Foliar Application of Zinc Oxide Nanoparticles and Zinc Sulfate Boosts the Content of Bioactive Compounds in Habanero Peppers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Characteristics of the ZnO NPs Used in This Experiment
2.3. Preparation of Suspensions
2.4. Foliar Exposure to Zn
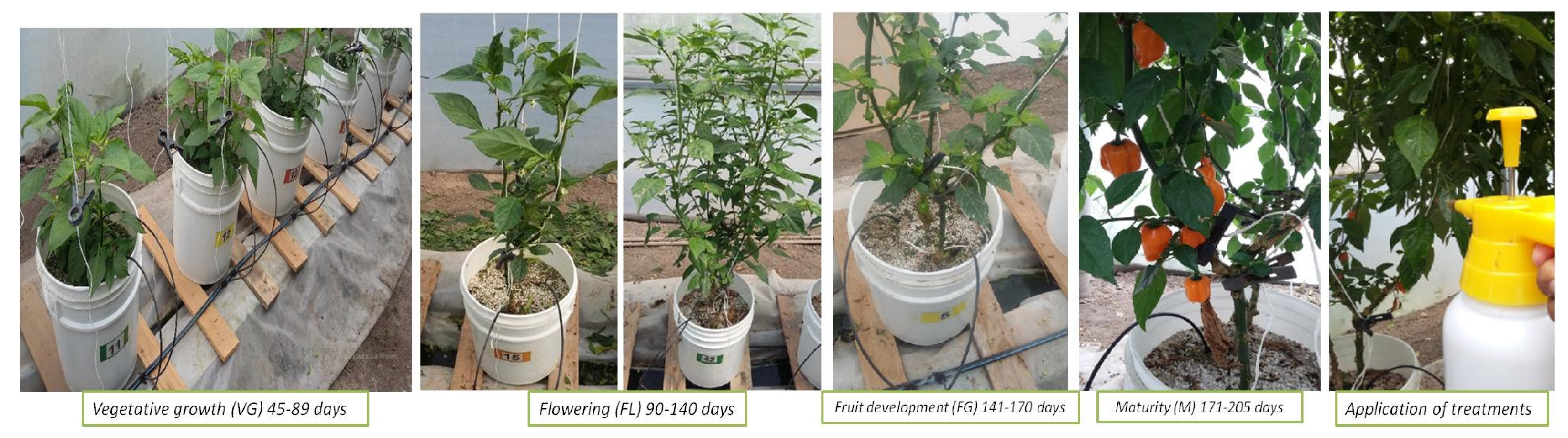
2.5. Growth of Habanero Pepper Plants and Greenhouse Conditions
2.6. Performance Variables and Relative Chlorophyll Content
2.7. Preparation for the Analysis of Habanero Pepper Fruits
2.7.1. Physicochemical Fruit Evaluations
2.7.2. Sample Preparation for Capsaicinoids, Phenolics and Antioxidant Capacity Analysis
2.7.3. Capsaicinoids Extraction
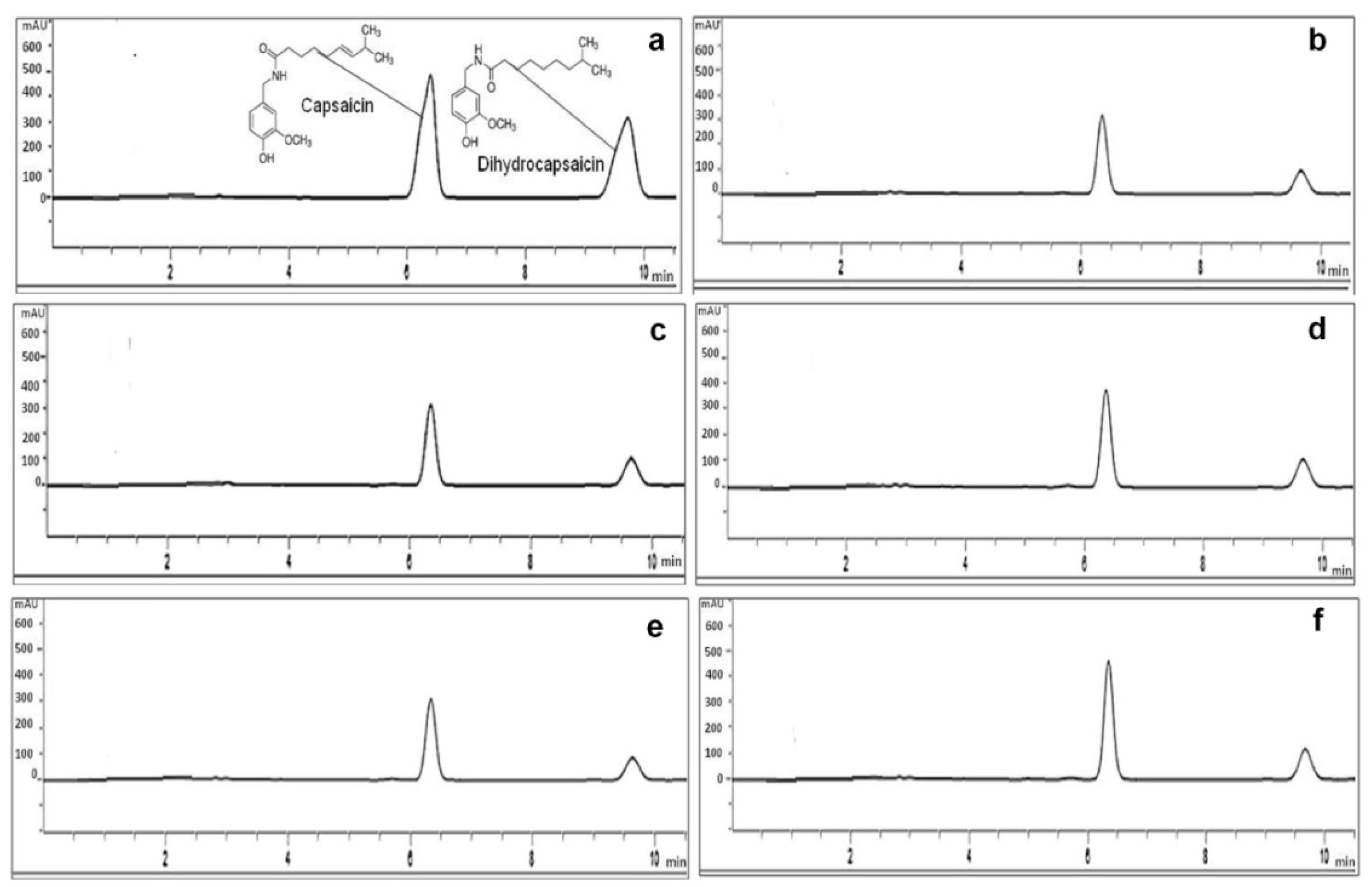
2.7.4. Quantification of Capsaicin and Dihydrocapsaicin by HPLC
2.7.5. Scoville Heat Units Calculation
2.8. Extraction of Soluble and Bound Phenolic Compounds
2.8.1. Determination of Total Phenols
2.8.2. Determination of Total Flavonoids
2.8.3. Determination of Condensed Tannins
2.8.4. Antioxidant Capacity
2.9. Experimental design and Statistical Analysis
3. Results and Discussion
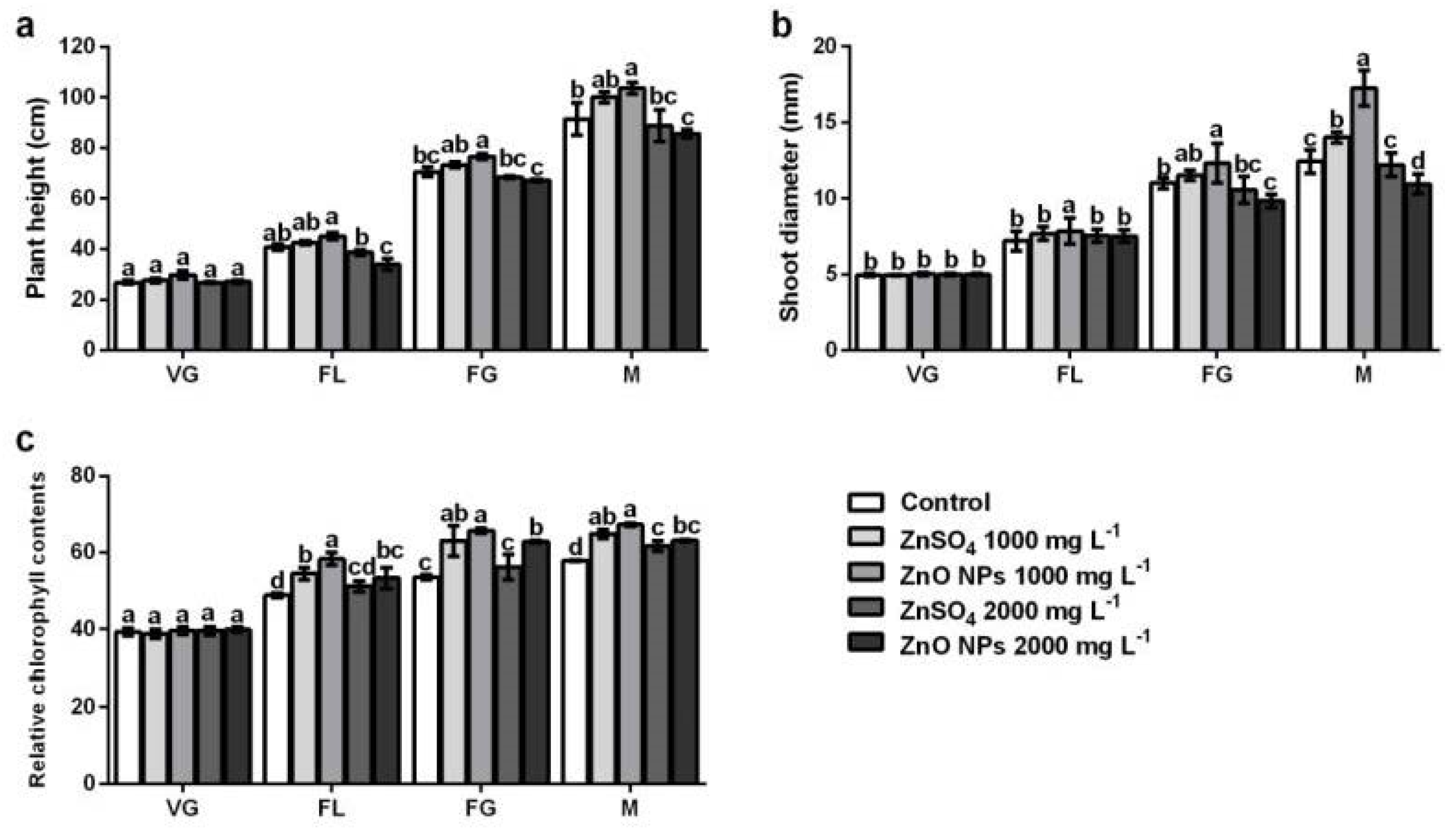
3.1. Growth of Pepper Plants
3.2. Relative Chlorophyll Content
3.3. Fruit Yield and Plant Biomass
3.4. Chromatic Characteristics
3.5. Quality of Habanero Pepper Fruit
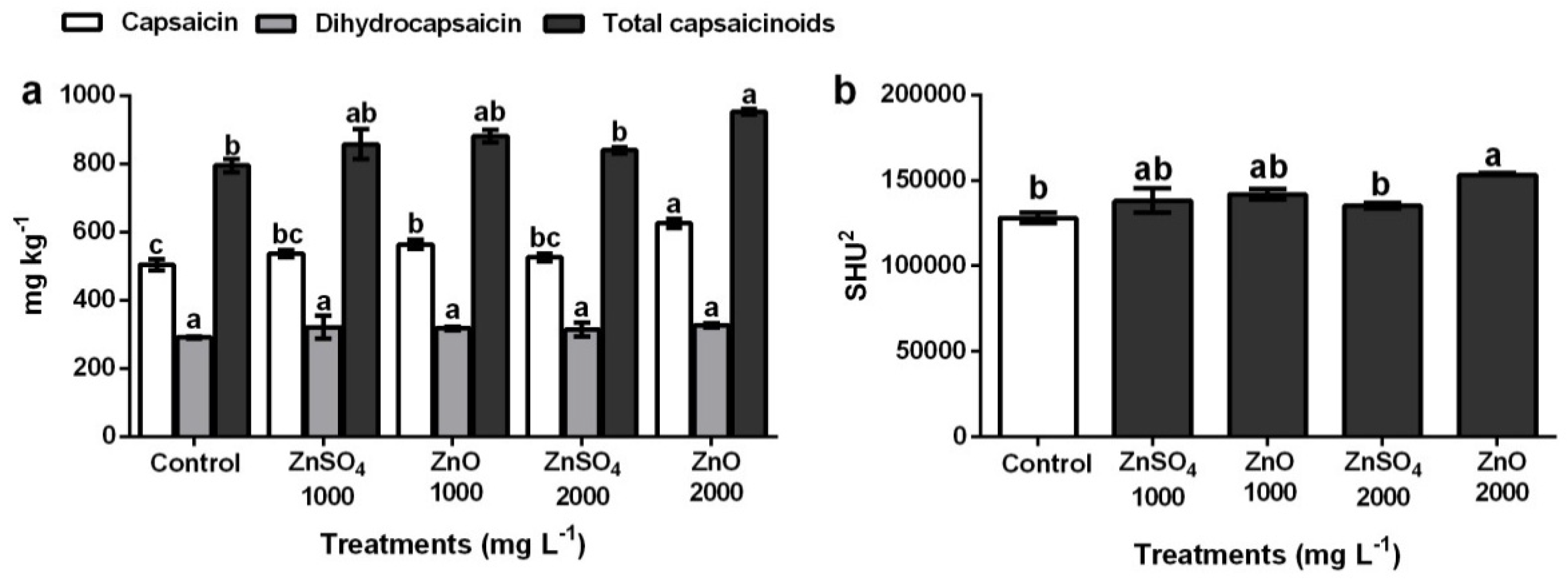
3.6. Capsaicin and Dihydrocapsaicin Content
3.7. Total Phenols, Total Flavonoids and Condensed Tannins
3.8. Antioxidant Capacity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davarpanah, S.; Tehranifar, A.; Davarynejad, G.; Abadía, J.; Khorasani, R. Effects of foliar applications of zinc and boron nano-fertilizers onpomegranate (Punica granatum cv. Ardestani) fruit yield and quality. Sci. Hort. 2016, 210, 57–64. [Google Scholar] [CrossRef]
- Narendhran, S.; Rajiv, P.; Sivaraj, R. Influence of zinc oxide nanoparticles on growth of Sesamum indicum L. in zinc deficient soil. Int. J. Pharm. Sci. 2016, 3, 365–371. [Google Scholar]
- Singh, R.S.; Ram, S. Studies on the use of palm growth substances for fruitretention in mango cv. Dashehair. Indian J. Hortic. 1983, 40, 188–194. [Google Scholar]
- Khan, N.; Malik, A.B.; Makbdoom, M.I.; Hag, A. Investigations on the efficiency of exogenous synthetic growth regulators on fruit drop in mango (Mangifera indica L.). Egypt J. Hortic. 1993, 20, 1–14. [Google Scholar]
- Cakmak, I. Role of zinc in protecting plant cells from reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef]
- Sadeghzadeh, B.; Rengel, Z. Zinc in soils and crop nutrition. In Molecular Basis of Nutrient Use Efficiency in Crops; Hawkesford, M.J., Barraclough, P., Eds.; Wiley: London, UK, 2011; pp. 335–376. [Google Scholar]
- Rengel, Z. Genotypic differences in micronutrient use efficiency in crops. Comm. Soil Sci. Plant Anal. 2001, 32, 1163–1186. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C.; Clark, R.B. Micronutrients in crop production. Adv. Agron. 2002, 77, 189–272. [Google Scholar]
- Doolette, C.L.; Read, T.L.; Li, C.; Scheckel, K.G.; Donner, E.; Kopittke, P.M.; Lombi, E. Foliar application of zinc sulphate and zinc EDTA to wheat leaves: Differences in mobility, distribution, and speciation. J. Exp. Bot. 2018, 69, 4469–4481. [Google Scholar] [CrossRef]
- Read, T.L.; Doolette, C.L.; Cresswell, T.; Howell, N.R.; Aughterson, R.; Karatchevtseva, I.; Lombi, E. Investigating the foliar uptake of zinc from conventional and nano-formulations: A methodological study. Environ. Chem. 2019, in press. [Google Scholar] [CrossRef]
- Tucuch, H.C.J.; Alcántar, G.G.; Ordaz-Chaparro, V.M.; Santizo, R.J.A.; Larqué, S.A. Production and quality of habanero pepper (Capsicum chinense Jacq.) with different NH4+/NO3− ratios and size of substrate particles. Terra Latinoam. 2012, 30, 9–15. [Google Scholar]
- Singh, A.; Singh, N.B.; Afzal, S.; Singh, T.; Hussain, I. Zinc oxide nanoparticles: A review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J. Mater. Sci. 2017, 53, 185–201. [Google Scholar] [CrossRef]
- Mengel, K.; Kirkby, E.A. Principles of Plant Nutrition, 5th ed.; International Potash Institute, Springer Science Business Media: Worblaufen, Switzerland, 1987; pp. 585–596. [Google Scholar]
- Fernández, V.; Brown, P.H. From plant surface to plant metabolism: The uncertain fate of foliar-applied nutrients. Front. Plant Sci. 2013, 4, 289. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Prasad, T.N.V.K.V.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Reddy, K.R.; Pradeep, T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Fedenia, L.N.; Sharifan, H.; Ma, X.; Lombardini, L. Effects of foliar application of zinc sulfate and zinc nanoparticles in coffee (Coffea arabica L.) plants. Plant Physiol. Biochem. 2018, 135, 160–166. [Google Scholar] [CrossRef]
- García-López, J.I.; Zavala-García, F.; Olivares-Sáenz, E.; Lira-Saldívar, R.; Barriga-Castro, E.; Ruiz-Torres, N.A.; Ramos-Cortez, E.; Vázquez-Alvarado, R.; Niño-Medina, G. Zinc oxide nanoparticles boosts phenolic compounds and antioxidant activity of Capsicum annuum L. during germination. Agronomy 2018, 8, 215. [Google Scholar] [CrossRef]
- Qados, A.M.S. Mechanism of nanosilicon-mediated alleviation of salinity stress in faba bean (Vicia faba L.) plants. AJEA 2015, 7, 78–95. [Google Scholar] [CrossRef]
- García-Gómez, C.; Obrador, A.; González, D.; Babína, M.; Dolores, M. Comparative effect of ZnO NPs, ZnO bulk and ZnSO4 in the antioxidant defences of two plant species growing in two agricultural soils under greenhouse conditions. Sci. Total Environ. 2017, 589, 11–24. [Google Scholar] [CrossRef]
- Nhan, L.V.; Ma, C.; Rui, Y.; Liu, S.; Li, X.; Xing, B. Phytotoxic mechanism of nanoparticles: Destruction of chloroplasts and vascular bundles and alteration of nutrient absorption. Sci. Rep. 2015, 5, 11618. [Google Scholar] [CrossRef]
- Laware, S.L.; Raskar, S. Influence of zinc oxide nanoparticles on growth, flowering and seed productivity in onion. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 874–881. [Google Scholar]
- Mukherjee, A.; Sun, Y.; Morelius, E.; Tamez, C.; Bandyopadhyay, S.; Niu, G.; White, J.C.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Diferential toxicity of bareand hybrid ZnO nanoparticles in green pea (Pisum sativum L.): A life cycle study. Front Plant Sci. 2016, 6, 1242. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Kumar, A. Impact of irrigation using water containing CuO and ZnO nanoparticles on Spinacia oleracea grown in soil media. Bull. Environ. Contam. Toxicol. 2016, 97, 548–553. [Google Scholar] [CrossRef] [PubMed]
- García-López, J.I.; Lira-Saldivar, R.H.; Zavala-García, F.; Olivares-Sáenz, E.; Niño-Medina, G.; Ruiz-Torres, N.A.; Méndez-Argüello, B.; Díaz-Barriga, E. Effects of zinc oxide nanoparticles on growth and antioxidant enzymes of Capsicum chínense. Toxicol. Environ. Chem. 2018, 100, 560–572. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C. ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef]
- Torabian, S.; Zahedi, M.; Khoshgoftar, A.H. Effects of foliar spray of two kinds of zinc oxide on the growth and ion concentration of sunflower cultivars under salt stress. J. Plant. Nutr. 2016, 39, 172–180. [Google Scholar] [CrossRef]
- Kisan, B.; Shruthi, H.; Sharanagouda, H.; Revanappa, S.B.; Pramod, N.K. Effect of nano-zinc oxide on the leaf physical and nutritional quality of spinach. Agrotechnology 2015, 5, 132–134. [Google Scholar]
- Steiner, A.A.A. Universal method for preparing nutrients solutions of a certain desired composition. Plant Soil. 1961, 15, 134–154. [Google Scholar] [CrossRef]
- López-Gómez, J.D.; Villegas-Torres, O.G.; Nava, H.S.; Rodríguez, M.A.; López, P.; Martínez, F. Yield and quality of habanero chili (Capsicum chinense Jacq.) by effect of nutritional régimen. REMEXCA 2017, 8, 1747–1758. [Google Scholar] [CrossRef]
- Zuverza-Mena, N.; Medina-Velo, I.; Barrios, A.C.; Tan, W.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Copper nanoparticles/compounds impact agronomic and physiological parameters in cilantro (Coriandrum sativum). Environ. Sci. Processes Impacts. 2015, 17, 1783–1793. [Google Scholar] [CrossRef]
- Simonovska, J.; Škerget, M.; Knez, Z.; Srbinoska, M.; Kavrakovski, Z.; Grozdanov, A.; Rafajlovska, V. Physicochemical characterization and bioactive compounds of stalk from hot fruits of Capsicum annuum L. Maced. J. Chem. Chem. Eng. 2016, 35, 199–208. [Google Scholar] [CrossRef]
- Elibox, W. Morphological changes associated with postharvest fruit deterioration and physical parameters for early determination of shelf life in Capsicum chinense Jacq. HortScience 2015, 50, 1537–1541. [Google Scholar] [CrossRef]
- Pinedo-Guerrero, Z.; Hernández-Fuentes, A.D.; Ortega-Ortiz, H.; Benavides-Mendoza, A.; Cadenas-Pliego, G.; Juárez-Maldonado, A. Cu nanoparticles in hydrogels of chitosan-PVA affects the characteristics of post-harvest and bioactive compounds of jalapeño pepper. Molecules 2017, 22, 926. [Google Scholar] [CrossRef] [PubMed]
- Commission Internationale De L’ecleirage. Cie 15: Technical Report: Colorimetry, Commission Internationale De L’ecleirage, 3rd ed.; CIE: Vienna, Austria, 2004. [Google Scholar]
- ColorHexa. Color Encyclopedia: Information and Conversion. Computer Software. 2019. Available online: https://www.colorhexa.com/ (accessed on 31 January 2019).
- AOAC. Vitamin and other nutrient. In Official Methods of Analysis of the Association of Official Analytical Chemists International, 17th ed.; Hoerwitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Ryu, W.K.; Kim, H.W.; Kim, G.D.; Rhee, H.I. Rapid determination of capsaicinoids by colorimetric method. J. Food Drug Anal. 2017, 25, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Todd, P.H.; Bensinger, M.G.; Biftu, T. Determination of pungency due to capsicum by gas-liquid chromatography. J. Food Sci. 1977, 42, 660–665. [Google Scholar] [CrossRef]
- López-Contreras, J.J.; Zavala-García, F.; Urías-Orona, V.; Martínez-Ávila, G.C.G.; Rojas, R.; Nino-Medina, G. Chromatic, phenolic and antioxidant properties of sorghum bicolor genotypes. Not. Bot. Horti. Agrobot. 2015, 43, 366–370. [Google Scholar] [CrossRef]
- Pavani, K.; Divya, V.; Veena, I.; Aditya, M.; Devakinandan, G. Influence of bioengineered zinc nanoparticles and zinc metal on Cicer arietinum seedlings growth. Asian J. Agric. Biol. 2014, 2, 216–223. [Google Scholar]
- De Rosa, M.C.; Monreal, C.; Schnitzer, M.; Walsh, R.; Sultan, Y. Nanotechnology in fertilizers. Nat. Nanotechnol. 2010, 5, 91. [Google Scholar] [CrossRef]
- Wang, P.; Lombi, E.; Sun, S.; Scheckel, K.G.; Malysheva, A.; McKenna, B.A.; Menzies, N.W.; Zhao, F.-J.; Kopittke, P.M. Characterizing the uptake, accumulation and toxicity of silver sulfide nanoparticles in plants. Environ. Sci. Nano. 2017, 4, 44. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Lombi, E.; Cheng, M.; Tang, C.; Howard, D.L.; Kopittke, P.M. Absorption of foliar-applied Zn fertilizers by trichomes in soybean and tomato. J. Exp. Bot. 2018, 69, 2717–2729. [Google Scholar] [CrossRef]
- Reed, R.B.; Ladner, D.A.; Higgins, C.P.; Westerhoff, P.; Ranville, J.F. Solubility of nano-zinc oxide in environmentally and biologically important matrices. Environ. Toxicol. Chem. 2012, 31, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Badger, M.R.; Price, G.D. The role of carbonic anhydrase in photosynthesis. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1994, 45, 369–392. [Google Scholar] [CrossRef]
- Pullagurala, V.L.R.; Adisa, I.O.; Rawat, S.; Kalagara, S.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. ZnO nanoparticles increase photosynthetic pigments and decrease lipid peroxidation in soil grown cilantro (Coriandrum sativum). Plant. Physiol. Biochem. 2018, 132, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant. Soil. 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Moura, D.J.; Péres, V.F.; Jacques, R.A.; Saffi, J. Heavy metal toxicity: Oxidative stress parameters and DNA repair. In Metal Toxicity in Plants: Perception, Signaling and Remediation; Springer: Berlin/Heidelberg, Germany, 2012; pp. 187–205. [Google Scholar]
- Chaney, R. Zinc phytotoxicity. In Zinc in Soils and Plants; Robson, A.D., Ed.; Kluwer Academic Publisher: Dordrecht, The Netherlands, 1993; pp. 135–150. [Google Scholar]
- Tirani, M.M.; Haghjou, M.M.; Sulieman, S.; Ismaili, A. Comparative evaluation of zinc oxide effects on tobacco (Nicotiana tabacum L.) grown in different media. J. Agric. Sci. Tech. 2018, 20, 787–802. [Google Scholar]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef]
- Khanm, H.; Vaishnavi, B.A.; Namratha, M.R.; Shankar, A.G. Nano zinc oxide boosting growth and yield in tomato: The rise of nano fertilizer era. IJASR 2017, 7, 197–206. [Google Scholar]
- Wang, P.; Menzies, N.W.; Lombi, E.; McKenna, B.A.; Johannessen, B.; Glover, C.J.; Kappen, P.; Kopittke, P.M. Fate of ZnO nanoparticles in soils and cowpea (Vigna unguiculata). Environ. Sci. Technol. 2013, 47, 13822–13830. [Google Scholar] [CrossRef]
- Eichert, T.; Kurtz, A.; Steiner, U.; Goldbach, H.E. Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueaus solutes and water suspended nanoparticles. Physiol. Plant. 2008, 134, 151–160. [Google Scholar] [CrossRef]
- Eichert, T.; Golbach, H.E. Equivalent pore raddi of hydrophilic foliar uptake routes in stomatous and astomatous leaf surfaces-further evidence for a stomatal pathway. Physiol. Plant. 2008, 132, 491–502. [Google Scholar] [CrossRef]
- Avellan, A.; Yun, J.; Zhang, Y.; Spielman-Sun, E.; Unrine, J.M.; Thieme, J.; Lowry, G.V. Nanoparticle size and coating chemistry control foliar uptake pathways, translocation and leaf-to-rhizosphere transport in wheat. ACS Nano 2019, 13, 5291–5305. [Google Scholar] [CrossRef] [PubMed]
- Larue, C.; Castillo-Michel, H.; Sobanska, S.; Trcera, N.; Sorieul, S.; Cécillon, L.; Sarret, G. Fate of pristine TiO2 nanoparticles and aged paint-containing TiO2 nanoparticles in lettuce crop after foliar exposure. J. Hazard. Mater. 2014, 273, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de-Luque, A. Interaction of nanomaterials with plants: What do we need for real applications in agriculture? Front. Environ. Sci. 2017, 5, 12. [Google Scholar] [CrossRef]
- Barceló, J.U.A.N.; Poschenrieder, C. Plant water relations as affected by heavy metal stress: A review. J. Plant. Nutr. 1990, 13, 1–37. [Google Scholar] [CrossRef]
- Kim, S.; Ha, T.Y.; Park, J. Characteristics of pigment composition and colour value by the difference of harvesting times in Korean red pepper varieties (Capsicum annuum L.). Int. J. Food Sci. Tech. 2008, 43, 915–920. [Google Scholar] [CrossRef]
- Lightbourn, G.J.; Griesbach, R.J.; Novotny, J.A.; Clevidence, B.A.; Rao, D.D.; Stommel, J.R. Effects of anthocyanin and carotenoid combinations on foliage and immature fruit color of Capsicum annuum L. J. Hered. 2008, 99, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Aloni, B.; Karni, L.; Deventurero, G.; Turhan, E.; Aktas, H. Changes in ascorbic acid concentration, ascorbate oxidase activity, and apoplastic pH in relation to fruit development in pepper (Capsicum annuum L.) and the occurrence of blossom-end rot. J. Hortic. Sci. Biotechnol. 2008, 83, 100–105. [Google Scholar] [CrossRef]
- Park, S.; Jeong, W.Y.; Lee, J.H.; Kim, Y.H.; Jeong, S.W.; Kim, G.S.; Bae, D.W.; Lim, C.S.; Jin, J.S.; Lee, S.J.; et al. Determination of polyphenol levels variation in Capsicum annuum L. cv. Chelsea (yellow bell pepper) infected by anthracnose (Colletotrichum gloeosporioides) using liquid chromatography-tandem mass spectrometry. Food Chem. 2012, 130, 981–985. [Google Scholar] [CrossRef]
- Wang, P.; Lombi, E.; Zhao, F.J.; Kopittke, P.M. Nanotechnology: A new opportunity in plant sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef]
- Pérez-Labrada, F.; López-Vargas, E.R.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Responses of tomato plants under saline stress to foliar application of copper nanoparticles. Plants 2019, 8, 151. [Google Scholar] [CrossRef]
- Yang, J.; Cao, W.; Rui, Y. Interactions between nanoparticles and plants: Phytotoxicity and defense mechanisms. J. Plant. Interact. 2017, 12, 158–169. [Google Scholar] [CrossRef]
- Sandmann, G.; Albrecht, M.; Schnurr, G.; Knörzer, O.; Böger, P. The biotechnological potential and design of novel carotenoids by gene combination in Escherichia coli. Trends Biotechnol. 1999, 17, 233–236. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, S. Compost as soil supplement enhanced plant growth and fruit quality of strawberry. J. Plant. Nutr. 2002, 25, 2243–2259. [Google Scholar] [CrossRef]
- Gupta, P.K.; Gupta, A.K. Studies of PGR and Micronutrient mixtures on vitamin ‘C’ content in tomato (Lycopersicon esculentum, Mill) products. Indian J. Hortic. 2004, 61, 102–103. [Google Scholar]
- Yogeratnam, N.; Greenham, D.W.P. The application of foliar sprayscontaining N, Mg, Zn and B to apple trees. I. Effect on fruit set and cropping. J. Hortic. Sci. 1982, 57, 151–154. [Google Scholar] [CrossRef]
- Welch, R.M.; Webb, M.J.; Loneragan, J.F. Zinc in membrane function and its role in phosphorus toxicity. In Proceedings of the 9th Plant Nutrition Colloquium, Coventry, UK, 22–27 August 1982; pp. 710–715. [Google Scholar]
- Weiss, E.A. Spice Crops; CABI Publishing International: New York, NY, USA, 2002; p. 411. [Google Scholar]
- Antonious, G.F.; Lobel, L.; Kochhar, T.; Jarret, R. Antioxidants in Capsicum chinense: Variation among countries of origin. J. Environ. Sci. Health. 2009, 6, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Sosan, A.; Svistunenko, D.; Straltsova, D.; Tsiurkina, K.; Smolich, I.; Lawson, S.; Golovko, V.; Anderson, D.; Sokolik, A.; Colbeck, I.; et al. Engineered silver nanoparticles are sensed at the plasma membrane and dramatically modify the physiology of Arabidopsis thaliana plants. Plant. J. 2016, 85, 245–257. [Google Scholar] [CrossRef]
- Castro-Concha, L.A.; Tuyub-Che, J.; Moo-Mukul, A.; Vacquez-Flota, F.A.; Miranda-Ham, L. Antioxidant capacity and total phenolic content in fruit tissues from accessions of Capsicum chinense Jacq. (Habanero pepper) at different stages of ripening. Sci. World J. 2014. [Google Scholar] [CrossRef]
- Campos, M.R.S.; Gómez, K.R.; Ordoñez, Y.M.; Ancona, D.B. Polyphenols, ascorbic acid and carotenoids contents and antioxidant properties of habanero pepper (Capsicum chinense) fruit. Food Nutr. Sci. 2013, 4, 47–54. [Google Scholar]
- Ma, H.; Williams, P.L.; Diamond, S.A. Ecotoxicity of manufactured ZnO nanoparticles—A review. Environ. Pollut. 2013, 172, 76–85. [Google Scholar] [CrossRef]
- Lopez-Moreno, M.L.; De La Rosa, G.; Hernandez-Viezcas, J.A.; Castillo-Michel, H.; Botez, C.E.; Peralta-Videa, J.R.; GardeaTorresdey, J.L. Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 NPs on soybean (Glycine max) plants. Environ. Sci. Technol. 2010, 44, 7315–7320. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Jana, A.; Sinha, S.; Jothiramajayam, M.; Nag, A.; Chakraborty, A.; Mukherjee, A.; Mukherjee, A. Effects of ZnO nanoparticles in plants: Cytotoxicity, genotoxicity, deregulation of antioxidant defenses, and cell-cycle arrest. Mutat Res. Genet. Toxicol. Environ. Mutagen. 2016, 807, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Kouhi, S.M.M.; Lahouti, M.; Ganjeali, A.; Entezari, M.H. Comparative effects of ZnO nanoparticles, ZnO bulk particles, and Zn2+ on Brassica napus after long-term exposure: Changes in growth, biochemical compounds, antioxidant enzyme activities, and Zn bioaccumulation. Water Air Soil Pollut. 2015, 226, 364. [Google Scholar] [CrossRef]
- García-Gómez, C.; Obrador, A.; González, D.; Babín, M.; Fernández, M.D. Comparative study of the phytotoxicity of ZnO nanoparticles and Zn accumulation in nine crops grown in a calcareous soil and an acidic soil. Sci. Total Environ. 2018, 644, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Ali, A.; Ali, J.S.; Haq, I.U.; Zia, M. Effect of ZnO nanoparticles on Brassica nigra seedlings and stem explants: Growth dynamics and antioxidative response. Front. Plant. Sci. 2016, 7, 535. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, S.; Zhu, H.; Colvin, V.L.; Alvarez, P.J. Quantum dot weathering results in microbial toxicity. Environ. Sci. Technol. 2008, 42, 9424–9430. [Google Scholar] [CrossRef]
- Bhumi, G.; Ratna, N.; Savithramma, N. Screening of zinc oxide nanoparticles for cell proliferation synthesized. Int. J. Drug Dev. Res. 2014, 6, 0975–9344. [Google Scholar]
- Sora, G.T.S.; Haminiuk, C.W.I.; Da Silva, M.V.; Zielinski, A.A.F.; Gonçalves, G.A.; Bracht, A.; Peralta, R.M. A comparative study of the capsaicinoid and phenolic contents and in vitro antioxidant activities of the peppers of the genus Capsicum: An application of chemometrics. J. Food Sci. Technol. 2015, 52, 8086–8094. [Google Scholar] [CrossRef]
- Alvarez-Parrilla, E.; De la Rosa, L.A.; Amarowicz, R.; Shahidi, F. Antioxidant activity of fresh and processed jalapeño and serrano peppers. J. Agric. Food Chem. 2011, 59, 163–173. [Google Scholar] [CrossRef]
- Parry, A.D.; Tiller, S.A.; Edwards, R. The effects of heavy metals and root immersion on isoflavonoid metabolism in alfalfa (Medicago sativa L). Plant. Physiol. 1994, 106–196. [Google Scholar] [CrossRef]
- Ghiassi-Tarzia, B.; Gharachorlooa, M.; Baharinia, M.; Mortazavic, S.A. The effect of germination on phenolic content and antioxidant activity of chickpea. Iran J. Pharm. Res. 2012, 11, 1137–1143. [Google Scholar]
- Zare, M.; Namratha, K.; Byrappa, K.; Surendra, D.M.; Yallappa, S.; Hungund, B. Surfactant assisted solvothermal synthesis of ZnO nanoparticles and study of their antimicrobial and antioxidant properties. J. Mater. Sci. Technol. 2018, 34, 1035–1043. [Google Scholar] [CrossRef]
| Treatments (mg L−1) | Number of Fruits | Average Fruit Weight (g) | Total Weight of Fruits (g) | Fresh Aerial Biomass (g) | Dry Aerial Biomass (g) |
|---|---|---|---|---|---|
| Control | 54.20 ± 2.28 c | 8.80 ± 0.22 ab | 476.97 ± 23.74 ab | 893.85 ± 11.00 b | 294.80 ± 11.33 c |
| ZnSO4 1000 | 58.50 ± 1.29 b | 9.12 ± 0.11 ab | 533.78 ± 11.93 ab | 905.09 ± 5.88 b | 310.81 ± 3.44 b |
| ZnO NPs 1000 | 64.00 ± 2.24 a | 9.46 ± 0.12 a | 605.30 ± 17.27 a | 925.64 ± 4.09 a | 324.91 ± 5.09 a |
| ZnSO4 2000 | 50.25 ± 1.50 d | 8.46 ± 0.07 b | 425.20 ± 11.19 b | 879.46 ± 5.99 c | 278.56 ± 8.59 d |
| ZnO NPs 2000 | 46.80 ± 2.39 d | 8.29 ± 0.11 b | 387.99 ± 18.71 b | 868.22 ± 2.42 c | 264.42 ± 4.92 e |
| Treatments (mg L−1) | Chromatic Parameter | |||||
|---|---|---|---|---|---|---|
| L* | a* | b* | C* | h | View | |
| Control | 50.02 ± 1.08 b | 30.57 ± 1.93 a | 38.92 ± 1.33 b | 49.37 ± 1.97 b | 51.77 ± 1.46 a |  |
| ZnSO4 1000 | 51.29 ± 1.76 b | 32.06 ± 0.85 a | 41.98 ± 1.11 a | 50.77 ± 1.32 b | 52.33 ± 1.00 a |  |
| ZnO NPs 1000 | 51.99 ± 1.11 ab | 32.33 ± 1.45 a | 42.48 ± 2.13 a | 51.81 ± 0.76 ab | 53.71 ± 1.24 a |  |
| ZnSO4 2000 | 51.92 ± 1.46 ab | 32.07 ± 0.84 a | 41.91 ± 1.42 a | 51.75 ± 1.01 ab | 53.30 ± 1.25 a |  |
| ZnO NPs 2000 | 53.46 ± 1.15 a | 33.45 ± 1.20 a | 43.59 ± 1.47 a | 53.43 ± 1.64 a | 53.84 ± 1.44 a |  |
| Treatments (mg L−1) | TA (%) | pH | Soluble Solids (%) | Firmness (N) | Cutting Force (N) |
|---|---|---|---|---|---|
| Control | 0.119 ± 0.0050 d | 5.63 ± 0.114 a | 9.32 ± 0.79 d | 14.79 ± 0.63 b | 11.35 ± 0.83 a |
| ZnSO4 1000 | 0.126 ± 0.0012 cd | 5.49 ± 0.030 b | 10.18 ± 0.38 c | 14.94 ± 0.65 ab | 11.39 ± 0.77 a |
| ZnO NPs 1000 | 0.134 ± 0.0025 b | 5.42 ± 0.048 b | 10.77 ± 0.60 b | 15.11 ± 0.68 ab | 11.43 ± 0.64 a |
| ZnSO4 2000 | 0.130 ± 0.0020 bc | 5.50 ± 0.025 b | 10.48 ± 0.52 bc | 15.20 ± 0.63 ab | 11.36 ± 0.72 a |
| ZnO NPs 2000 | 0.155 ± 0.0055 a | 5.40 ± 0.040 b | 11.33 ± 0.72 a | 15.35 ± 0.54 a | 11.32 ± 0.47 a |
| Treatments (mg L−1) | Total Phenolics (mgGAE kg−1) | Total Flavonoids (mgCatE kg−1) | ||||
|---|---|---|---|---|---|---|
| Free | Bound | Total | Free | Bound | Total | |
| Control | 1154.85 ± 10.55 b | 113.50 ± 4.30 b | 1286.35 b | 114.35 ± 5.52 b | 69.30 ± 2.71 c | 183.65 b |
| ZnSO4 1000 | 1168.44 ± 17.76 b | 122.11 ± 6.55 b | 1290.55 b | 116.95 ± 10.22 b | 79.28 ± 5.83 bc | 196.24 b |
| ZnO NPs 1000 | 1293.42 ± 28.30 a | 149.34 ± 6.45 a | 1442.76 a | 144.63 ± 9.53 a | 92.54 ± 5.39 ab | 237.17 a |
| ZnSO4 2000 | 1176.24 ± 15.61 b | 123.68 ± 4.98 b | 1299.93 b | 119.73 ± 6.89 a | 76.62 ± 4.43 c | 196.35 b |
| ZnO NPs 2000 | 1347.41 ± 30.06 a | 157.18 ± 7.58 a | 1504.60 a | 155.01 ± 8.04 a | 96.50 ± 6.20 a | 251.50 a |
| Treatments (mg L−1) | ABTS (mmolTE kg−1) | DPPH (mmolTE kg−1) | FRAP (mmolTE kg−1) | |||
|---|---|---|---|---|---|---|
| Free | Bound | Free | Bound | Free | Bound | |
| Control | 76.60 ± 3.19 b | 6.68. ± 4.30 c | 112.97 ± 5.52 b | 32.32 ± 2.71 c | 172.75 ± 4.68 b | 61.50 ± 3.25 b |
| ZnSO4 1000 | 83.33 ± 3.18 ab | 7.95 ± 6.55 b | 121.67 ± 10.22 b | 35.60 ± 5.83 c | 180.86 ± 5.19 b | 65.19 ± 3.51 b |
| ZnO NPs 1000 | 86.77 ± 2.75 a | 8.75 ± 6.45 ab | 154.98 ± 9.53 a | 49.70 ± 5.39 b | 198.22 ± 5.14 a | 80.51 ± 0.66 a |
| ZnSO4 2000 | 81.08 ± 3.18 ab | 8.12 ± 4.98 b | 130.31 ± 6.89 b | 38.19 ± 4.43 c | 180.30 ± 6.73 b | 67.61 ± 3.08 b |
| ZnO NPs 2000 | 89.14 ± 2.96 a | 9.35 ± 7.58 a | 156.55 ± 8.04 a | 56.61 ± 6.20 a | 212.21 ± 8.35 a | 83.85 ± 1.97 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-López, J.I.; Niño-Medina, G.; Olivares-Sáenz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Vázquez-Alvarado, R.; Rodríguez-Salinas, P.A.; Zavala-García, F. Foliar Application of Zinc Oxide Nanoparticles and Zinc Sulfate Boosts the Content of Bioactive Compounds in Habanero Peppers. Plants 2019, 8, 254. https://doi.org/10.3390/plants8080254
García-López JI, Niño-Medina G, Olivares-Sáenz E, Lira-Saldivar RH, Barriga-Castro ED, Vázquez-Alvarado R, Rodríguez-Salinas PA, Zavala-García F. Foliar Application of Zinc Oxide Nanoparticles and Zinc Sulfate Boosts the Content of Bioactive Compounds in Habanero Peppers. Plants. 2019; 8(8):254. https://doi.org/10.3390/plants8080254
Chicago/Turabian StyleGarcía-López, Josué I., Guillermo Niño-Medina, Emilio Olivares-Sáenz, Ricardo H. Lira-Saldivar, Enrique Díaz Barriga-Castro, Rigoberto Vázquez-Alvarado, Pablo A. Rodríguez-Salinas, and Francisco Zavala-García. 2019. "Foliar Application of Zinc Oxide Nanoparticles and Zinc Sulfate Boosts the Content of Bioactive Compounds in Habanero Peppers" Plants 8, no. 8: 254. https://doi.org/10.3390/plants8080254
APA StyleGarcía-López, J. I., Niño-Medina, G., Olivares-Sáenz, E., Lira-Saldivar, R. H., Barriga-Castro, E. D., Vázquez-Alvarado, R., Rodríguez-Salinas, P. A., & Zavala-García, F. (2019). Foliar Application of Zinc Oxide Nanoparticles and Zinc Sulfate Boosts the Content of Bioactive Compounds in Habanero Peppers. Plants, 8(8), 254. https://doi.org/10.3390/plants8080254





