High-Throughput Sequencing Reveals Bell Pepper Endornavirus Infection in Pepper (Capsicum annum) in Slovakia and Enables Its Further Molecular Characterization
Abstract
1. Introduction
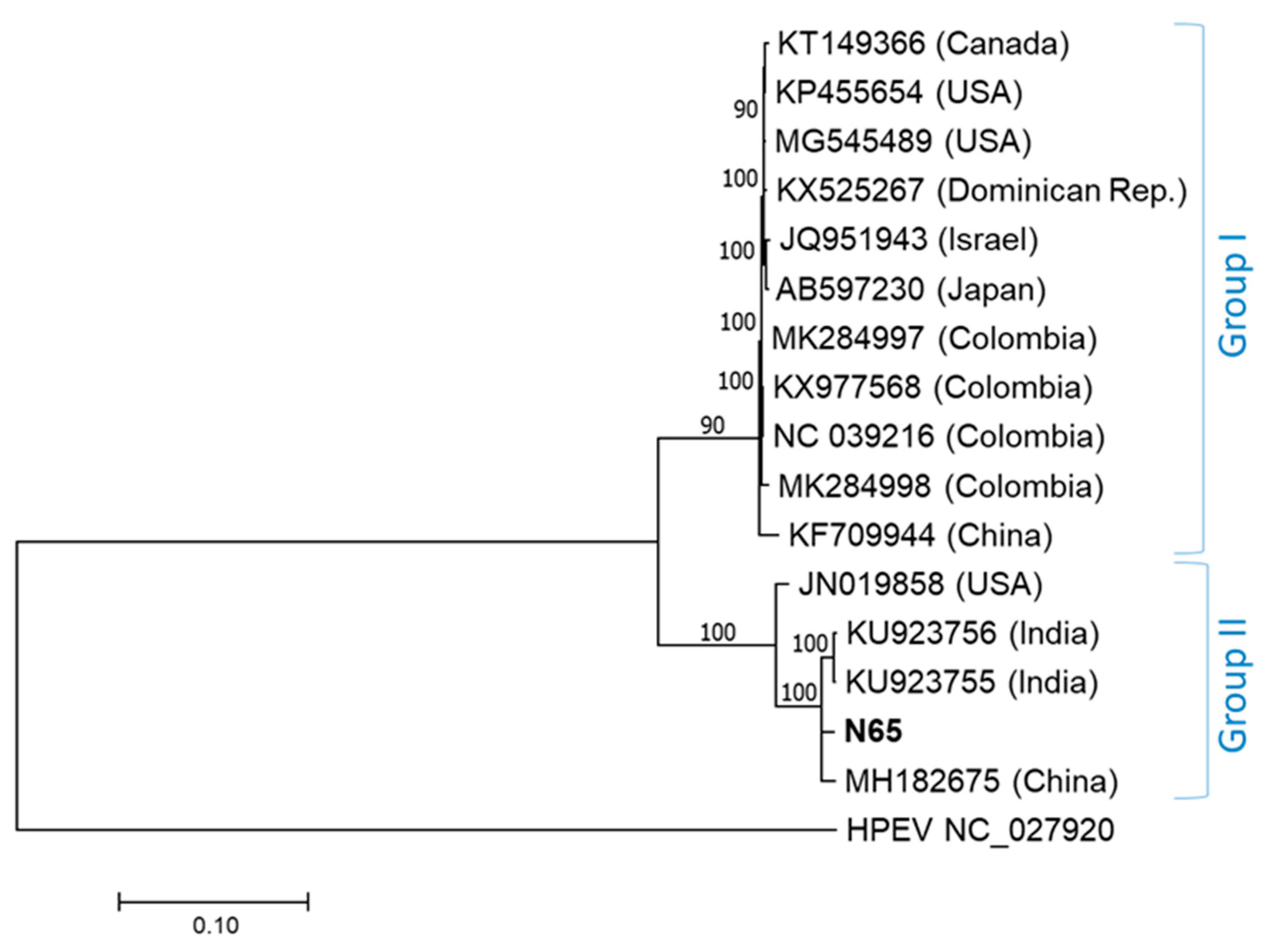
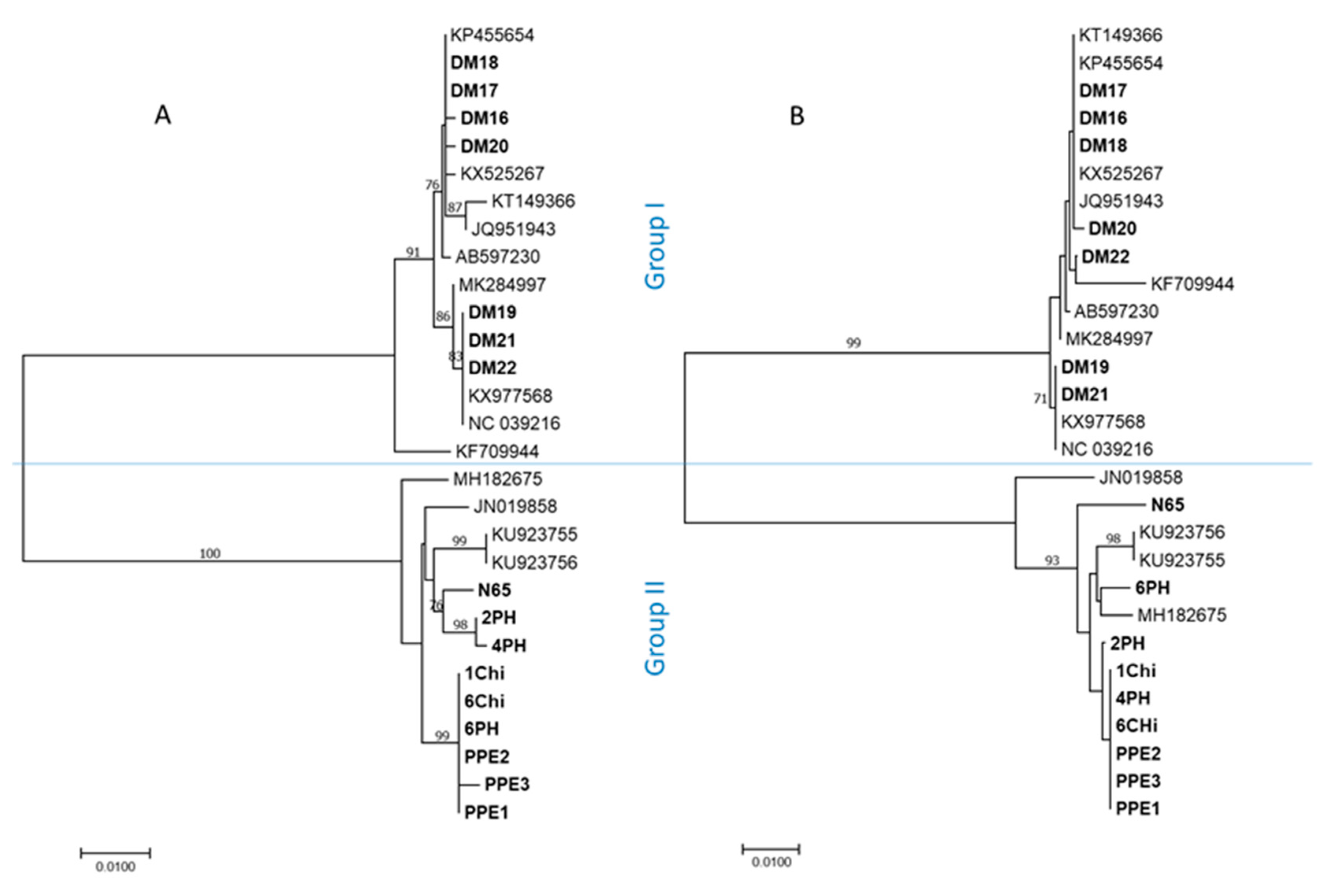
2. Results
3. Discussion
- -
- The nt coding region 4832–5542 (major parent N65, minor parent KX977568, overall probability 2.9*e−83, supported by 7/7 programs);
- -
- The nt coding region 6320–7134 (major parent MH182675, minor parent KX977568, overall probability 1.3*e−91, supported by 7/7 programs).
4. Materials and Methods
4.1. Analysis of the Virome and Determination of the BPEV Full-Length Genome Sequence by HTS
4.2. RT-PCR Detection and Partial Molecular Characterisation of Additional BPEV Isolates
4.3. Sequence Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Aguilar-Meléndez, A.; Morrell, P.L.; Roose, M.L.; Kim, S.-C. Genetic diversity and structure in semi wild and domesticated Chiles (Capsicum annuum; Solanaceae) from Mexico. Am. J. Bot. 2009, 96, 1190–1202. [Google Scholar] [CrossRef] [PubMed]
- Penella, C.; Calatayud, A. Pepper crop under climate change: Grafting as an environmental friendly strategy. In Climate Resilient Agriculture—Strategies and Perspectives; Shanker, A., Ed.; IntechOpen: London, UK, 2018; pp. 129–155. ISBN 978-953-51-3896-9. [Google Scholar] [CrossRef]
- Kenyon, L.; Kumar, S.; Tsai, W.S.; Hughes, J. Virus diseases of peppers (Capsicum spp.) and their control. Adv. Virus Res. 2014, 90, 297–354. [Google Scholar] [CrossRef] [PubMed]
- Roossinck, M.J. Metagenomics of plant and fungal viruses reveals an abundance of persistent lifestyles. Front. Microbiol. 2015, 5, 767. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Choi, H.; Yoon, J.Y.; Choi, S.K.; Cho, W.K. In silico identification of bell pepper endornavirus from pepper transcriptomes and their phylogenetic and recombination analyses. Gene 2016, 575, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.J.; Lefkowitz, E.J.; King, A.M.Q.; Harrach, B.; Harrison, R.L.; Knowles, N.J.; Kropinski, A.M.; Krupovic, M.; Kuhn, J.H.; Mushegian, A.R.; et al. Ratification vote on taxonomic proposals to the international committee on taxonomy of viruses. Arch. Virol. 2016, 161, 2921–2949. [Google Scholar] [CrossRef]
- Zheng, L.; Shu, C.; Zhang, M.; Yang, M.; Zhou, E. Molecular characterization of a novel endornavirus conferring hypovirulence in rice sheath blight fungus Rhizoctonia solani AG-1 IA Strain GD-2. Viruses 2019, 11, 178. [Google Scholar] [CrossRef]
- Valverde, R.A.; Khalifa, M.E.; Okada, R.; Fukuhara, T.; Sabanadzovic, S. ICTV Virus Taxonomy Profile: Endornaviridae. ICTV Report Consortium. J. Gen Virol. 2019, 100, 1204–1205. [Google Scholar] [CrossRef]
- Safari, M.; Roossinck, M.J. Coevolution of a persistent plant virus and its pepper hosts. Mol. Plant-Microbe Interact. 2018, 31, 766–776. [Google Scholar] [CrossRef]
- Okada, R.; Kiyota, E.; Sabanadzovic, S.; Moriyama, H.; Fukuhara, T.; Saha, P.; Roossinck, M.J.; Severin, A.; Valverde, R.A. Bell pepper endornavirus: Molecular and biological properties, and occurrence in the genus Capsicum. J. Gen. Virol. 2011, 92, 2664–2673. [Google Scholar] [CrossRef]
- Fukuhara, T. Endornaviruses: Persistent dsRNA viruses with symbiotic properties in diverse eukaryotes. Virus Genes 2019, 55, 165–173. [Google Scholar] [CrossRef]
- Roossinck, M.J.; Sabanadzovic, S.; Okada, R.; Valverde, R.A. The remarkable evolutionary history of endornaviruses. J. Gen. Virol. 2011, 92, 2674–2678. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Cho, W.K.; Park, S.H.; Jo, Y.; Kim, K.H. Evolution of and horizontal gene transfer in the Endornavirus genus. PLoS ONE 2013, 8, e64270. [Google Scholar] [CrossRef] [PubMed]
- Khankhum, S.; Sela, N.; Osorno, J.M.; Valverde, R.A. RNAseq analysis of endornavirus-infected vs. endornavirus-free common bean (Phaseolus vulgaris) cultivar Black Turtle Soup. Front. Microbiol. 2016, 7, 1905. [Google Scholar] [CrossRef] [PubMed]
- Khankhum, S.; Valverde, R.A. Physiological traits of endornavirus-infected and endornavirus-free common bean (Phaseolus vulgaris) cv Black Turtle Soup. Arch. Virol. 2018, 163, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Sabanadzovic, S.; Wintermantel, W.; Valverde, R.A.; McCreight, J.D.; Abou Ghanem-Sabanadzovic, N. Cucumis melo endornavirus: Genome organization, host range and co-divergence with the host. Virus Res. 2016, 214, 49–58. [Google Scholar] [CrossRef]
- Villamor, D.E.V.; Ho, T.; Al Rwahnih, M.; Martin, R.R.; Tzanetakis, I.E. High throughput sequencing for plant virus detection and discovery. Phytopathology 2019, 109, 716–725. [Google Scholar] [CrossRef]
- Maree, H.J.; Fox, A.; Al Rwahnih, M.; Boonham, N.; Candresse, T. Application of HTS for routine plant virus diagnostics: State of the art and challenges. Front. Plant Sci. 2018, 9, 1082. [Google Scholar] [CrossRef]
- Wu, Q.; Ding, S.-W.; Zhang, Y.; Zhu, S. Identification of viruses and viroids by next-generation sequencing and homology-dependent and homology-independent algorithms. Annu. Rev. Phytopathol. 2015, 53, 425–444. [Google Scholar] [CrossRef]
- Maliogka, V.I.; Minafra, A.; Saldarelli, P.; Ruiz-García, A.B.; Glasa, M.; Katis, N.; Olmos, A. Recent advances on detection and characterization of fruit tree viruses using High-Throughput Sequencing technologies. Viruses 2018, 10, 436. [Google Scholar] [CrossRef]
- Knierim, D.; Menzel, W.; Winter, S. Immunocapture of virions with virus-specific antibodies prior to high-throughput sequencing effectively enriches for virus-specific sequences. PLoS ONE 2019, 14, e0216713. [Google Scholar] [CrossRef]
- Viral Genomes. Available online: ftp://ftp.ncbi.nih.gov/genomes/Viruses/all.fna.tar.gz (accessed on 28 November 2019).
- Glasa, M.; Šoltys, K.; Predajňa, L.; Sihelská, N.; Budiš, J.; Mrkvová, M.; Kraic, J.; Mihálik, D.; Ruiz-Garcia, A.B. High-throughput sequencing of potato virus M from tomato in Slovakia reveals a divergent variant of the virus. Plant Protect. Sci. 2019, 55, 159–166. [Google Scholar] [CrossRef]
- GenBank. Available online: https://www.ncbi.nlm.nih.gov/genbank/ (accessed on 28 November 2019).
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015, 43, D222–D226. [Google Scholar] [CrossRef]
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015, 1, vev003. [Google Scholar] [CrossRef]
| Virus Acronym and Genome Segment | Reference Genome Sequence | Reads Mapped against Reference | Percentage of Genome Covered | Coverage Depth |
|---|---|---|---|---|
| BPEV * | NC_039216 | 2535 | 99.8% | 23.2 x |
| CMV RNA1 | NC_002034 | 2498 | 100% | 97.9 x |
| CMV RNA2 | NC_002035 | 1868 | 100% | 83.9 x |
| CMV RNA3 | NC_001440 | 3770 | 100% | 207.6 x |
| WMV * | NC_006262 | 411 | 93.0% | 6.3 x |
| PCV2 RNA1 | NC_034159 | 3378 | 98.3% | 300.3 x |
| PCV2 RNA2 | NC_034167 | 5514 | 99.7% | 523.5 x |
| Sample | Locality | Type of Plantation/Pepper Type/Year of Sampling | Symptoms Observed | Detection of Additional Viruses | BPEV GenBank Accession Numbers | |
|---|---|---|---|---|---|---|
| PVY | CMV | |||||
| N65 | Čachtice | Garden/sweet pepper cv. Promotor/2017 | M, Mot, De | − | + | MN580384 a |
| DM16 | Piešťany | Garden/sweet pepper/2018 | M | − | − | MN580386 b, MN580405 c |
| DM17 | Piešťany | Garden/sweet pepper/2018 | 0 | − | + | MN580390 b, MN580406 c |
| DM18 | Piešťany | Garden/sweet pepper/2018 | M | − | - | MN580385 b, MN580407 c |
| DM19 | Piešťany | Garden/sweet pepper/2018 | M, De | − | − | MN580387 b, MN580408 c |
| DM20 | Piešťany | Garden/sweet pepper/2018 | Mot | − | + | MN580389 b, MN580409 c |
| DM21 | Piešťany | Garden/sweet pepper/2018 | Mot | − | + | MN580388 b, MN580410 c |
| DM22 | Piešťany | Garden/sweet pepper cv. Anka/2018 | 0 | − | − | MN580391 b, MN580411 c |
| PPE1 | Pezinok | Garden/sweet pepper cv. Slovakia/2019 | De | − | − | MN580399 b, MN580413 c |
| PPE2 | Pezinok | Garden/sweet pepper cv. Slovakia/2019 | 0 | − | − | MN580397 b, MN580412 c |
| PPE3 | Pezinok | Garden/sweet pepper cv. Slovakia/2019 | M | - | + | MN580398 b, MN580414 c |
| 2PH | Bratislava | Retail shop/chili pepper/2019 | 0 | − | + | MN580393 b, MN580401 c |
| 4PH | Bratislava | Retail shop/chili pepper/2019 | M | + | − | MN580395 b, MN580402 c |
| 6PH | Bratislava | Retail shop/sweet pepper/2019 | M | + | - | MN580392 b, MN580404 c |
| 1Chi | Bratislava | Greenhouse/chili pepper/2018 | M, Mot, De | + | MN580394 b, MN580400 c | |
| 6Chi | Bratislava | Greenhouse/chili pepper cv. Habanero Lemon/2018 | M, Mot, De | + | + | MN580396 b, MN580403 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomašechová, J.; Hančinský, R.; Predajňa, L.; Kraic, J.; Mihálik, D.; Šoltys, K.; Vávrová, S.; Böhmer, M.; Sabanadzovic, S.; Glasa, M. High-Throughput Sequencing Reveals Bell Pepper Endornavirus Infection in Pepper (Capsicum annum) in Slovakia and Enables Its Further Molecular Characterization. Plants 2020, 9, 41. https://doi.org/10.3390/plants9010041
Tomašechová J, Hančinský R, Predajňa L, Kraic J, Mihálik D, Šoltys K, Vávrová S, Böhmer M, Sabanadzovic S, Glasa M. High-Throughput Sequencing Reveals Bell Pepper Endornavirus Infection in Pepper (Capsicum annum) in Slovakia and Enables Its Further Molecular Characterization. Plants. 2020; 9(1):41. https://doi.org/10.3390/plants9010041
Chicago/Turabian StyleTomašechová, Jana, Richard Hančinský, Lukáš Predajňa, Ján Kraic, Daniel Mihálik, Katarína Šoltys, Silvia Vávrová, Miroslav Böhmer, Sead Sabanadzovic, and Miroslav Glasa. 2020. "High-Throughput Sequencing Reveals Bell Pepper Endornavirus Infection in Pepper (Capsicum annum) in Slovakia and Enables Its Further Molecular Characterization" Plants 9, no. 1: 41. https://doi.org/10.3390/plants9010041
APA StyleTomašechová, J., Hančinský, R., Predajňa, L., Kraic, J., Mihálik, D., Šoltys, K., Vávrová, S., Böhmer, M., Sabanadzovic, S., & Glasa, M. (2020). High-Throughput Sequencing Reveals Bell Pepper Endornavirus Infection in Pepper (Capsicum annum) in Slovakia and Enables Its Further Molecular Characterization. Plants, 9(1), 41. https://doi.org/10.3390/plants9010041







