Insight into Composition of Bioactive Phenolic Compounds in Leaves and Flowers of Green and Purple Basil
Abstract
1. Introduction
2. Results
2.1. Evaluation of the Main Phenotypic Traits in Plants, Leaves and Flowers
2.2. Total Phenolic Compounds, Anthocyanins Contents and Antioxidant Capacity in Leaves, Flowers and Corollas
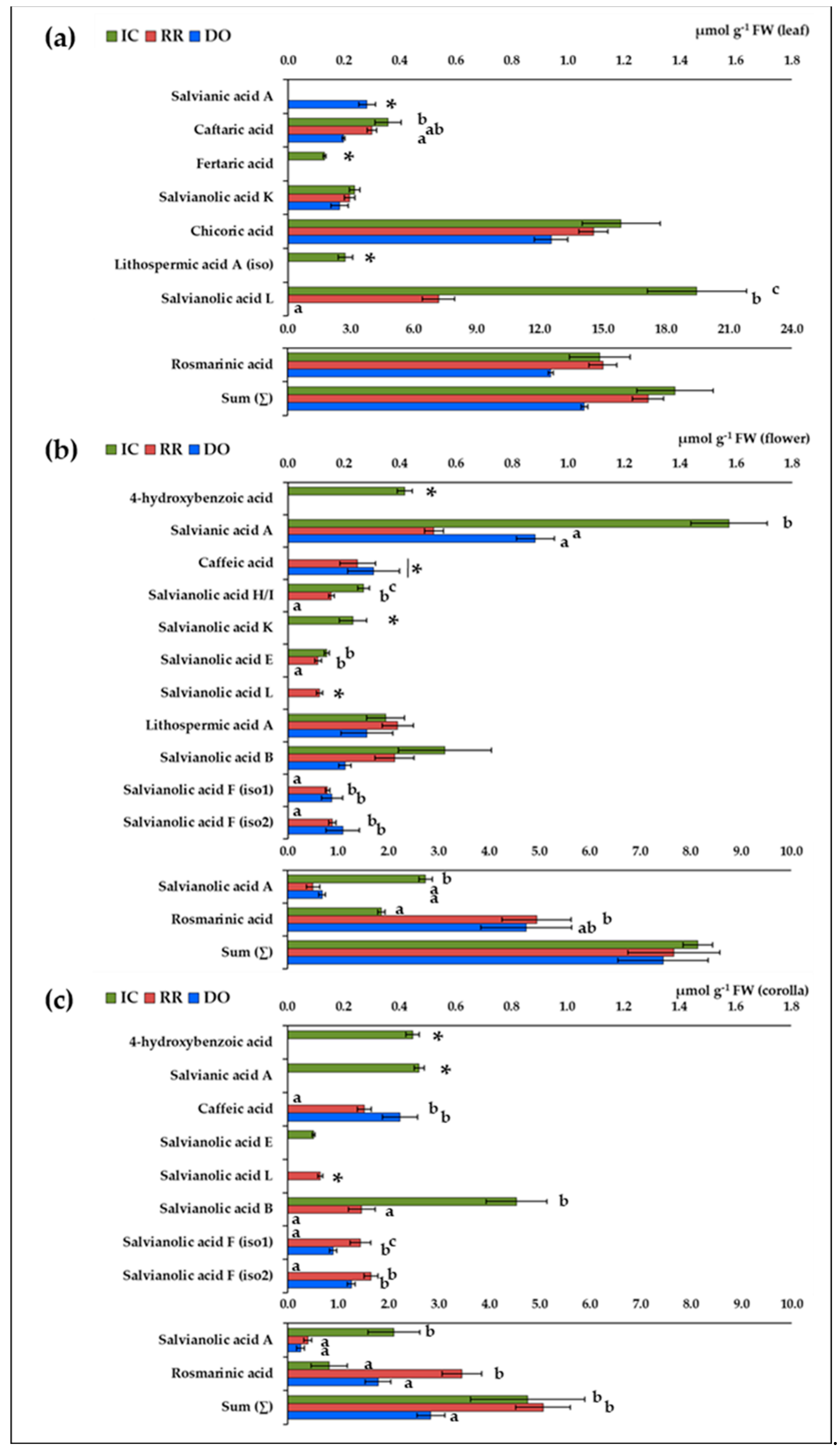
2.3. Identification and Quantification of Individual (Poly)phenolic Acids
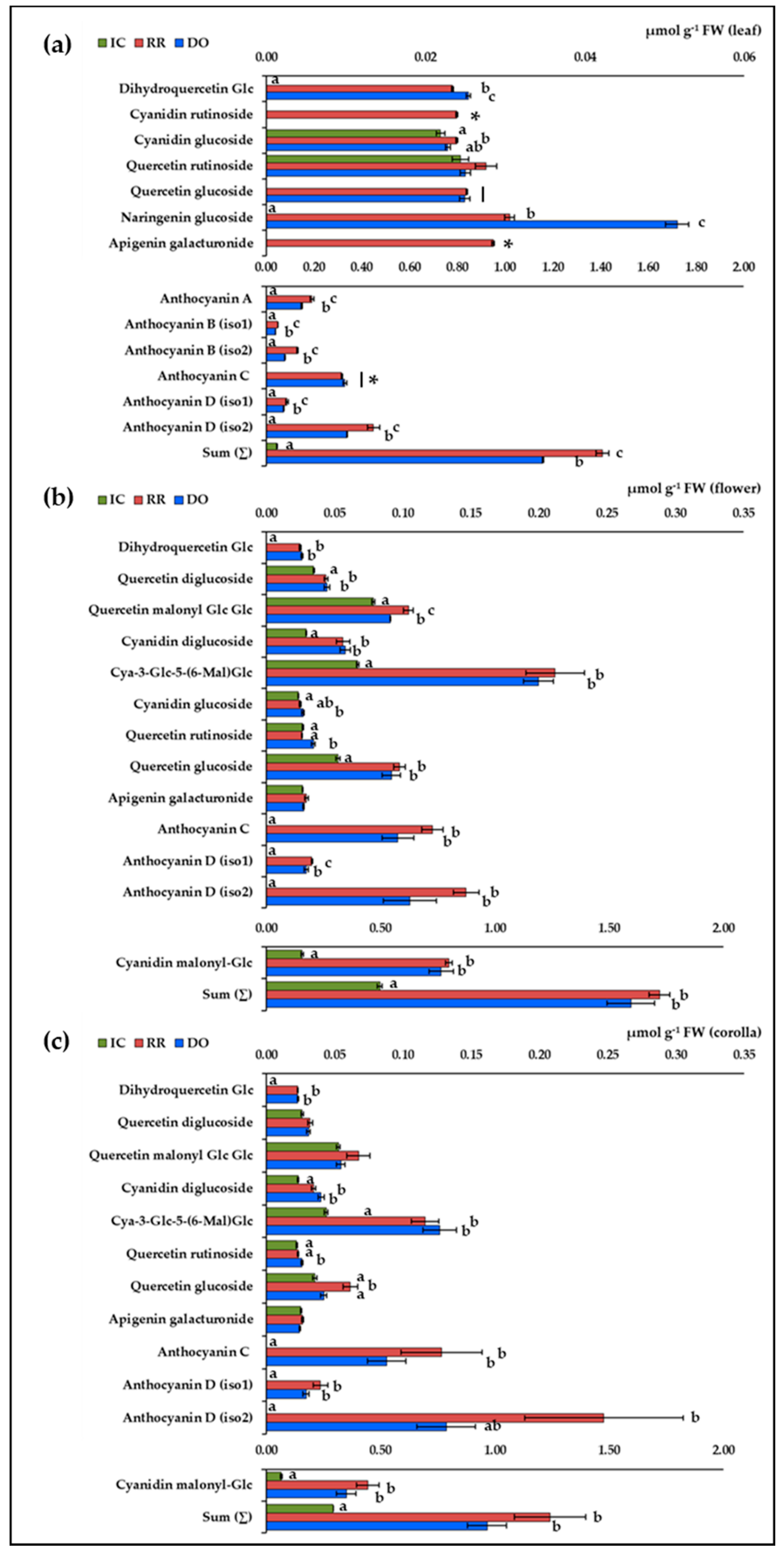
2.4. Identification and Quantification of Individual Flavonoids
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growing Conditions
4.2. Determination of the Total Phenol Compounds and Anthocyanins Contents
4.3. Quantification of Individual (Poly)Phenolic Acids and Flavonoids
Author Contributions
Funding
Conflicts of Interest
References
- Simon, J.E.; Morales, M.R.; Phippen, W.B.; Vieira, R.F.; Hao, Z. Basil: A Source of Aroma Compounds and a Popular Culinary and Ornamental Herb. In Perspective on New Crops and New Uses; Janick, J., Ed.; ASHS Press: Alexandria, VA, USA, 1999; pp. 499–505. [Google Scholar]
- Makri, O.; Kintzios, S. Ocimum Sp. (Basil): Botany, Cultivation, Pharmaceutical Properties, and Biotechnology. J. Herbs Spices Med. Plants 2008, 13, 123–150. [Google Scholar] [CrossRef]
- Phippen, W.B.; Simon, J.E. Anthocyanins in Basil (Ocimum basilicum L.). J. Agric. Food Chem. 1998, 46, 1734–1738. [Google Scholar] [CrossRef]
- Mastaneh, M.; Ahamd, M.; Taher, N.; Mehrdad, H. Antioxidant Effect of Purple Basil (Lamiaceae) Phenolics. Orient. J. Chem. 2014, 30, 1965–1969. [Google Scholar] [CrossRef]
- Ch, M.; Naz, S.; Sharif, A.; Akram, M.; Saeed, M. Biological and Pharmacological Properties of the Sweet Basil (Ocimum basilicum). Br. J. Pharm. Res. 2015, 7, 330–339. [Google Scholar] [CrossRef]
- Jayasinghe, C.; Gotoh, N.; Aoki, T.; Wada, S. Phenolics Composition and Antioxidant Activity of Sweet Basil (Ocimum basilicum L.). J. Agric. Food Chem. 2003, 51, 4442–4449. [Google Scholar] [CrossRef]
- Lee, J.; Scagel, C.F. Chicoric Acid Found in Basil (Ocimum basilicum L.) Leaves. Food Chem. 2009, 115, 650–656. [Google Scholar] [CrossRef]
- Petersen, M. Rosmarinic Acid: New Aspects. Phytochem. Rev. 2013, 12, 207–227. [Google Scholar] [CrossRef]
- Miguel, M.G. Anthocyanins: Antioxidant and/or Anti-Inflammatory Activities. J. Appl. Pharm. Sci. 2011, 1, 7–15. [Google Scholar]
- Luna, M.C.; Bekhradi, F.; Ferreres, F.; Jordán, M.J.; Delshad, M.; Gil, M.I. Effect of Water Stress and Storage Time on Anthocyanins and Other Phenolics of Different Genotypes of Fresh Sweet Basil. J. Agric. Food Chem. 2015, 63, 9223–9231. [Google Scholar] [CrossRef]
- Szymanowska, U.; Złotek, U.; Karaś, M.; Baraniak, B. Anti-Inflammatory and Antioxidative Activity of Anthocyanins from Purple Basil Leaves Induced by Selected Abiotic Elicitors. Food Chem. 2015, 172, 71–77. [Google Scholar] [CrossRef]
- Mol, J.; Grotewold, E.; Koes, R. How Genes Paint Flowers and Seeds. Trends Plant Sci. 1998, 3, 212–217. [Google Scholar] [CrossRef]
- Hatier, J.-H.B.; Gould, K.S. Anthocyanin Function in Vegetative Organs. In Anthocyanins; Winefield, C., Davies, K., Gould, K., Eds.; Springer: New York, NY, USA, 2008; pp. 1–19. [Google Scholar] [CrossRef]
- Landi, M.; Guidi, L.; Pardossi, A.; Tattini, M.; Gould, K.S. Photoprotection by Foliar Anthocyanins Mitigates Effects of Boron Toxicity in Sweet Basil (Ocimum basilicum). Planta 2014, 240, 941–953. [Google Scholar] [CrossRef]
- Tattini, M.; Landi, M.; Brunetti, C.; Giordano, C.; Remorini, D.; Gould, K.S.; Guidi, L. Epidermal Coumaroyl Anthocyanins Protect Sweet Basil against Excess Light Stress: Multiple Consequences of Light Attenuation. Physiol. Plant. 2014, 152, 585–598. [Google Scholar] [CrossRef]
- Javanmardi, J.; Khalighi, A.; Kashi, A.; Bais, H.P.; Vivanco, J.M. Chemical Characterization of Basil (Ocimum basilicum L.) Found in Local Accessions and Used in Traditional Medicines in Iran. J. Agric. Food Chem. 2002, 50, 5878–5883. [Google Scholar] [CrossRef] [PubMed]
- Kwee, E.M.; Niemeyer, E.D. Variations in Phenolic Composition and Antioxidant Properties among 15 Basil (Ocimum basilicum L.) Cultivars. Food Chem. 2011, 128, 1044–1050. [Google Scholar] [CrossRef]
- Flanigan, P.M.; Niemeyer, E.D. Effect of Cultivar on Phenolic Levels, Anthocyanin Composition, and Antioxidant Properties in Purple Basil (Ocimum basilicum L.). Food Chem. 2014, 164, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Ferrante, C.; Gnapi, D.E.; Sinan, K.I.; Orlando, G.; Recinella, L.; Diuzheva, A.; Jekő, J.; Cziáky, Z.; Chiavaroli, A.; et al. Comprehensive Approaches on the Chemical Constituents and Pharmacological Properties of Flowers and Leaves of American Basil (Ocimum americanum L). Food Res. Int. 2019, 125, 108610. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Barreira, J.C.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Incorporation of Natural Colorants Obtained from Edible Flowers in Yogurts. LWT 2018, 97, 668–675. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible Flowers: Emerging Components in the Diet. Trends Food Sci. Technol. 2019, 93, 244–258. [Google Scholar] [CrossRef]
- Mačukanović-Jocić, M.P.; Rančić, D.V.; Dajić Stevanović, Z.P. Floral Nectaries of Basil (Ocimum basilicum): Morphology, Anatomy and Possible Mode of Secretion. S. Afr. J. Bot. 2007, 73, 636–641. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R. Sweet Basil (Ocimum basilicum L.) Flowering Affected by Foliar Nitrogen Application. Acta Agrobot. 2011, 64, 57–64. [Google Scholar] [CrossRef]
- Lee, J.; Chan, B.L.S.; Mitchell, A.E. Identification/Quantification of Free and Bound Phenolic Acids in Peel and Pulp of Apples (Malus domestica) Using High Resolution Mass Spectrometry (HRMS). Food Chem. 2017, 215, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Fu, S.; Du, W.; Wang, B.; Li, L.; Zhu, M.; Liu, C.; Zhang, J. Validation and Application of an Rapid HPLC–MS Method for the Determination of Salvianic Acid A in Human Plasma. J. Chromatogr. Sci. 2015, 53, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Khoza, B.S.; Gbashi, S.; Steenkamp, P.A.; Njobeh, P.B.; Madala, N.E. Identification of Hydroxylcinnamoyl Tartaric Acid Esters in Bidens Pilosa by UPLC-Tandem Mass Spectrometry. S. Afr. J. Bot. 2016, 103, 95–100. [Google Scholar] [CrossRef]
- Ruan, M.; Li, Y.; Li, X.; Luo, J.; Kong, L. Qualitative and Quantitative Analysis of the Major Constituents in Chinese Medicinal Preparation Guan-Xin-Ning Injection by HPLC–DAD–ESI-MSn. J. Pharm. Biomed. Anal. 2012, 59, 184–189. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, Y. Rosmarinic Acid Derivatives from Salvia officinalis. Phytochemistry 1999, 51, 91–94. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Dias, M.I.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic Profiles of Cultivated, In Vitro Cultured and Commercial Samples of Melissa officinalis L. Infusions. Food Chem. 2013, 136, 1–8. [Google Scholar] [CrossRef]
- Prinsi, B.; Negri, A.S.; Quattrocchio, F.M.; Koes, R.E.; Espen, L. Proteomics of Red and White Corolla Limbs in Petunia Reveals a Novel Function of the Anthocyanin Regulator ANTHOCYANIN1 in Determining Flower Longevity. J. Proteom. 2016, 131, 38–47. [Google Scholar] [CrossRef]
- Abad-García, B.; Garmón-Lobato, S.; Berrueta, L.A.; Gallo, B.; Vicente, F. A Fragmentation Study of Dihydroquercetin Using Triple Quadrupole Mass Spectrometry and Its Application for Identification of Dihydroflavonols in Citrus Juices. Rapid Commun. Mass Spectrom. 2009, 23, 2785–2792. [Google Scholar] [CrossRef]
- Nováková, L.; Vildová, A.; Mateus, J.P.; Gonçalves, T.; Solich, P. Development and Application of UHPLC–MS/MS Method for the Determination of Phenolic Compounds in Chamomile Flowers and Chamomile Tea Extracts. Talanta 2010, 82, 1271–1280. [Google Scholar] [CrossRef]
- Lozovaya, V.V.; Lygin, A.V.; Zernova, O.V.; Ulanov, A.V.; Li, S.; Hartman, G.L.; Widholm, J.M. Modification of Phenolic Metabolism in Soybean Hairy Roots through down Regulation of Chalcone Synthase or Isoflavone Synthase. Planta 2007, 225, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Chwil, M. Flowering Pattern, The Structure of Nectary Surface and Nectar Secretion in Two Varieties of Ocimum basilicum L. Acta Agrobot. 2007, 60, 55–65. [Google Scholar] [CrossRef][Green Version]
- Hakkim, F.L.; Shankar, C.G.; Girija, S. Chemical Composition and Antioxidant Property of Holy Basil (Ocimum sanctum L.) Leaves, Stems, and Inflorescence and Their in Vitro Callus Cultures. J. Agric. Food Chem. 2007, 55, 9109–9117. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Scagel, C.F. Chicoric Acid: Chemistry, Distribution, and Production. Front. Chem. 2013, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, J.; Gong, X.; Yang, M.; Zhang, C.; Li, M. Biosynthesis, Chemistry, and Pharmacology of Polyphenols from Chinese Salvia Species: A Review. Molecules 2019, 24, 155. [Google Scholar] [CrossRef]
- Yao, Y.-F.; Wang, C.-S.; Qiao, J.; Zhao, G.-R. Metabolic Engineering of Escherichia Coli for Production of Salvianic Acid A via an Artificial Biosynthetic Pathway. Metab. Eng. 2013, 19, 79–87. [Google Scholar] [CrossRef]
- Habtemariam, S. Molecular Pharmacology of Rosmarinic and Salvianolic Acids: Potential Seeds for Alzheimer’s and Vascular Dementia Drugs. Int. J. Mol. Sci. 2018, 19, 458. [Google Scholar] [CrossRef]
- Liu, R.H. Health Benefits of Fruit and Vegetables Are from Additive and Synergistic Combinations of Phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517–520. [Google Scholar] [CrossRef]
- Park, C.; Park, S.-Y.; Lee, S.; Kim, J.; Park, S. Analysis of Metabolites in White Flowers of Magnolia Denudata Desr. and Violet Flowers of Magnolia Liliiflora Desr. Molecules 2018, 23, 1558. [Google Scholar] [CrossRef]
- Zhang, F.-P.; Yang, Q.-Y.; Zhang, S.-B. Dual Effect of Phenolic Nectar on Three Floral Visitors of Elsholtzia Rugulosa (Lamiaceae) in SW China. PLoS ONE 2016, 11, 0154381. [Google Scholar] [CrossRef]
- Kovinich, N.; Kayanja, G.; Chanoca, A.; Otegui, M.S.; Grotewold, E. Abiotic Stresses Induce Different Localizations of Anthocyanins in Arabidopsis. Plant Signal Behav. 2015. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L. Determination of Total Phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1.1.1–I1.1.8. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Prinsi, B.; Negri, A.S.; Espen, L.; Piagnani, M.C. Proteomic Comparison of Fruit Ripening between ‘Hedelfinger’ Sweet Cherry (Prunus avium L.) and Its Somaclonal Variant ‘HS’. J. Agric. Food Chem. 2016, 64, 4171–4181. [Google Scholar] [CrossRef] [PubMed]
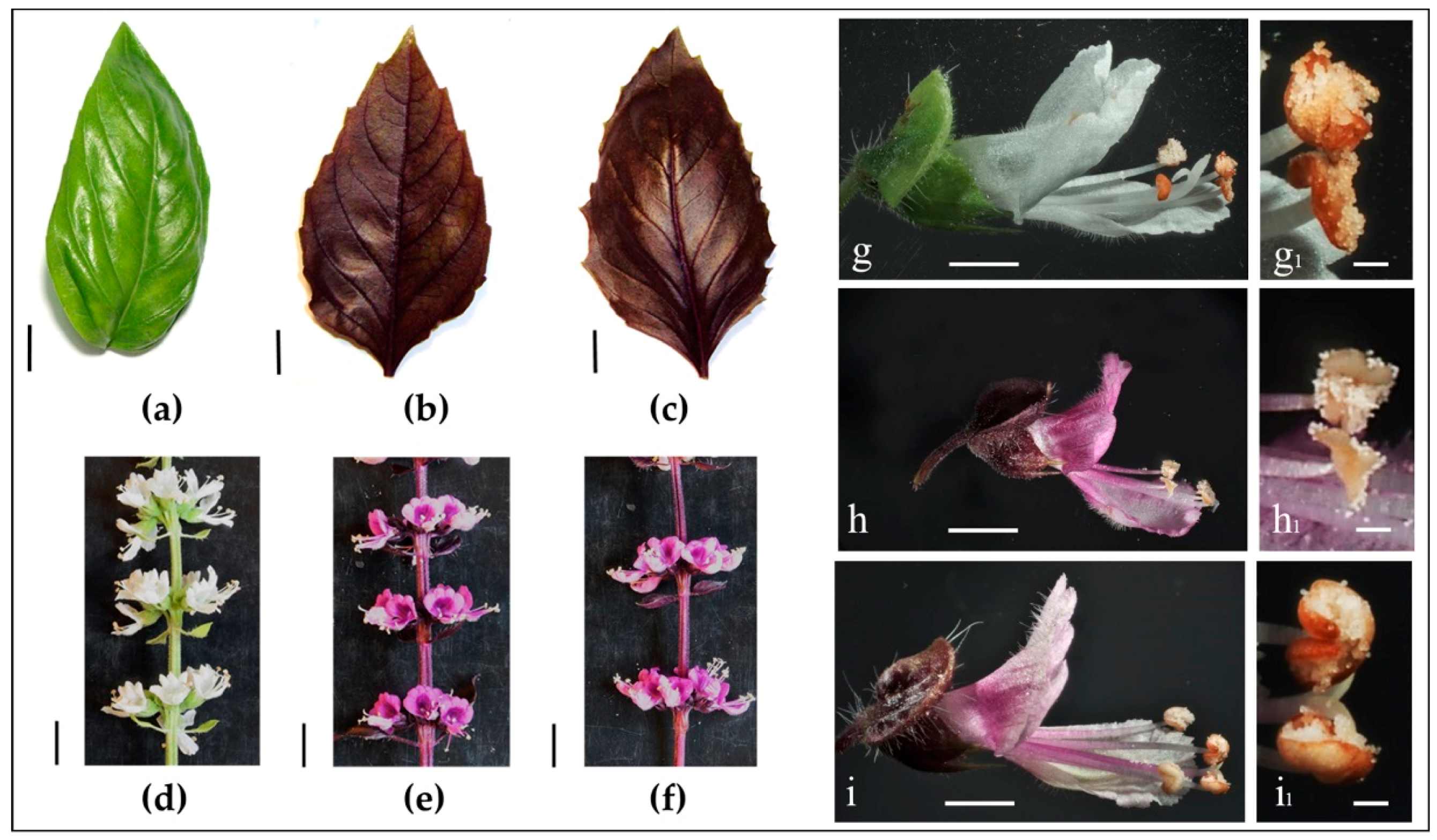
| Plant Characteristic | Variety | ||
|---|---|---|---|
| ‘Italiano Classico’ | ‘Red Rubin’ | ‘Dark Opal’ | |
| Plant height 1 (cm) | 18.83 ± 0.58 (b) | 13.58 ± 0.47 (a) | 18.00 ± 0.39 (b) |
| Leaf fresh weight (g) | 0.53 ± 0.05 (b) | 0.38 ± 0.03 (a) | 0.35 ± 0.03 (a) |
| Days to flowering | 54 ± 1 (a) | 59 ± 1 (b) | 55 ± 1 (a) |
| Flower fresh weight (mg) | 11.73 ± 0.13 (b) | 9.80 ± 0.13 (a) | 10.04 ± 0.22 (a) |
| Corolla fresh weight (mg) | 7.94 ± 0.17 (a) | 7.51 ± 0.16 (a) | 7.04 ± 0.44 (a) |
| Organ | Variety | Total Phenols (mg GAE g−1 FW) | Dvar | Dorg | Anthocyanins (µmol CGE g−1 FW) | Dvar | Dorg | Antioxidant Capacity (µmol AAE g−1 FW) | Dvar | Dorg |
|---|---|---|---|---|---|---|---|---|---|---|
| Leaf | IC | 5.57 ± 0.29 | a | c | 0.01 ± 0.01 | a | a | 63.48 ± 2.00 | a | a |
| RR | 7.11 ± 0.14 | b | C | 3.96 ± 0.05 | b | B | 68.98 ± 2.12 | a | A | |
| DO | 6.07 ± 0.22 | a | c | 3.68 ± 0.20 | b | b | 61.45 ± 0.62 | a | a | |
| Flower | IC | 3.58 ± 0.06 | A | b | 0.02 ± 0.01 | A | a | 148.18 ± 2.51 | C | b |
| RR | 4.35 ± 0.10 | B | B | 0.87 ± 0.11 | B | A | 133.45 ± 7.86 | B | B | |
| DO | 4.54 ± 0.33 | B | b | 0.75 ± 0.12 | B | a | 106.44 ± 4.45 | A | b | |
| Corolla | IC | 2.30 ± 0.16 | a | a | 0.03 ± 0.02 | a | a | 147.59 ± 1.23 | b | b |
| RR | 3.32 ± 0.03 | b | A | 1.30 ± 0.27 | b | A | 135.41 ± 2.44 | b | B | |
| DO | 3.74 ± 0.25 | b | a | 1.00 ± 0.14 | b | a | 99.96 ± 3.00 | a | b |
| n. | Compound | RT (min) | Formula | [M−H]−(m/z) | MS2 Fragmentation Profile (m/z) 1 | Ref. |
|---|---|---|---|---|---|---|
| 1 | 4-hydroxybenzoic acid | 1.5 | C7H6O3 | 137.02 | 137.02 (5), 93.03 (100) 2 | [24] |
| 2 | Salvianic acid A | 2.5 | C9H10O5 | 197.04 | 197.04 (14), 179.03 (70), 135.04 (84), 123.04 (57), 72.99 (100) | [24,25] |
| 3 | Caftaric acid | 5.3 | C13H12O9 | 311.04 | 179.03 (100), 149.01 (79), 135.04 (14) | [26] |
| 4 | Caffeic acid | 8.6 | C9H8O4 | 179.03 | 179.03 (25), 135.04 (100) | [27] 2 |
| 5 | Fertaric acid | 9.0 | C14H14O9 | 325.06 | 193.05 (100), 134.04 (12) | [7,26] |
| 6 | Salvianolic acid H/I | 11.3 | C27H22O12 | 537.10 | 537.10 (5), 493.11 (66), 339.05 (100), 313.07 (8), 295.06 (34), 197.04 (27), 179.03 (8) | [27] |
| 7 | Salvianolic acid K | 11.7 | C27H24O13 | 555.11 | 537.10 (10), 493.11 (56), 295.06 (100) | [28,29] 3 |
| 8 | Chicoric acid | 12.0 | C22H18O12 | 473.07 | 311.04 (100), 293.03 (24), 179.03 (48), 149.01 (82) | [26] 2 |
| 9 | Lithospermic acid A (iso) | 12.3 | C27H22O12 | 537.10 | 537.10 (48), 493.11 (77), 295.06 (100) | [29] 3 |
| 10 | Salvianolic acid E | 13.9 | C36H30O16 | 717.15 | 717.15 (100), 519.09 (73), 475.10 (19), 339.05 (7) | [27,29] 3 |
| 11 | Salvianolic acid L | 14.8 | C36H30O16 | 717.15 | 717.15 (68), 673.16 (10), 537.10 (26), 519.09 (71), 321.04 (5), 295.06 (5) | [27,29] 3 |
| 12 | Rosmarinic acid | 15.2 | C18H16O8 | 359.08 | 359.08 (13), 197.04 (35), 179.03 (8), 161.02 (100) | [27] 2 |
| 13 | Salvianolic acid A | 15.6 | C26H22O10 | 493.11 | 493.11 (21), 313.07 (7), 295.06 (100), 185.02 (17) | [27] |
| 14 | Lithospermic acid A | 16.7 | C27H22O12 | 537.10 | 493.11 (99), 359.08 (99), 313.07 (13), 295.06 (28), 197.04 (9), 179.03 (14), 161.02 (29), 135.04 (9) | [29] 3 |
| 15 | Salvianolic acid B | 17.4 | C36H30O16 | 717.15 | 717.15 (23), 519.09 (100), 321.04 (10) | [27] 2 |
| 16 | Salvianolic acid F (iso1) | 22.0 | C17H14O6 | 313.07 | 161.02 (100) | [29] 3 |
| 17 | Salvianolic acid F (iso2) | 23.7 | C17H14O6 | 313.10 | 161.02 (100) | [29] 3 |
| n. | Compound | RT (min) | Formula | [M+H]+ (m/z) | MS2 Fragmentation Profile (m/z) 1 | Ref. |
|---|---|---|---|---|---|---|
| 18 | Dihydroquercetin glucoside | 7.9 | C21H22O12 | 467.12 | 287.06 (9), 259.06 (80), 231.07 (56), 167.03 (22), 153.02 (86), 149.02 (69), 123.04 (26) | [30,31] |
| 19 | Quercetin diglucoside | 8.7 | C27H30O17 | 627.17 | 303.05 (100) | [30] |
| 20 | Quercetin malonyl Glc Glc | 9.1 | C30H32O20 | 713.16 | 465.10 (10), 303.05 (100) | |
| 21 | Cyanidin diglucoside | 9.2 | C27H31O16 | 611.16 | 287.06 (100) | [30] |
| 22 | Cyanidin rutinoside | 9.5 | C27H31O15 | 595.17 | 287.06 (100) | 2 |
| 23 | Cyanidin-3-Glc-5-(6-Mal)Glc | 9.8 | C30H33O19 | 697.16 | 449.11 (12), 287.06 (100) | [10] |
| 24 | Cyanidin glucoside | 11.1 | C21H21O11 | 449.11 | 287.06 (100) | [30] |
| 25 | Quercetin rutinoside | 11.6 | C27H30O16 | 611.16 | 303.05 (100) | [32] |
| 26 | Quercetin glucoside | 12.0 | C21H20O12 | 465.10 | 303.05 (100) | [30,32] |
| 27 | Anthocyanin A * | 12.1 | C51H53O26 | 1081.28 | 1081.28 (100), 919.23 (79), 449.11 (24), 287.06 (45) | [10,18] |
| 28 | Anthocyanin B (iso1) * | 12.9 | C54H55O29 | 1167.28 | 1167.28 (100), 1005.25 (11), 919.23 (8), 535.11 (15), 287.06 (19) | [10,18] |
| 29 | Anthocyanin B (iso2) * | 13.4 | C54H55O29 | 1167.28 | 1167.28 (100), 1005.23 (57), 449.11 (9), 287.06 (9) | [10,18] |
| 30 | Anthocyanin C * | 13.5 | C51H53O25 | 1065.29 | 1065.29 (99), 903.23 (96), 449.11 (28), 287.06 (62) | [10,18] |
| 31 | Naringenin glucoside | 13.8 | C21H22O10 | 435.13 | 273.08 (100), 153.02 (68), 147.04 (44) | [33] |
| 32 | Apigenin galacturonide | 14.1 | C21H18O11 | 447.09 | 271.06 (100) | [32] |
| 33 | Anthocyanin D (iso1) * | 14.3 | C54H55O28 | 1151.29 | 1151.29 (100), 903.23 (13), 535.11 (24), 287.06 (16) | [10,18] |
| 34 | Cyanidin malonylglucoside | 14.8 | C24H30O14 | 535.11 | 287.06 (100) | [10] |
| 35 | Anthocyanin D (iso2) * | 14.9 | C54H55O28 | 1151.29 | 1151.29 (100), 989.24 (68), 449.11 (11), 287.06 (29) | [10,18] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prinsi, B.; Morgutti, S.; Negrini, N.; Faoro, F.; Espen, L. Insight into Composition of Bioactive Phenolic Compounds in Leaves and Flowers of Green and Purple Basil. Plants 2020, 9, 22. https://doi.org/10.3390/plants9010022
Prinsi B, Morgutti S, Negrini N, Faoro F, Espen L. Insight into Composition of Bioactive Phenolic Compounds in Leaves and Flowers of Green and Purple Basil. Plants. 2020; 9(1):22. https://doi.org/10.3390/plants9010022
Chicago/Turabian StylePrinsi, Bhakti, Silvia Morgutti, Noemi Negrini, Franco Faoro, and Luca Espen. 2020. "Insight into Composition of Bioactive Phenolic Compounds in Leaves and Flowers of Green and Purple Basil" Plants 9, no. 1: 22. https://doi.org/10.3390/plants9010022
APA StylePrinsi, B., Morgutti, S., Negrini, N., Faoro, F., & Espen, L. (2020). Insight into Composition of Bioactive Phenolic Compounds in Leaves and Flowers of Green and Purple Basil. Plants, 9(1), 22. https://doi.org/10.3390/plants9010022








