [50]. This disparity is considered to be attributed to the fact that photosynthetic capacity rises linearly with increasing N until limitation of other factors [1,2]. Although accurate linear relations of Psat–N in both short and long shoots were recorded, this difference became negligible when the Pmax took place of Psat, indicating that CO2 diffusion may be a reason for this disparity (Figure 3) We should know which photosynthetic processes are most affected by this CO2 diffusion differences, such as mesophyll conductance or cell wall resistance [40,41].
Vcmax linearly increased with leaf N, and no differences in Vcmax–N were found between short and long shoots (Figure 5a). On the other hand, Jmax of long-shoot needles increased steeper with an increase in leaf N compared with that of the short ones (Figure 5b). Thus, although the efficiency of leaf N in carboxylation enzymes of short and long shoots was quite similar, the less efficiently in RuBP regeneration for short-shoot needles decreased the Jmax, finally resulting in the apparent differences observed in Psat–N (Figure 3a). Surely, the diffusion difference between short and long shoots strongly affects the N use in RuBP regeneration, but not the carboxylation processes.
Given that N could be equally allocated between carboxylation and RuBP regeneration, one possible reason for the less N efficiency in RuBP regeneration may be attributed to the shortage of other resources, such as CO2. It has been proved that Psat at CO2 and light saturation (=Pmax) may indicate maximum photosynthetic without any limitation of stomata; therefore, there should be clear positive correlation between N and Pmax (Figure 3b).
We found that N remobilized at different rates in short and long shoots; for instance, short shoots tended to remobilize less N during leaf senescence compared with long shoots (Figure 6), especially in 2003 when we had relatively high temperature and dry spring (Figure 1). Moreover, long-shoot needles are positioned at the branch top [17], where elongation in the next year will occur. Therefore, we considered that this process is essential in the development of long shoot as it can store more nutrients for the next year’s growth at the shoot top [29].
LMA stands for leaf level function with changes in N allocation to photosynthetic protein [9,51,52]. Therefore, yearly variation of LMA, mainly attributed to temperature and humidity changes, could affect the annual variation of PNUE and Psat.
The photosynthesis–N relation of larch needles with different shoot types is considered to be surely affected by several factors [53]: CO2 diffusion, leaf N, stomatal conductance, and kinetics of Rubisco (Figure 3). Hikosaka et al. [54] also suggested that PNUE differences between evergreen broadleaved trees and annual herbaceous plants may be caused not by a single factor but a combination of several factors. Their conclusion agrees with our findings, i.e., yearly variation of Psat–N and Psat–N can be induced by a combination of changes brought about by LMA, N content, N allocated to photosynthetic protein [49,50,53,54,55,56,57], etc. in the needles.
In conclusion, as a typical heterophyllous conifer, Japanese larch trees display no potential difference in photosynthetic rate (Psat) between short and long shoots, which only shows that climate factors, such as high temperature and soil moisture during leaf development rather than shoot types, affect the Psat. However, the Psat–N of short- and long-shoot needles was different, although this difference was reduced under conditions of saturation with CO2 and light (i.e., Pmax–N). No differences were observed in Vcmax–N; however, Jmax–N showed that Jmax of short-shoot needles was suppressed, and this trend was presented as a gentler slope than that of the long ones. Moreover, much higher N remobilization rates were found in long shoots for the successive growth the following year.
Therefore, climate factors can affect the morphological (LMA) and physiological traits (N, Vcmax, Jmax) of shoot needles [3,38,48,58,59,60,61]; thus, a combination of these external and internal factors can result in the yearly variation of Psat and Psat–N relationship in short- and long-shoot needles. With the changing climate, Japanese larch trees would be able to cope with high temperature and drought to some extent via high plasticity in LMA of both short- and long-shoot needles.
4. Materials and Methods
4.1. Study Site
The study was conducted in a larch plantation (50 years old as of 2003) located at Tomakomai National Forest, northern Japan (42°40′ N, 141°36′ E, 200–300 m a.s.l.) from 2001 to 2003. The soil is comprised of immature volcanic ash (vitric andosols) and is very shallow (around 15–20 cm), which is an aftereffect of the eruption of Mt. Tarumae in 1739. The mean annual precipitation is about 1250 mm, and the mean monthly air temperature ranges from −3.2 °C to 19.1 °C. Typical rainy season is from mid-July to September. Typical freezing season is from December to March, and the coldest season is from late January to early February.
4.2. Plant Materials
A 20 m tower with walkway was built to reach the shady and sunny crown of the three mature trees of larch (Larix kaempferi (Lamb.) Carrière) with heterophyllous shoots (48 years old as of 2001). From late April to early May, buds of short-shoot needles flushed, and 2–3 weeks later, long-shoot needles started succeeding growth. On late August, the growth of long-shoot needles stopped. At late September to early October, both short- and long-shoot needles start to color, and by mid to late October, larch trees started shedding of their needles. As shown in the previous studies [16,29], separated needles of long shoots grow directly on current-year branches, and these needles are found to be generally thick and had a large connection interface between leaves and branches. Meanwhile, short shoots grow on older branches with bundles of needle leaves, and these needles generally had a thin leaf blade and small connection interface [30,31]. The average height of the larch plantation was 12.3 m. From 2001 to 2003, three replications in each for short and long shoots from the upper canopy of three individual trees were selected for the measurement. For 1 year, we measured the photosynthetic rates of the short-shoot needles five times (a total of 15 shoots was used as sample) and of the long-shoot needles four times (a total of 12 shoots). The total number of short and long shoots was 45 and 36, respectively, for 3 years.
4.3. Air Temperature and Soil Moisture Monitoring
Temperatures at the study site were monitored through auto-logged climatic monitors (HMP45D, VAISALA, Helsinki, Finland) at a height of 8 m aboveground. The soil moisture was measured by the TDR sensor (CS615, Campbell Scientific Inc., Logan, UT, USA) at a depth of 0.2 m. The data were recorded twice per hour (Asia Flux web: http://asiaflux.net/index.php?page_id=113).
4.4. Photosynthesis and Nitrogen Measurement of Needles
All of the gas exchanges of short and long shoots were measured using a portable gas analyzer LI-6400 (LI-Cor, Lincoln, NE, USA) with transparent conifer chamber (6400-05), equipped with a 2050 HB illumination system (Walz, Effeltrich, Germany) to determine light-saturated photosynthetic rates at 360 ppm CO2 (Psat). Today, CO2 concentrations reach to 410 ppm, and 50 ppm differences are present; however we think that the effect of the differences to photosynthetic rates would be minor, based on the results of photosynthetic responses of larch species to the elevated CO2 [58]. Approximately 5 cm shoots enclosed the chamber. Measurements of Psat were made under steady-state conditions at a leaf temperature of 24 –28 °C and the photosynthetic photon flux density (PPFD, µmol m−2s−1) of about 2000 µmol m−2s−1. After determining Psat, we calculated the Pmax by increasing the CO2 concentration in increments of 100 ppm up to 1500–1800 ppm. Stomatal conductance was maintained above 0.05 mol m−2s−1 during the measurement of Pmax.
After the each gas exchange measurement, the measured shoots were harvested and brought back to the laboratory. The leaf area (A) was then measured using a LI-3000 leaf area system (LI-Cor); the result was used to calculate the photosynthetic rate per unit area by recalculation program of LI-6400. Following this area determination, the needle dry mass (M) was measured at 65 °C for 48 h, and the LMA was calculated according to Pérez-Harguindeguy et al. [59], namely:
LMA = M/A
Foliar nitrogen content (N) was determined using an NC analyzer (NC-900, Shimadzu, Kyoto, Japan). The number of needles ranged between six and ten for each time. The N was calibrated and checked against a standard (acetanilide: N = 10.36%, C = 71.09%; Wako, Osaka, Japan).
4.5. Analysis of A-Ci Curves
A-Ci curves can be used to estimate Vcmax (maximum rate of carboxylation allowed by Rubisco), and Jmax (maximum electron transportation rates) was estimated by the model [42,43,51] displayed as follows:
where Wc is the Rubisco-limited net photosynthetic rate (µmol m−2 s−1) and Wj is the RuBP regeneration rate-limited net photosynthetic rate (µmol m−2 s−1). The Wc and Wj are provided by Equations (3) and (4):
Pn = min (Wc, Wj)
In Equation (4) at light saturation, J is equal to Jmax [42]; therefore:
Here, Pn is the net photosynthetic rate, Vcmax is the maximum rate of carboxylation allowed by Rubisco, J is the potential electron transport rate, Ci is the intercellular concentration of CO2 (µmol m−2s−1) and O that of O2, Kc is the Michaelis–Menten constant for carboxylation and Ko that for oxygenation, Γ* is the CO2 compensation point (µmol m−2s−1), Rd denotes day respiration (µmol m−2 s−1), Wc is the Rubisco-limited rate (potential rate limited by the activity of Rubisco and the concentration of CO2 and O2), Wj is RuBP-limited rates (the rate of RuBP carboxylation in photosynthesis is either equal to the potential rate allowed by the concentration of RuBP), and Γ* = 3.69 (KPa), Kc = 40.4 (KPa), Ko = 24.8 (KPa) [42,56]. In this study, we calculated the apparent Vcmax and Jmax because we did not estimate mesophyll conductance for CO2 diffusion, the same as the other studies [48,49].
4.6. Calculation of Nitrogen Remobilization During Autumn Senescence
Several studies have examined N remobilization from aged leaves [8,29,59,60,61]. We express the N remobilization rate (NRMR) as:
where NLSP is the mean value of N (g m−2) in leaf stable period (LSP, [36]) and Ndied is the N content of leaves (g m−2) collected in late October, just before leaf shedding [8].
4.7. Statistical Analysis
All statistical tests were carried out using the R language (R developing core team, Vienna). To compare the Psat–N linear relation, the Vcmax–linear relation, and Jmax–N linear relation, we used analysis of covariance (ANCOVA). To compare the Psat for long and short shoots, we use t-tests. To compare the seasonal and yearly fluctuations in photosynthesis and leaf characteristics, and to compare the difference in photosynthetic capacity and the NRMR between short and long shoots, we used component analysis of variance.
Author Contributions
S.K., M.W. and T.K. conceptualized the study; S.K. and Q.L. are responsible of planning this study and S.K., Y.W. and M.W. for field researches. S.K. prepared the original draft. S.K., M.W., Y.W., Q.L. and T.K. are responsible for writing, reviewing, and editing. T.K. supervised this study, and Q.L., T.W. and T.K. are responsible for funding. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support throughout our research: the Ministry of Environment of Japan, JSPS, and JST Grant No. JPMJSC18HB (to T.W. and T.K.) and the National Key Research and Development Program of China (2017YFE0127700 to Q.L.). This study was a part of responses of larch forests carbon sink capacities to environmental changes, supported by the Global Environment Research Fund (0134) of the Ministry of the Environment, from 2001 to 2003.
Acknowledgments
We thank the members of the Tomakomai Flux Research Site and R. Hirata (National Institute for Environmental Studies), professor T. Hirano of Hokkaido University, T. Ichie (JSPS-PD at the time) of Kochi University, and Yuko Sakuma for their valuable suggestions and comments and support during the field survey. We are also grateful to Enago for the English proofreading of our manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lambers, H.; Chapin, F.; Stuart, I.; Pons, T.L. Plant Physiological Ecology, 2nd ed.; Springer: New York, NY, USA, 2008; ISBN 978-0-387-78340-6. [Google Scholar]
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups, 4th ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2003; ISBN 3-540-43516-6. [Google Scholar]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. (Eds.) Climate Change 2013: The Physical Science Basis; Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Japan Meteorological Agency. Available online: http://www.jma.go.jp/jma/indexe.html (accessed on 17 May 2020).
- Schwartz, M.D.; Ahas, R.; Aasa, A. Onset of spring starting earlier across the Northern Hemisphere. Glob. Chang. Biol. 2006, 12, 343–351. [Google Scholar] [CrossRef]
- Abrams, M.D.; Kubiske, M.E. Photosynthesis and water relations during drought in Acer rubrum L. genotypes from contrasting sites in central Pennsylvania. Funct. Ecol. 1990, 4, 727–733. [Google Scholar] [CrossRef]
- Kitaoka, S. Ecophysiological study on the growth and photosynthetic responses of deciduous broad-leaved tree seedlings invading into unmanaged larch [Larix leptolepis] plantations. Res. Bull. Hokkaido Univ. For. 2007, 64, 37–90. [Google Scholar]
- Kitaoka, S.; Koike, T. Seasonal and yearly variations in light use and nitrogen use by seedlings of four deciduous broad-leaved tree species invading larch plantations. Tree Physiol. 2005, 25, 467–475. [Google Scholar] [CrossRef]
- Evans, J.R.; Poorter, H. Photosynthetic acclimation of plants to growth irradiance: The relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Environment 2001, 24, 755–767. [Google Scholar] [CrossRef]
- Kikuzawa, K.; Lechowicz, M.J. Ecology of Leaf Longevity; Ecological Research Monographs; Springer: Tokyo, Japan, 2011; ISBN 978-4-431-53917-9. [Google Scholar]
- Kozlowski, T.T.; Clausen, J.J. Shoot growth characteristics of heterophyllous woody plants. Can. J. Bot. 1966, 44, 827–843. [Google Scholar] [CrossRef]
- Matsuki, S.; Sano, Y.; Koike, T. Chemical and physical defence in early and late leaves in three heterophyllous birch species native to northern Japan. Ann. Bot. 2004, 93, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Gower, S.T.; Richards, J.H. Larches: Deciduous conifers in an evergreen world. BioScience 1990, 40, 818–826. [Google Scholar] [CrossRef]
- Kitaoka, S.; Koike, T.; Quoreshi, A.M.; Takagi, K.; Wang, W.; Shi, F.; Kayama, M.; Ishida, N.; Mamiya, H.; Sasa, K. Seasonal changes in the photosynthetic capacity of Japanese larch trees planted on the Tomakomai National Forest, Northern Japan. Proc. Asia Flux Netw. 2001, 1, 109–112. [Google Scholar]
- Kurachi, N.; Hagihara, A.; Hozumi, K. Photosynthetic production in a Larix leptolepis plantation (IV). Photosynthetic characteristics of long and short shoot leaves. Trans. Jpn. Soc. Chubu Br. 1984, 32, 131–134. [Google Scholar]
- Kurachi, N. Distribution of Leaf—And Branch-Biomass Density within a Crown of Japanese Larch and its Relationship to Primary Production: Analysis by Sainome Cutting. In Crown and Canopy Structure in Relation to Productivity; Fujimori, T., Whitehead, D., Eds.; Forestry and Forest Products Research Institute: Tsukuba, Japan, 1986; pp. 308–322. [Google Scholar]
- Dörken, V.M. The Evolutionary Relevance of Vegetative Long-Shoot/Short-Shoot Differentiation in Gymnospermous Tree Species. Available online: https://www.schweizerbart.de/publications/detail/isbn/9783510480326/The_evolutionary_relevance_of_vegetative_long_shoot_short_shoot_differentiation_in_gymnospermous_tree_species (accessed on 18 May 2020).
- Takenaka, A. Structural variation in current-year shoots of broad-leaved evergreen tree saplings under forest canopies in warm temperate Japan. Tree Physiol. 1997, 17, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Koike, T.; Kitaoka, S.; Ichie, T.; Lei, T.T.; Kitao, M. Photosynthetic characteristics of mixed deciduous-broadleaf forests from leaf to stand. Glob. Environ. Chang. Ocean Land Tokyo Terrapub 2004, 453–472. [Google Scholar]
- Koike, T.; Yazaki, K.; Eguchi, N.; Kitaoka, S.; Funada, R. Effects of elevated CO2 on ecophysiological responses of larch species native to northeast Eurasia. In Permafrost Ecosystems: Siberian Larch Forests; Ecological Studies (Analysis and Synthesis); Osawa, A., Zyryanova, O.A., Matsuura, Y., Kajimoto, T., Wein, R., Eds.; Springer: Dordrecht, The Netherland, 2010; Volume 209, pp. 447–456. ISBN 978-1-4020-9692-1. [Google Scholar]
- Nelson, G.C.; Valin, H.; Sands, R.D.; Havlík, P.; Ahammad, H.; Deryng, D.; Elliott, J.; Fujimori, S.; Hasegawa, T.; Heyhoe, E.; et al. Climate change effects on agriculture: Economic responses to biophysical shocks. Proc. Natl. Acad. Sci. USA 2014, 111, 3274–3279. [Google Scholar] [CrossRef]
- Hoshika, Y.; Paoletti, E.; Agathokleous, E.; Sugai, T.; Koike, T. Developing ozone risk assessment for larch species. Front. For. Glob. Chang. 2020, 3. [Google Scholar] [CrossRef]
- Fry, D.J.; Phillips, I.D.J. Photosynthesis of conifers in relation to annual growth cycles and dry matter production: I. some C4 characteristics in photosynthesis of Japanese larch (Larix leptolepis). Physiol. Plant. 1976, 37, 185–190. [Google Scholar] [CrossRef]
- Kurachi, N.; Hagihara, A.; Hozumi, K. Canopy photosynthetic production in a Japanese larch stand. I. Seasonal and vertical changes of leaf characteristics along the light gradient in a canopy. Ecol. Res. 1992, 7, 255–265. [Google Scholar] [CrossRef]
- Matyssek, R. Carbon, water and nitrogen relations in evergreen and deciduous conifers. Tree Physiol. 1986, 2, 177–187. [Google Scholar] [CrossRef]
- Matyssek, R.; Schulze, E.-D. Heterosis in hybrid larch (Larix decidua x leptolepis) I. The role of leaf characteristics. Trees 1987, 1, 219–224. [Google Scholar] [CrossRef]
- Matyssek, R.; Schulze, E.-D. Heterosis in hybrid larch (Larix decidua x leptolepis) II. Growth characteristics. Trees 1987, 1, 225–231. [Google Scholar] [CrossRef]
- Richards, J.H.; Teeri, J.A. Re-evaluation of proposed C4 photosynthetic characteristics in the genus Larix. Physiol. Plant. 1982, 55, 117–120. [Google Scholar] [CrossRef]
- Hozumi, K.; Kurachi, N. Estimation of seasonal changes in translocation rates in leaves of a Japanese larch stand. Bot. Mag. Shokubutsu-Gaku-Zasshi 1991, 104, 25–36. [Google Scholar] [CrossRef]
- Fujimoto, S. Studies on the shoot formation in Larix leptolepis Gordon. Res. Bull. Hokkaido Univ. For. Jpn. 1978, 35, 1–28. [Google Scholar]
- Wang, W.; Zu, Y.; Wang, H.; Matsuura, Y.; Sasa, K.; Koike, T. Plant biomass and productivity of Larix gmelinii forest ecosystems in Northeast China: Intra- and inter-species comparison. Eurasian J. For. Res. 2005, 8, 21–41. [Google Scholar]
- Ellsworth, D.S. Seasonal CO2 assimilation and stomatal limitations in a Pinus taeda canopy. Tree Physiol. 2000, 20, 435–445. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wright, I.J.; Reich, P.B.; Westoby, M. Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats. Funct. Ecol. 2001, 15, 423–434. [Google Scholar] [CrossRef]
- Wright, I.J.; Westoby, M. Leaves at low versus high rainfall: Coordination of structure, lifespan and physiology. New Phytol. 2002, 155, 403–416. [Google Scholar] [CrossRef]
- Bassow, S.L.; Bazzaz, F.A. Intra- and inter-specific variation in canopy photosynthesis in a mixed deciduous forest. Oecologia 1997, 109, 507–515. [Google Scholar] [CrossRef]
- Koike, T. Leaf structure and photosynthetic performance as related to the forest succession of deciduous broad-leaved trees. Plant Species Biol. 1988, 3, 77–87. [Google Scholar] [CrossRef]
- Nobel, P.S.; Zaragoza, L.J.; Smith, W.K. Relation between mesophyll surface area, photosynthetic rate, and illumination level during development for leaves of Plectranthus parviflorus Henckel. Plant Physiol. 1975, 55, 1067–1070. [Google Scholar] [CrossRef]
- Terashima, I.; Miyazawa, S.-I.; Hanba, Y.T. Why are sun leaves thicker than shade leaves?—Consideration based on analyses of CO2 diffusion in the leaf. J. Plant Res. 2001, 114, 93–105. [Google Scholar] [CrossRef]
- Evans, J.R. Leaf anatomy enables more equal access to light and CO2 between chloroplasts. New Phytol. 1999, 143, 93–104. [Google Scholar] [CrossRef]
- Adams, W.W., III; Terashima, I. (Eds.) The Leaf: A Platform for Performing Photosynthesis; Advances in photosynthesis and respiration; Springer International Publishing: New York, NY, USA, 2018; ISBN 978-3-319-93592-8. [Google Scholar]
- Evans, J.R.; von Caemmerer, S. Carbon dioxide diffusion inside leaves. Plant Physiol. 1996, 110, 339–346. [Google Scholar] [CrossRef]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef]
- Manter, D.K.; Kerrigan, J. A/C(i) curve analysis across a range of woody plant species: Influence of regression analysis parameters and mesophyll conductance. J. Exp. Bot. 2004, 55, 2581–2588. [Google Scholar] [CrossRef]
- Field, C.B.; Mooney, H.A. The photosynthesis-nitrogen relationship in wild plants. In On the Economy of Plant Form and Function; Givnish, T.J., Ed.; Cambridge University Press: Cambridge, UK, 1986; pp. 25–55. ISBN 978-0-521-02249-1. [Google Scholar]
- Kitaoka, S.; Koike, T. Invasion of broad-leaf tree species into a larch plantation: Seasonal light environment, photosynthesis and nitrogen allocation. Physiol. Plant. 2004, 121, 604–611. [Google Scholar] [CrossRef]
- Koike, T.; Miyashita, N.; Toda, H. Effects of shading on leaf structural characteristics in successional deciduous broadleaved tree seedlings and their silvicultural meaning. For. Resour. Environ. Tokyo Univ. Agric. Technol. 1997, 35, 9–25. [Google Scholar]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Marchi, S.; Tognetti, R.; Minnocci, A.; Borghi, M.; Sebastiani, L. Variation in mesophyll anatomy and photosynthetic capacity during leaf development in a deciduous mesophyte fruit tree (Prunus persica) and an evergreen sclerophyllous Mediterranean shrub (Olea europaea). Trees 2008, 22, 559. [Google Scholar] [CrossRef]
- Hikosaka, K.; Nagamatsu, D.; Ishii, H.S.; Hirose, T. Photosynthesis-nitrogen relationships in species at different altitudes on Mount Kinabalu, Malaysia. Ecol. Res. 2002, 17, 305–313. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Kloeppel, B.D.; Ellsworth, D.S. Different photosynthesis-nitrogen relations in deciduous hardwood and evergreen coniferous tree species. Oecologia 1995, 104, 24–30. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Tenhunen, J.D. A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant Cell Environ. 1997, 20, 845–866. [Google Scholar] [CrossRef]
- Niinemets, Ü. Stomatal conductance alone does not explain the decline in foliar photosynthetic rates with increasing tree age and size in Picea abies and Pinus sylvestris. Tree Physiol. 2002, 22, 515–535. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü. Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 2001, 82, 453–469. [Google Scholar] [CrossRef]
- Hikosaka, K.; Hanba, Y.T.; Hirose, T.; Terashima, I. Photosynthetic nitrogen-use efficiency in leaves of woody and herbaceous species. Funct. Ecol. 1998, 12, 896–905. [Google Scholar] [CrossRef]
- Kenzo, T.; Ichie, T.; Yoneda, R.; Kitahashi, Y.; Watanabe, Y.; Ninomiya, I.; Koike, T. Interspecific variation of photosynthesis and leaf characteristics in canopy trees of five species of Dipterocarpaceae in a tropical rain forest. Tree Physiol. 2004, 24, 1187–1192. [Google Scholar] [CrossRef]
- Onoda, Y.; Hirose, T.; Hikosaka, K. Effect of elevated CO2 levels on leaf starch, nitrogen and photosynthesis of plants growing at three natural CO2 springs in Japan. Ecol. Res. 2007, 22, 475–484. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Watanabe, M.; Watanabe, Y.; Kitaoka, S.; Utsugi, H.; Kita, K.; Koike, T. Growth and photosynthetic traits of hybrid larch F1 (Larix gmelinii var. japonica x L. kaempferi) under elevated CO2 concentration with low nutrient availability. Tree Physiol. 2011, 31, 965–975. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Kedrowski, R.A. Seasonal changes in nitrogen and phosphorus fractions and autumn retranslocation in evergreen and deciduous taiga trees. Ecology 1983, 64, 376–391. [Google Scholar] [CrossRef]
- Herrick, J.D.; Thomas, R.B. Leaf senescence and late-season net photosynthesis of sun and shade leaves of overstory sweetgum (Liquidambar styraciflua) grown in elevated and ambient carbon dioxide concentrations. Tree Physiol. 2003, 23, 109–118. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).