Modulation of the Functional Components of Growth, Photosynthesis, and Anti-Oxidant Stress Markers in Cadmium Exposed Brassica juncea L.
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material
2.2. Morphological Parameters
2.3. Oxidative Stress Markers
2.3.1. Malondialdehyde Content
2.3.2. H2O2 Content
2.4. Pigment Analysis
2.4.1. Chlorophyll Content
2.4.2. Anthocyanin Content
2.5. Determination of Sodium and Potassium Ions
2.6. Determination of Cd Content and Bioconcentration Factor (BCF)
2.7. Estimation of Antioxidant Enzyme Activities
2.7.1. CAT
2.7.2. SOD
2.7.3. GR
2.7.4. DHAR
2.7.5. MDHAR
2.8. Non-Enzymatic Antioxidants
2.8.1. Ascorbic Acid Content
2.8.2. Total Phenolic Content
2.9. Statistical Analysis
3. Results
3.1. Morphological Parameters
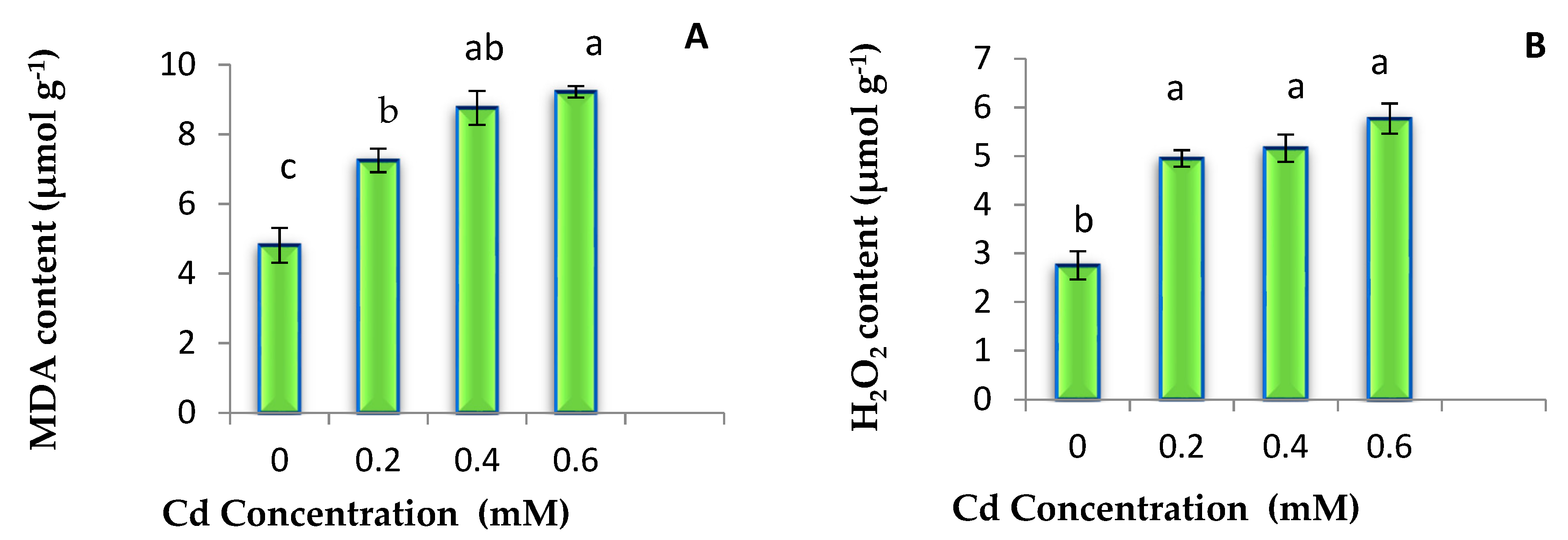
3.2. MDA and H2O2 Content
3.3. Pigments
3.3.1. Chlorophyll Content
3.3.2. Anthocyanin Content
3.4. Cd Uptake and Bioconcentration Factor (BCF) Studies
3.5. Sodium and Potassium Ion Analysis
3.6. Antioxidant Enzyme Activities
3.7. Level of Non-Enzymatic Antioxidants
3.8. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of interest
Abbreviations
| H2O2 | hydrogen peroxide |
| CAT | catalase |
| SOD | superoxide dismutase |
| GR | glutathione reductase |
| DHAR | dehydroascorbate reductase |
| MDHAR | monodehydroascorbate reductase |
References
- Di Salvatore, M.; Carafa, A.M.; Carratu, G. Assessment of heavy metals phytotoxicity using seed germination and root elongation tests: A comparison of two growth substrates. Chemosphere 2008, 73, 1461–1464. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, B.; Tanveer, M.; Rehman, A.; Cheema, S.A.; Fahad, S.; Rehman, S.; Sharma, A. Nickel; whether toxic or essential for plants and environment-A review. Plant Physiol. Biochem. 2018, 132, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Interaction of 24-epibrassinolide and salicylic acid regulates pigment contents, antioxidative defense responses, and gene expression in Brassica juncea L. seedlings under Pb stress. Environ. Sci. Pollut. Res. Int. 2018, 25, 15159–15173. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, B.; Tanveer, M.; Che, Z.; Rehman, A.; Cheema, S.A.; Sharma, A.; Song, H.; ur Rehman, S.; Zhaorong, D. Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: A review. Ecotoxicol. Environ. Saf. 2018, 147, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Soares, C.; Sousa, B.; Martins, M.; Kumar, V.; Shahzad, B.; Sidhu, G.P.S.; Bali, A.S.; Asgher, M.; Bhardwaj, R.; et al. Nitric oxide-mediated regulation of oxidative stress in plants under metal stress: A review on molecular and biochemical aspects. Physiol. Plant. 2019. [Google Scholar] [CrossRef] [PubMed]
- Tomescu, D.; Şumălan, R.; Copolovici, L.; Copolovici, D. The influence of soil salinity on volatile organic compounds emission and photosynthetic parameters of Solanum lycopersicum L. varieties. Open Life Sci. 2017, 12, 135–142. [Google Scholar] [CrossRef]
- Tang, W.; Luo, C. Overexpression of Zinc Finger Transcription Factor ZAT6 Enhances Salt Tolerance. Open Life Sci. 2018, 13, 431–445. [Google Scholar] [CrossRef]
- Guo, H.; Feng, X.; Hong, C.; Chen, H.; Zeng, F.; Zheng, B.; Jiang, D. Malate secretion from the root system is an important reason for higher resistance of Miscanthus sacchariflorus to cadmium. Physiol. Planta 2017, 159, 340–353. [Google Scholar] [CrossRef]
- Guo, H.; Chen, H.; Hong, C.; Jiang, D.; Zheng, B. Exogenous malic acid alleviates cadmium toxicity in Miscanthus sacchariflorus through enhancing photosynthetic capacity and restraining ROS accumulation. Ecotoxicol. Environ. Saf. 2017, 141, 119–128. [Google Scholar] [CrossRef]
- Chen, X.; Qiu, L.; Guo, H.; Wang, Y.; Yuan, H.; Yan, D.; Zheng, B. Spermidine induces physiological and biochemical changes in southern highbush blueberry under drought stress. Braz. J. Bot. 2017, 40, 841–851. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Herawati, N.; Suzuki, S.; Hayashi, K.; Rivai, I.; Koyama, H. Cadmium, copper, and zinc levels in rice and soil of Japan, Indonesia, and China by soil type. Bull. Environ. Contam. Toxicol. 2000, 64, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sharma, A.; Kaur, P.; Sidhu, G.P.S.; Bali, A.S.; Bhardwaj, R.; Thukral, A.K.; Cerda, A. Pollution assessment of heavy metals in soils of India and ecological risk assessment: A state-of-the-art. Chemosphere 2018, 216, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Villiers, F.; Ducruix, C.; Hugouvieux, V.; Jarno, N.; Ezan, E.; Garin, J.; Junot, C.; Bourguignon, J. Investigating the plant response to cadmium exposure by proteomic and metabolomic approaches. Proteomics 2011, 11, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Combined effect of 24-epibrassinolide and salicylic acid mitigates lead (Pb) toxicity by modulating various metabolites in Brassica juncea L. seedlings. Protoplasma 2018, 255, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Handa, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Abd_Allah, E.F.; Alqarawi, A.A.; Ahmad, P. Selenium modulates dynamics of antioxidative defence expression, photosynthetic attributes and secondary metabolites to mitigate chromium toxicity in Brassica juncea L. plants. Environ. Exp. Bot. 2019, 161, 180–192. [Google Scholar] [CrossRef]
- Kaur, R.; Yadav, P.; Sharma, A.; Kumar Thukral, A.; Kumar, V.; Kaur Kohli, S.; Bhardwaj, R. Castasterone and citric acid treatment restores photosynthetic attributes in Brassica juncea L. under Cd(II) toxicity. Ecotoxicol. Environ. Saf. 2017, 145, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Bali, S.; Sharma, A.; Vig, A.P.; Bhardwaj, R. Effect of earthworms on growth, photosynthetic efficiency and metal uptake in Brassica juncea L. plants grown in cadmium-polluted soils. Environ. Sci. Pollut. Res. Int. 2017, 24, 13452–13465. [Google Scholar] [CrossRef]
- Anjum, N.A.; Ahmad, I.; Mohmood, I.; Pacheco, M.; Duarte, A.C.; Pereira, E.; Umar, S.; Ahmad, A.; Khan, N.A.; Iqbal, M. Modulation of glutathione and its related enzymes in plants’ responses to toxic metals and metalloids—A review. Environ. Exp. Bot. 2012, 75, 307–324. [Google Scholar] [CrossRef]
- Kaur, P.; Bali, S.; Sharma, A.; Kohli, S.K.; Vig, A.P.; Bhardwaj, R.; Thukral, A.K.; Abd-Allah, E.F.; Wijaya, L.; Alyemeni, M.N. Cd induced generation of free radical species in Brassica juncea is regulated by supplementation of earthworms in the drilosphere. Sci. Total Environ. 2019, 655, 663–675. [Google Scholar] [CrossRef]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy metals and living systems: An overview. Indian J. Pharmacol. 2011, 43, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.A.; Ali, B.; Hayat, S.; Ahmad, A. Cadmium-induced changes in the growth and carbonic anhydrase activity of chickpea. Turk. J. Biol. 2007, 31, 137–140. [Google Scholar]
- Mishra, S.; Srivastava, S.; Tripathi, R.D.; Govindarajan, R.; Kuriakose, S.V.; Prasad, M.N. Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L. Plant Physiol. Biochem. 2006, 44, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Al-Yemeni, M.N. Effect of cadmium, mercury and lead on seed germination and early seedling growth of Vigna ambacensis L. Indian J. Plant Physiol. 2001, 6, 147–151. [Google Scholar]
- Seth, C.S.; Chaturvedi, P.K.; Misra, V. The role of phytochelatins and antioxidants in tolerance to Cd accumulation in Brassica juncea L. Ecotoxicol. Environ. Saf. 2008, 71, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Gozubenli, H. Seed vigor of maize grown on the contaminated soils by cadmium. Asian J. Plant Sci. 2010, 9, 168. [Google Scholar] [CrossRef]
- Wahid, A.; Ghani, A.; Ali, I.; Ashraf, M. Effects of cadmium on carbon and nitrogen assimilation in shoots of mungbean [Vigna radiata (L.) Wilczek] seedlings. J. Agron. Crop Sci. 2007, 193, 357–365. [Google Scholar] [CrossRef]
- Kovalchuk, O.; Titov, V.; Hohn, B.; Kovalchuk, I. A sensitive transgenic plant system to detect toxic inorganic compounds in the environment. Nat. Biotechnol. 2001, 19, 568–572. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Bao, M.; Wang, L.; Khan, I.; Ullah, E.; Tung, S.A.; Samad, R.A.; Shahzad, B. Cadmium toxicity in Maize (Zea mays L.): Consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ. Sci. Pollut. Res. 2015, 22, 17022–17030. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Shahzad, B.; Ashraf, U.; Fahad, S.; Hassan, W.; Jan, S.; Khan, I.; Saleem, M.F. Osmoregulation and antioxidant production in maize under combined cadmium and arsenic stress. Environ. Sci. Pollut. Res. 2016, 23, 11864–11875. [Google Scholar] [CrossRef]
- Amari, T.; Ghnaya, T.; Abdelly, C. Nickel, cadmium and lead phytotoxicity and potential of halophytic plants in heavy metal extraction. S. Afr. J. Bot. 2017, 111, 99–110. [Google Scholar] [CrossRef]
- Anuradha, S.; Rao, S.S.R. Effect of 24-epibrassinolide on the photosynthetic activity of radish plants under cadmium stress. Photosynthetica 2009, 47, 317–320. [Google Scholar] [CrossRef]
- Shekhawat, K.; Rathore, S.; Premi, O.; Kandpal, B.; Chauhan, J. Advances in agronomic management of Indian mustard (Brassica juncea (L.) Czernj. Cosson): An overview. Int. J. Agron. 2012, 2012. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Mancinelli, A.L. Photoregulation of anthocyanin synthesis: VIII. Effect of light pretreatments. Plant Physiol. 1984, 75, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Chapman, S.B. Methods in Plant Ecology; Blackwell Scientific Publications: London, UK, 1976. [Google Scholar]
- Retamal-Salgado, J.; Hirzel, J.; Walter, I.; Matus, I. Bioabsorption and bioaccumulation of cadmium in the straw and grain of maize (Zea mays L.) in growing soils contaminated with cadmium in different environment. Int. J. Environ. Res. Public Health 2017, 14, 1399. [Google Scholar] [CrossRef]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 1974; pp. 673–684. [Google Scholar]
- Kono, Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch. Biochem. Biophys. 1978, 186, 189–195. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 1975, 250, 5475–5480. [Google Scholar]
- Dalton, D.A.; Russell, S.A.; Hanus, F.; Pascoe, G.A.; Evans, H.J. Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc. Natl. Acad. Sci. USA 1986, 83, 3811–3815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.A.; Nakano, Y.; Asada, K. Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol. 1984, 25, 385–395. [Google Scholar]
- Roe, J.H.; Kuether, C.A. The determination of ascorbic acid in whole blood and urine through the 2, 4-dinitrophenylhydrazine derivavative of dehydroascorbic acid. J. Biol. Chem. 1943, 147, 399–407. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Hashem, H. Cadmium toxicity induces lipid peroxidation and alters cytokinin content and antioxidant enzyme activities in soybean. Botany 2013, 92, 1–7. [Google Scholar] [CrossRef]
- Kaur, P.; Bali, S.; Sharma, A.; Vig, A.P.; Bhardwaj, R. Role of earthworms in phytoremediation of cadmium (Cd) by modulating the antioxidative potential of Brassica juncea L. Appl. Soil Ecol. 2018, 124, 306–316. [Google Scholar] [CrossRef]
- Khanna, K.; Jamwal, V.L.; Sharma, A.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R.; Al-Huqail, A.A.; Siddiqui, M.H.; Ali, H.M.; Ahmad, P. Supplementation with plant growth promoting rhizobacteria (PGPR) alleviates cadmium toxicity in Solanum lycopersicum by modulating the expression of secondary metabolites. Chemosphere 2019, 230, 628–639. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, G.; Bao, M.; Wang, L.; Xie, X. Exogenous application of ascorbic acid mitigates cadmium toxicity and uptake in Maize (Zea mays L.). Environ. Sci. Pollut. Res. 2019, 26, 19261–19271. [Google Scholar] [CrossRef]
- Kaya, C.; Okant, M.; Ugurlar, F.; Alyemeni, M.N.; Ashraf, M.; Ahmad, P. Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere 2019, 225, 627–638. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, S.; Liu, R.; Zhou, W.; Jin, J. Changes of photosynthetic activities of maize (Zea mays L.) seedlings in response to cadmium stress. Photosynthetica 2009, 47, 277–283. [Google Scholar] [CrossRef]
- Pandey, P.; Tripathi, A. Effect of heavy metals on morphological and biochemical characteristics of Albizia procera (Roxb.) Benth. seedlings. Int. J. Environ. Sci. 2011, 1, 1009. [Google Scholar]
- Aydinalp, C.; Marinova, S. The effects of heavy metals on seed germination and plant growth on alfalfa plant (Medicago sativa). Bulg. J. Agric. Sci. 2009, 15, 347–350. [Google Scholar]
- Verma, K.; Shekhawat, G.S.; Sharma, A.; Mehta, S.K.; Sharma, V. Cadmium induced oxidative stress and changes in soluble and ionically bound cell wall peroxidase activities in roots of seedling and 3–4 leaf stage plants of Brassica juncea (L.) czern. Plant Cell Rep. 2008, 27, 1261–1269. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Shi, Y.; Zhao, M.; Chi, G. Effects of cadmium on growth and photosynthetic activities in pakchoi and mustard. Bot. Stud. 2011, 52, 41–46. [Google Scholar]
- Andresen, E.; Küpper, H. Cadmium toxicity in plants. In Cadmium: From Toxicity to Essentiality; Springer: New York, NY, USA, 2013; pp. 395–413. [Google Scholar]
- Sandalio, L.M.; Dalurzo, H.C.; Gomez, M.; Romero-Puertas, M.C.; del Rio, L.A. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J. Exp. Bot. 2001, 52, 2115–2126. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Ying, R.-R.; Qiu, R.-L.; Tang, Y.-T.; Hu, P.-J.; Qiu, H.; Chen, H.-R.; Shi, T.-H.; Morel, J.-L. Cadmium tolerance of carbon assimilation enzymes and chloroplast in Zn/Cd hyperaccumulator Picris divaricata. J. Plant Physiol. 2010, 167, 81–87. [Google Scholar] [CrossRef]
- Keilig, K.; Ludwig-Mueller, J. Effect of flavonoids on heavy metal tolerance in Arabidopsis thaliana seedlings. Bot. Stud. 2009, 50, 311–318. [Google Scholar]
- Dai, L.-P.; Dong, X.-J.; Ma, H.-H. Antioxidative and chelating properties of anthocyanins in Azolla imbricata induced by cadmium. Pol. J. Environ. Stud. 2012, 21, 837–844. [Google Scholar]
- Zhang, H.; Xia, Y.; Wang, G.; Shen, Z. Excess copper induces accumulation of hydrogen peroxide and increases lipid peroxidation and total activity of copper–zinc superoxide dismutase in roots of Elsholtzia haichowensis. Planta 2008, 227, 465–475. [Google Scholar] [CrossRef]
- Nehnevajova, E.; Lyubenova, L.; Herzig, R.; Schröder, P.; Schwitzguébel, J.-P.; Schmülling, T. Metal accumulation and response of antioxidant enzymes in seedlings and adult sunflower mutants with improved metal removal traits on a metal-contaminated soil. Environ. Exp. Bot. 2012, 76, 39–48. [Google Scholar] [CrossRef]
- Najeeb, U.; Jilani, G.; Ali, S.; Sarwar, M.; Xu, L.; Zhou, W. Insights into cadmium induced physiological and ultra-structural disorders in Juncus effusus L. and its remediation through exogenous citric acid. J. Hazard. Mater. 2011, 186, 565–574. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The roles of reactive oxygen metabolism in drought: Not so cut and dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef]
- Zheng, G.; Lv, H.; Gao, S.; Wang, S. Effects of cadmium on growth and antioxidant responses in Glycyrrhiza uralensis seedlings. Plantsoil Environ. 2010, 56, 508–515. [Google Scholar] [CrossRef]
- Foyer, C.H. Regulation of glutathione synthesis and its role in abiotic and biotic stress defence. In Sulfur Nutrition and Sulfur Assimilation in Higher Plants; SPB Academic Publishing: The Hague, The Netherlands, 2000; pp. 127–153. [Google Scholar]
- Romero-Puertas, M.C.; Corpas, F.J.; Rodríguez-Serrano, M.; Gómez, M.; Luis, A.; Sandalio, L.M. Differential expression and regulation of antioxidative enzymes by cadmium in pea plants. J. Plant Physiol. 2007, 164, 1346–1357. [Google Scholar] [CrossRef]
- Nazar, R.; Iqbal, N.; Masood, A.; Khan, M.I.R.; Syeed, S.; Khan, N.A. Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Am. J. Plant Sci. 2012, 3, 1476. [Google Scholar] [CrossRef]
- Mithofer, A.; Schulze, B.; Boland, W. Biotic and heavy metal stress response in plants: Evidence for common signals. Febs Lett. 2004, 566, 1–5. [Google Scholar] [CrossRef]
| Cd Conc. (mM) | Root Length (cm) | Shoot Length (cm) |
|---|---|---|
| 0.0 | 11.7 ± 1.57 a | 4.37 ± 0.22 a |
| 0.2 | 7.63 ± 0.41 b | 4.17 ± 0.2 ab |
| 0.4 | 5.97 ± 0.12 b | 3.43 ± 0.2 ab |
| 0.6 | 3.93 ± 0.5 b | 3.33 ± 0.26 b |
| Cd Conc. (mM) | Total Chl (mg g−1 FW) | Chl a (mg g−1 FW) | Chl b (mg g−1 FW) | Anthocyanin (mg g−1 FW) |
|---|---|---|---|---|
| 0.0 | 26.75 ± 0.77 a | 16.78 ± 0.56 a | 7.54 ± 0.47 a | 2.41 ± 0.28 b |
| 0.2 | 21.69 ± 0.9 b | 15.04 ± 0.93 a | 5.33 ± 1.29 ab | 3.6 ± 0.32 b |
| 0.4 | 18.56 ± 0.39 c | 11.59 ± 0.44 b | 6.12 ± 0.41 a | 5.09 ± 0.41 a |
| 0.6 | 13.38 ± 0.39 d | 7.1 ± 0.23 c | 2.24 ± 0.19 b | 6.02 ± 0.11 a |
| Cd Conc. (mM) | Cd Accumulation in Seedlings (μg g−1 DW) | BCF |
|---|---|---|
| 0.2 | 69.13 ± 5.14 b | 1.85 |
| 0.4 | 83.39 ± 2.35 ab | 1.13 |
| 0.6 | 89.68 ± 2.87 a | 0.82 |
| Cd Conc. (mM) | Sodium Ion (ppm) | Potassium Ion (ppm) |
|---|---|---|
| 0.0 | 6.38 ± 0.57 a | 5.82 ± 0.59 a |
| 0.2 | 5.89 ± 0.59 a | 5.25 ± 0.29 ab |
| 0.4 | 4.99 ± 0.49 b | 5.5 ± 0.62 ab |
| 0.6 | 4.42 ± 0.36 b | 4.24 ± 0.79 b |
| Cd Conc. (mM) | CAT (UA mg−1 Protein) | SOD (UA mg−1 Protein) | GR (UA mg−1 Protein) | DHAR (UA mg−1 Protein) | MDHAR (UA mg−1 Protein) |
|---|---|---|---|---|---|
| 0.0 | 6.43 ± 0.19 b | 5.43 ± 0.35 b | 7.63 ± 0.27 b | 8.33 ± 0.49 c | 11.29 ± 0.33 c |
| 0.2 | 8.27 ± 0.35 a | 6.38 ± 0.21 b | 9.66 ± 0.40 a | 10.25 ± 0.33 b | 14.65 ± 0.44 b |
| 0.4 | 8.51 ± 0.53 a | 7.75 ± 0.25 a | 10.87 ± 0.54 a | 11.25 ± 0.26 ab | 16.94 ± 0.62 a |
| 0.6 | 8.94 ± 0.32 a | 8.39 ± 0.24 a | 9.93 ± 0.47 a | 12.21 ± 0.29 a | 18.46 ± 0.55 a |
| Cd Conc. (mM) | Ascorbic Acid (mg g−1 FW) | Total Phenolic Content (mg g−1 FW) |
|---|---|---|
| 0.0 | 8.63 ± 0.25 d | 6.86 ± 0.20 c |
| 0.2 | 10.34 ± 0.49 c | 7.89 ± 0.49b c |
| 0.4 | 12.97 ± 0.31 b | 8.89 ± 0.35 ab |
| 0.6 | 15.23 ± 0.41 a | 9.96 ± 0.15 a |
| Parameter | Correlation Coefficient | p Value |
|---|---|---|
| Root length | −0.8738 | 0.0002 |
| Shoot length | −0.6936 | 0.0123 |
| MDA content | 0.9189 | 2.39 × 10−5 |
| H2O2 content | 0.9347 | 8.38 × 10−6 |
| Total chlorophyll content | −0.8791 | 0.0001 |
| Anthocyanin content | 0.8566 | 0.0003 |
| Sodium ion content | −0.6271 | 0.0291 |
| Potassium ion content | −0.3413 | 0.2775 |
| CAT activity | 0.8703 | 0.0002 |
| SOD activity | 0.8365 | 0.0006 |
| GR activity | 0.8729 | 0.0002 |
| DHAR activity | 0.8856 | 0.0001 |
| MDHAR activity | 0.9227 | 1.89 × 10−5 |
| Ascorbic acid content | 0.8186 | 0.0011 |
| Total phenolic content | 0.8241 | 0.0009 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapoor, D.; Singh, M.P.; Kaur, S.; Bhardwaj, R.; Zheng, B.; Sharma, A. Modulation of the Functional Components of Growth, Photosynthesis, and Anti-Oxidant Stress Markers in Cadmium Exposed Brassica juncea L. Plants 2019, 8, 260. https://doi.org/10.3390/plants8080260
Kapoor D, Singh MP, Kaur S, Bhardwaj R, Zheng B, Sharma A. Modulation of the Functional Components of Growth, Photosynthesis, and Anti-Oxidant Stress Markers in Cadmium Exposed Brassica juncea L. Plants. 2019; 8(8):260. https://doi.org/10.3390/plants8080260
Chicago/Turabian StyleKapoor, Dhriti, Mahendra P. Singh, Satwinderjeet Kaur, Renu Bhardwaj, Bingsong Zheng, and Anket Sharma. 2019. "Modulation of the Functional Components of Growth, Photosynthesis, and Anti-Oxidant Stress Markers in Cadmium Exposed Brassica juncea L." Plants 8, no. 8: 260. https://doi.org/10.3390/plants8080260
APA StyleKapoor, D., Singh, M. P., Kaur, S., Bhardwaj, R., Zheng, B., & Sharma, A. (2019). Modulation of the Functional Components of Growth, Photosynthesis, and Anti-Oxidant Stress Markers in Cadmium Exposed Brassica juncea L. Plants, 8(8), 260. https://doi.org/10.3390/plants8080260






