Evolutionary Analysis of Calcium-Dependent Protein Kinase in Five Asteraceae Species
Abstract
:1. Introduction
2. Results
2.1. Identification of CPKs in Five Asteraceae Species
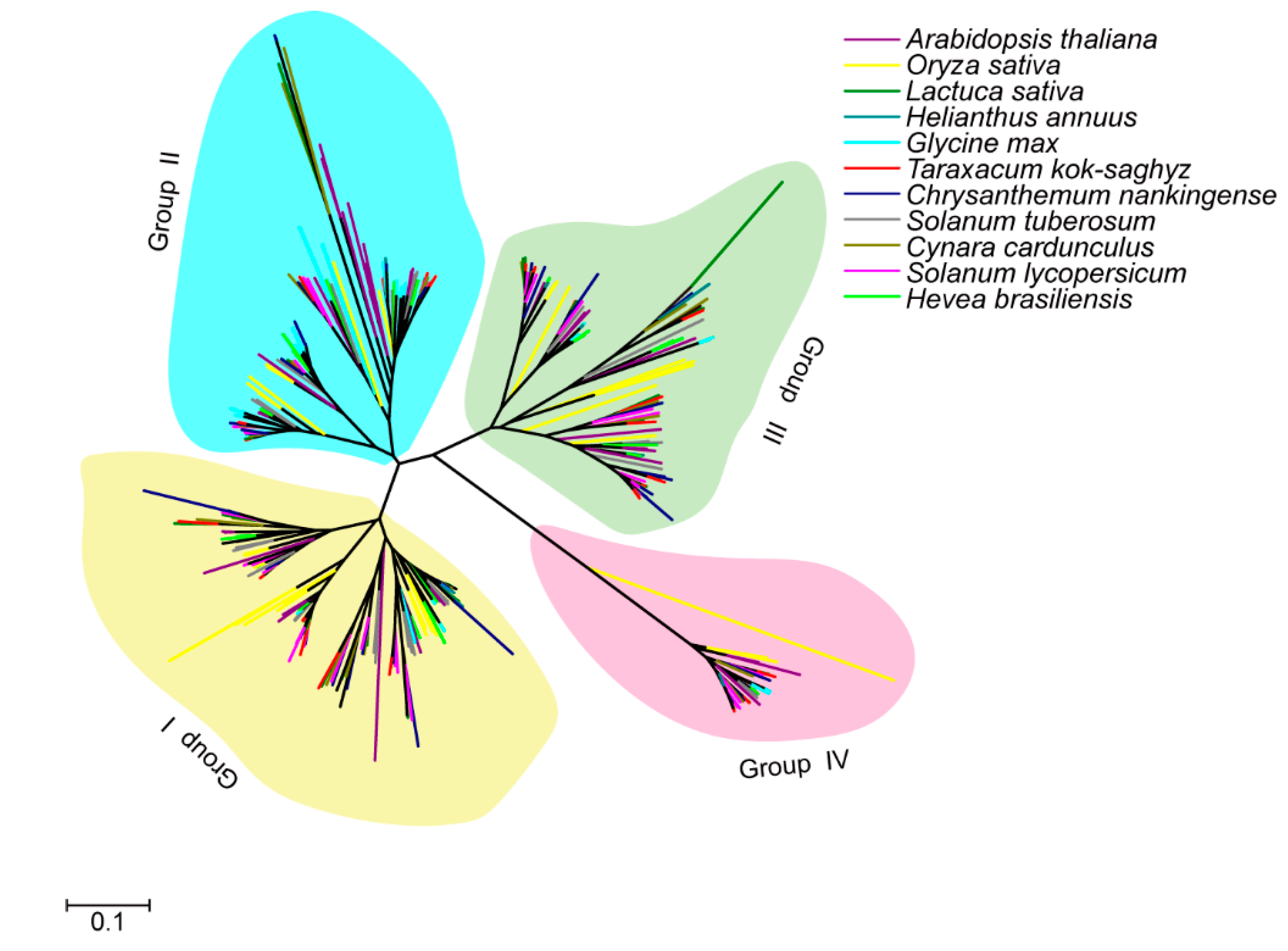
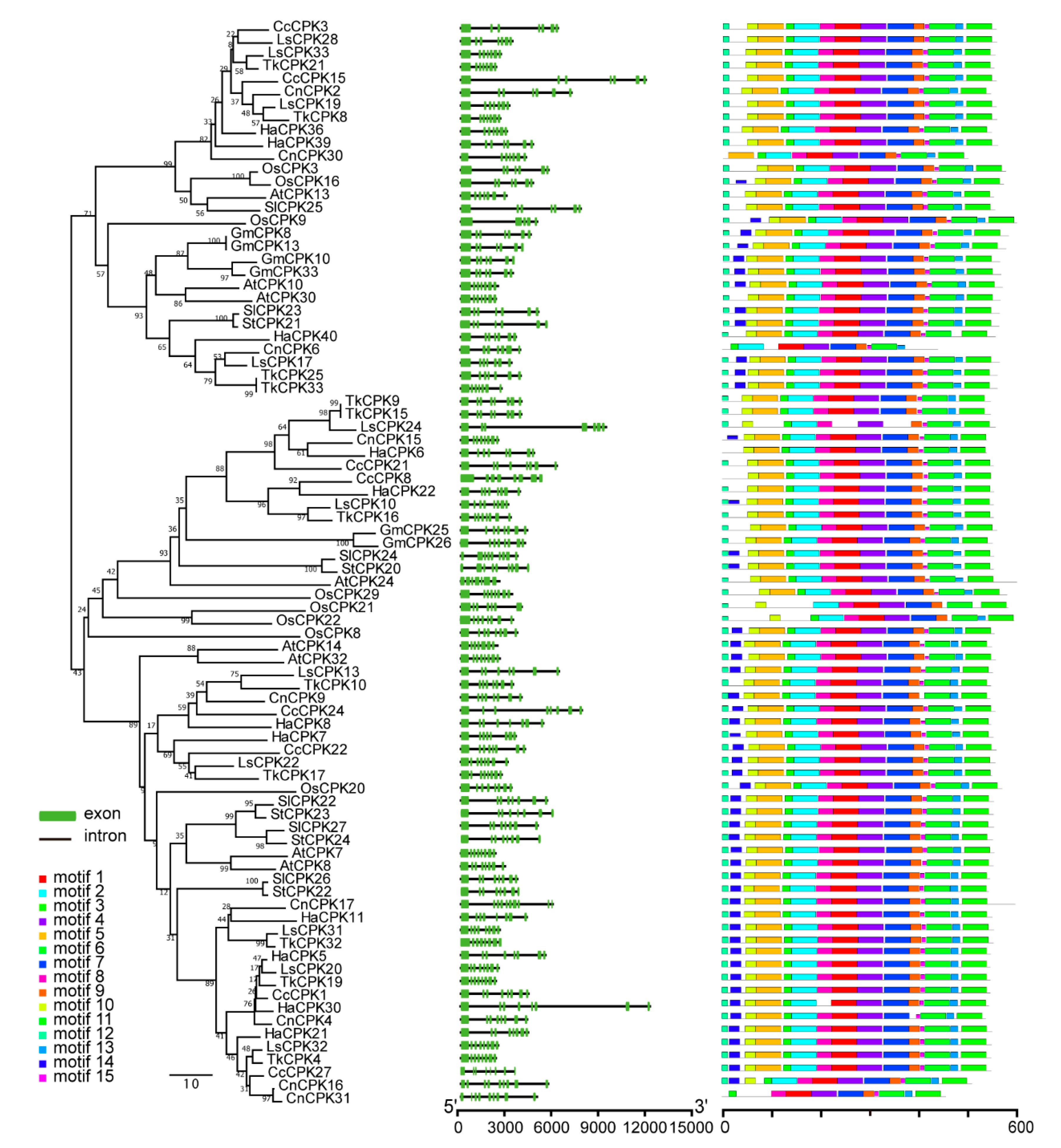
2.2. Phylogenetic Analysis of CPK Members
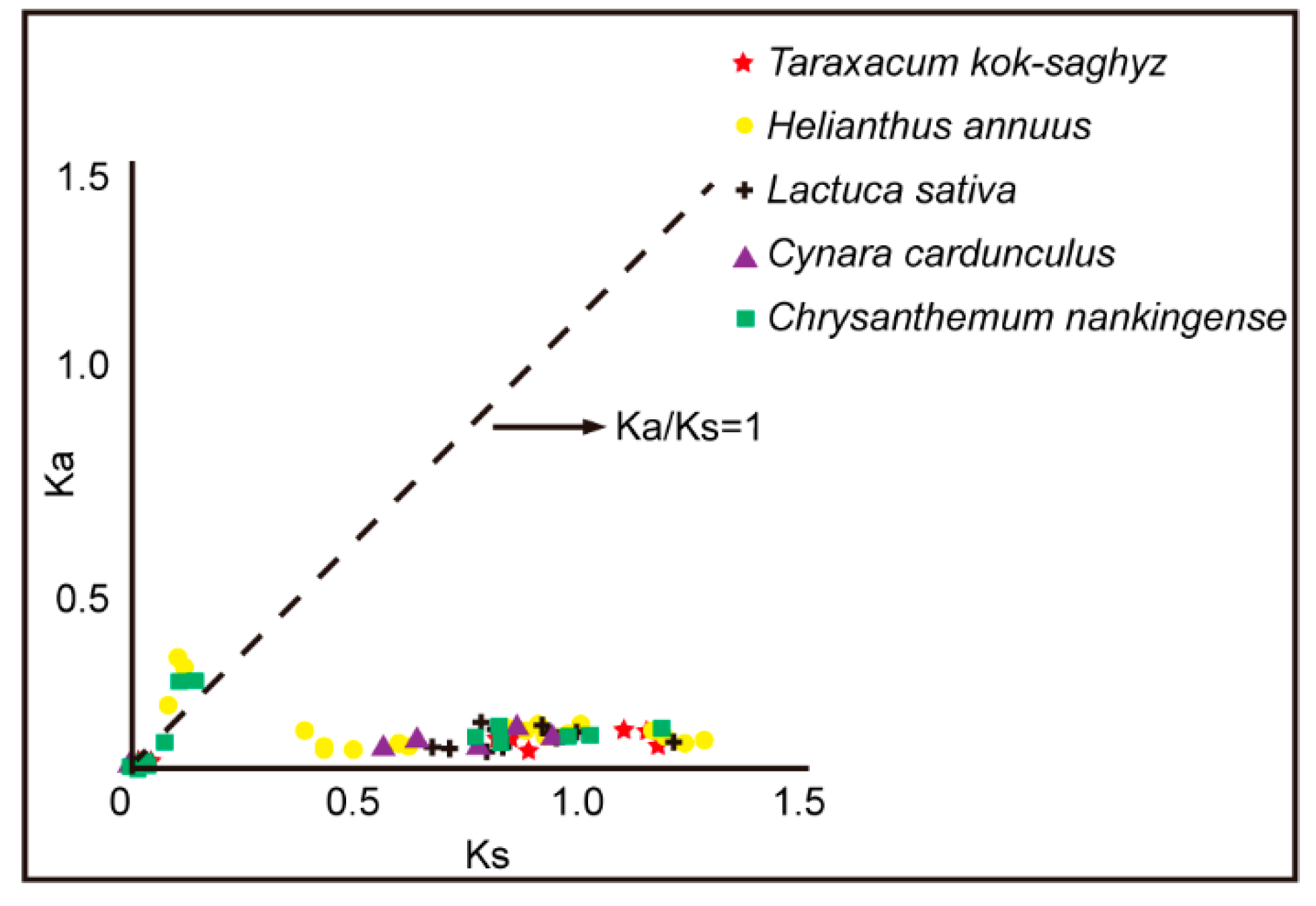
2.3. Evolutionary Analyses of Duplicated Gene Pairs in Asteraceas Species
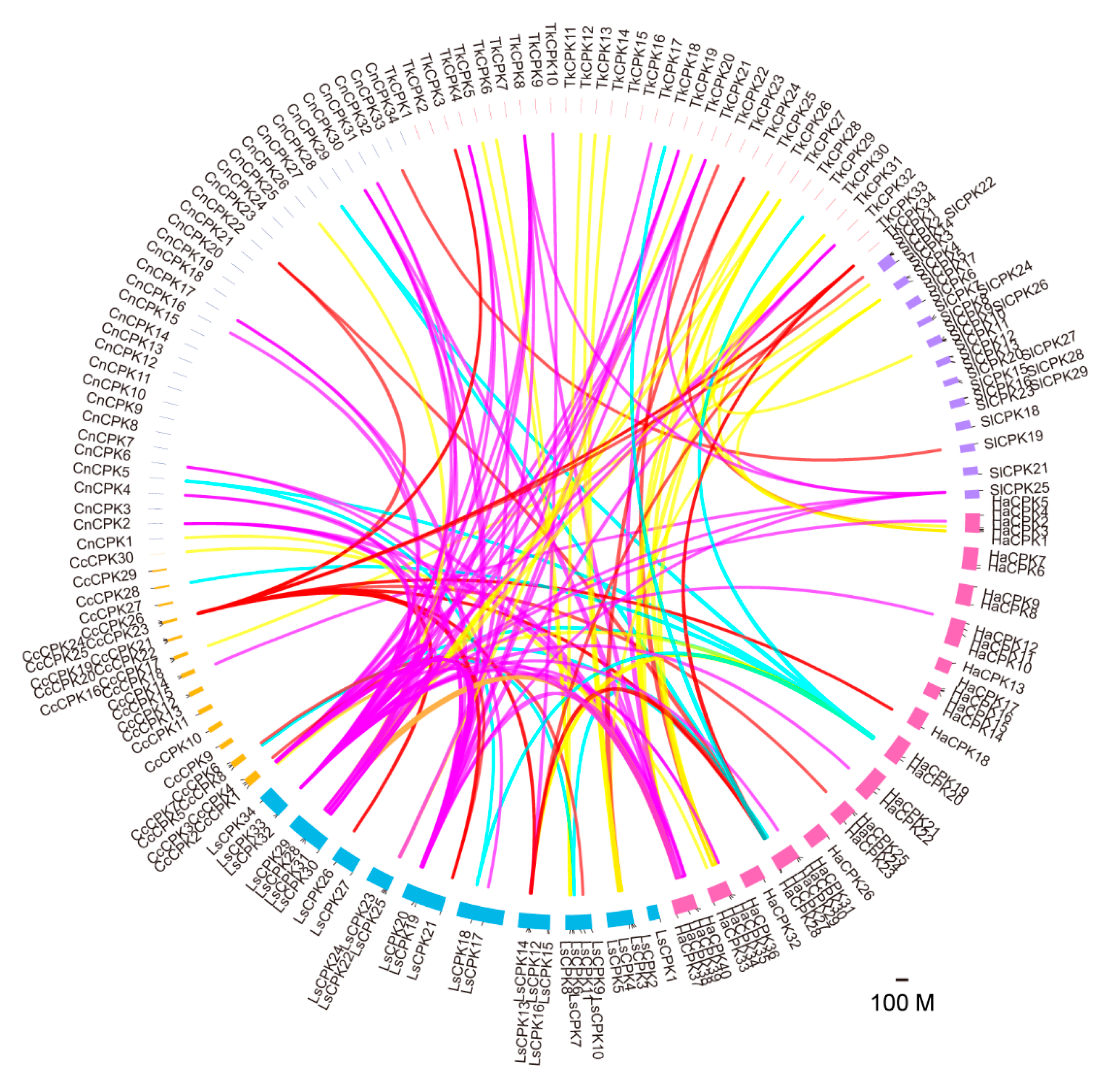
2.4. Syntenic Analysis of CPKs from Five Asteraceae Species
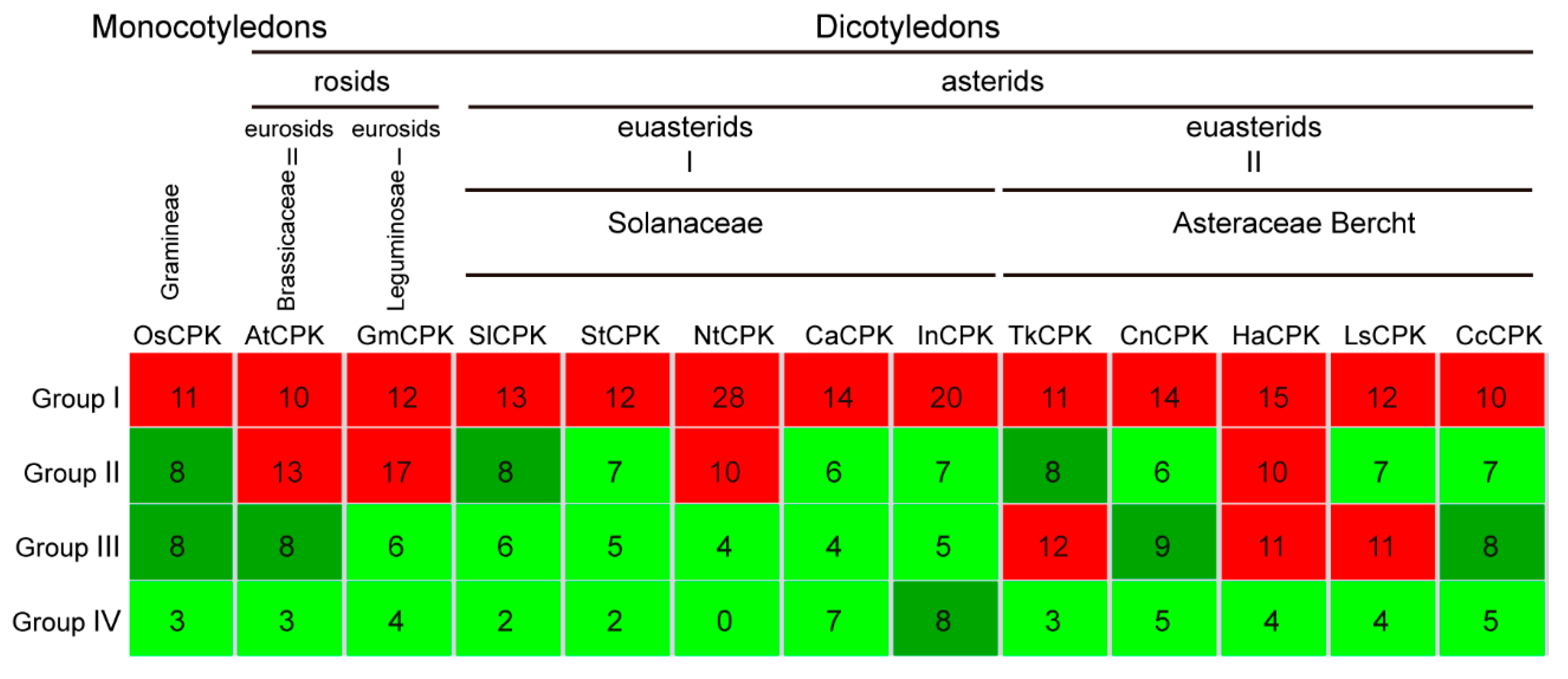
2.5. The CPKs in Group II and Group III Are Expanded
2.6. Gene Structure and Motif Distribution of CPKs
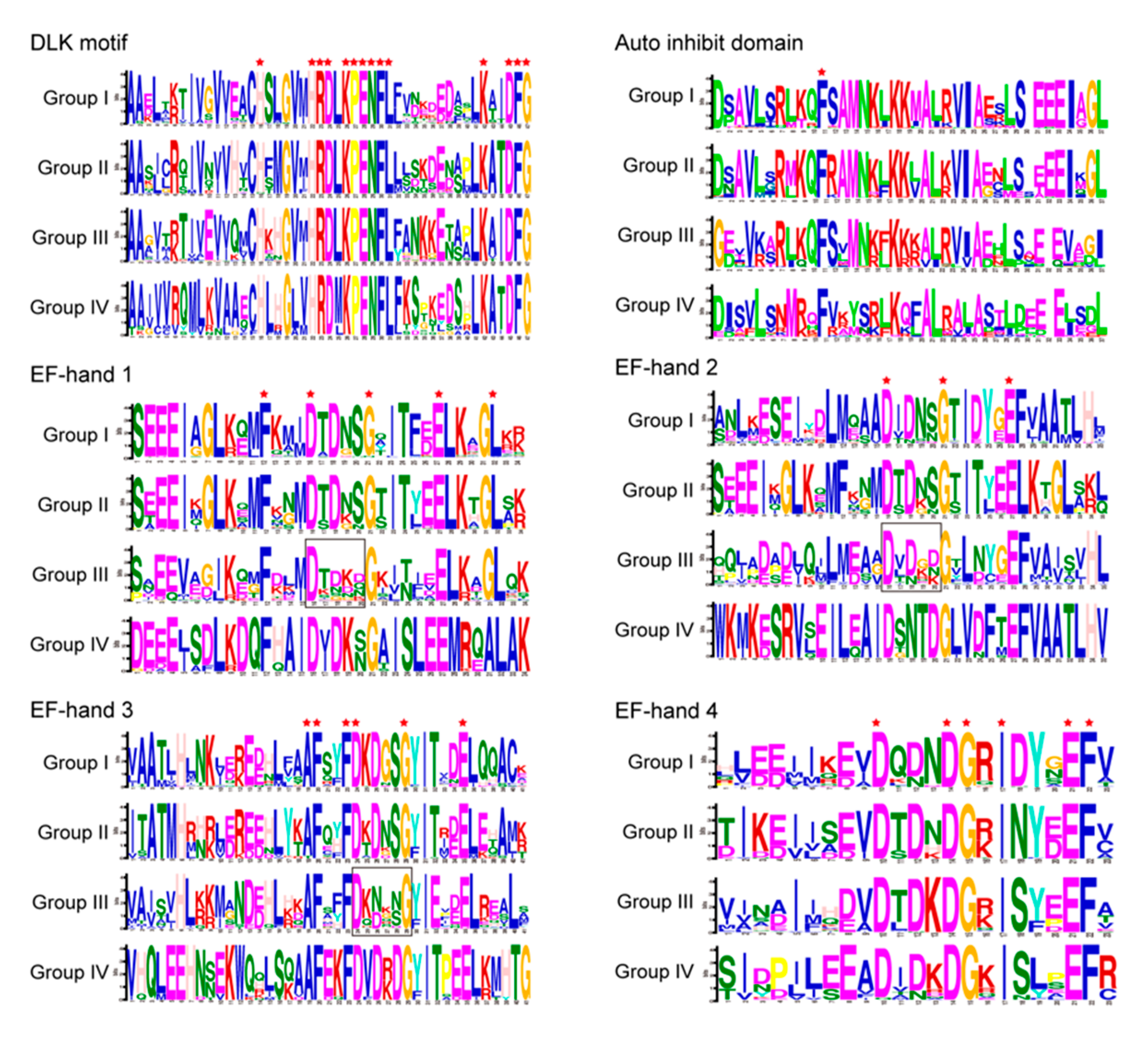
2.7. Motif Sequence Analysis of CPKs in Group III from Asteraceae
3. Discussion
3.1. Identification and Characteristics of CPKs in Asteraceae Species
3.2. Phylogenetic Analysis and Group-Specific Expansion of CPKs in Asteraceae Species
4. Materials and Methods
4.1. Identification and Characteristics of CPK Members in Five Representative Asteraceae Species
4.2. Phylogenetic Tree Construction
4.3. Duplication Event and Syntenic Analysis
4.4. Gene Structure and Motif Distribution Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CPK | calcium-dependent protein kinases |
| CaM | calmodulins |
| CaML | caM-like proteins |
| CBL | calcineurin B-like proteins |
| NR | natural rubber |
| cPTs | cis-prenyltransferases |
| SRPPs | small rubber particle proteins |
| SNP | single nucleotide polymorphism |
| Pal | palmitoylation |
| Myr | myristoylation |
| CDS | coding sequence |
| Ka | nonsynonymous substitution rate |
| Ks | synonymous substitutions rate |
| NJ | Neighbour-Joining |
| MEME | Multiple Expectation Maximization for Motif Elicitation |
| WGD | whole-genome duplication |
Appendix A
References
- White, P.; Broadley, M. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Luan, S. The CBL-CIPK network in plant calcium signaling. Trends Plant Sci. 2009, 14, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.A.; Romeis, T.; Jones, J.D. CDPK-mediated signalling pathways: Specificity and cross-talk. J. Exp. Bot. 2004, 55, 181–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harper, J.F.; Sussman, M.R.; Schaller, G.E.; Putnam-Evans, C.; Charbonneau, H.; Harmon, A.C. A calcium-dependent protein kinase with a regulatory domain similar to calmodulin. Science 1991, 252, 951–954. [Google Scholar] [CrossRef]
- Cheng, S.H.; Willmann, M.R.; Chen, H.C.; Sheen, J. Calcium signalling through protein kinases. The Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol. 2002, 129, 469–485. [Google Scholar] [CrossRef] [Green Version]
- Saito, S.; Hamamoto, S.; Moriya, K.; Matsuura, A.; Sato, Y.; Muto, J.; Noguchi, H.; Yamauchi, S.; Tozawa, Y.; Ueda, M.; et al. N-myristoylation and S-acylation are common modifications of Ca2+-regulated Arabidopsis kinases and are required for activation of the SLAC1 anion channel. New Phytol. 2018, 218, 1504–1521. [Google Scholar] [CrossRef] [Green Version]
- Yip Delormel, T.; Boudsocq, M. Properties and functions of calcium-dependent protein kinases and their relatives in Arabidopsis thaliana. New Phytol. 2019, 224, 585–604. [Google Scholar] [CrossRef] [Green Version]
- Boudsocq, M.; Droillard, M.J.; Regad, L.; Laurière, C. Characterization of Arabidopsis calcium-dependent protein kinases: Activated or not by calcium? Biochem. J. 2012, 447, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Franz, S.; Ehlert, B.; Liese, A.; Kurth, J.; Cazalé, A.C.; Romeis, T. Calcium-dependent protein kinase CPK21 functions in abiotic stress response in Arabidopsis thaliana. Mol. Plant 2011, 4, 83–96. [Google Scholar] [CrossRef]
- Tähtiharju, S.; Rijpkema, A.S.; Vetterli, A.; Albert, V.A.; Teeri, T.H.; Elomaa, P. Evolution and diversification of the CYC/TB1 gene family in Asteraceae—A comparative study in Gerbera (Mutisieae) and sunflower (Heliantheae). Mol. Biol. Evol. 2012, 29, 1155–1166. [Google Scholar] [CrossRef] [Green Version]
- Barker, M.S.; Kane, N.C.; Matvienko, M.; Kozik, A.; Michelmore, R.W.; Knapp, S.J.; Rieseberg, L.H. Multiple paleopolyploidizations during the evolution of the Compositae reveal parallel patterns of duplicate gene retention after millions of years. Mol. Biol. Evol. 2008, 25, 2445–2455. [Google Scholar] [CrossRef] [Green Version]
- Funk, V.A.; Bayer, R.J.; Keeley, S.; Chan, R.; Watson, L.; Gemeinholzer, B.; Schilling, E.; Panero, J.L.; Baldwin, B.G.; Garcia-jacas, N.; et al. Everywhere but Antarctica: Using a supertree to understand the diversity and distribution of the Compositae. Biol. Skr. 2005, 55, 343–374. [Google Scholar]
- Judd, W.S.; Campbell, E.A. Kellogg. Plant systematics: A phylogenetic approach. Cladistics Int. J. Willi Hennig Soc. 2010, 24, 848–850. [Google Scholar]
- Badouin, H.; Gouzy, J.; Grassa, C.J.; Murat, F.; Staton, S.E.; Cottret, L.; Lelandais-Brière, C.; Owens, G.L.; Carrère, S.; Mayjonade, B.; et al. The sunflower genome provides insights into oil metabolism, flowering and Asterid evolution. Nature 2017, 546, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Chin-Wo, S.; Wang, Z.; Yang, X.; Kozik, A.; Arikit, S.; Song, C.; Xia, L.; Froenicke, L.; Lavelle, D.O.; Truco, M.J.; et al. Genome assembly with in vitro proximity ligation data and whole-genome triplication in lettuce. Nat. Commun. 2017, 8, 14953. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Xu, X.; Ruan, J.; Liu, S.; Wu, S.; Shao, X.; Wang, X.; Gan, L.; Qin, B.; Yang, Y.; et al. Genome analysis of Taraxacum kok-saghyz rodin provides new insights into rubber biosynthesis. Natl. Sci. Rev. 2018, 5, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Scaglione, D.; Reyes-Chin-Wo, S.; Acquadro, A.; Froenicke, L.; Portis, E.; Beitel, C.; Tirone, M.; Mauro, R.; Lo, M.A.; Mauromicale, G.; et al. The genome sequence of the outbreeding globe artichoke constructed de novo incorporating a phase-aware low-pass sequencing strategy of F1 progeny. Sci. Rep. 2016, 6, 19427. [Google Scholar]
- Song, C.; Liu, Y.; Song, A.; Dong, G.; Zhao, H.; Sun, W.; Ramakrishnan, S.; Wang, Y.; Wang, S.; Li, T.; et al. The Chrysanthemum nankingense genome provides insights into the evolution and diversification of chrysanthemum flowers and medicinal traits. Mol Plant. 2018, 11, 1482–1491. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Yang, M.; Fang, Y.; Luo, Y.; Gao, S.; Xiao, X.; An, Z.; Zhou, B.; Zhang, B.; Tan, X.; et al. The rubber tree genome reveals new insights into rubber production and species adaptation. Nat. Plants 2016, 2, 16073. [Google Scholar] [CrossRef] [Green Version]
- Stipanovic, R.; O’Brien, D.; Rogers, C.; Hanlon, K. Natural rubber from sunflower. J. Agric. Food Chem. 1980, 28, 1322–1323. [Google Scholar] [CrossRef]
- Schmidt, T.; Hillebrand, A.; Wurbs, D.; Wahler, D.; Lenders, M.; Gronover, C.S.; Prüfer, D. Molecular cloning and characterization of rubber biosynthetic genes from Taraxacum kok-saghyz. Plant Mol. Biol. Rep. 2010, 28, 277–284. [Google Scholar] [CrossRef]
- Collins-Silva, J.; Nural, A.T.; Skaggs, A.; Scott, D.; Hathwaik, U.; Woolsey, R.; Schegg, K.; McMahan, C.; Whalen, M.; Cornish, K.; et al. Altered levels of the Taraxacum kok-saghyz (Russian dandelion) small rubber particle protein, TkSRPP3, result in qualitative and quantitative changes in rubber metabolism. Phytochemistry 2012, 79, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Chakrabarty, R.; Tran, H.T.; Kwon, E.J.; Kwon, M.; Nguyen, T.D.; Ro, D.K. A lettuce (Lactuca sativa) homolog of human Nogo-B receptor interacts with cis-prenyltransferase and is necessary for natural rubber biosynthesis. J. Biol. Chem. 2015, 290, 1898–1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epping, J.; Deenen, N.V.; Niephaus, E.; Stolze, A.; Fricke, J.; Huber, C.; Eisenreich, W.; Twyman, R.M.; Prüfer, D.; Gronover, C.S. A rubber transferase activator is necessary for natural rubber biosynthesis in dandelion. Nat. Plants 2015, 1, 15048. [Google Scholar] [CrossRef]
- Luo, Z.; Iaffaldano, B.J.; Zhuang, X.; Fresnedo-Ramírez, J.; Cornish, K. Analysis of the first Taraxacum kok-saghyz transcriptome reveals potential rubber yield related SNPs. Sci. Rep. 2017, 7, 9939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Jin, X.; Xie, Q.; Yao, Q.; Wang, X.; Li, H. Calcium-dependent protein kinase family genes involved in ethylene-induced natural rubber production in different Hevea brasiliensis cultivars. Int. J. Mol. Sci. 2018, 19, 947. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Zhu, L.; Yao, Q.; Meng, X.; Ding, G.; Wang, D.; Xie, Q.; Tong, Z.; Tao, C.; Yu, L.; et al. Expression profiling of mitogen-activated protein kinase genes reveals their evolutionary and functional diversity in different rubber tree (Hevea brasiliensis) cultivars. Genes 2017, 8, 261. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.; Agarwal, P.; Arora, R.; Kapoor, S.; Tyagi, A.K. Expression analysis of calcium-dependent protein kinase gene family during reproductive development and abiotic stress conditions in rice (Oryza sativa L. ssp. indica). Mol. Genet. Genom. 2007, 278, 493–505. [Google Scholar] [CrossRef]
- Liu, H.; Che, Z.; Zeng, X.; Zhou, X.; Sitoe, H.M.; Wang, H.; Yu, D. Genome-wide analysis of calcium-dependent protein kinases and their expression patterns in response to herbivore and wounding stresses in soybean. Func. Integr. Genom. 2016, 16, 481–493. [Google Scholar] [CrossRef]
- Hu, Z.; Lv, X.; Xia, X.; Zhou, J.; Shi, K.; Yu, J.; Zhou, Y. Genome-wide identification and expression analysis of calcium-dependent protein kinase in tomato. Front. Plant Sci. 2016, 7, 469. [Google Scholar] [CrossRef] [Green Version]
- Fantino, E.; Segretin, M.E.; Santin, F.; Mirkin, F.G.; Ulloa, R.M. Analysis of the potato calcium-dependent protein kinase family and characterization of StCDPK7, a member induced upon infection with Phytophthora infestans. Plant Cell Rep. 2017, 36, 1137–1157. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.H.; Yang, M.; Sui, J.L.; Qi, J.Y.; Fang, Y.J.; Hu, S.N.; Tang, C.R. The calcium-dependent protein kinase (CDPK) and CDPK-related kinase gene families in Hevea brasiliensis-comparison with five other plant species in structure, evolution, and expression. FEBS Open 2016, 7, 4–24. [Google Scholar] [CrossRef] [PubMed]
- Boudet, N.; Aubourg, S.; Toffano-Nioche, C.; Kreis, M.; Lecharny, A. Evolution of intron/exon structure of DEAD helicase family genes in Arabidopsis, Caenorhabditis, and Drosophila. Genome Res. 2001, 11, 2101–2114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, K.; Chen, J.; Wang, Y.; Chen, Y.; Chen, S.; Lin, Y.; Pan, S.; Zhong, X.; Xie, D. Genome-wide analysis of bZIP-encoding genes in maize. DNA Res. 2012, 19, 463–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, S.; Li, S.; Asim, M.; Mao, J.; Xu, D.; Ullah, Z.; Liu, G.; Wang, Q.; Liu, H. The Arabidopsis calcium-dependent protein kinases (CDPKs) and their roles in plant growth regulation and abiotic stress responses. Int. J. Mol. Sci. 2018, 19, 1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, X.; Lv, W.; Jiang, S.; Zhang, D.; Cai, G.; Pan, J.; Li, D. Genome-wide identification and expression analysis of calcium-dependent protein kinase in maize. BMC Genom. 2013, 14, 433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Han, Y.; Zhao, F.; Hu, Y.; Gao, Y.; Ma, Y.; Zheng, Y.; Wang, Y.; Wen, Y. Genome-wide identification and expression analysis of the CDPK gene family in grape, Vitis spp. BMC Plant Biol. 2015, 15, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Liu, M.; Lu, L.; He, M.; Qu, W.; Xu, Q.; Qi, X.; Chen, X. Genome-wide analysis and expression of the calcium-dependent protein kinase gene family in cucumber. Mol. Genet. Genom. 2015, 290, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, W.; He, Q.; Daud, M.K.; Chen, J.; Zhu, S. Genome-wide survey and expression analysis of calcium-dependent protein kinase in Gossypium raimondii. PLoS ONE 2014, 9, e98189. [Google Scholar] [CrossRef] [Green Version]
- Valmonte, G.R.; Arthur, K.; Higgins, C.M.; MacDiarmid, R.M. Calcium-dependent protein kinases in plants: Evolution, expression and function. Plant Cell Physiol. 2014, 55, 551–569. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.H.; Niu, Y.; Konishi, M.; Wu, Y.; Du, H.; Sun, C.H.; Li, L.; Boudsocq, M.; McCormack, M.; Maekawa, S.; et al. Discovery of nitrate-CPK-NLP signalling in central nutrient-growth networks. Nature 2017, 18, 311–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, J.J.; Li, X.D.; Ratnasekera, D.; Wang, C.; Liu, W.X.; Song, L.F.; Zhang, W.Z.; Wu, W.H. Arabidopsis calcium-dependent protein kinase 8 and catalase 3 function in abscisic acid-mediated signaling and H2O2 homeostasis in stomatal guard cells under drought stress. Plant Cell 2015, 27, 1445–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.N.; Shen, L.K.; Zhang, W.Z.; Zhang, W.; Wang, Y.; Wu, W.H. Ca2+-dependent protein kinase11 and 24 modulate the activity of the inward rectifying K+ channels in Arabidopsis pollen tubes. Plant Cell. 2013, 25, 649–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boudsocq, M.; Sheen, J. CDPKs in immune and stress signaling. Trends Plant Sci. 2013, 18, 30–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronzier, E.; Corratgé-Faillie, C.; Sanchez, F.; Prado, K.; Brière, C.; Leonhardt, N.; Thibaud, J.B.; Xiong, T.C. CPK13, a noncanonical Ca2+-dependent protein kinase, specifically inhibits KAT2 and KAT1 shaker K+ channels and reduces stomatal opening. Plant Physiol. 2014, 166, 314–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [Green Version]
- Tajima, F. Simple methods for testing the molecular evolutionary clock hypothesis. Genetics 1993, 135, 599–607. [Google Scholar]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [Green Version]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Xia, R.; Chen, H.; He, Y. TBtools, a Toolkit for Biologists integrating various HTS-data handling tools with a user-friendly interface. bioRxiv 2018, 289660. [Google Scholar] [CrossRef]
| CPKs a | Species | Exon Number | CDS | aa | Pal b | Myr b | |||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | ||||||
| OsCPKs | Oryza sativa | 3–8 | 6–8 | 5–8 | 11–12 | 1539–1839 | 513–613 | 14 | 22 |
| AtCPKs | Arabidopsis thaliana | 6–7 | 7–9 | 7–8 | 12–13 | 1455–1941 | 484–646 | 21 | 28 |
| GmCPKs | Glycine max | 7 | 5–8 | 7–8 | 10–13 | 1377–1788 | 459–596 | 21 | 28 |
| SlCPKs | Solanum lycopersicum | 7–8 | 5–9 | 7–9 | 12 | 1290–1797 | 430–599 | 15 | 23 |
| StCPKs | Solanum tuberosum | 7–8 | 8–9 | 7–9 | 12 | 1530–2626 | 510–638 | 13 | 22 |
| TkCPKs | Taraxacum koksaghyz | 6–8 | 8 | 7–8 | 12 | 145–1779 | 483–592 | 22 | 28 |
| CnCPKs | Chrysanthemum nankingense | 6–9 | 8–9 | 7–10 | 8–12 | 1230–1815 | 409–604 | 11 | 22 |
| HaCPKs | Helianthus annuus | 7–9 | 7–8 | 7–8 | 8–12 | 1470–1896 | 490–632 | 25 | 35 |
| LsCPKs | Lactuca sativa | 6–7 | 7–8 | 7–8 | 8–12 | 1464–1800 | 488–600 | 20 | 28 |
| CcCPKs | Cynara cardunculus | 7 | 7–8 | 7–8 | 8–12 | 1470–1824 | 489–607 | 18 | 25 |
| HbCPKs | Hevea brasiliensis | 7 | 8–9 | 7–10 | 12 | 1110–1773 | 369–590 | 18 | 25 |
| Testing Group a | Group | Mt b | M1 c | M2 d | χ2 | pe |
|---|---|---|---|---|---|---|
| TkCPK7/TkCPK11 with SlCPK20 | II | 408 | 0 | 0 | 0.00 | 1.00000 |
| TkCPK9/TkCPK15 with SlCPK24 | III | 357 | 0 | 0 | 0.00 | 1.00000 |
| TkCPK25/TkCPK33 with SlCPK23 | III | 452 | 0 | 1 | 1.00 | 0.31731 |
| TkCPK26/TkCPK31 with SlCPK8 | I | 445 | 0 | 0 | 0.00 | 1.00000 |
| TkCPK8/TkCPK21 with SlCPK25 | III | 454 | 13 | 13 | 0.00 | 1.00000 |
| TkCPK26/TkCPK27 with SlCPK8 | I | 417 | 8 | 28 | 11.11 | 0.00086 |
| TkCPK27/TkCPK31 with SlCPK8 | I | 417 | 8 | 28 | 11.11 | 0.00086 |
| TkCPK4/TkCPK19 with SlCPK26 | III | 425 | 16 | 11 | 0.93 | 0.33592 |
| TkCPK4/TkCPK32 with SlCPK26 | III | 409 | 16 | 27 | 2.81 | 0.09345 |
| TkCPK3/TkCPK28 with SlCPK18 | II | 380 | 21 | 20 | 0.02 | 0.87590 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Zheng, B.; Song, W.; Li, H.; Jin, X. Evolutionary Analysis of Calcium-Dependent Protein Kinase in Five Asteraceae Species. Plants 2020, 9, 32. https://doi.org/10.3390/plants9010032
Zhu L, Zheng B, Song W, Li H, Jin X. Evolutionary Analysis of Calcium-Dependent Protein Kinase in Five Asteraceae Species. Plants. 2020; 9(1):32. https://doi.org/10.3390/plants9010032
Chicago/Turabian StyleZhu, Liping, Bowen Zheng, Wangyang Song, Hongbin Li, and Xiang Jin. 2020. "Evolutionary Analysis of Calcium-Dependent Protein Kinase in Five Asteraceae Species" Plants 9, no. 1: 32. https://doi.org/10.3390/plants9010032





