Enzymatic Properties of Recombinant Phospho-Mimetic Photorespiratory Glycolate Oxidases from Arabidopsis thaliana and Zea mays
Abstract
1. Introduction
2. Results
2.1. Phosphopeptides and Phosphorylated Residues of Arabidopsis GOX1 and GOX2
2.2. Phospho-Mimetic AtGOX1, AtGOX2 and ZmGO1 Exhibit Altered Glycolate Oxidase Activities
2.3. Phospho-site Mutations at T4/5 and T158/159 Have a Limited Effect on Substrate Specificity
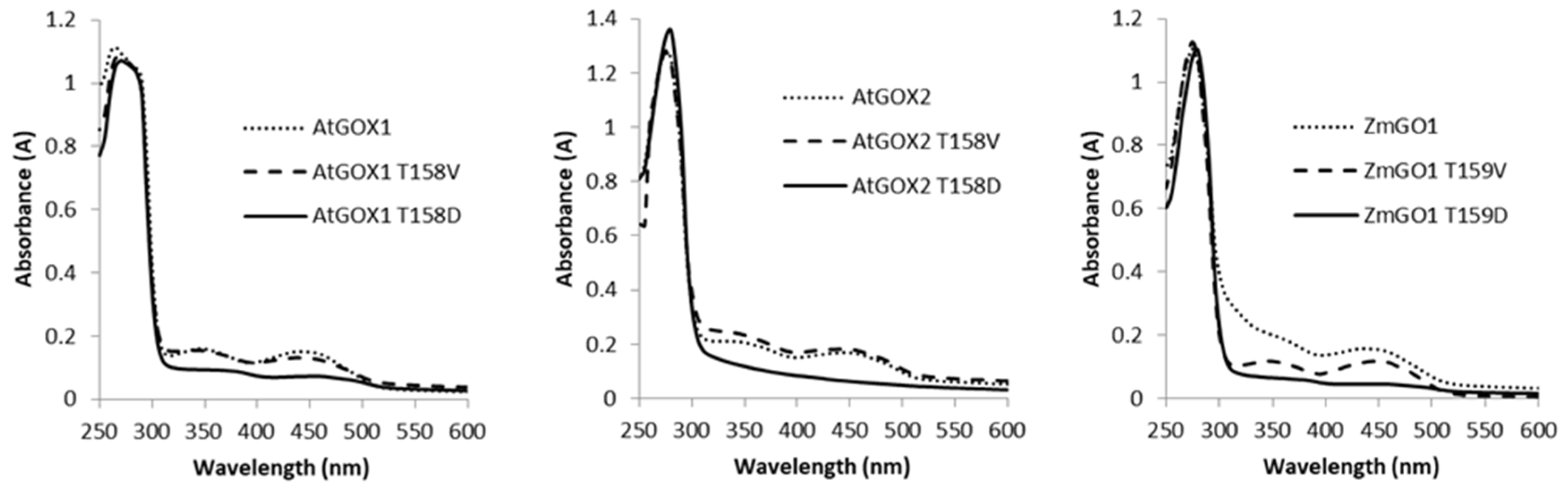
2.4. Phospho-Mimetic T4/5D and T158/159D Recombinant Proteins Lack FMN
3. Discussion
3.1. Phospho-Mimetic GOX and Inhibition of Enzyme Activity
3.2. Phospho-Site Mutations Suggest Differences between Photorespiratory GOX Proteins
3.3. Discovering Conditions Inducing GOX Phosphorylation and Identifying GOX Kinases
4. Materials and Methods
4.1. Plasmid Constructions and Site-Directed Mutagenesis
4.2. Purification of Recombinant GOX Proteins, SDS-PAGE and Determination of FMN Content
4.3. GOX Activity Measurements
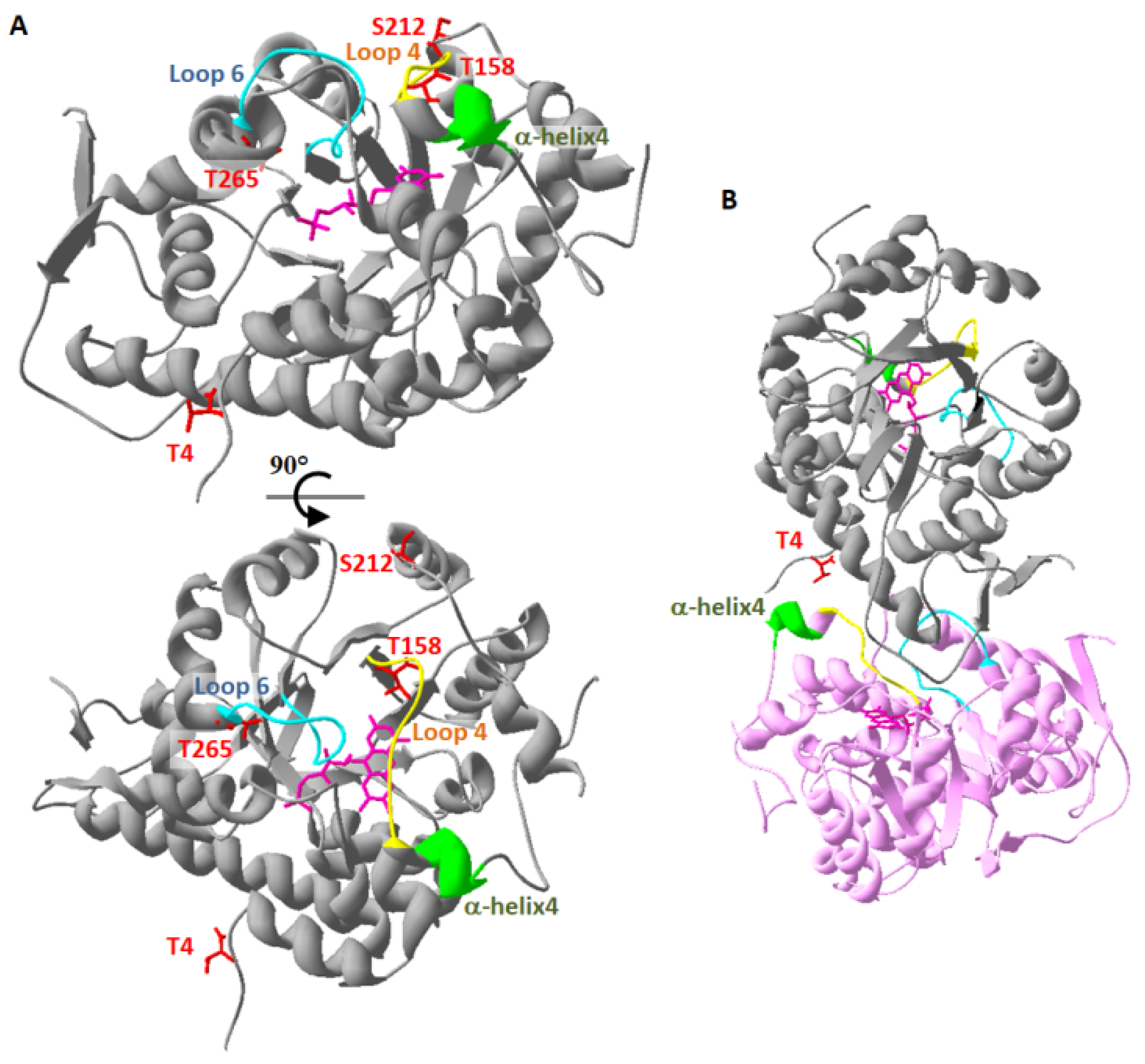
4.4. Structural Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bauwe, H.; Hagemann, M.; Fernie, A.R. Photorespiration: Players, partners and origin. Trends Plant Sci. 2010, 15, 330–336. [Google Scholar] [CrossRef] [PubMed]
- South, P.F.; Cavanagh, A.P.; Liu, H.W.; Ort, D.R. Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field. Science 2019, 363, eaat9077. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.R.; Wang, L.M.; Lin, X.L.; Yao, Z.; Xu, H.W.; Zhu, C.H.; Teng, H.Y.; Cui, L.L.; Liu, E.E.; Zhang, J.J.; et al. Engineering a New Chloroplastic photorespiratory bypass to increase photosynthetic efficiency and productivity in rice. Mol. Plant 2019, 12, 199–214. [Google Scholar] [CrossRef]
- Flügel, F.; Timm, S.; Arrivault, S.; Florian, A.; Stitt, M.; Fernie, A.R.; Bauwe, H. The photorespiratory metabolite 2-Phosphoglycolate regulates photosynthesis and starch accumulation in Arabidopsis. Plant Cell 2017, 29, 2537–2551. [Google Scholar] [CrossRef] [PubMed]
- Dellero, Y.; Jossier, M.; Schmitz, J.; Maurino, V.G.; Hodges, M. Photorespiratory glycolate-glyoxylate metabolism. J. Exp. Bot. 2016, 67, 3041–3052. [Google Scholar] [CrossRef]
- Esser, C.; Kuhn, A.; Groth, G.; Lercher, M.J.; Maurino, V.G. Plant and animal glycolate oxidases have a common eukaryotic ancestor and convergently duplicated to evolve long-chain 2-hydroxy acid oxidases. Mol. Biol. Evol. 2014, 31, 1089–1101. [Google Scholar] [CrossRef]
- Dellero, Y.; Jossier, M.; Glab, N.; Oury, C.; Tcherkez, G.; Hodges, M. Decreased glycolate oxidase activity leads to altered carbon allocation and leaf senescence after a transfer from high CO2 to ambient air in Arabidopsis thaliana. J. Exp. Bot. 2016, 67, 3149–3163. [Google Scholar] [CrossRef]
- Engqvist, M.K.M.; Schmitz, J.; Gertzmann, A.; Florian, A.; Jaspert, N.; Arif, M.; Balazadeh, S.; Mueller-Roeber, B.; Fernie, A.R.; Maurino, V.G. GLYCOLATE OXIDASE3, a glycolate oxidase homolog of yeast L-lactate cytochrome c oxidoreductase, supports L-lactate oxidation in roots of arabidopsis. Plant Physiol. 2015, 169, 1042–1061. [Google Scholar] [CrossRef]
- Rojas, C.M.; Senthil-Kumar, M.; Wang, K.; Ryu, C.-M.; Kaundal, A.; Mysore, K.S. Glycolate oxidase modulates reactive oxygen species-mediated signal transduction during nonhost resistance in Nicotiana benthamiana and Arabidopsis. Plant Cell 2012, 24, 336–352. [Google Scholar] [CrossRef]
- Saji, S.; Bathula, S.; Kubo, A.; Tamaoki, M.; Aono, M.; Sano, T.; Tobe, K.; Timm, S.; Bauwe, H.; Nakajima, N.; et al. Ozone-sensitive arabidopsis mutants with deficiencies in photorespiratory enzymes. Plant Cell Physiol. 2017, 58, 914–924. [Google Scholar] [CrossRef]
- Kerchev, P.; Waszczak, C.; Lewandowska, A.; Willems, P.; Shapiguzov, A.; Li, Z.; Alseekh, S.; Mühlenbock, P.; Hoeberichts, F.A.; Huang, J.; et al. Lack of GLYCOLATE OXIDASE1, but not GLYCOLATE OXIDASE2, attenuates the photorespiratory phenotype of CATALASE2-deficient Arabidopsis. Plant Physiol. 2016, 171, 1704–1719. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Nishimura, M. Reduction to below threshold levels of glycolate oxidase activities in transgenic tobacco enhances photoinhibition during irradiation. Plant Cell Physiol. 2000, 41, 1397–1406. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, J.; Zeng, J.; Jiang, L.; Liu, E.; Peng, C.; He, Z.; Peng, X. Inducible antisense suppression of glycolate oxidase reveals its strong regulation over photosynthesis in rice. J. Exp. Bot. 2009, 60, 1799–1809. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Y.; Yang, Q.; Zhang, Z.; Chen, Y.; Zhang, S.; Peng, X.X. Suppression of glycolate oxidase causes glyoxylate accumulation that inhibits photosynthesis through deactivating Rubisco in rice. Physiol. Plant. 2014, 150, 463–476. [Google Scholar] [CrossRef]
- Zelitch, I.; Schultes, N.P.; Peterson, R.B.; Brown, P.; Brutnell, T.P. High glycolate oxidase activity is required for survival of maize in normal air. Plant Physiol. 2009, 149, 195–204. [Google Scholar] [CrossRef]
- Hodges, M.; Dellero, Y.; Keech, O.; Betti, M.; Raghavendra, A.S.; Sage, R.; Zhu, X.-G.; Allen, D.K.; Weber, A.P.M. Perspectives for a better understanding of the metabolic integration of photorespiration within a complex plant primary metabolism network. J. Exp. Bot. 2016, 67, 3015–3026. [Google Scholar] [CrossRef]
- Timm, S.; Florian, A.; Wittmiß, M.; Jahnke, K.; Hagemann, M.; Fernie, A.R.; Bauwe, H.; Rostock, D.; Germany, S.T. Serine acts as a metabolic signal for the transcriptional control of photorespiration-related genes. Plant Physiol. 2013, 162, 379–389. [Google Scholar] [CrossRef]
- Sandalio, L.M.; Gotor, C.; Romero, L.C.; Romero-Puertas, M.C. Multilevel regulation of peroxisomal proteome by post-translational modifications. Int. J. Mol. Sci. 2019, 20, 4881. [Google Scholar] [CrossRef]
- Ortega-Galisteo, A.P.; Rodríguez-Serrano, M.; Pazmiño, D.M.; Gupta, D.K.; Sandalio, L.M.; Romero-Puertas, M.C. S-Nitrosylated proteins in pea (Pisum sativum L.) leaf peroxisomes: Changes under abiotic stress. J. Exp. Bot. 2012, 63, 2089–2103. [Google Scholar] [CrossRef]
- Palmieri, M.C.; Lindermayr, C.; Bauwe, H.; Steinhauser, C.; Durner, J. Regulation of plant glycine decarboxylase by S-nitrosylation and glutathionylation. Plant Physiol. 2010, 152, 1514–1528. [Google Scholar] [CrossRef]
- Bartsch, O.; Mikkat, S.; Hagemann, M.; Bauwe, H. An autoinhibitory domain confers redox regulation to maize glycerate kinase. Plant Physiol. 2010, 153, 832–840. [Google Scholar] [CrossRef]
- Da Fonseca-Pereira, F.; Geigenberger, P.; Thormählen, I.; Souza, P.V.L.; Nesi, A.N.; Timm, S.; Fernie, A.R.; Araújo, W.L.; Daloso, D.M. Thioredoxin h2 contributes to the redox regulation of mitochondrial photorespiratory metabolism. Plant Cell Environ. 2019. [Google Scholar] [CrossRef]
- Reinholdt, O.; Schwab, S.; Zhang, Y.; Reichheld, J.; Alisdair, R. Redox-regulation of photorespiration through mitochondrial thioredoxin o1. Plant Physiol. 2019, 181, 442–457. [Google Scholar] [CrossRef]
- Hodges, M.; Jossier, M.; Boex-Fontvieille, E.; Tcherkez, G. Protein phosphorylation and photorespiration. Plant Biol. 2013, 15, 694–706. [Google Scholar] [CrossRef]
- Wu, X.N.; Rodriguez, C.S.; Pertl-Obermeyer, H.; Obermeyer, G.; Schulze, W.X. Sucrose-induced receptor kinase SIRK1 regulates a plasma membrane aquaporin in Arabidopsis. Mol. Cell. Proteom. 2013, 12, 2856–2873. [Google Scholar] [CrossRef]
- Umezawa, T.; Sugiyama, N.; Takahashi, F.; Anderson, J.C.; Ishihama, Y.; Peck, S.C.; Shinozaki, K. Genetics and phosphoproteomics reveal a protein phosphorylation network in the abscisic acid signaling pathway in Arabidopsis thaliana. Sci. Signal. 2013, 6, rs8. [Google Scholar] [CrossRef]
- Choudhary, M.K.; Nomura, Y.; Wang, L.; Nakagami, H.; Somers, D.E. Quantitative Circadian Phosphoproteomic analysis of Arabidopsis reveals extensive clock control of key components in physiological, metabolic, and signaling pathways. Mol. Cell. Proteom. 2015, 14, 2243–2260. [Google Scholar] [CrossRef]
- Abadie, C.; Mainguet, S.; Davanture, M.; Hodges, M.; Zivy, M.; Tcherkez, G. Concerted changes in the phosphoproteome and metabolome under different CO2/O2 gaseous conditions in Arabidopsis rosettes. Plant Cell Physiol. 2016, 57, 1544–1556. [Google Scholar]
- Aryal, U.K.; Krochko, J.E.; Ross, A.R.S. Identification of phosphoproteins in arabidopsis thaliana leaves using polyethylene glycol fractionation, immobilized metal-ion affinity chromatography, two-dimensional gel electrophoresis and mass spectrometry. J. Proteome Res. 2012, 11, 425–437. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, W.; Chen, Z. Structures of glycolate oxidase from Nicotiana benthamiana reveal a conserved pH sensor affecting the binding of FMN. Biochem. Biophys. Res. Commun. 2018, 503, 3050–3056. [Google Scholar] [CrossRef]
- Lindqvist, Y.; Brändén, C.-I. The structure of glycolate oxidase from spinach. Proc. Natl. Acad. Sci. USA 1985, 82, 6855–6859. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, Y.; Brändén, C.I. The active site of spinach glycolate oxidase. J. Biol. Chem. 1989, 264, 3624–3628. [Google Scholar] [PubMed]
- Macheroux, P.; Kieweg, V.; Massey, V.; Söderlind, E.; Stenberg, K.; Lindqvist, Y. Role of tyrosine 129 in the active site of spinach glycolate oxidase. Eur. J. Biochem. 1993, 213, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Dellero, Y.; Mauve, C.; Boex-Fontvieille, E.; Flesch, V.; Jossier, M.; Tcherkez, G.; Hodges, M. Experimental evidence for a hydride transfer mechanism in plant glycolate oxidase catalysis. J. Biol. Chem. 2015, 290, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-P.; Yang, J.; Cai, X.-Z. Glycolate oxidase gene family in Nicotiana benthamiana: Genome-wide identification and functional analyses in disease resistance. Sci. Rep. 2018, 8, 8615. [Google Scholar] [CrossRef]
- Chern, M.; Bai, W.; Chen, X.; Canlas, P.E.; Ronald, P.C. Reduced expression of glycolate oxidase leads to enhanced disease resistance in rice. PeerJ 2013, 1, e28. [Google Scholar] [CrossRef]
- Fujita, Y.; Nakashima, K.; Yoshida, T.; Katagiri, T.; Kidokoro, S.; Kanamori, N.; Umezawa, T.; Fujita, M.; Maruyama, K.; Ishiyama, K.; et al. Three SnRK2 protein kinases are the main positive regulators of abscisic acid signaling in response to water stress in arabidopsis. Plant Cell Physiol. 2009, 50, 2123–2132. [Google Scholar] [CrossRef]
- Liu, Y.; Mauve, C.; Lamothe-Sibold, M.; Guerard, F.; Glab, N.; Hodges, M.; Jossier, M. Photorespiratory serine hydroxymethyltransferase 1 activity impacts abiotic stress tolerance and stomatal closure. Plant Cell Environ. 2019, 42, 2567–2583. [Google Scholar] [CrossRef]
- Lingner, T.; Kataya, A.R.; Antonicelli, G.E.; Benichou, A.; Nilssen, K.; Chen, X.-Y.; Siemsen, T.; Morgenstern, B.; Meinicke, P.; Reumann, S. Identification of novel plant peroxisomal targeting signals by a combination of machine learning methods and in vivo subcellular targeting analyses. Plant Cell 2011, 23, 1556–1572. [Google Scholar] [CrossRef]
- Pan, R.; Hu, J. Proteomics of peroxisomes. Subcell. Biochem. 2018, 89, 3–45. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
| Gene Name, Locus | Phosphopeptide | Peptide Location | Sample Type, Age | Treatment | References |
|---|---|---|---|---|---|
| AtGOX1, At3g14420 orAtGOX2, At3g14415 | MEI(pT4)NVTEYDAIAK | 1–14/367 | Leaves | Oxygen depletion | PhosPhAt 4.0 1 |
| AIAL(pT155)VDTPRL | 151-161/367 | Seedlings, 11 days | Sucrose depletion | [25] | |
| AIALTVD(pT158)PRL | 151-161/367 | Seedling, 2 weeks | ABA and dehydration | [26] | |
| Seedlings, 10 days | Continuous light for 24 h | [27] | |||
| Rosette | Varying O2/CO2 conditions | [28] | |||
| TL(pS212)WK | 210-214/367 | Seedlings, 2 weeks | ABA and dehydration | [26] | |
| QLDYVPA(pT265)ISALEEVVK | 258-273/367 | Cauline leaves, 2 months | [29] | ||
| AtGOX1 At3g14420 | NHI(pT355)TEWDTPR | 352-362/367 | Seedlings, 10 days | Continuous light for 24 h | [27] |
| KM | kcat | KM | kcat | KM | kcat | |||
|---|---|---|---|---|---|---|---|---|
| AtGOX1 | (µM) | (s−1) | AtGOX2 | (µM) | (s−1) | ZmGO1 | (µM) | (s−1) |
| WT | 210 ± 90 | 11.12 ± 3.08 | WT | 279 ± 30 | 10.93 ± 3.48 | WT | 126 ± 34 | 11.45 ± 1.86 |
| T4V | 123 ± 35 | 9.59 ± 1.57 | T4V | 251 ± 70 | 12.09 ± 1.50 | T5V | 135 ± 56 | 8.67 ± 2.32 |
| T4D | 151 ± 44 | 0.61 ± 0.46 * | T4D | 390 ± 215 | 1.40 ± 0.42 * | T5D | 157 ± 31 | 0.53 ± 0.22 * |
| T158V | 100 ± 19 * | 2.36 ± 0.47 * | T158V | 133 ± 83 * | 2.49 ± 0.60 * | T159V | 46 ± 20 * | 2.40 ± 0.35 * |
| T158D | no activity | T158D | no activity | T159D | no activity | |||
| S212A | 257 ± 31 | 12.13 ± 2.15 | S212A | 249 ± 14 | 6.10 ± 0.64 * | S213A | 89 ± 13 | 7.19 ± 0.46 * |
| S212D | 277 ± 24 | 11.46 ± 2.31 | S212D | 325 ± 13 | 5.23 ± 0.41 * | S213D | 129 ± 28 | 6.39 ± 1.45 * |
| T265A | 276 ± 112 | 14.02 ± 2.65 | T265A | 237 ± 67 | 11.33 ± 3.27 | T266A | 91 ± 18 | 11.75 ± 2.94 |
| T265D | 531 ± 50 * | 0.14 ± 0.06 * | T265D | 388 ± 159 | 2.06 ± 1.09 * | T266D | 173 ± 65 | 0.62 ± 0.77 * |
| L-lactate | 2-hydroxy-octanoate | |||
|---|---|---|---|---|
| Enzyme | KM | kcat | KM | kcat |
| (µM) | (s−1) | (µM) | (s−1) | |
| AtGOX1WT | 1664 ± 293 | 9.09 ± 1.37 | 757 ± 82 | 5.95 ± 0.58 |
| AtGOX1T4V | 1844 ± 911 | 9.01 ± 3.59 | 350 ± 221 * | 4.66 ± 1.56 |
| AtGOX1T4D | 2377 ± 270 * | 0.30 ± 0.07 * | 1255 ± 818 | 0.39 ± 0.17 * |
| AtGOX1T158V | 549 ± 160 * | 2.30 ± 0.56 * | 191 ± 51 * | 2.89 ± 0.89 * |
| AtGOX1T158D | no activity | no activity | ||
| AtGOX2 WT | 2094 ± 791 | 6.81 ± 1.54 | 487 ± 99 | 3.78 ± 1.26 |
| AtGOX2 T4V | 2119 ± 453 | 6.65 ± 1.17 | 951 ± 91 * | 4.05 ± 2.94 |
| AtGOX2 T4D | 2019 ± 169 | 0.67 ± 0.17 * | 1721 ± 677 * | 0.59 ± 0.30 * |
| AtGOX2 T158V | 439 ± 171 * | 1.71 ± 0.53 * | 501 ± 116 | 2.46 ± 0.83 |
| AtGOX2 T158D | no activity | no activity | ||
| ZmGO1 WT | 495 ± 75 | 10.85 ± 0.08 | 136 ± 19 | 5.89 ± 0.14 |
| ZmGO1 T4V | 488 ± 233 | 11.99 ± 1.68 | 168 ± 59 | 5.88 ± 1.59 |
| ZmGO1 T4D | 719 ± 233 | 0.57 ± 0.26 * | 414 ± 84 * | 0.53 ± 0.40 * |
| ZmGO1 T158V | 161 ± 29 * | 3.55 ± 0.53 * | 48 ± 36 * | 3.22 ± 0.39 * |
| ZmGO1 T158D | no activity | no activity | ||
| AtGOX1 | Ratio 280/450 nm | AtGOX2 | Ratio 280/450 nm | ZmGO1 | Ratio 280/450 nm |
|---|---|---|---|---|---|
| WT | 8.7 ± 2.1 | WT | 8.3 ± 0.7 | WT | 8.1 ± 1.8 |
| T4V | 10.7 ± 4.1 | T4V | 6.9 ± 2.2 | T5V | 6.7 ± 3.0 |
| T4D | 28.3 ± 6.1 * | T4D | 36.5 ± 7.3 * | T5D | 25.7 ± 6.2 * |
| T158V | 8.7 ± 2.3 | T158V | 8.2 ± 1.7 | T159V | 7.9 ± 1.1 |
| T158D | 23.0 ± 8.3 * | T158D | 20.5 ± 9.3 * | T159D | 19.5 ± 7.1 * |
| S212A | 6.9 ± 3.3 | S212A | 12.4 ± 5.1 | S213A | 6.6 ± 3.5 |
| S212D | 6.6 ± 4.0 | S212D | 10.4 ± 2.2 | S213D | 6.1 ± 1.7 |
| T265A | 9 ± 3.3 | T265A | 9.6 ± 2.4 | T266A | 7.0 ± 0.1 |
| T265D | 8.5 ± 2.5 | T265D | 11.8 ± 4.5 | T266D | 21.2 ± 12.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jossier, M.; Liu, Y.; Massot, S.; Hodges, M. Enzymatic Properties of Recombinant Phospho-Mimetic Photorespiratory Glycolate Oxidases from Arabidopsis thaliana and Zea mays. Plants 2020, 9, 27. https://doi.org/10.3390/plants9010027
Jossier M, Liu Y, Massot S, Hodges M. Enzymatic Properties of Recombinant Phospho-Mimetic Photorespiratory Glycolate Oxidases from Arabidopsis thaliana and Zea mays. Plants. 2020; 9(1):27. https://doi.org/10.3390/plants9010027
Chicago/Turabian StyleJossier, Mathieu, Yanpei Liu, Sophie Massot, and Michael Hodges. 2020. "Enzymatic Properties of Recombinant Phospho-Mimetic Photorespiratory Glycolate Oxidases from Arabidopsis thaliana and Zea mays" Plants 9, no. 1: 27. https://doi.org/10.3390/plants9010027
APA StyleJossier, M., Liu, Y., Massot, S., & Hodges, M. (2020). Enzymatic Properties of Recombinant Phospho-Mimetic Photorespiratory Glycolate Oxidases from Arabidopsis thaliana and Zea mays. Plants, 9(1), 27. https://doi.org/10.3390/plants9010027






