Gene Expression and K+ Uptake of Two Tomato Cultivars in Response to Sub-Optimal Temperature
Abstract
1. Introduction
2. Results
2.1. Plant Dry Weight and Root Morphology
2.2. MDA Content and POD Activity
2.3. K+ Content in Different Organs and K+ Uptake by Whole Plants
2.4. K+ Net Uptake Rate and K+ Transportation Ratio
2.5. K+ Flux Rate
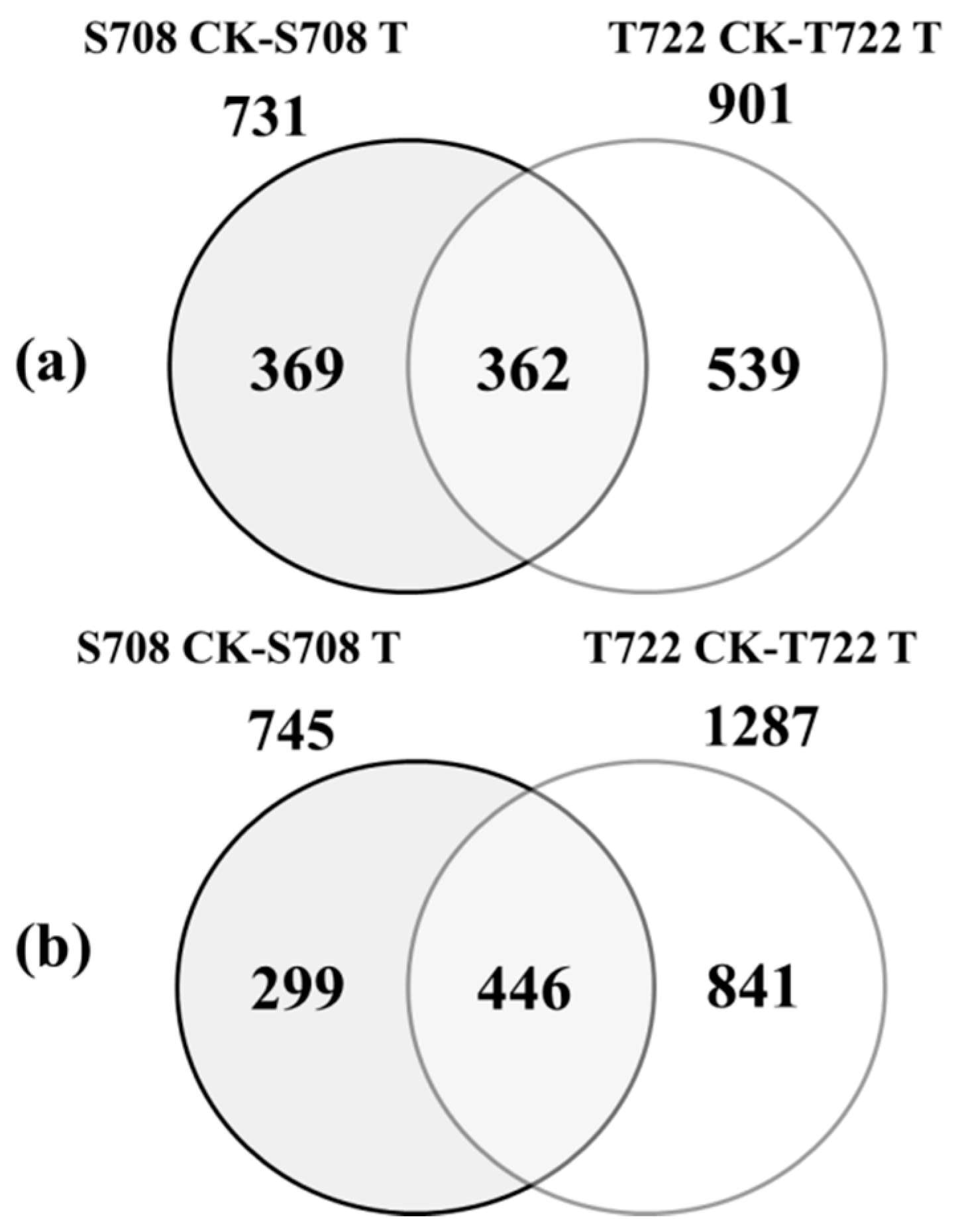
2.6. Summary of RNA-Seq Data
2.6.1. DEGs in Response to Sub-Optimal Temperature
2.6.2. GO and KEGG Enrichment Analysis of DEGs
2.6.3. Expression Analysis of Genes Related to K+ Uptake and Translocation
2.6.4. Expression Analysis of Aquaporin Genes and Cold Tolerance-Related Genes
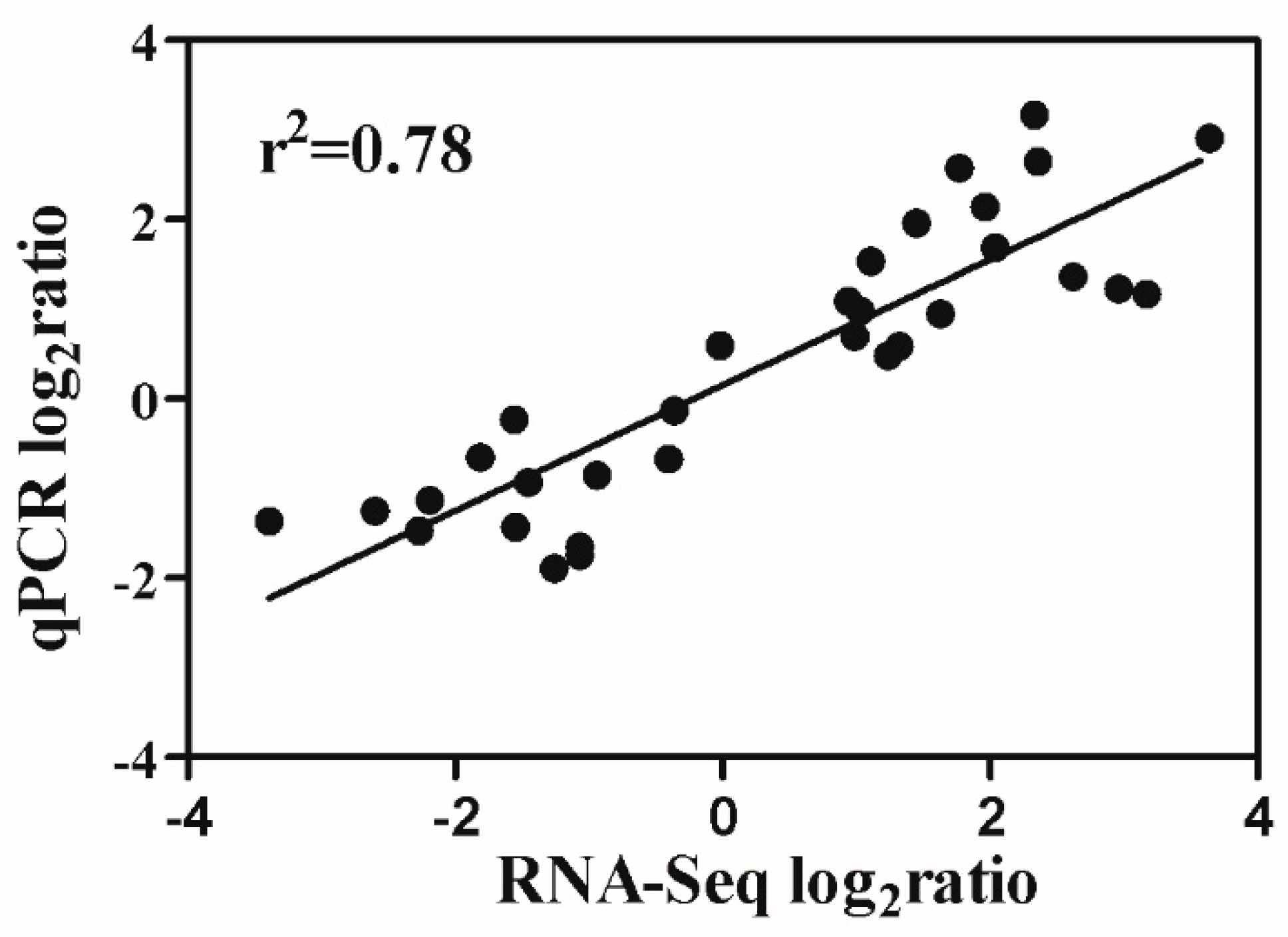
2.7. Verification of RNA-Seq Data
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Sub-Optimal Temperature Treatment
4.2. Measurements of Plant Height, Dry Weight and Root Morphology
4.3. Measurements of MDA Content and POD Activity
4.4. Measurements of K+ Content, K+ Amount, K+ Net Uptake Rate and K+ Transportation Ratio
4.5. Measurement of Net K+ Flux
4.6. RNA Preparation
4.7. Illumina Sequencing
4.7.1. Transcriptome Assembly and Annotation
4.7.2. Analysis of Differential Gene Expression
4.8. Verification of RNA-Seq Results by Quantitative Real-Time PCR (qRT-PCR)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ploeg, V.D.; Heuvelink, E. Influence of sub-optimal temperature on tomato growth and yield: A review. J. Hortic. Sci. Biotechnol. 2005, 80, 652–659. [Google Scholar] [CrossRef]
- Zhang, X.; Fowler, S.G.; Cheng, H.M.; Lou, Y.G.; Rhee, S.Y.; Stockinger, E.J.; Thomashow, M.F. Freezing-sensitive tomato has a functional CBF cold response pathway, but a CBF regulon that differs from that of freezing-tolerant Arabidopsis. Plant J. 2004, 39, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.F.; Chen, X.H.; Wang, S.; Zhang, X.C. Transcriptome sequencing and analysis of chilling tolerance mutant tomato under low temperature. Russ. J. Plant Physiol. 2019, 66, 110–118. [Google Scholar] [CrossRef]
- Criddle, R.S.; Smith, B.N.; Hansen, L.D. A respiration based description of plant growth rate responses to temperature. Planta 1997, 201, 441–445. [Google Scholar] [CrossRef]
- Schwarz, D.; Rouphael, Y.; Colla, G.; Venema, J.H. Grafting as a tool to improve tolerance of vegetables to abiotic stresses: Thermal stress, water stress and organic pollutants. Sci. Hortic. 2010, 127, 162–171. [Google Scholar] [CrossRef]
- Ntatsi, G.; Savvas, D.; Druege, U.; Schwarz, D. Contribution of phytohormones in alleviating the impact of sub-optimal temperature stress on grafted tomato. Sci. Hortic. 2013, 149, 28–38. [Google Scholar] [CrossRef]
- Calzadilla, P.I.; Maiale, S.J.; Ruiz, O.A.; Escaray, F.J. Transcriptome response mediated by cold stress in Lotus japonicus. Front. Plant Sci. 2016, 7, 374. [Google Scholar] [CrossRef]
- Steponkus, P.L. Role of the plasma membrane in freezing injury and cold acclimation. Annu. Rev. Plant Biol. 1984, 35, 543–584. [Google Scholar] [CrossRef]
- Khan, T.A.; Fariduddin, Q.; Yusuf, M.; Ahmad, M. Low temperature triggered varied antioxidant responses in tomato. Int. J. Veg. Sci. 2015, 21, 329–343. [Google Scholar] [CrossRef]
- Yoshida, K.; Kaothien, P.; Matsui, T.; Kawaoka, A.; Shinmyo, A. Molecular biology and application of plant peroxidase genes. Appl. Microbiol. Biotechnol. 2003, 60, 665. [Google Scholar] [CrossRef]
- Passardi, F.; Cosio, C.; Penel, C.; Dunand, C. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 2005, 24, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Khaledian, Y.; Maali-Amiri, R.; Talei, A. Phenylpropanoid and antioxidant changes in chickpea plants during cold stress. Russ. J. Plant Physiol. 2015, 62, 772–778. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Tittal, M.; Mir, R.A.; Agarwal, R.M. Alleviation of water and osmotic stress-induced changes in nitrogen metabolizing enzymes in Triticum aestivum L. cultivars by potassium. Protoplasma 2017, 254, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Devi, B.S.; Kim, Y.J.; Selvi, S.K.; Gayathri, S.; Altanzul, K.; Parvin, S.; Yang, D.; Lee, O.R.; Yang, D.C. Influence of potassium nitrate on antioxidant level and secondary metabolite genes under cold stress in Panax ginseng. Russ. J. Plant Physiol. 2012, 59, 318–325. [Google Scholar] [CrossRef]
- Römheld, V.; Kirkby, E.A. Research on potassium in agriculture: Needs and prospects. Plant Soil 2010, 335, 155–180. [Google Scholar] [CrossRef]
- Wang, X.G.; Zhao, X.H.; Jiang, C.J.; Li, C.H.; Cong, S.; Wu, D.; Chen, Y.Q.; Yu, H.Q.; Wang, C.Y. Effects of potassium deficiency on photosynthesis and photoprotection mechanisms in soybean (Glycine max (L.) Merr.). J. Integr. Agric. 2015, 14, 856–863. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Nahar, K.; Hossain, M.; Mahmud, J.; Hossen, M.; Masud, A.; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Kanai, S.; Moghaieb, R.E.; El-Shemy, H.A.; Panigrahi, R.; Mohapatra, P.K.; Ito, J.; Nguyen, N.T.; Saneoka, H.; Fujita, K. Potassium deficiency affects water status and photosynthetic rate of the vegetative sink in green house tomato prior to its effects on source activity. Plant Sci. 2011, 180, 368–374. [Google Scholar] [CrossRef]
- Very, A.A.; Sentenac, H. Molecular mechanisms and regulation of K+ transport in higher plants. Annu. Rev. Plant Biol. 2003, 54, 575–603. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, W.H. Regulation of potassium transport and signaling in plants. Curr. Opin. Plant Biol. 2017, 39, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Nieves-ordones, M.; Lara, A.; Ródenas, R.; Amo, J.; Rivero, R.M.; Martine, V.; Rubio, F. Modulation of K+ translocation by AKT1 and AtHAK5 in Arabidopsis plant. Plant Cell Environ. 2019, 42, 2357–2371. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plant. 2008, 133, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Pottosin, I. Regulation of potassium transport in plants under hostile conditions: Implications for abiotic and biotic stress tolerance. Physiol. Plant. 2014, 151, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.N.; Lew, R.R. Turgor regulation in osmotically stressed Arabidopsis epidermal root cells. Direct support for the role of inorganic ion uptake as revealed by concurrent flux and cell turgor measurements. Plant Physiol. 2002, 129, 290–299. [Google Scholar] [CrossRef]
- Yan, Q.Y.; Duan, Z.Q.; Mao, J.D.; Li, X.; Dong, F. Effects of root-zone temperature and N, P, and K supplies on nutrient uptake of cucumber (Cucumis sativus L.) seedlings in hydroponics. Soil Sci. Plant Nutr. 2012, 58, 707–717. [Google Scholar] [CrossRef]
- Ding, M.; Hou, P.; Shen, X.; Wang, M.; Deng, S.; Sun, J.; Xiao, F.; Wang, R.; Zhou, X.; Lu, C. Salt-induced expression of genes related to Na+/K+ and ROS homeostasis in leaves of salt-resistant and salt-sensitive poplar species. Plant Mol. Biol. 2010, 73, 251–269. [Google Scholar] [CrossRef]
- Fu, X.; Wu, X.; Zhou, X.; Liu, S.; Shen, Y.; Wu, F. Companion cropping with potato onion enhances the disease resistance of tomato against Verticillium dahliae. Front. Plant Sci. 2015, 6, 726. [Google Scholar] [CrossRef]
- Cui, J.; Luan, Y.; Jiang, N.; Bao, H.; Meng, J. Comparative transcriptome analysis between resistant and susceptible tomato allows the identification of lncRNA16397 conferring resistance to Phytophthora infestans by co-expressing glutaredoxin. Plant J. 2017, 89, 577–589. [Google Scholar] [CrossRef]
- Chen, H.Y.; Chen, X.L.; Chen, D.; Li, J.F.; Zhang, Y.; Wang, A.X. A comparison of the low temperature transcriptomes of two tomato genotypes that differ in freezing tolerance: Solanum lycopersicum and Solanum habrochaites. BMC Plant Biol. 2015, 15, 132. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.H.; Zhao, T.T.; Jiang, J.B.; Zhang, H.; Chen, X.L.; Liu, G.; Zhang, D.Y.; Du, C.; Wang, S.B.; Xu, X.Y. Transcriptome analysis of the Sm-mediated hypersensitive response to Stemphylium lycopersici in tomato. Front. Plant Sci. 2017, 8, 1257. [Google Scholar] [CrossRef] [PubMed]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Huang, Y.; Zhong, Y.; Kong, Q.; Xie, J.; Niu, M.; Xu, Y.; Bie, Z. Comparative transcriptome profiling of potassium starvation responsiveness in two contrasting watermelon genotypes. Planta 2014, 239, 397–410. [Google Scholar] [CrossRef]
- Liu, H.; Ouyang, B.; Zhang, J.; Wang, T.; Li, H.; Zhang, Y.; Yu, C.; Ye, Z. Differential modulation of photosynthesis, signaling, and transcriptional regulation between tolerant and sensitive tomato genotypes under cold stress. PLoS ONE 2012, 7, e50785. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Ishitani, M.; Zhu, J.K. Interaction of osmotic stress, temperature, and abscisic acid in the regulation of gene expression in Arabidopsis. Plant Physiol. 1999, 119, 205–212. [Google Scholar] [CrossRef]
- Wang, J.; Song, K.; Sun, L.; Qin, Q.; Sun, Y.; Pan, J.; Xue, Y. Morphological and transcriptome analysis of wheat seedlings response to low nitrogen stress. Plants 2019, 8, 98. [Google Scholar] [CrossRef]
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef]
- Tian, Q.; Reed, J.W. Control of auxin regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development 1999, 126, 711–721. [Google Scholar]
- Randall, R.S.; Miyashima, S.; Blomster, T.; Jing, Z.; Elo, A.; Karlberg, A.; Immanen, J.; Nieminen, K.; Lee, J.Y.; Kakimoto, T. AINTEGUMENTA and the D-type cyclin CYCD3;1 regulate root secondary growth and respond to cytokinins. Biol. Open 2015, 4, 1229–1236. [Google Scholar] [CrossRef]
- Yadav, V.; Mallappa, C.; Gangappa, S.N.; Bhatia, S.; Chattopadhyay, S. A basic helix-loop-helix transcription factor in Arabidopsis, MYC2, acts as a repressor of blue light–mediated photomorphogenic growth. Plant Cell 2005, 17, 1953–1966. [Google Scholar] [CrossRef] [PubMed]
- Dombrecht, B.; Xue, G.P.; Sprague, S.J.; Kirkegaard, J.A.; Ross, J.J.; Reid, J.B.; Fitt, G.P.; Sewelam, N.; Schenk, P.M.; Manners, J.M. MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell 2007, 19, 2225–2245. [Google Scholar] [CrossRef] [PubMed]
- Janská, A.; Marsík, P.; Zelenková, S.; Ovesná, J. Cold stress and acclimation-what is important for metabolic adjustment? Plant Biol. 2010, 12, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Jia, F.F.; Zhang, X.M.; Qiao, Y.X.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Temperature effects on the reactive oxygen species formation and antioxidant defence in roots of two cucurbit species with contrasting root zone temperature optima. Acta Physiol. Plant 2012, 34, 713–720. [Google Scholar] [CrossRef]
- Qu, Y.; Bai, X.; Zhu, Y.J.; Qi, R.; Tian, G.; Wang, Y.; Li, Y.H.; Zhang, K.M. Reactive oxygen species acts as an important inducer in low-temperature-induced anthocyanin biosynthesis in Begonia semperflorens. J. Am. Soc. Hort. Sci. 2018, 143, 486. [Google Scholar] [CrossRef]
- Zong, X.J.; Li, D.P.; Gu, L.K.; Li, D.Q.; Liu, L.X.; Hu, X.L. Abscisic acid and hydrogen peroxide induce a novel maize group C MAP kinase gene, ZmMPK7, which is responsible for the removal of reactive oxygen species. Planta 2009, 229, 485–495. [Google Scholar] [CrossRef]
- Demidchik, V. Mechanisms and physiological roles of K+ efflux from root cells. J. Plant Physiol. 2014, 171, 696–707. [Google Scholar] [CrossRef]
- Chen, Z.; Newman, I.; Zhou, M.; Mendham, N.; Zhang, G.; Shabala, S. Screening plants for salt tolerance by measuring K+ flux: A case study for barley. Plant Cell Environ. 2005, 28, 1230–1246. [Google Scholar] [CrossRef]
- Shigeto, J.; Tsutsumi, Y. Diverse functions and reactions of class III peroxidases. New Phytol. 2016, 209, 1395–1402. [Google Scholar] [CrossRef]
- Roxas, V.P.; Lodhi, S.A.; Garrett, D.K. Stress tolerance in transgenic tobacco seedlings that overexpress glutathione S-transferase/glutathione peroxidase. Plant Cell Physiol. 2000, 41, 1229–1234. [Google Scholar] [CrossRef]
- Rausch, T.; Wachter, A. Sulfur metabolism: A versatile platform for launching defence operations. Trends Plant Sci. 2005, 10, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, R.E.; Lewis, B.D.; Spalding, E.P.; Sussman, M.R. A role for the AKT1 potassium channel in plant nutrition. Science 1998, 280, 918–921. [Google Scholar] [CrossRef] [PubMed]
- Gierth, M.; Maser, P.; Schroeder, J.I. The potassium transporter AtHAK5 functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+ uptake kinetics in Arabidopsis roots. Plant Physiol. 2005, 137, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, W.H. Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 2013, 64, 451–476. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, H.D.; Chen, L.Q.; Wang, Y.; Liu, L.L.; He, L.; Wu, W.H. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 2006, 125, 1347–1360. [Google Scholar] [CrossRef]
- Lee, S.C.; Lan, W.Z.; Kim, B.G.; Li, L.; Cheong, Y.H.; Pandey, G.K.; Lu, G.; Buchanan, B.B.; Luan, S. A protein phosphorylation/dephosphorylation network regulates a plant potassium channel. Proc. Natl. Acad. Sci. USA 2007, 104, 15959–15964. [Google Scholar] [CrossRef]
- Scherzer, S.; Böhm, J.; Krol, E.; Shabala, L.; Kreuzer, I.; Larisch, C.; Bemm, F.; Al-Rasheid, K.A.; Shabala, S.; Rennenberg, H. Calcium sensor kinase activates potassium uptake systems in gland cells of Venus flytraps. Proc. Natl. Acad. Sci. USA 2015, 112, 7309–7314. [Google Scholar] [CrossRef]
- Ragel, P.; Ródenas, R.; García-Martín, E.; Andrés, Z.; Villalta, I.; Nieves-Cordones, M.; Rivero, R.M.; Martínez, V.; Pardo, J.M.; Quintero, F.J. CIPK23 regulates HAK5-mediated high-affinity K+ uptake in Arabidopsis roots. Plant Physiol. 2015, 169, 2863. [Google Scholar] [CrossRef]
- Drechsler, N.; Zheng, Y.; Bohner, A.; Nobmann, B.; Wirén, N.V.; Kunze, R.; Rausch, C. Nitrate-dependent control of shoot K homeostasis by NPF7.3/NRT1.5 and SKOR in Arabidopsis. Plant Physiol. 2015, 169, 2842–2847. [Google Scholar] [CrossRef]
- Li, H.; Yu, M.; Du, X.Q.; Wang, Z.F.; Wu, W.H.; Quintero, F.J.; Jin, X.H.; Li, H.D.; Wang, Y. NRT1.5/NPF7.3 functions as a proton-coupled H+/K+ antiporter for K+ loading into the xylem in Arabidopsis. Plant Cell 2017, 29, 2016–2026. [Google Scholar] [CrossRef]
- Liu, H.Y.; Sun, W.N.; Su, W.A.; Tang, Z.C. Co-regulation of water channels and potassium channels in rice. Physiol. Plant. 2006, 128, 58–69. [Google Scholar] [CrossRef]
- Duan, H.R.; Ma, Q.; Zhang, J.L.; Hu, J.; Bao, A.K.; Wei, L.; Wang, Q.; Luan, S.; Wang, S.M. The inward-rectifying K+ channel SsAKT1 is a candidate involved in K+ uptake in the halophyte Suaeda salsa under saline condition. Plant Soil 2015, 395, 173–187. [Google Scholar] [CrossRef]
- Ma, Q.; Hu, J.; Zhou, X.R.; Yuan, H.J.; Kumar, T.; Luan, S.; Wang, S.M. ZxAKT1 is essential for K+ uptake and K+/Na+ homeostasis in the succulent xerophyte Zygophyllum xanthoxylum. Plant J. 2017, 90, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Li, C.; Zhou, X.; Liu, S.; Wu, F. Physiological response and sulfur metabolism of the V. dahliae-infected tomato plants in tomato/potato onion companion cropping. Sci. Rep. 2016, 6, 36445. [Google Scholar] [CrossRef] [PubMed]
- Beadle, C.L.; Hall, D.O.; Scurlock, J.M.O. Growth analysis. In Photosynthesis and Production in a Changing Environment: A Field and Laboratory MANUAL; Bohr-Nordenkampf, H.R., Leegood, R.C., Long, S.P., Eds.; Chapman and Hall: London, UK, 1993; pp. 36–46. [Google Scholar]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutaseand catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Khanna-Chopra, R.; Chauhan, S. Wheat cultivars differing in heat tolerance show a differential response to oxidative stress during monocarpic senescence under high temperature stress. Protoplasma 2015, 252, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Chachar, K.M.; Chachar, Q.I.; Chachar, N.A. Genotypic variation for potassium uptake and utilization efficiency in Wheat (Triticum aestivum L.). Int. J. Agric. Technol. 2015, 11, 839–853. [Google Scholar]
- Nieves-Cordones, M.; Aleman, F.; Martinez, V.; Rubio, F. The Arabidopsis thaliana HAK5 K+ transporter is required for plant growth and K+ acquisition from low K+ solutions under saline conditions. Mol. Plant 2010, 3, 326–333. [Google Scholar] [CrossRef]
- Chen, G.; Hu, Q.D.; Luo, L.; Yang, T.Y.; Zhang, S.; Hu, Y.B.; Yu, L.; Xu, G.H. Rice potassium transporter OsHAK1 is essential for maintaining potassium-mediated growth and functions in salt tolerance over low and high potassium concentration ranges. Plant Cell Environ. 2015, 38, 2747–2765. [Google Scholar] [CrossRef]
- Lei, B.; Huang, Y.; Sun, J.Y.; Xie, J.J.; Niu, M.L.; Liu, Z.X.; Fan, M.L.; Bie, Z.L. Scanning ion-selective electrode technique and X-ray microanalysis provide direct evidence of contrasting Na+ transport ability from root to shoot in salt-sensitive cucumber and salt-tolerant pumpkin under NaCl stress. Physiol. Plant. 2014, 152, 738–748. [Google Scholar] [CrossRef]
- Pattemore, J.A. RNA extraction from cereal vegetative tissue. Methods Mol. Biol. 2014, 1099, 17–21. [Google Scholar] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Wang, X.; Bowers, J.E.; Ming, R.; Alam, M.; Paterson, A.H. Unraveling ancient hexaploidy through multiply-aligned angiosperm gene maps. Genome Res. 2008, 18, 1944–1954. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Furumichi, M.; Tanabe, M.; Hirakawa, M. KEGG for representation and analysis of molecular networks involving diseases and drugs. Nucleic Acids Res. 2010, 38, D355–D360. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Miller, A.J.; Aleman, F.; Martinez, V.; Rubio, F. A putative role for the plasma membrane potential in the control of the expression of the gene encoding the tomato high-affinity potassium transporter HAK5. Plant Mol. Biol. 2008, 68, 521–532. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
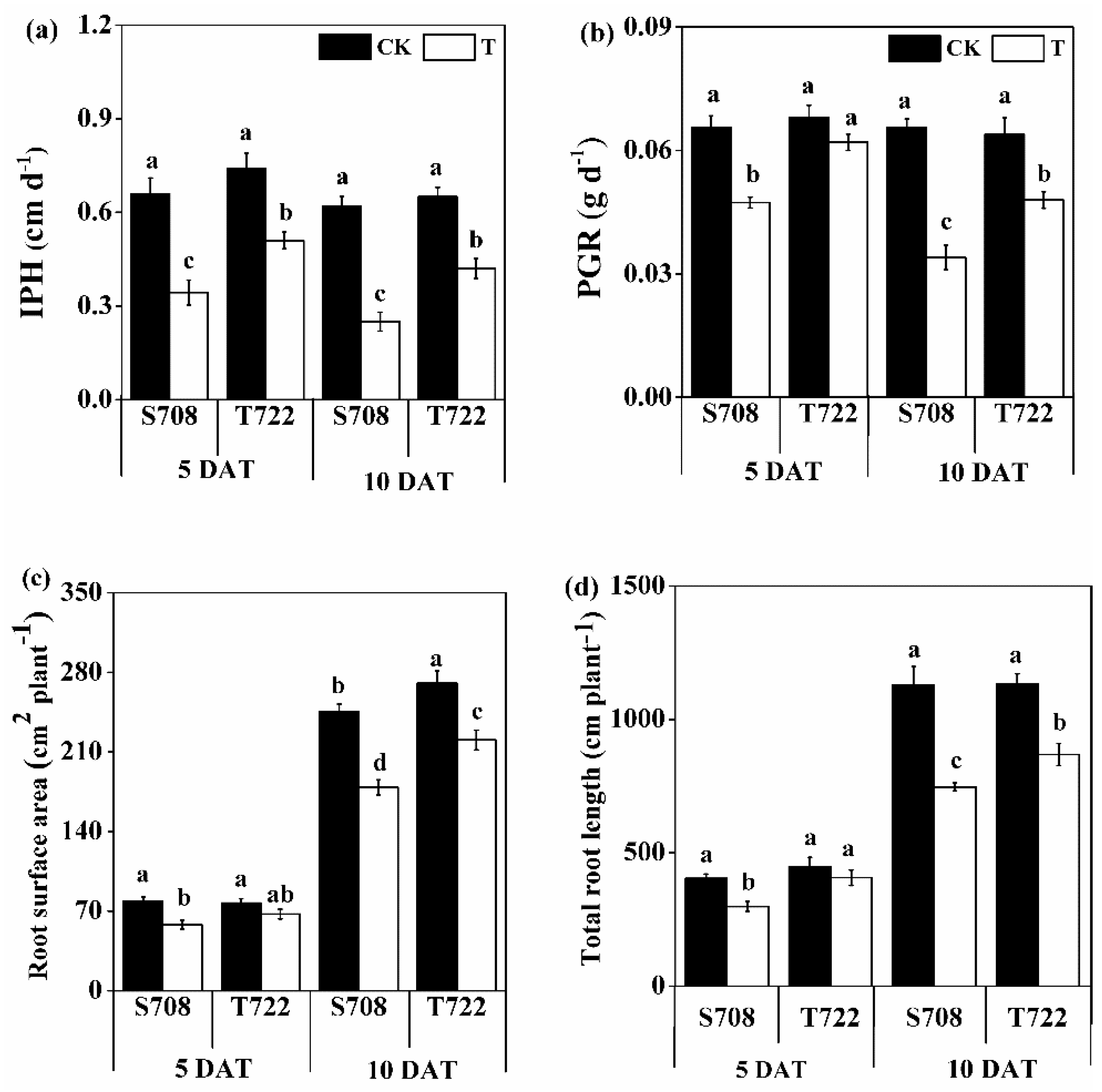
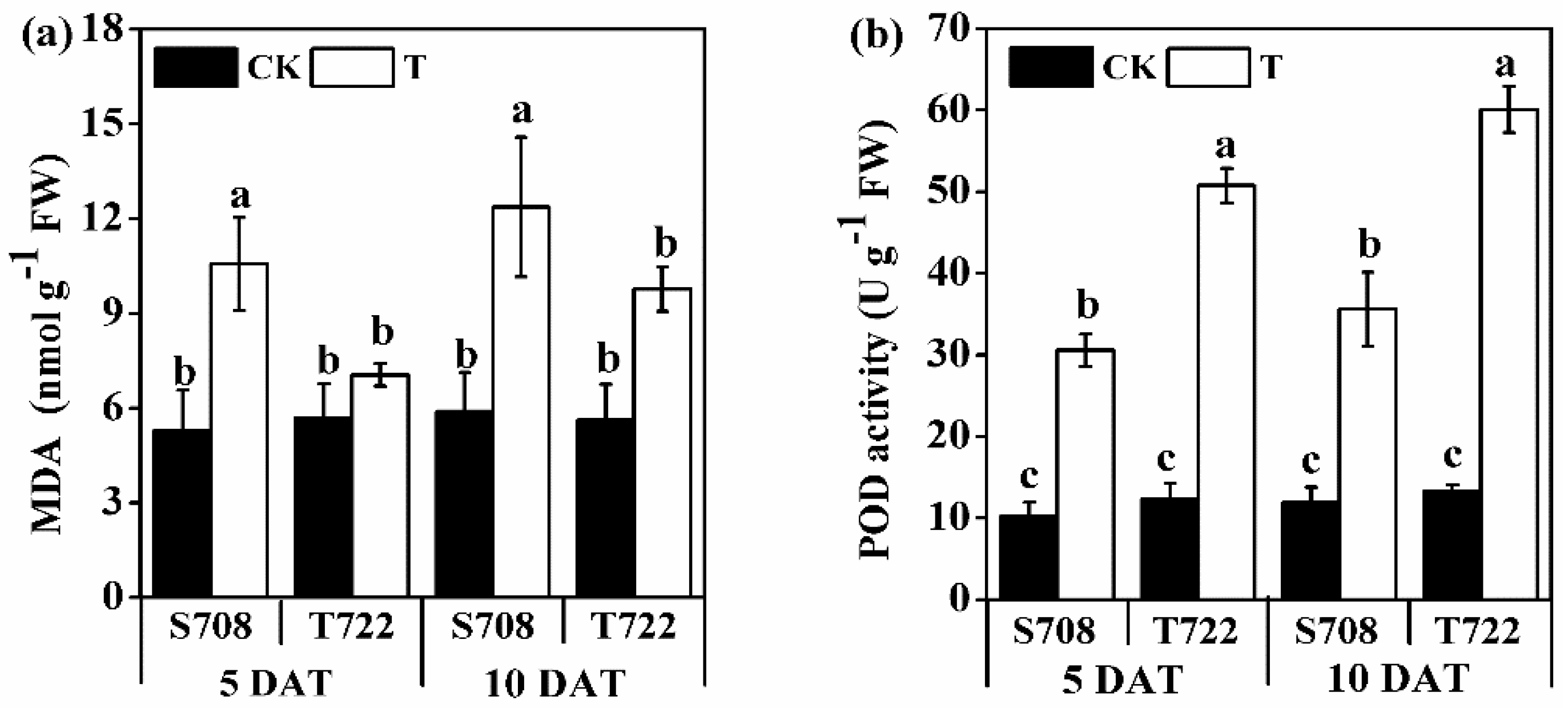
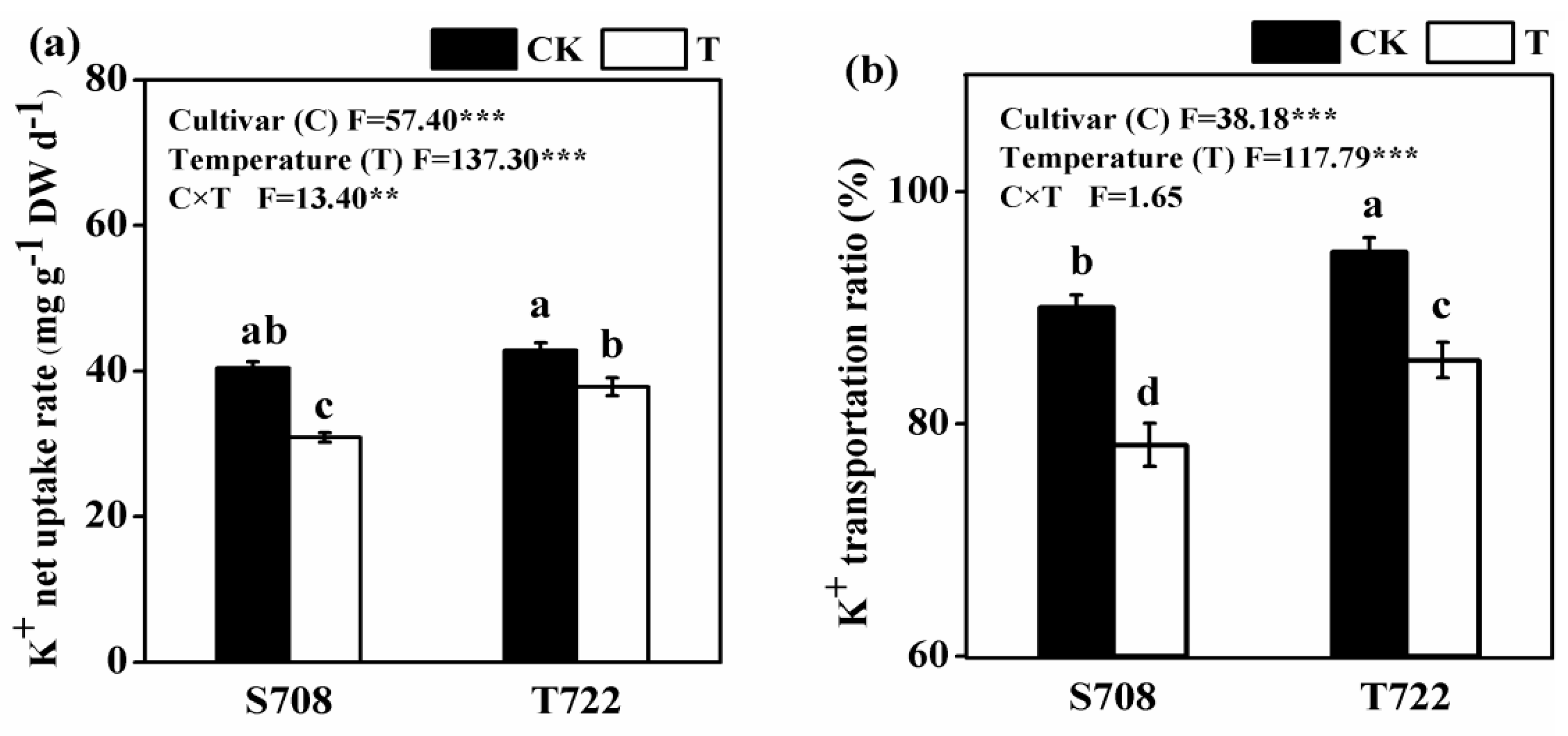
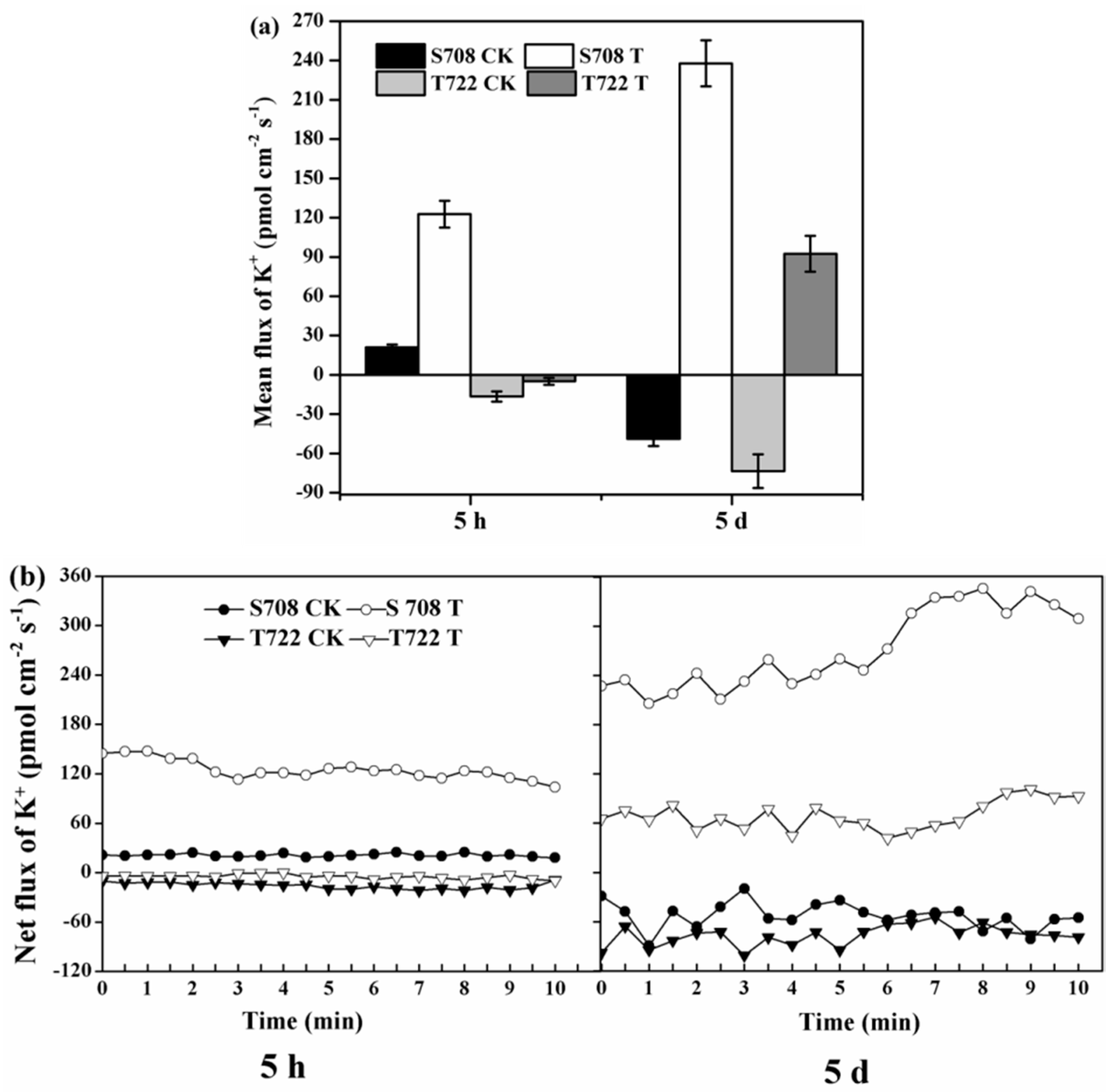
| DAT | Variables | IPH | PGR | Root Surface Area | Total Root Length | MDA Content | POD Activity |
|---|---|---|---|---|---|---|---|
| 5 | Cultivar (C) | 23.74 ** | 26.60 ** | 2.81 ns | 77.94 *** | 13.64 * | 107.19 *** |
| Temperature (T) | 113.77 *** | 53.86 *** | 40.71 *** | 72.83 *** | 62.28 ** | 740.64 *** | |
| C × T | 3.40 ns | 12.75 * | 4.88 ns | 13.48 ** | 23.23 ** | 70.22 *** | |
| 10 | Cultivar (C) | 30.77 ** | 11.34 * | 30.09 ** | 4.30 ns | 4.25 ns | 52.14 *** |
| Temperature (T) | 296.34 *** | 181.36 *** | 93.44 *** | 114.79 *** | 34.88 ** | 390.29 *** | |
| C × T | 16.13 ** | 16.93 ** | 1.91 ns | 3.79 ns | 3.81 ns | 42.14 *** |
| DAT | Treatments | K+ Content (mg g−1) | K+ Amount (mg Plant−1) | ||
|---|---|---|---|---|---|
| Root | Stem | Leaf | |||
| 5 | S708 CK | 23.76 ± 0.60 b | 46.69 ± 0.55 b | 25.11 ± 1.55 ab | 15.45 ± 0.50 c |
| S708 T | 28.17 ± 1.31 a | 42.62 ± 1.30 c | 22.68 ± 0.52 b | 8.48 ± 0.70 d | |
| T722 CK | 23.69 ± 1.60 b | 52.38 ± 0.96 a | 26.55 ± 1.73 a | 26.01 ± 1.01 a | |
| T722 T | 27.40 ± 0.98 a | 49.24 ± 0.92 b | 26.18 ± 1.15 ab | 21.52 ± 0.87 b | |
| Analysis of variance | |||||
| Cultivar (C) | ns | *** | * | *** | |
| Temperature (T) | ** | *** | ns | *** | |
| C × T | ns | ns | ns | ns | |
| 10 | S708 CK | 26.70 ± 2.14 | 50.76 ± 2.38 a | 27.46 ± 0.78 a | 27.85 ± 0.98 c |
| S708 T | 29.07 ± 1.44 | 43.96 ± 1.51 b | 22.90 ± 1.11b | 17.55 ± 0.54 d | |
| T722 CK | 24.68 ± 1.18 | 51.61 ± 0.74 a | 27.66 ± 0.78 a | 41.44 ± 0.39 a | |
| T722 T | 26.17 ± 0.79 | 49.62 ± 0.74 a | 26.48 ± 1.22 a | 33.53 ± 1.23 b | |
| Analysis of variance | |||||
| Cultivar (C) | ns | * | * | *** | |
| Temperature (T) | ns | ** | ** | *** | |
| C × T | ns | ns | * | ns | |
| Pathway | Number of Up-Regulated Genes | Number of Down-Regulated Genes | Pathway ID | |
|---|---|---|---|---|
| S708 CK-S708 T | Plant hormone signal transduction | 17 | 7 | ko04075 |
| Phenylpropanoid biosynthesis | 13 | 15 | ko00940 | |
| Sulfur metabolism | 8 | 0 | ko00920 | |
| Cytochrome P450 | 8 | 5 | ko00982 | |
| T722 CK-T722 T | Plant hormone signal transduction | 21 | 17 | ko04075 |
| Phenylpropanoid biosynthesis | 24 | 18 | ko00040 | |
| Sulfur metabolism | 8 | 2 | ko00920 | |
| Cytochrome P450 | 8 | 8 | ko00982 |
| Gene ID | S708 | T722 | Annotation |
|---|---|---|---|
| log2(T/CK) | log2(T/CK) | ||
| Auxin | |||
| Solyc09g065850.2 | 0.72 | 1.40 | IAA3 protein, AUX/IAA (S. lycopersicum) |
| Solyc04g081270.1 | 0.03 | 1.28 | Indole-3-acetic acid-induced protein, ARG7 SAUR (S. lycopersicum) |
| Gibberellin | |||
| Solyc09g074270.2 | 0.93 | 1.05 | Gibberellin receptor, GID1 (S. lycopersicum) |
| Abscisic acid | |||
| Solyc10g050210.1 | 0.56 | 1.44 | Abscisic acid insensitive, ABF (S. lycopersicum) |
| Ethylene | |||
| Solyc12g009560.1 | 0.87 | 1.18 | EIN3-binding F-box protein, EBF1 (A. thaliana) |
| Brassinosteroid | |||
| Solyc04g078470.2 | 0.56 | 1.16 | CycD3;2 protein (A. thaliana) |
| Jasmonic acid | |||
| Solyc08g008600.2 | 0.46 | 2.19 | Transcription factor, MYC2 (S. lycopersicum) |
| Salicylic acid | |||
| Solyc01g106640.2 | 0.06 | 1.50 | Pathogenesis-related protein, PR1 (S. lycopersicum) |
| Solyc10g085960.1 | 0.64 | 1.22 | Pathogenesis-related protein, PR1 (S. tuberosum) |
| Solyc09g007020.1 | −0.31 | 1.24 | Pathogenesis-related protein, PR1 (S. lycopersicum) |
| Gene ID | S708 | T722 | Annotation |
|---|---|---|---|
| log2(T/CK) | log2(T/CK) | ||
| Solyc11g018800.1 | 0.86 | 1.17 | Lignin-forming anionic peroxidase (S. lycopersicum) |
| Solyc10g078890.1 | 0.07 | 1.30 | Peroxidase (S. lycopersicum) |
| Solyc05g050880.2 | 0.02 | 1.86 | Cationic peroxidase (S. lycopersicum) |
| Solyc12g096530.1 | 0.92 | 1.29 | Peroxidase (A. thaliana) |
| Solyc02g085930.2 | 0.99 | 1.36 | Peroxidase (A. thaliana) |
| Solyc02g077300.1 | 0.61 | 1.02 | Peroxidase (A. thaliana) |
| Solyc03g120800.2 | 0.61 | 1.55 | Peroxidase (A. thaliana) |
| Solyc10g076210.1 | 0.35 | 1.14 | Peroxidase (S. lycopersicum) |
| Solyc01g058520.2 | 0.57 | 1.16 | Peroxidase (S. lycopersicum) |
| Solyc01g101050.2 | 0.57 | 1.55 | Peroxidase (S. lycopersicum) |
| Solyc05g050870.2 | −0.09 | 1.48 | Cationic peroxidase (S. tuberosum) |
| Solyc05g050890.1 | 0.38 | 1.92 | Cationic peroxidase (S. lycopersicum) |
| Gene ID | S708 | T722 | Annotation |
|---|---|---|---|
| log2(T/CK) | log2(T/CK) | ||
| Solyc09g042660.2 | 0.99 | 1.03 | CBL-interacting protein kinase 18 OsCIPK18 (O. sativa) |
| Solyc03g006110.2 | −0.02 | 2.04 | CBL-interacting protein kinase 5 OsCIPK05 (O. sativa) |
| Solyc03g083320.2 | 0.94 | 1.24 | Calcineurin B-like protein 7 OsCBL7 (O. sativa) |
| Solyc09g005220.1 | −1.56 | −0.40 | Potassium channel AKT1 (A. thaliana) |
| Solyc12g006850.1 | −0.94 | −0.36 | Potassium channel LKT1 (S. lycopersicum) |
| Solyc12g005670.1 | −1.07 | −1.07 | Potassium transporter 5 SIHAK5 (S. lycopersicum) |
| Solyc06g051830.1 | −3.39 | −1.45 | Potassiumtransporter HAK26 (O. sativa) |
| Solyc08g007060.2 | −1.26 | −1.55 | Nitrate transporter 1.5 AtNRT1.5 (A. thaliana) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Yang, W.; Li, C.; Zhou, X.; Gao, D.; Khashi u Rahman, M.; Li, N.; Wu, F. Gene Expression and K+ Uptake of Two Tomato Cultivars in Response to Sub-Optimal Temperature. Plants 2020, 9, 65. https://doi.org/10.3390/plants9010065
Gao H, Yang W, Li C, Zhou X, Gao D, Khashi u Rahman M, Li N, Wu F. Gene Expression and K+ Uptake of Two Tomato Cultivars in Response to Sub-Optimal Temperature. Plants. 2020; 9(1):65. https://doi.org/10.3390/plants9010065
Chicago/Turabian StyleGao, Huan, Wanji Yang, Chunxia Li, Xingang Zhou, Danmei Gao, Muhammad Khashi u Rahman, Naihui Li, and Fengzhi Wu. 2020. "Gene Expression and K+ Uptake of Two Tomato Cultivars in Response to Sub-Optimal Temperature" Plants 9, no. 1: 65. https://doi.org/10.3390/plants9010065
APA StyleGao, H., Yang, W., Li, C., Zhou, X., Gao, D., Khashi u Rahman, M., Li, N., & Wu, F. (2020). Gene Expression and K+ Uptake of Two Tomato Cultivars in Response to Sub-Optimal Temperature. Plants, 9(1), 65. https://doi.org/10.3390/plants9010065





