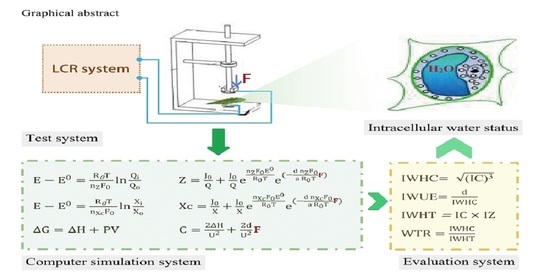
A Plant’s Electrical Parameters Indicate Its Physiological State: A Study of Intracellular Water Metabolism
Abstract
1. Introduction
2. Theories
2.1. Intrinsic Mechanism Relationships of Clamping Force (F) and Leaf Z, Xc, and C
2.2. Intracellular Water Utilization Parameters
3. Results
3.1. Intracellular Water Utilization of B. Papyrifera Grown in Two Habitats
3.2. Intracellular Water Utilization of Herbaceous and Woody Plants
3.3. Intracellular Water Utilization of Solanum Tuberosum and Capsicum Annuum
4. Discussion
5. Materials and Methods
5.1. Plant Materials
5.2. Lea f Z, Xc, and C Measurement
5.3. Data Analyses
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| C | capacitance |
| Z | impedance |
| Xc | capacitive reactance |
| F | clamping force |
| IC | intrinsic capacitance |
| IZ | intrinsic impedance |
| IXc | intrinsic capacitive reactance, d: specific effective thickness |
| IWHC | intracellular water-holding capacity, |
| IWUE | intracellular water-use efficiency |
| IWHT | intracellular water-holding time |
| WTR | water transfer rate |
References
- Hopkins, W.G.; Huner, N.P.A. Introduction to Plant Physiology, 3rd ed.; John Wiley & Sons Inc.: New York, NY, USA, 2004; p. 27. [Google Scholar]
- Yan, X.; Wang, Z.; Huang, L.; Wang, C.; Hou, R.; Xu, Z.; Qiao, X. Research progress on electrical signals in higher plants. Progr. Nat. Sci. Mater. Int. 2009, 19, 531–541. [Google Scholar] [CrossRef]
- Choi, W.G.; Hilleary, R.; Swanson, S.J.; Kim, S.H.; Gilroy, S. Rapid, long-distance electrical and calcium signaling in plants. Annu. Rev. Plant. Biol. 2016, 67, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Gil, P.M.; Gurovich, L.; Schaffer, B.; Alcayaga, J.; Rey, S.; Iturriaga, R. Root to leaf electrical signaling in avocado in response to light and soil water content. J. Plant. Physiol. 2008, 165, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Hedrich, R.; Salvador-Recatala, V.; Dreyer, I. Electrical wiring and long-distance plant communication. Trends. Plant. Sci. 2016, 21, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Favre, P.; Greppin, H.; Agosti, R.D. Accession-dependent action potentials in Arabidopsis. J. Plant. Physiol. 2011, 168, 653–660. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Kurenda, A.; Stolz, S.; Chetelat, A.; Farmer, E.E. Identification of cell populations necessary for leaf-to-leaf electrical signaling in a wounded plant. Proc. Natl. Acad. Sci. USA 2018, 115, 10178–10183. [Google Scholar] [CrossRef]
- Gallé, A.; Lautner, S.; Flexas, J.; Fromm, J. Environmental stimuli and physiological responses: The current view on electrical signaling. Environ. Exp. Bot. 2015, 114, 15–21. [Google Scholar] [CrossRef]
- Volkov, A.G. Plant Electrophysiology: Theory and Methods; Springer: Berlin/Heidelberg, Germany, 2006; p. 25. [Google Scholar]
- Wang, Z.Y.; Qin, X.H.; Li, J.H.; Fan, L.F.; Zhou, Q.; Wang, Y.Q.; Zhao, X.; Xie, C.J.; Wang, Z.Y.; Huang, L. Highly reproducible periodic electrical potential changes associated with salt tolerance in wheat plants. Environ. Exp. Bot. 2019, 160, 120–130. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, D.J.; Wang, Z.Y.; Wang, Z.Y.; Tang, G.; Huang, L. Plant electrical signal classification based on waveform similarity. Algorithms 2016, 9, 70. [Google Scholar] [CrossRef]
- Zhao, D.J.; Wang, Z.Y.; Li, J.; Wen, X.; Liu, A.; Wang, X.D.; Hou, R.F.; Wang, C.; Huang, L. Recording extracellular signals in plants: A modeling and experimental study. Math. Comput. Model. 2013, 58, 556–563. [Google Scholar] [CrossRef]
- Harker, F.R.; Dunlop, J. Electrical impedance studies of nectarines during coolstorage and fruit ripening. Postharvest Biol. Tec. 1994, 4, 125–134. [Google Scholar] [CrossRef]
- Kertész, Á.; Hlaváčová, Z.; Vozáry, E.; Staroňová, L. Relationship between moisture content and electrical impedance of carrot slices during drying. Int. Agrophys. 2015, 29, 61–66. [Google Scholar] [CrossRef]
- Ibba, P.; Falco, A.; Abera, B.D.; Cantarella, G.; Petti, L.; Lugli, P. Bio-impedance and circuit parameters: An analysis for tracking fruit ripening. Postharvest Biol. Tec. 2020, 159, 110978. [Google Scholar] [CrossRef]
- Zhang, M.M.; Wu, Y.Y.; Xing, D.K.; Zhao, K.; Yu, R. Rapid measurement of drought resistance in plants based on electrophysiological properties. Trans. ASABE 2015, 58, 1441–1446. [Google Scholar] [CrossRef]
- Xing, D.K.; Xu, X.J.; Wu, Y.Y.; Liu, Y.J.; Wu, Y.S.; Ni, J.H.; Azeem, A. Leaf tensity: A method for rapid determination of water requirement in formation in Brassica napus L. J. Plant. Interact. 2018, 13, 380–387. [Google Scholar] [CrossRef]
- Javed, Q.; Wu, Y.Y.; Xing, D.K.; Azeem, A.; Ullah, I.; Zaman, M. Re-watering: An effective measure to recover growth and photosynthetic characteristics in salt-stressed Brassica napus L. Chil. J. Agric. Res. 2017, 77, 78–86. [Google Scholar] [CrossRef]
- Buckley, D.J.; Lefebvre, M.; Meijer, E.G.M.; Brown, D.C.W. A signal generator for electrofusion of plant protoplasts. Comput. Electron. Agric. 1990, 5, 179–185. [Google Scholar] [CrossRef]
- Lodish, H.; Berk, A.; Kaiser, C.A.; Krieger, M. Molecular Cell Biology, 5th ed.; Freeman and Company: New York, NY, USA, 2004; pp. 35–41. [Google Scholar]
- Beilby, M.J.; Coster, H.G.L. The action potential in Chara coralline: III. The Hodgkin-Huxley parameters for the plasmalemma. Funct. Plant Biol. 1979, 6, 355–365. [Google Scholar] [CrossRef]
- Philip, N. Biological Physics: Energy, Information Life; Freeman and Company: New York, NY, USA, 2003; pp. 413–448. [Google Scholar]
- Guo, W.C.; Liu, D.X.; Zhou, C.C.; Han, W.T. Non-destructive moisture detector for plant leaves based on capacitance. Trans. Chin. Soc. Agric. Mach. 2014, 45, 287–293. (In Chinese) [Google Scholar] [CrossRef]
- De Jong, S.M.; Addink, E.A.; Doelman, J.C. Detecting leaf-water content in Mediterranean trees using high-resolution spectrometry. Int. J. Appl. Earth. Obs. 2014, 27, 128–136. [Google Scholar] [CrossRef]
- De Jong, S.M.; Addink, E.A.P.; Hoogenboom Nijland, W. The spectral response of Buxus sempervirens to different types of environmental stress, a laboratory experiment. ISPRS J. Photogramm. 2012, 74, 56–65. [Google Scholar] [CrossRef]
- González-Fernández, A.B.; Rodríguez-Pérez, J.R.; Marcelo, V.; Valenciano, J.B. Using field spectrometry and a plant probe accessory to determine leaf water content in commercial vineyards. Agric. Water. Manag. 2015, 156, 43–50. [Google Scholar] [CrossRef]
- Kahmen, A.; Simonin, K.; Tu, K.P.; Merchant, A.; Arndt, S.K. Effects of environmental parameters, leaf physiological properties and leaf water relations on leaf water δ18O enrichment in different eucalyptus species. Plant. Cell. Environ. 2008, 31, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Seelig, H.D.; Hoehn, A.; Stodieck, L.S.; Klaus, D.M.; Adams, W.W.; Emery, W.J. Relations of remote sensing leaf water indices to leaf water thickness in cowpea, bean, and sugarbeet plants. Remote Sens. Environ. 2008, 112, 445–455. [Google Scholar] [CrossRef]
- Drew, D.H. Mineral nutrition and the water relations of plants. Plant Soil. 1967, 26, 469–480. [Google Scholar] [CrossRef]
- Xing, D.K.; Chen, X.L.; Wu, Y.Y.; Xu, X.J.; Chen, Q.; Li, L.; Zhang, C. Rapid prediction of the re-watering time point of Orychophragmus violaceus L. based on the online monitoring of electrophysiological indexes. Sci. Hortic. 2019, 256, 108642. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Xing, D.K.; Hang, H.T.; Zhao, K. Principles and Technology of Determination on Plant’ Adaptation to Karst Environment; Science Press: Beijing, China, 2019; p. 191. [Google Scholar]
- Ma, Z.; Guo, D.; Xu, X.; Lu, M.; Bardgett, R.D.; Eissenstat, D.M.; McCormack, M.L.; Hedin, L.O. Evolutionary history resolves global organization of root functional traits. Nature 2018, 555, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Huang, K.; Hu, S. Distinct fine-root responses to precipitation changes in herbaceous and woody plants: A meta-analysis. New Phytol. 2020, 225, 1491–1499. [Google Scholar] [CrossRef]
- Chatterjee, C.; Gopal, R.; Dube, B.K. Impact of iron stress on biomass, yield, metabolism and quality of potato (Solanum tuberosum L.). Sci. Hortic. 2006, 108, 1–6. [Google Scholar] [CrossRef]
- Thapliyal, A.; Vasudevan, P.; Dastidar, M.G.; Mamta, T.; Mishra, S. Effects of irrigation with domestic wastewater on productivity of green chili and soil status. Commun. Soil. Sci. Plant. 2013, 44, 1–17. [Google Scholar] [CrossRef]
- Fromm, J.; Fei, H. Electrical signaling and gas exchange in maize plants of drying soil. Plant. Sci. 1998, 132, 203–213. [Google Scholar] [CrossRef]
- Jin, S.D.; Zhang, S.F.; Zheng, R.L. Diagnosis of drought from maize physiological electrical characteristics. Trans. CSAE 1999, 15, 91–95. (In Chinese) [Google Scholar]
- Hamid, A.; Agata, W.; Kawamitsu, Y. Photosynthesis, transpiration and water use efficiency in four cultivars of mungbean, Vigna radiata (L.) Wilczek. Photosynthetica 1990, 24, 96–101. [Google Scholar]





| Habitats | IZ (MΩ) | IXc (MΩ) | IC (pF) | d | IWUE | IWHT | WTR |
|---|---|---|---|---|---|---|---|
| AS-B | 0.50 ± 0.34 b | 1.13 ± 0.97 b | 99.48 ± 76.61 a | 67.60 ± 50.36 a | 0.12 ± 0.11 a | 27.22 ± 6.71 b | 40.80 ± 33.63 a |
| MRDS-B | 12.98 ± 10.75 a | 7.41 ± 0.83 a | 7.23 ± 0.70 b | 1.39 ± 0.77 b | 0.08 ± 0.06 a | 92.63 ± 84.12 a | 0.31 ± 0.13 b |
| Plants | IZ (MΩ) | IXc (MΩ) | IC (pF) | d | IWUE | IWHT | WTR |
|---|---|---|---|---|---|---|---|
| W-A | 4.26 ± 1.53 b | 4.36 ± 1.50 b | 13.22 ± 3.65 d | 5.15 ± 1.43 e | 0.12 ± 0.06 c | 51.67 ± 1.10 a | 0.96 ± 0.40 d |
| W-R | 3.27 ± 0.93 c | 3.54 ± 0.82 c | 15.63 ± 3.18 c | 9.54 ± 2.30 c | 0.16 ± 0.06 bc | 48.49 ± 4.67 a | 1.33 ± 0.50 cd |
| W-G | 5.17 ± 1.14 a | 5.25 ± 1.08 a | 10.50 ± 2.11 e | 3.14 ± 0.94 f | 0.10 ± 0.04 c | 52.09 ± 1.62 a | 0.67 ± 0.21 d |
| H-I | 1.85 ± 0.44 e | 1.92 ± 0.50 e | 29.59 ± 8.62 a | 33.83 ± 14.89 a | 0.23 ± 0.13 b | 52.11 ± 9.86 a | 3.14 ± 1.07 a |
| H-S | 2.55 ± 0.89 d | 2.65 ± 1.00 d | 22.28 ± 7.12 b | 27.97 ± 8.12 b | 0.33 ± 0.21 a | 51.26 ± 1.71 a | 2.12 ± 1.00 bc |
| H-B | 2.82 ± 1.81 d | 3.31 ± 1.68 c | 19.68 ± 9.57 c | 6.00 ± 2.56 d | 0.09 ± 0.06 c | 43.79 ± 9.43 b | 2.38 ± 2.17 ab |
| Plants | IZ (MΩ) | IXc (MΩ) | IC (pF) | d | IWHC | IWUE | IWHT | WTR |
|---|---|---|---|---|---|---|---|---|
| S. tuberosum | 0.24 ± 0.10 b | 0.37 ± 0.21 b | 163.88 ± 49.86 a | 307.88 ± 94.13 a | 2164.13 ± 847.71 a | 0.19 ± 0.16 b | 34.99 ± 5.76 b | 62.23 ± 27.37 a |
| C. annuum | 4.03 ± 1.44 a | 4.12 ± 1.41 a | 14.26 ± 4.92 b | 72.29 ± 11.87 b | 55.84 ± 28.03 b | 1.69 ± 1.04 a | 51.70 ± 0.84 a | 1.09 ± 0.56 b |
| Plants | Places | Age (Years) | Habitats | Soil Properties | Measurement Conditions | Sampling Weather | |||
|---|---|---|---|---|---|---|---|---|---|
| pH | Organic Matter Content (g/kg) | Soil Moisture Content (%) | Time | Temperature (°C) | |||||
| B. papyrifera 1 | Puding County | 1 | AS | 6.27 ± 0.03 | 4.35 ± 0.65 | 18.46 ± 0.02 | 2018.08.25 a.m. | 25.0 ± 2.0 | Sunny |
| B. papyrifera 2 | 1 | MRDS | 6.85 ± 0.03 | 3.58 ± 0.33 | 15.51 ± 0.02 | ||||
| A. persica | 3 | MRDS | 6.74 ± 0.02 | 3.63 ± 0.15 | 15.66 ± 0.09 | 2018.08.24 a.m. | |||
| R. chinensis | 3 | MRDS | 6.81 ± 0.03 | 3.64 ± 0.27 | 16.13 ± 0.15 | ||||
| G. biloba | 3 | MRDS | 6.75 ± 0.04 | 3.57 ± 0.31 | 15.82 ± 0.12 | 2018.08.26 a.m. | |||
| I. batatas | 1 | AS | 6.31 ± 0.01 | 4.63 ± 0.21 | 18.53 ± 0.42 | ||||
| S. scandens | 1 | AS | 6.44 ± 0.02 | 4.98 ± 0.34 | 19.17 ± 0.21 | 2018.08.27 a.m. | |||
| B. penduliflora | 1 | AS | 6.47 ± 0.05 | 4.75 ± 0.36 | 19.21 ± 0.32 | ||||
| S. tuberosum | Qingzhen County | 1 | PAS | 6.32 ± 0.05 | 4.82 ± 0.53 | 19.65 ± 0.21 | 2018.08.15 a.m. | ||
| C. annuum | 1 | PAS | 6.34 ± 0.07 | 4.86 ± 0.31 | 19.72 ± 0.13 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Wu, Y.; Su, Y.; Xing, D.; Dai, Y.; Wu, Y.; Fang, L. A Plant’s Electrical Parameters Indicate Its Physiological State: A Study of Intracellular Water Metabolism. Plants 2020, 9, 1256. https://doi.org/10.3390/plants9101256
Zhang C, Wu Y, Su Y, Xing D, Dai Y, Wu Y, Fang L. A Plant’s Electrical Parameters Indicate Its Physiological State: A Study of Intracellular Water Metabolism. Plants. 2020; 9(10):1256. https://doi.org/10.3390/plants9101256
Chicago/Turabian StyleZhang, Cheng, Yanyou Wu, Yue Su, Deke Xing, Yi Dai, Yansheng Wu, and Lei Fang. 2020. "A Plant’s Electrical Parameters Indicate Its Physiological State: A Study of Intracellular Water Metabolism" Plants 9, no. 10: 1256. https://doi.org/10.3390/plants9101256
APA StyleZhang, C., Wu, Y., Su, Y., Xing, D., Dai, Y., Wu, Y., & Fang, L. (2020). A Plant’s Electrical Parameters Indicate Its Physiological State: A Study of Intracellular Water Metabolism. Plants, 9(10), 1256. https://doi.org/10.3390/plants9101256