Effects of Arbuscular Mycorrhizal Fungi on Yield, Biochemical Characteristics, and Elemental Composition of Garlic and Onion under Selenium Supply
Abstract
1. Introduction
2. Results and Discussion
2.1. Root Mycorrhizal Colonization, Bulb Yield, and Dry Matter
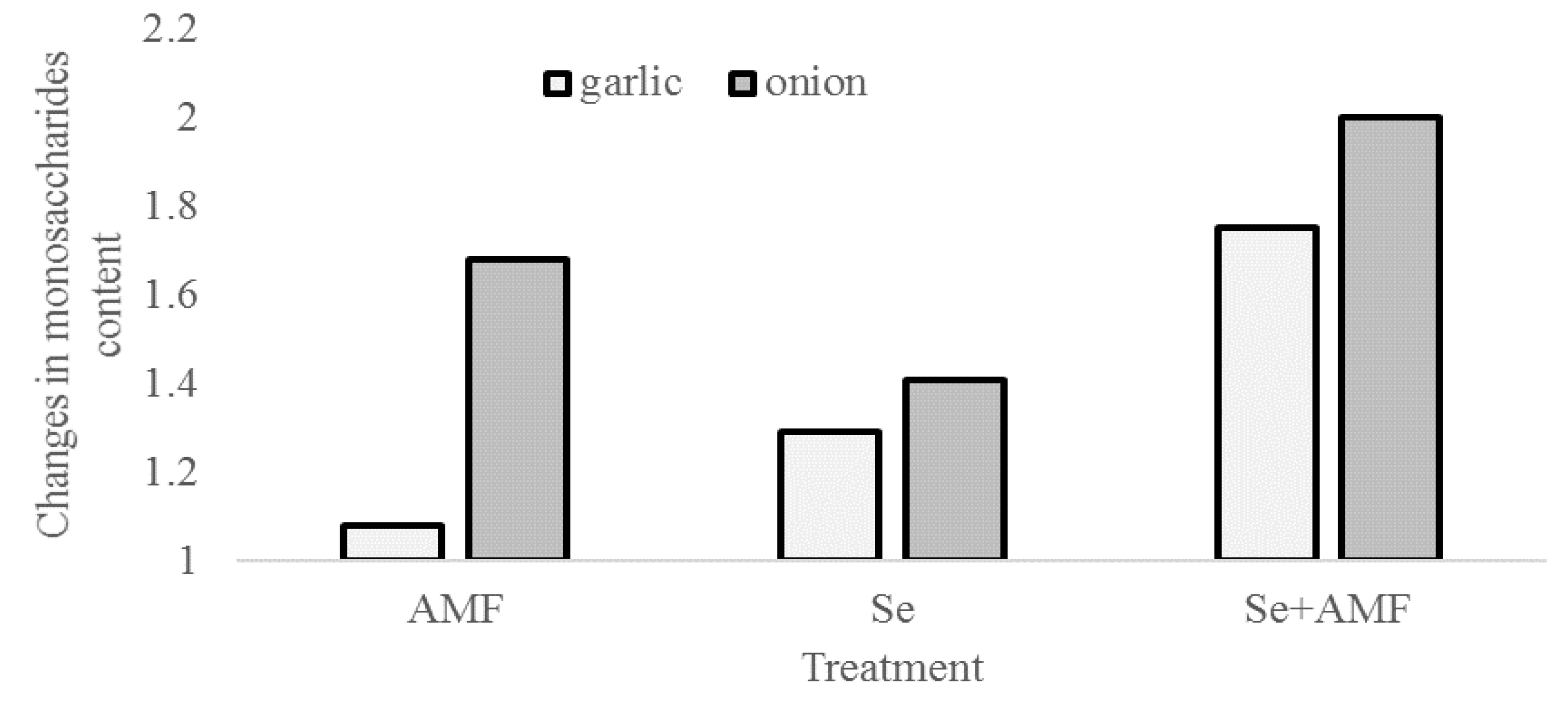
2.2. Carbohydrates and Organic Acids Content
2.3. Antioxidants
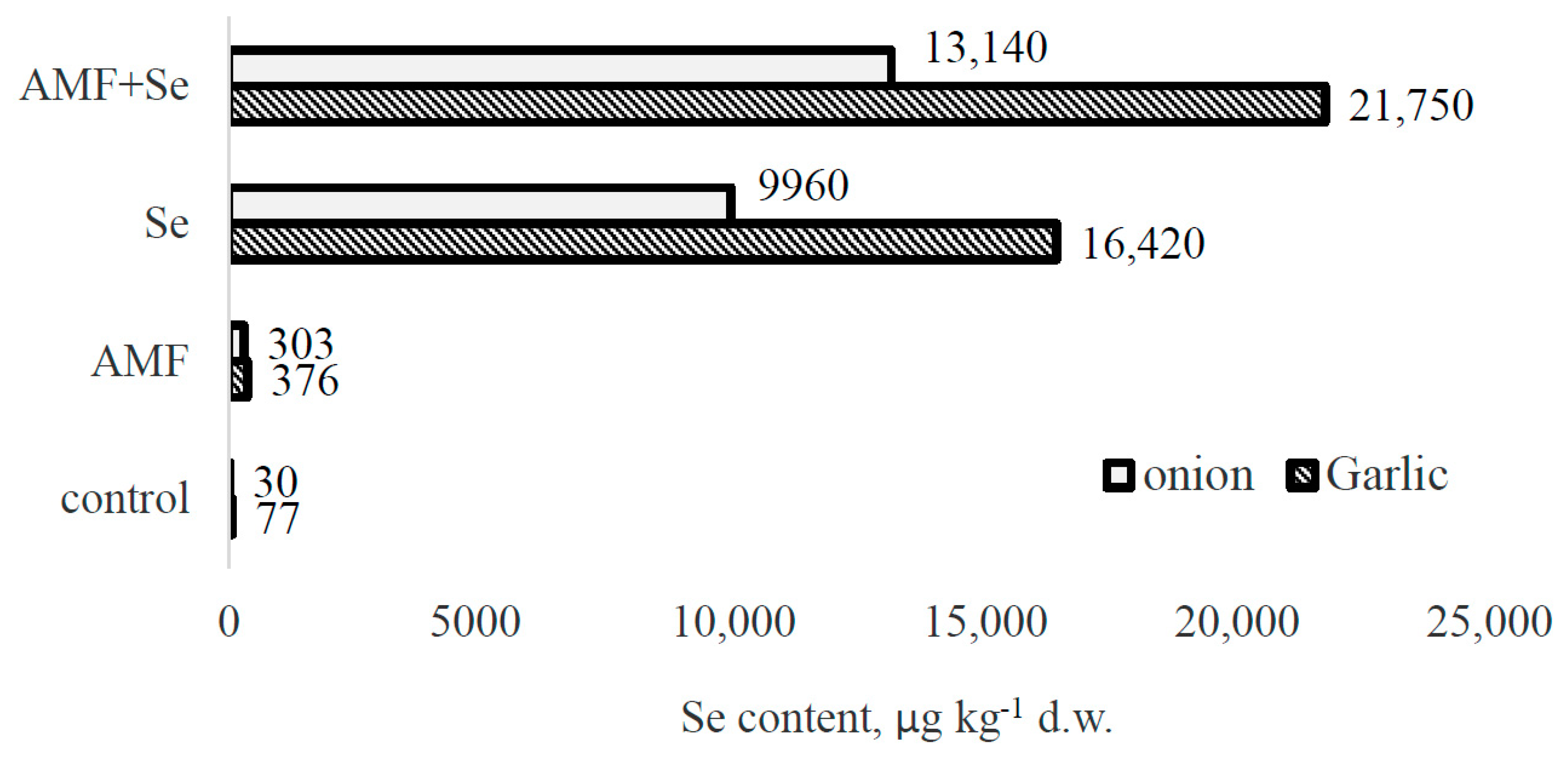
2.4. Selenium Accumulation
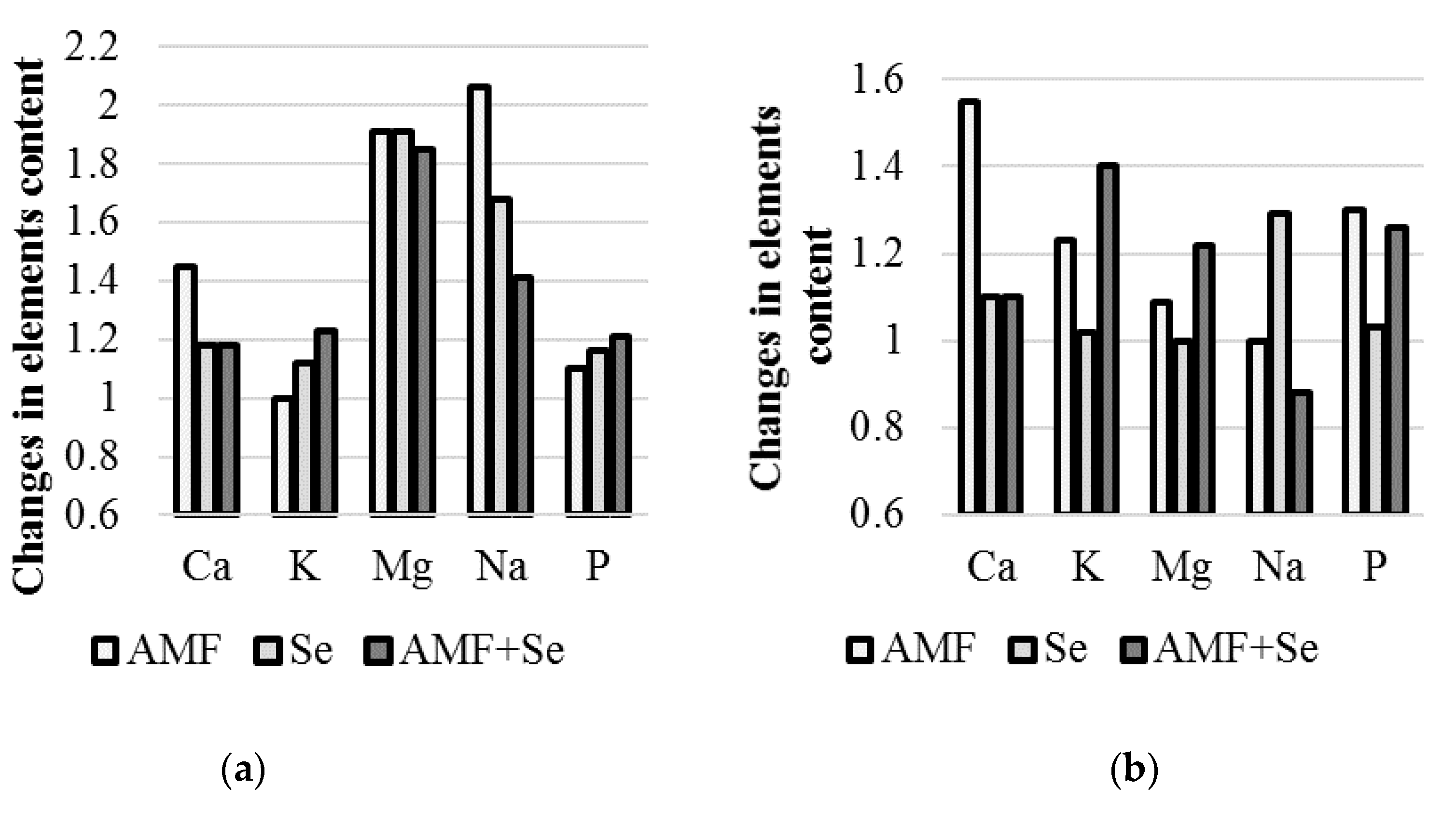
2.5. Macroelements
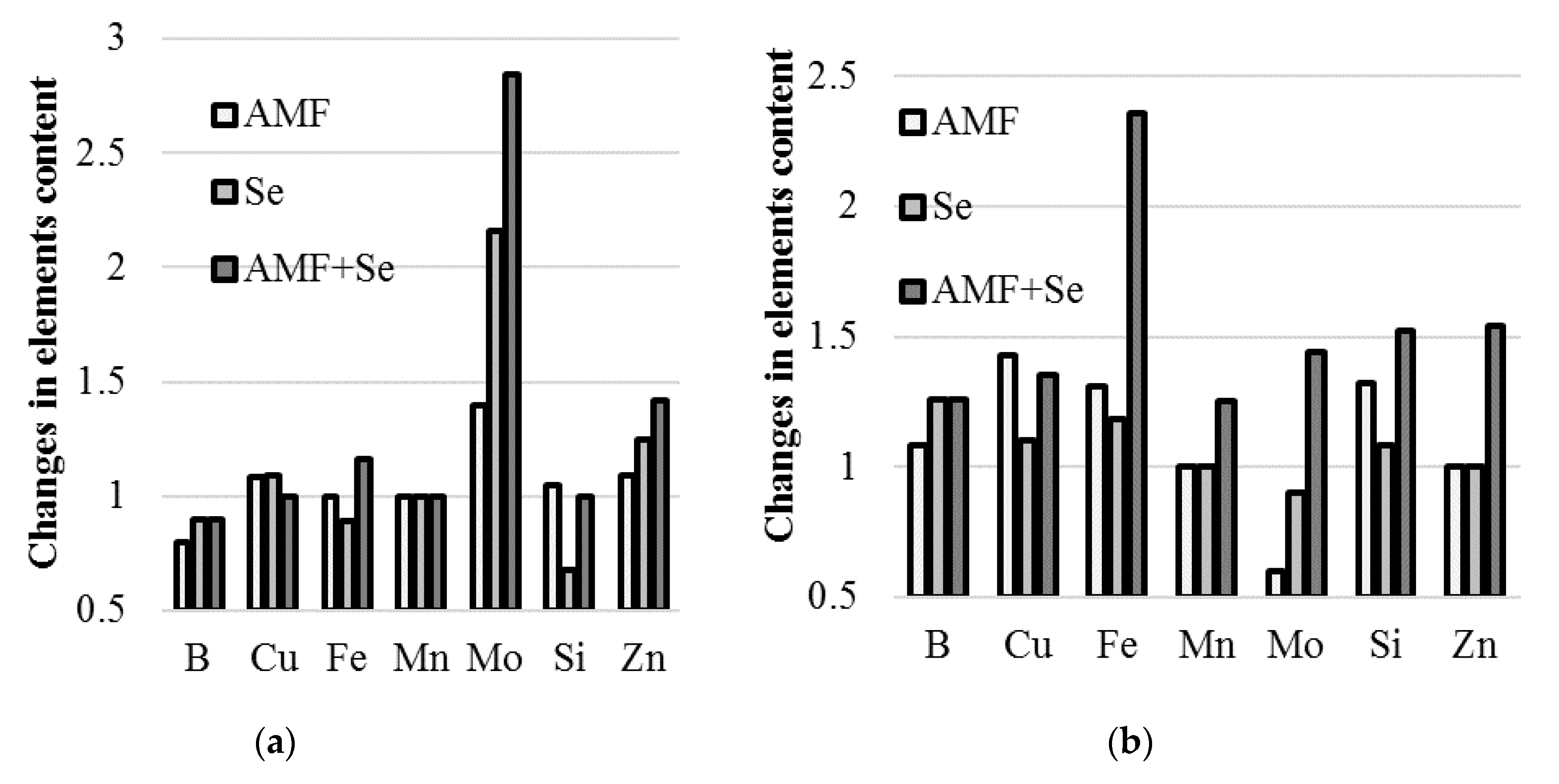
2.6. Microelements
3. Material and Methods
3.1. Plant Material and Experimental Conditions
3.2. Sample Preparation
3.3. Antioxidants
3.3.1. Ascorbic Acid
3.3.2. Polyphenols
3.3.3. Flavonoids
3.3.4. Antioxidant Activity (AOA)
3.4. Nitrates
3.5. Sugars
3.6. Selenium
3.7. Elemental Composition
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zaburaeva, K.S. Geoecological problems of land management in the Chechen Republic. Vestn. Krasn. Agrar. Univ. 2012, 5, 196–200. [Google Scholar]
- Bhattacharya, A.; Toth, K.; Sen, A. Inhibition of colon cancer growth by methylselenocysteine-induced angiogenic chemomodulation is influenced by histologic characteristics of the tumor. Clin. Colorectal Cancer 2009, 8, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, E.A.H. On the ecology of selenium accumulation in plants. Plants 2019, 8, 197. [Google Scholar] [CrossRef] [PubMed]
- Golubkina, N.A.; Papazyan, T.T. Selenium in nutrition. Plants, animals, human beings; Pechatny Gorod: Moscow, Russia, 2006. [Google Scholar]
- White, P.J.; Broadley, M.R. Biofortifying crops with essential mineral elements. Trends Plant Sci. 2005, 10, 586–593. [Google Scholar] [CrossRef]
- Colella, T.; Candido, V.; Campanelli, G.; Camele, I.; Battaglia, D. Effect of irrigation regimes and artificial mycorrhization on insect pest infestations and yield in tomato crop. Phytoparasitica 2014, 42, 235–246. [Google Scholar] [CrossRef]
- Chen, M.; Arato, M.; Borghi, L.; Nouri, E.; Reinhardt, D. Beneficial services of AMF- from ecology to application. Front. Plant Sci. 2018, 9, 1270. [Google Scholar] [CrossRef]
- Caruso, G.; Golubkina, N.; Seredin, T.; Selitto, B. Utilization of AMF in production of Allium species. Veg. Crop. Russ. 2018, 13, 85–90. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, H.; Zou, C.; Li, Y.; Chen, Y.; Wang, Z.; Jiang, Y.; Liu, A.; Zhao, P.; Wang, M.; et al. Combined inoculation with multiple arbuscular mycorrhizal fungi improves growth, nutrient uptake and photosynthesis in cucumber seedlings. Front. Microbiol. 2017, 8, 2516. [Google Scholar] [CrossRef]
- Larsen, E.H.; Lobinski, R.; Burger-Meÿer, K.; Hansen, M.; Ruzik, R.; Mazurowska, L.; Rasmussen, P.H.; Sloth, J.J.; Scholten, O.; Kik, C. Uptake and speciation of selenium in garlic cultivated in soil amended with symbiotic fungi (mycorrhiza) and selenate. Anal. Bioanal. Chem. 2006, 385, 1098–1108. [Google Scholar] [CrossRef]
- Patharajan, S.; Raaman, N. Influence of arbuscular mycorrhizal fungi on growth and selenium uptake by garlic plants. Arch. Phytopathol. Plant Prot. 2012, 45, 138–151. [Google Scholar] [CrossRef]
- Durán, P.; Acuña, J.J.; Jorquera, M.A.; Azcón, R.; Borie, F.; Cornejo, P.; Mora, M.L. Enhanced selenium content in wheat grain by co-inoculation of selenobacteria and arbuscular mycorrhizal fungi: A preliminary study as a potential selenium biofortification strategy. J. Cereal Sci. 2013, 57, 227–280. [Google Scholar] [CrossRef]
- Golubkina, N.; Zamana, S.; Seredin, T.; Poluboyarinov, P.; Sokolov, S.; Baranova, H.; Krivenkov, L.; Pietrantonio, L.; Caruso, G. Effect of selenium biofortification and arbuscular mycorrhizal fungi on yield, quality and antioxidant properties of shallot bulbs. Plants 2019, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, C.; Garmendia, I.; Romano, B.; Díaz, M.; Palop, J.A.; Goicoechea, N. Mycorrhizal inoculation affected growth, mineral composition, proteins and sugars in lettuces biofortified with organic or inorganic selenocompounds. Sci. Hortic. 2014, 180, 40–51. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, S.; Wen, B.; Huang, H.; Luo, L. Accumulation and speciation of selenium in plants as affected by arbuscular mycorrhizal fungus Glomus Mosseae. Biol. Trace Elem. Res. 2011, 143, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Bolandnazar, S.; Neishabury, M.R.; Aliasgharzad, N.; Chaparzadeh, N. Effects of mycorrhizal colonization on growth parameters of onion under different irrigation and soil conditions. Pak. J. Biol. Sci. 2007, 10, 1491–1495. [Google Scholar] [CrossRef]
- Bolandnazar, S.; Aliasgharzad, N.; Neishabury, M.R.; Chaparzadeh, N. Mycorrhizal colonization improves onion (Allium cepa L.) yield and water use efficiency under water deficit condition. Sci. Hortic. 2007, 114, 11–15. [Google Scholar] [CrossRef]
- Bolandnazar, S. The effect of mycorrhizal fungi on onion (Allium cepa L.) growth and yield under three irrigation intervals at field condition. J. Food Agric. Environ 2009, 7, 360–362. [Google Scholar]
- Martin, C.A.; Stutz, J.C. Interactive effects of temperature and arbuscular mycorrhizal fungi on growth, P uptake and root respiration of Capsicum annuum L. Mycorrhiza 2004, 4, 241–244. [Google Scholar] [CrossRef]
- Zhu, X.C.; Song, F.B.; Xu, H.W. Influence of arbuscular mycorrhizae on lipid peroxidation and antioxidant enzyme activity of maize plants under temperature stress. Mycorrhiza 2010, 20, 325–332. [Google Scholar] [CrossRef]
- Shuab, R.; Lone, R.; Naidu, J.; Sharma, V.; Imtiyaz, S.; Koul, K.K. Benefits of inoculation of arbuscular mycorrhizal fungi on growth and development of onion (Allium cepa) plant. American-Eurasian. J. Agric. Environ. Sci. 2014, 14, 527–535. [Google Scholar] [CrossRef]
- Bona, E.; Cantamessa, S.; Massa, N.; Manassero, P.; Marsano, F.; Copetta, A.; Lingua, G.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads improve yield, quality and nutritional value of tomato: A field study. Mycorrhiza 2016, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Ann. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [PubMed]
- Abdel, F.G.; Mohamedin, A.H. Interactions between a vesicular-arbuscular mycorrhizal fungus and Streptomyces and their effects on sorghum plants. Biol. Fertil. Soils. 2000, 32, 401–409. [Google Scholar] [CrossRef]
- Franco, D.A.; Cano, G.I. Arbuscular mycorrhizal colonization and growth of buffel grass (Cenchrus ciliaris) genotypes. Rev. Fitotec. 2006, 29, 203–206. [Google Scholar]
- Zuccarini, P. Mycorrhizal infection ameliorates chlorophyll content and nutrient uptake of lettuce exposed to saline irrigation. Plant Soil Environ. 2007, 53, 283–289. [Google Scholar] [CrossRef]
- Regvar, M.; Vogel-Mikuš, K.; Ševerkar, T. Effect of AMF inoculum from field isolates on the yield of green pepper, parsley, carrot, and tomato. Folia Geobot. 2003, 38, 223. [Google Scholar] [CrossRef]
- Rozpądek, P.; Rąpała-Kozik, M.; Węzowicz, K.; Karlsson, A.G.S.; Wązny, R.; Anielska, T.; Turnau, K. Arbuscular mycorrhiza improves yield and nutritional properties of onion (Allium cepa). Plant Physiol. Biochem. 2016, 107, 264–272. [Google Scholar] [CrossRef]
- Albrechtova, J.; Latr, A.; Nedorost, L.; Pokluda, R.; Posta, K.; Vosatka, M. Dual inoculation with mycorrhizal and saprotrophic fungi applicable in sustainable cultivation improves the yield and nutritive value of onion. Sci. World J. 2012, 2012, 374091. [Google Scholar] [CrossRef]
- Mollavali, M.; Perner, H.; Rohn, S.; Riehle, P.; Hanschen, F.S.; Schwarz, D. Nitrogen form and mycorrhizal inoculation amount and timing affect flavonol biosynthesis in onion (Allium cepa L). Mycorrhiza 2018, 28, 59–70. [Google Scholar] [CrossRef]
- Bloem, E.; Haneklaus, S.; Schnug, E. Influence of nitrogen and sulfur fertilization on the alliin content of onions and garlic. J. Plant Nutr. 2004, 27, 1827–1839. [Google Scholar] [CrossRef]
- Ip, C.; Lisk, D.J. Enrichment of selenium in Allium vegetables for cancer prevention. Carcinogenesis 1994, 15, 1881–1885. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.; Eweda, W.E.E.; Heggo, A.M.; Hassan, E.A. Effect of dual inoculation with arbuscular mycorrhizal fungi and sulphur-oxidizing bacteria on onion (Allium cepa L.) and maize (Zea mays L.) grown in sandy soil under green house conditions. Ann. Agric. Sci. 2014, 59, 109–118. [Google Scholar] [CrossRef]
- Nori, M.; Aali, J.; Shafizi, R. Effect of different sources and levels of nitrogen fertilizer on yield and nitrate accumulation in garlic (Allium sativum L). Int. J. Agric. Crop Sci. 2012, 4, 1878–1880. [Google Scholar]
- Kucova, L. Effect of mycorrhizal inoculation of leek A. porrum L on mineral nitrogen leaching. Hortic. Sci. 2016, 43, 195–202. [Google Scholar] [CrossRef]
- Carling, D.E.; Riehle, W.G.; Brown, M.F.; Johnson, D.R. Physiology and biochemistry effects of a vesicular-arbuscular mycorrhizal fungus on nitrate reductase and nitrogenase activities in nodulating and non-nodulating soybeans. Phytopathology 1978, 68, 1590–1596. [Google Scholar] [CrossRef]
- Shannon, M.C.; Griev, C.M. Tolerance of vegetable crops to salinity. Sci. Hortic. 1999, 78, 5–38. [Google Scholar] [CrossRef]
- Golubkina, N.A.; Kekina, H.; Caruso, G. Yield, Quality and antioxidant properties of Indian mustard (Brassica juncea L.) in response to foliar biofortification with selenium and iodine. Plants 2018, 7, 80. [Google Scholar] [CrossRef]
- Shireen, F.; Nawaz, M.A.; Chen, C.; Zhang, Q.; Zheng, Z.; Sohail, H.; Sun, J.; Cao, H.; Huang, Y.; Bie, Z. Boron: Functions and approaches to enhance its availability in plants for sustainable agriculture. Int. J. Mol. Sci. 2018, 19, 1856. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, J.; Wang, F.; Li, K.; Yuan, W.; Liu, J. Arbuscular mycorrhizal inoculation increases molybdenum accumulation but decreases molybdenum toxicity in maize plants grown in polluted soil. RSC Adv. 2018, 8, 37069–37076. [Google Scholar] [CrossRef]
- Caruso, G.; Conti, S.; Villari, G.; Borrelli, C.; Minutolo, M.; Russo, G.; Amalfitano, C. Effects of transplanting time and plant density on yield, quality and antioxidant content of onion (Allium cepa L.) in southern Italy. Sci. Hortic. 2014, 166, 111–120. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular-arbuscular mycorrhiza in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- AOAC Association Official Analytical Chemists. The Official Methods of Analysis of AOAC International; 22 Vitamin C; AOAC: Rockville, MD, USA, 2012. [Google Scholar]
- Golubkina, N.A.; Kosheleva, O.V.; Krivenkov, L.V.; Dobrutskaya, H.G.; Nadezhkin, S.; Caruso, G. Intersexual differences in plant growth, yield, mineral composition and antioxidants of spinach (Spinacia oleracea L.) as affected by selenium form. Sci. Hortic. 2017, 225, 350–358. [Google Scholar] [CrossRef]
- Da Silva, L.A.L.; Pezzini, B.R.; Soares, L. Spectrophotometric determination of the total flavonoid content in Ocimum basilicum L. (Lamiaceae) leaves. Pharmacogn. Mag. 2015, 11, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Maximova, T.V.; Nikulina, I.N.; Pakhomov, V.P.; Shkarina, H.I.; Chumakova, Z.V.; Arzamastsev, A.P. Method of Antioxidant Activity. Determination. Patent RU 2170930, 20 July 2001. [Google Scholar]
- Srivastava, S.; Adholeya, A.; Conlan, X.A.; Cahill, D.M. Acidic potassium permanganate chemiluminescence for the determination of antioxidant potential in three cultivars of Ocimum basilicum. Plant Foods Hum. Nutr. 2015, 71, 72–80. [Google Scholar] [CrossRef]
- Castronuovo, D.; Russo, D.; Libonati, R.; Faraone, I.; Candido, V.; Picuno, P.; Andrade, P.; Valentao, P.; Milella, L. Influence of shading treatment on yield, morphological traits and phenolic profile of sweet basil (Ocimum basilicum L.). Sci. Hortic. 2019, 254, 91–98. [Google Scholar] [CrossRef]
- Zhan, M.G.; Liu, N.; Liu, H. Determination of the total mass of antioxidant substances and antioxidant capacity per unit mass in serum using redox titration. Bioinorg. Chem. Appl. 2014, 2014, 928595. [Google Scholar] [CrossRef]
- Swamy, P.M. Laboratory Manual on Biotechnology; Rastogi Publications: Meerut, India, 2008; p. 617. [Google Scholar]
- Alfthan, G.V. A micromethod for the determination of selenium in tissues and biological fluids by single-test-tube fluorimetry. Anal. Chim. Acta 1984, 65, 187–194. [Google Scholar] [CrossRef]
- Skalny, A.V.; Lakarova, H.V.; Kuznetsov, V.V.; Skalnaya, M.G. Analytical Methods in Bioelementology; Saint Petersburg-Science: St. Petersburg, Russia, 2009. [Google Scholar]
| Species | Treatment | Root Mycorrhizal Colonization (%) | Yield (t·ha−1) | Mean Bulb Weight (g) | Dry Matter (%) |
|---|---|---|---|---|---|
| Garlic | Control | 21.3 ± 2.2 b | 11.5 ± 1.3 c | 18.4 ± 2.1 c | 34.8 ± 0.1 c |
| AMF | 65.7 ± 3.0 a | 16.1 ± 1.9 b | 25.8 ± 4.3 b | 37.6 ± 0.2 a | |
| Se | 21.5 ± 2.0 b | 11.8 ± 1.8 c | 18.9 ± 3.7 c | 38.0 ± 0.1 a | |
| AMF + Se | 66.4 ± 2.8 a | 17.9 ± 2.2 a | 28.7 ± 4.7 a | 36.1 ± 0.3 b | |
| Onion | Control | 21.1 ± 2.3 b | 22.7 ± 3.0 b | 45.3 ± 7.8 b | 14.3 ± 0.2 b |
| AMF | 68.6 ± 2.7 a | 33.0 ± 3.5 a | 65.9 ± 7.2 a | 17.0 ± 0.4 a | |
| Se | 21.7 ± 2.2 b | 22.8 ± 2.9 b | 45.5 ± 7.6 b | 15.0 ± 0.4 b | |
| AMF + Se | 69.2 ± 3.1 a | 34.1 ± 3.5 a | 68.1 ± 7.0 a | 16.3 ± 0.1 a |
| Species | Treatment | Monosaccharides (% d.w.) | Total Sugars (% d.w.) | Titratable Acidity (mg-eq Citric Acid 100 g−1 d.w.) |
|---|---|---|---|---|
| Garlic | Control | 15.7 ± 1.1 c | 50.9 ± 3.1 c | 4.9 ± 0.2 b |
| AMF | 17.0 ± 1.2 c | 60.9 ± 3.6 ab | 4.3 ± 0.2 c | |
| Se | 20.3 ± 1.3 b | 59.8 ± 3.2 b | 4.5 ± 0.2 bc | |
| AMF + Se | 27.5 ± 1.6 a | 62.0 ± 3.7 a | 5.8 ± 0.3 a | |
| Onion | Control | 3.4 ± 0.3 c | 61.8 ± 2.8 c | 13.4 ± 1.0 b |
| AMF | 5.7 ± 0.5 ab | 68.9 ± 3.1 a | 19.4 ± 1.2 a | |
| Se | 4.8 ± 0.4 b | 63.7 ± 3.5 bc | 17.6 ± 1.2 a | |
| AMF + Se | 6.8 ± 0.5 a | 65.2 ± 3.7 b | 14.9 ± 1.1 b |
| Species | Treatment | AOA (mg-eq GA g−1 d.w.) | Phenolics (mg-eq GA g−1 d.w.) | Flavonoids (mg-eq Q 100 g−1 d.w.) | Ascorbic Acid (mg 100 g−1 d.w.) |
|---|---|---|---|---|---|
| Garlic | Control | 39.0 ± 1.1 b | 5.3 ± 0.4 | 1.1 ± 0.1 | 59.8 ± 1.7 b |
| AMF | 38.8 ± 1.0 b | 4.4 ± 0.4 | 1.1 ± 0.1 | 61.4 ± 1.8 b | |
| Se | 40.5 ± 1.1 ab | 5.2 ± 0.4 | 1.2 ± 0.1 | 62.9 ± 1.8 ab | |
| AMF + Se | 42.2 ± 1.1 a | 5.0 ± 0.3 | 1.3 ± 0.1 | 66.0 ± 2.1 a | |
| n.s. | n.s. | ||||
| Onion | Control | 31.7 ± 0.7 c | 11.5 ± 0.5 b | 3.2 ± 0.1 b | 59.4 ± 1.4 c |
| AMF | 36.4 ± 0.9 b | 15.2 ± 0.6 a | 3.4 ± 0.1 b | 58.0 ± 1.3 c | |
| Se | 33.0 ± 0.7 c | 14.9 ± 0.6 a | 3.2 ± 0.1 b | 66.7 ± 1.5 b | |
| AMF + Se | 43.4 ± 0.6 a | 13.0 ± 0.5 b | 5.4 ± 0.1 a | 73.6 ± 1.5 a |
| Treatment | Bulb Weight | Dry Matter | Monosaccharides | Total Sugars | TA | AA | Fl | PP | AOA |
|---|---|---|---|---|---|---|---|---|---|
| Garlic | |||||||||
| AMF | * | * | |||||||
| Se | * | * | |||||||
| AMF + Se | ** | * | * | * | |||||
| Onion | |||||||||
| AMF | * | * | * | * | * | ||||
| Se | * | * | * | * | |||||
| AMF + Se | ** | * | * | ** | ** | * | * | ||
| Species | Treatment | Nitrates (mg·kg−1 d.w.) | Ca | K | Mg | Na | P |
|---|---|---|---|---|---|---|---|
| (g·kg−1 d.w.) | |||||||
| Garlic | Control | 1483 ± 58 a | 0.51 b | 15.5 c | 0.90 b | 403 c | 4.22 b |
| AMF | 1172 ± 37 b | 0.74 a | 14.2 c | 1.72 a | 832 a | 4.63 ab | |
| Se | 1404 ± 54 a | 0.60 b | 17.4 b | 1.72 a | 679 b | 4.92 ab | |
| AMF + Se | 1479 ± 55 a | 0.60 b | 19.1 a | 1.67 a | 567 b | 5.13 a | |
| Onion | Control | 1310 ± 30 a | 1.92 b | 16.7 c | 1.97 b | 809 b | 3.93 b |
| AMF | 1144 ± 39 b | 2.97 a | 20.6 b | 2.15 b | 748 b | 5.10 a | |
| Se | 1293 ± 42 a | 2.13 b | 17.0 c | 1.93 b | 1046 a | 4.05 b | |
| AMF + Se | 1261 ± 40 a | 2.08 b | 23.4 a | 2.43 a | 708 b | 4.97 a | |
| Species | Treatment | B | Cu | Fe | Mn | Mo | Si | Zn |
|---|---|---|---|---|---|---|---|---|
| (mg·kg−1 d.w.) | ||||||||
| Garlic | Control | 8.9 a | 6.4 | 58.2 ab | 11.8 | 0.25 d | 19.8 a | 38.2 c |
| AMF | 7.0 b | 6.9 | 57.9 ab | 12.4 | 0.35 c | 13.5 b | 41.7 bc | |
| Se | 8.0 ab | 7.0 | 51.7 b | 11.8 | 0.54 b | 20.8 a | 47.9 ab | |
| AMF + Se | 8.0 ab | 6.6 | 67.7 a | 10.4 | 0.71 a | 18.4 a | 54.1 a | |
| n.s. | n.s. | |||||||
| Onion | Control | 8.7 b | 6.3 b | 40.7 c | 9.1 ab | 0.25 b | 16.5 b | 28.5 b |
| AMF | 9.4 b | 9.0 a | 53.5 b | 8.9 b | 0.15 c | 17.9 b | 30.0 b | |
| Se | 11.0 a | 6.9 b | 48.1 c | 8.8 b | 0.22 b | 21.7 a | 27.8 b | |
| AMF + Se | 11.0 a | 8.5 a | 96.1 a | 11.4 a | 0.36 a | 25.0 a | 43.9 a | |
| Treatment | B | Cu | Fe | Mn | Mo | Si | Zn | Ca | K | Mg | Na | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Garlic | ||||||||||||
| AMF | * | * | * | |||||||||
| Se | * | * | * | * | ||||||||
| AMF + Se | * | * | * | * | * | |||||||
| Onion | ||||||||||||
| AMF | * | * | * | * | * | |||||||
| Se | * | * | * | |||||||||
| AMF + Se | * | * | * | * | * | * | * | * | * | * | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubkina, N.; Amagova, Z.; Matsadze, V.; Zamana, S.; Tallarita, A.; Caruso, G. Effects of Arbuscular Mycorrhizal Fungi on Yield, Biochemical Characteristics, and Elemental Composition of Garlic and Onion under Selenium Supply. Plants 2020, 9, 84. https://doi.org/10.3390/plants9010084
Golubkina N, Amagova Z, Matsadze V, Zamana S, Tallarita A, Caruso G. Effects of Arbuscular Mycorrhizal Fungi on Yield, Biochemical Characteristics, and Elemental Composition of Garlic and Onion under Selenium Supply. Plants. 2020; 9(1):84. https://doi.org/10.3390/plants9010084
Chicago/Turabian StyleGolubkina, Nadezhda, Zarema Amagova, Visita Matsadze, Svetlana Zamana, Alessio Tallarita, and Gianluca Caruso. 2020. "Effects of Arbuscular Mycorrhizal Fungi on Yield, Biochemical Characteristics, and Elemental Composition of Garlic and Onion under Selenium Supply" Plants 9, no. 1: 84. https://doi.org/10.3390/plants9010084
APA StyleGolubkina, N., Amagova, Z., Matsadze, V., Zamana, S., Tallarita, A., & Caruso, G. (2020). Effects of Arbuscular Mycorrhizal Fungi on Yield, Biochemical Characteristics, and Elemental Composition of Garlic and Onion under Selenium Supply. Plants, 9(1), 84. https://doi.org/10.3390/plants9010084