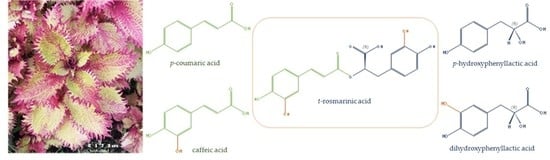
Green Ultrasound Assisted Extraction of trans Rosmarinic Acid from Plectranthus scutellarioides (L.) R.Br. Leaves
Abstract
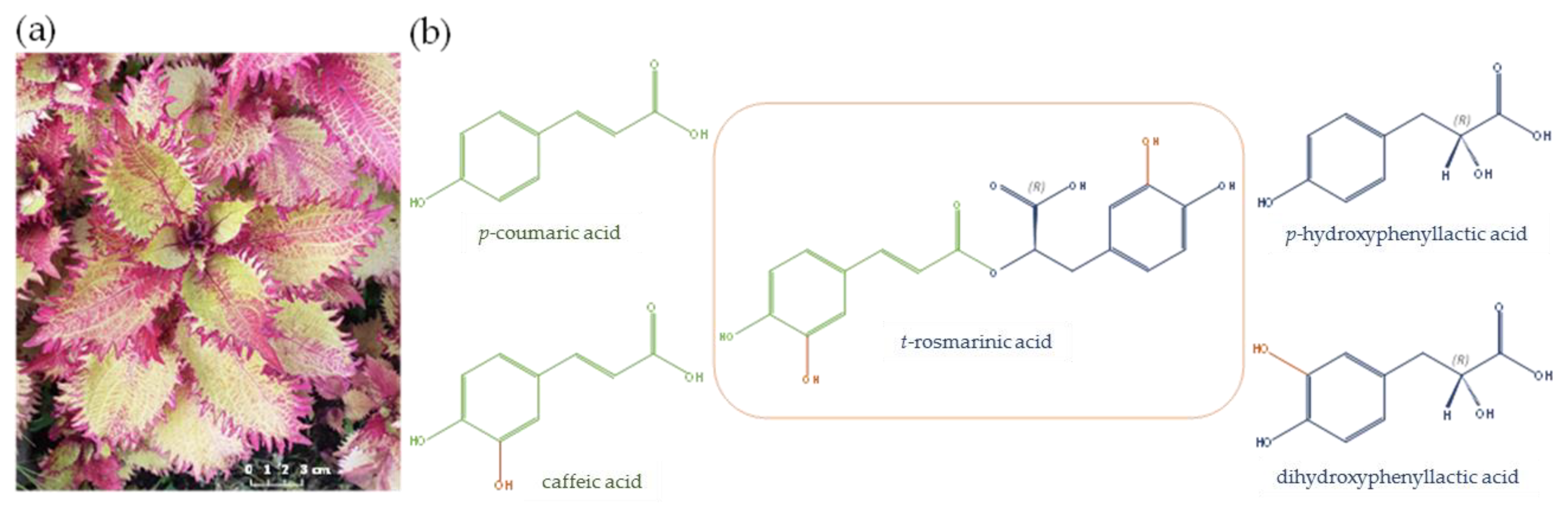
1. Introduction
2. Results and Discussion
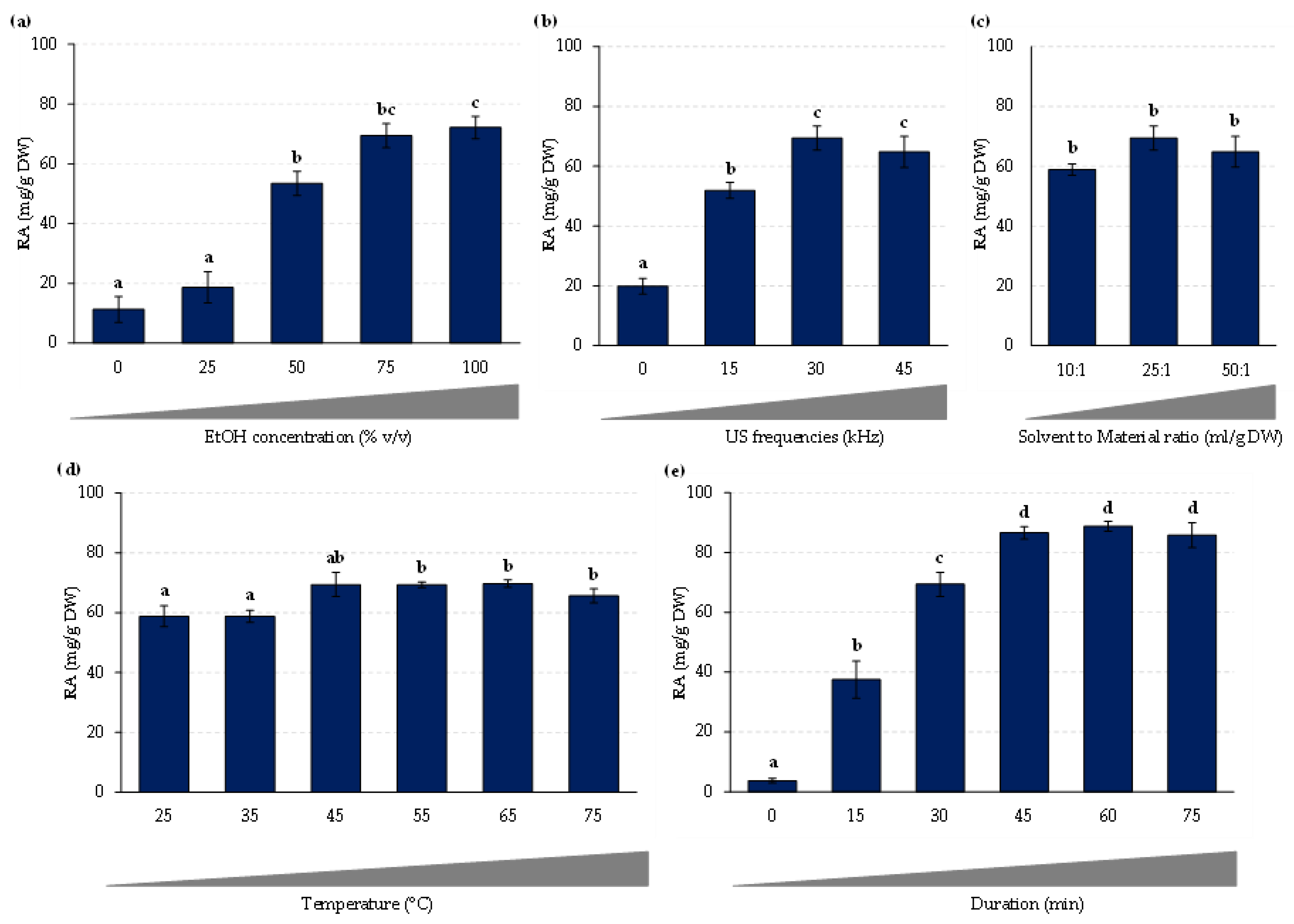
2.1. Preliminary Single Factor Experiments
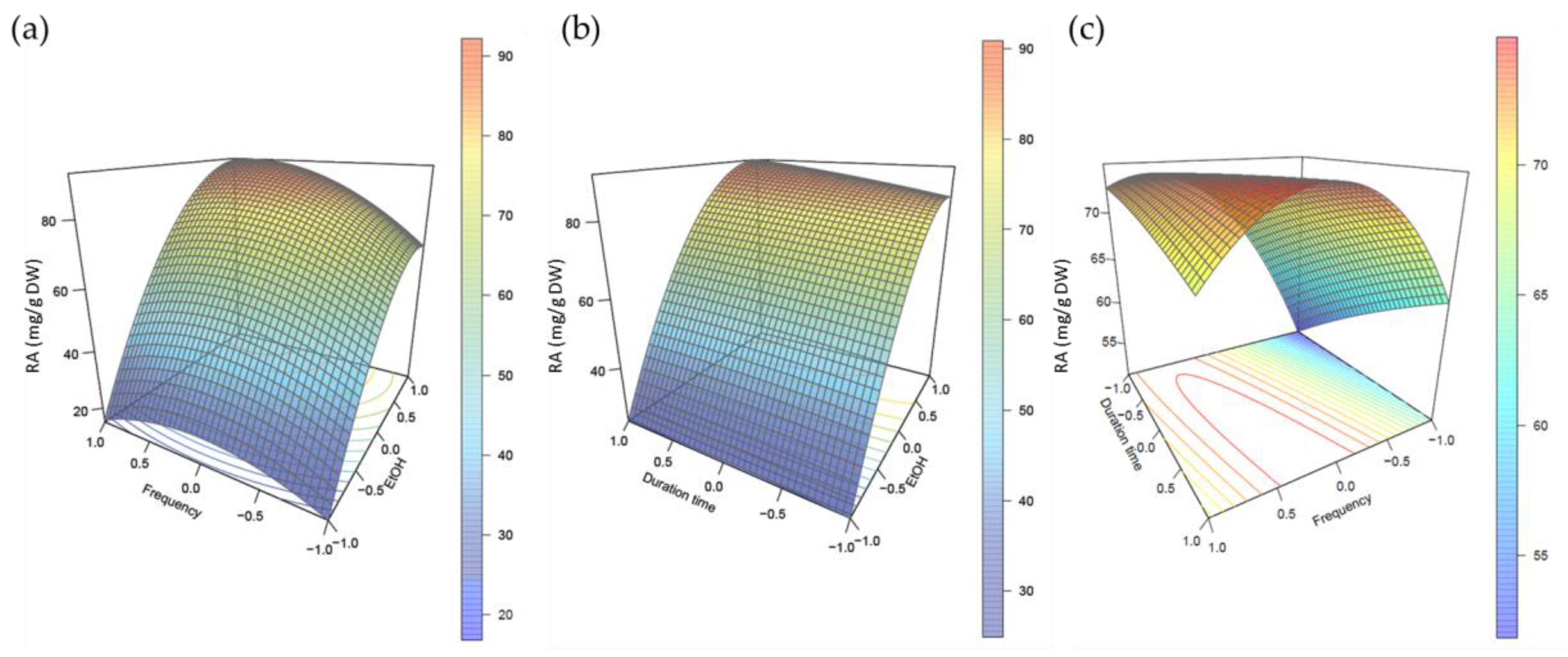
2.2. Develoment of a Multifactorial Approach
2.3. Validation of the Extraction Method
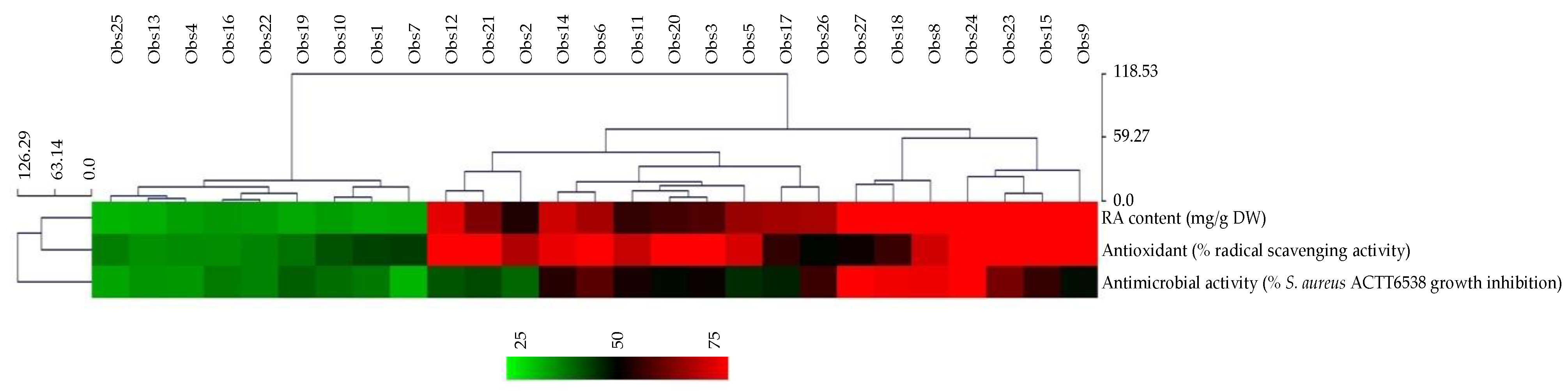
2.4. Biological Activities of the Extracts
2.5. Comparison with Conventional Extraction Protocol and Other Biotechnological Sources
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Materials
3.3. Ultrasound-Assisted Extraction (USAE) Optimization
3.4. Conventional Solid/Liquid Extraction
3.5. High-Performance Liquid Chromatography (HPLC) Analysis
3.6. Experimental Design
3.7. Method Validation
3.8. Antioxidant DPPH assay
3.9. Antibacterial Activity
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Codd, L.E. Plectranthus (Labiatae) and allied genera in Southern Africa. Bothalia Afr. Biodivers. Conserv. 1975, 11, 371–442. [Google Scholar] [CrossRef]
- Lukhoba, C.W.; Simmonds, M.S.J.; Paton, A.J. Plectranthus: A review of ethnobotanical uses. J. Ethnopharmacol. 2006, 103, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Namsa, N.D.; Tag, H.; Mandal, M.; Kalita, P.; Das, A.K. An ethnobotanical study of traditional anti-inflammatory plants used by the Lohit community of Arunachal Pradesh, India. J. Ethnopharmacol. 2009, 125, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Cetto, A. Ethnobotanical study of the medicinal plants from Tlanchinol, Hidalgo, México. J. Ethnopharmacol. 2009, 122, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.D.; Park, Y.S.; Jin, Y.H.; Park, C.S. Production and applications of rosmarinic acid and structurally related compounds. Appl. Microbiol. Biotechnol. 2015, 99, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Khojasteh, A.; Mirjalili, M.H.; Hidalgo, D.; Corchete, P.; Palazon, J. New trends in biotechnological production of rosmarinic acid. Biotechnol. Lett. 2014, 36, 2393–2406. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Simmonds, M.S. Rosmarinic Acid. Phytochemistry 2013, 62, 43–50. [Google Scholar] [CrossRef]
- Petersen, M.; Abdullah, Y.; Benner, J.; Eberle, D.; Gehlen, K.; Hücherig, S.; Janiak, V.; Kim, K.H.; Sander, M.; Weitzel, C.; et al. Evolution of rosmarinic acid biosynthesis. Phytochemistry 2009, 70, 1663–1679. [Google Scholar] [CrossRef] [PubMed]
- Falé, P.L.; Borges, C.; Madeira, P.J.A.; Ascensão, L.; Araújo, M.E.M.; Florêncio, M.H.; Serralheiro, M.L.M. Rosmarinic acid, scutellarein 4′-methyl ether 7-O-glucuronide and (16S)-coleon E are the main compounds responsible for the antiacetylcholinesterase and antioxidant activity in herbal tea of Plectranthus barbatus (“falso boldo”). Food Chem. 2009, 114, 798–805. [Google Scholar] [CrossRef]
- Falé, P.L.V.; Madeira, P.J.A.; Florêncio, M.H.; Ascensão, L.; Serralheiro, M.L.M. Function of Plectranthus barbatus herbal tea as neuronal acetylcholinesterase inhibitor. Food Funct. 2011, 2, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Falé, P.L.; Ascensão, L.; Serralheiro, M.L.M. Effect of luteolin and apigenin on rosmarinic acid bioavailability in Caco-2 cell monolayers. Food Funct. 2013, 4, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Rijo, P.; Matias, D.; Fernandes, A.S.; Simões, M.F.; Nicolai, M.; Reis, C.P. Antimicrobial plant extracts encapsulated into polymeric beads for potential application on the skin. Polymers 2014, 6, 479–490. [Google Scholar] [CrossRef]
- Kubínová, R.; Pořízková, R.; Navrátilová, A.; Farsa, O.; Hanáková, Z.; Bačinská, A.; Čížek, A.; Valentová, M. Antimicrobial and enzyme inhibitory activities of the constituents of Plectranthus madagascariensis (Pers.) Benth. J. Enzym. Inhib. Med. Chem. 2014, 29, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Yu, H.M.; Shie, J.J.; Cheng, T.J.R.; Wu, C.Y.; Fang, J.M.; Wong, C.H. Chemical constituents of Plectranthus amboinicus and the synthetic analogs possessing anti-inflammatory activity. Bioorg. Med. Chem. 2014, 22, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Medrado, H.; dos Santos, E.; Ribeiro, E.; David, J.; David, J.; Araújo, J.F.; do Vale, A.; Bellintani, M.; Brandão, H.; Meira, P. Rosmarinic and Cinnamic Acid Derivatives of in vitro Tissue Culture of Plectranthus ornatus: Overproduction and Correlation with Antioxidant Activities. J. Braz. Chem. Soc. 2016, 28, 2017–2019. [Google Scholar] [CrossRef]
- Qiao, S.; Li, W.; Tsubouchi, R.; Haneda, M.; Murakami, K.; Takeuchi, F.; Nisimoto, Y.; Yoshino, M. Rosmarinic acid inhibits the formation of reactive oxygen and nitrogen species in RAW264.7 macrophages. Free Radic. Res. 2005, 39, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, N.; Yasuda, A.; Natsume, M.; Sanbongi, C.; Kato, Y.; Osawa, T.; Yoshikawa, T. Rosmarinic acid, a major polyphenolic component of Perilla frutensis, reduces liposaccharide (LPS)-induced liver injury in D-glucosamine (D-GalN)-sensitized mice. Free Radic. Biol. Med. 1997, 5, 637–647. [Google Scholar]
- Fallarini, S.; Miglio, G.; Paoletti, T.; Minassi, A.; Amoruso, A.; Bardelli, C.; Brunelleschi, S.; Lombardi, G. Clovamide and rosmarinic acid induce neuroprotective effects in in vitro models of neuronal death. Br. J. Pharmacol. 2009, 157, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Ono, K.; Murase, A.; Yamada, M. Phenolic compounds prevent Alzheimer’s pathology through different effects on the amyloid-β aggregation pathway. Am. J. Pathol. 2009, 175, 2557–2565. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Hano, C.; Corbin, C.; Drouet, S.; Quéro, A.; Rombaut, N.; Savoire, R.; Molinié, R.; Thomasset, B.; Mesnard, F.; Lainé, E. The lignan (+)-secoisolariciresinol extracted from flax hulls is an effective protectant of linseed oil and its emulsion against oxidative damage. Eur. J. Lipid Sci. Technol. 2017, 119, 1–9. [Google Scholar] [CrossRef]
- Lopez, T.; Corbin, C.; Falguieres, A.; Doussot, J.; Montguillon, J.; Hagège, D.; Hano, C.; Lainé, É. Influence de la composition du milieu de culture sur la production de métabolites secondaires et les activités antioxydantes et antibactériennes des extraits produits í partir de cultures in vitro de Clidemia hirta L. C. R. Chim. 2016, 19, 1071–1076. [Google Scholar] [CrossRef]
- Bourgeois, C.; Leclerc, É.A.; Corbin, C.; Doussot, J.; Serrano, V.; Vanier, J.R.; Seigneuret, J.M.; Auguin, D.; Pichon, C.; Lainé, É.; et al. L’ortie (Urtica dioica L.), une source de produits antioxidants et phytochimiques anti-âge pour des applications en cosmétique. C. R. Chim. 2016, 19, 1090–1100. [Google Scholar] [CrossRef]
- Lavilla, I.; Bendicho, C. Fundamentals of Ultrasound-Assisted Extraction. Water Extr. Bioact. Compd. 2017, 291–316. [Google Scholar] [CrossRef]
- Corbin, C.; Fidel, T.; Leclerc, E.A.; Barakzoy, E.; Sagot, N.; Falguiéres, A.; Renouard, S.; Blondeau, J.P.; Ferroud, C.; Doussot, J.; et al. Development and validation of an efficient ultrasound assisted extraction of phenolic compounds from flax (Linum usitatissimum L.) seeds. Ultrason. Sonochem. 2015, 26, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound Assisted Extraction for the Recovery of Phenolic Compounds from Vegetable Sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Bauer, N.; Leljak-Levanic, D.; Jelaska, S. Rosmarinic Acid Synthesis in Transformed Callus Culture of Coleus blumei Benth. Z. Naturforsch. C 2004, 59, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Häusler, E.; Meinhard, J.; Karwatzki, B.; Gertlowski, C. The biosynthesis of rosmarinic acid in suspension cultures of Coleus blumei. Plant Cell Tissue Organ Cult. 1994, 38, 171–179. [Google Scholar] [CrossRef]
- Martinez, B.C.; Park, C. Characteristics of Batch Suspension Cultures of Preconditioned Coleus blumei Cells: Sucrose Effect. Biotechnol. Prog. 1993, 9, 97–100. [Google Scholar] [CrossRef]
- Furukawa, M.; Makino, M.; Ohkoshi, E.; Uchiyama, T.; Fujimoto, Y. Terpenoids and phenethyl glucosides from Hyssopus cuspidatus (Labiatae). Phytochemistry 2011, 72, 2244–2252. [Google Scholar] [CrossRef] [PubMed]
- Hajimehdipoor, H.; Saeidnia, S.; Gohari, A.; Hamedani, M.; Shekarchi, M. Comparative study of rosmarinic acid content in some plants of Labiatae family. Pharmacogn. Mag. 2012, 8, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Jacotet-Navarro, M.; Rombaut, N.; Fabiano-Tixier, A.S.; Danguien, M.; Bily, A.; Chemat, F. Ultrasound versus microwave as green processes for extraction of rosmarinic, carnosic and ursolic acids from rosemary. Ultrason. Sonochem. 2015, 27, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Caleja, C.; Barros, L.; Prieto, M.A.; Barreiro, M.F.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Extraction of rosmarinic acid from Melissa officinalis L. by heat-, microwave- and ultrasound-assisted extraction techniques: A comparative study through response surface analysis. Sep. Purif. Technol. 2017, 186, 297–308. [Google Scholar] [CrossRef]
- Adham, A.N. Comparative extraction methods, phytochemical constituents, fluorescence analysis and HPLC validation of rosmarinic acid content in Mentha piperita, Mentha longifolia and Osimum basilicum. J. Pharmacogn. Phytochem. 2015, 3, 130–139. [Google Scholar]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Tandon, S.; Ahmad, A. Comparative Extraction and Downstream Processing Techniques for Quantitative Analysis of Rosmarinic Acid in Rosmarinus officinalis. Asian J. Chem. 2014, 26, 4313–4318. [Google Scholar] [CrossRef]
- Tabaraki, R.; Nateghi, A. Optimization of ultrasonic-assisted extraction of natural antioxidants from rice bran using response surface methodology. Ultrason. Sonochem. 2011, 18, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Rakić, T.; Kasagić-Vujanović, I.; Jovanović, M.; Jančić-Stojanović, B.; Ivanović, D. Comparison of Full Factorial Design, Central Composite Design, and Box-Behnken Design in Chromatographic Method Development for the Determination of Fluconazole and Its Impurities. Anal. Lett. 2014, 47, 1334–1347. [Google Scholar] [CrossRef]
- Amoah, S.; Sandjo, L.; Kratz, J.; Biavatti, M. Rosmarinic Acid–Pharmaceutical and Clinical Aspects. Planta Med. 2016, 82, 388–406. [Google Scholar] [CrossRef] [PubMed]
- Ngo, Y.L.; Lau, C.H.; Chua, L.S. Review on rosmarinic acid extraction, fractionation and its anti-diabetic potential. Food Chem. Toxicol. 2018, 121, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, Y.; Schneider, B.; Petersen, M. Occurrence of rosmarinic acid, chlorogenic acid and rutin in Marantaceae species. Phytochem. Lett. 2008, 1, 199–203. [Google Scholar] [CrossRef]
- Snyder, L.R.; Kirkland, J.J.; Glajch, J.L. Practical HPLC Method Development; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1997; ISBN 9781118592014. [Google Scholar]
- Es-Safi, N.E.; Ghidouche, S.; Ducrot, P.H. Flavonoids: Hemisynthesis, reactivity, characterization and free radical scavenging activity. Molecules 2007, 12, 2228–2258. [Google Scholar] [CrossRef] [PubMed]
- El Abdellaoui, S.; Destandau, E.; Krolikiewicz-Renimel, I.; Cancellieri, P.; Toribio, A.; Jeronimo-Monteiro, V.; Landemarre, L.; André, P.; Elfakir, C. Centrifugal partition chromatography for antibacterial bio-guided fractionation of Clidemia hirta roots. Sep. Purif. Technol. 2014, 123, 221–228. [Google Scholar] [CrossRef]
- Fliniaux, O.; Corbin, C.; Ramsay, A.; Renouard, S.; Beejmohun, V.; Doussot, J.; Falguières, A.; Ferroud, C.; Lamblin, F.; Lainé, E.; et al. Microwave-assisted extraction of herbacetin diglucoside from flax (Linum usitatissimum L.) seed cakes and its quantification using an RP-HPLC-UV system. Molecules 2014, 19, 3025–3037. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, G.; Gismondi, A.; Panzanella, L.; Canuti, L.; Impei, S.; Leonardi, D.; Canini, A. Botanical influence on phenolic profile and antioxidant level of Italian honeys. J. Food Sci. Technol. 2018, 55, 4042–4050. [Google Scholar] [CrossRef] [PubMed]
| Independent variable | Code unit | Coded variable levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Ethanol concentration (% v/v) 1 | X1 | 50 | 75 | 100 |
| US frequency (kHz) | X2 | 15 | 30 | 45 |
| Extraction duration (min) | X3 | 15 | 30 | 45 |
| Run ID | Run order | X1 | X2 | X3 | RA (mg/g DW) |
|---|---|---|---|---|---|
| Obs1 | 17 | −1 | −1 | −1 | 16.9 ± 2.6 |
| Obs2 | 24 | 0 | −1 | −1 | 53.2 ± 0.5 |
| Obs3 | 26 | +1 | −1 | −1 | 57.8 ± 6.0 |
| Obs4 | 21 | −1 | 0 | −1 | 18.7 ± 3.8 |
| Obs5 | 22 | 0 | 0 | −1 | 65.2 ± 5.0 |
| Obs6 | 6 | +1 | 0 | −1 | 66.5 ± 2.4 |
| Obs7 | 10 | −1 | +1 | −1 | 17.6 ± 4.3 |
| Obs8 | 27 | 0 | +1 | −1 | 78.2 ± 1.1 |
| Obs9 | 7 | +1 | +1 | −1 | 102.2 ± 1.15 |
| Obs10 | 18 | −1 | −1 | 0 | 19.2 ± 2.2 |
| Obs11 | 12 | 0 | −1 | 0 | 55.1 ± 2.5 |
| Obs12 | 8 | +1 | −1 | 0 | 72.6 ± 0.6 |
| Obs13 | 25 | −1 | 0 | 0 | 15.6 ± 0.1 |
| Obs14 | 1 | 0 | 0 | 0 | 70.4 ± 7.4 |
| Obs15 | 16 | +1 | 0 | 0 | 91.6 ± 3.1 |
| Obs16 | 23 | −1 | +1 | 0 | 20.3 ± 2.5 |
| Obs17 | 11 | 0 | +1 | 0 | 66.3 ± 2.6 |
| Obs18 | 14 | +1 | +1 | 0 | 90.2 ± 1.3 |
| Obs19 | 15 | −1 | −1 | +1 | 16.9 ± 4.3 |
| Obs20 | 3 | 0 | −1 | +1 | 56.7 ± 1.9 |
| Obs21 | 13 | +1 | −1 | +1 | 62.6 ± 1.6 |
| Obs22 | 9 | −1 | 0 | +1 | 19.5 ± 0.5 |
| Obs23 | 5 | 0 | 0 | +1 | 91.1 ± 4.4 |
| Obs24 | 19 | +1 | 0 | +1 | 110.8 ± 4.5 * |
| Obs25 | 4 | −1 | +1 | +1 | 14.4 ± 1.1 |
| Obs26 | 20 | 0 | +1 | +1 | 66.7 ± 3.0 |
| Obs27 | 2 | +1 | +1 | +1 | 75.9 ± 2.6 |
| Source | Value | SD | t | P > |t| |
|---|---|---|---|---|
| Constant | 73.22 | 5.279 | 13.871 | < 0.0001 *** |
| X1 | 31.74 | 2.444 | 12.988 | < 0.0001 *** |
| X2 | 6.70 | 2.444 | 2.743 | 0.014 * |
| X3 | 2.15 | 2.444 | 0.879 | 0.392 ns |
| X12 | −17.60 | 4.232 | −4.159 | 0.001 ** |
| X22 | −8.68 | 4.232 | −2.052 | 0.046 * |
| X32 | −0.65 | 4.232 | −0.153 | 0.880 ns |
| X1X2 | 6.34 | 2.993 | 2.118 | 0.049 * |
| X1X3 | 2.11 | 2.993 | 0.707 | 0.489 ns |
| X2X3 | −4.11 | 2.993 | −1.373 | 0.188 ns |
| Source | Sum of square | df | Mean of square | F-value | p-value |
|---|---|---|---|---|---|
| Model | 22074.5 | 9 | 2452.7 | 22.72 | < 0.0001 |
| Lack of fit | 1592.8 | 17 | 93.7 | 0.87 | 0.266 |
| Residual | 1827.1 | 17 | 107.5 | - | - |
| Pure Error | 234.3 | 0 | - | - | - |
| Cor. Error | 23901.6 | 26 | - | - | - |
| R2 | 0.924 | ||||
| R2 adj | 0.883 | ||||
| CV % | 0.79 |
| Equation | R2 | LOD (ng) | LOQ (ng) | Precision (%RSD) | Repeatability (%RSD) | Recovery (%RSD) | |
|---|---|---|---|---|---|---|---|
| Intraday | Interday | ||||||
| y = 4.872x − 0.123 | 0.9998 | 1.8 | 5.3 | 0.48 | 0.94 | 3.5 | 2.8 |
| RA content as a function of extraction method and time | Conventional Heat Reflux Extraction | USAE | ||
|---|---|---|---|---|
| Extraction duration | 45 min | 90 min | 180 min | 45 min |
| RA content (mg/g DW) | 55.6 ± 3.1d | 79.7 ± 1.5c | 95.6 ± 2.2b | 110.8 ± 4.5a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tungmunnithum, D.; Garros, L.; Drouet, S.; Renouard, S.; Lainé, E.; Hano, C. Green Ultrasound Assisted Extraction of trans Rosmarinic Acid from Plectranthus scutellarioides (L.) R.Br. Leaves. Plants 2019, 8, 50. https://doi.org/10.3390/plants8030050
Tungmunnithum D, Garros L, Drouet S, Renouard S, Lainé E, Hano C. Green Ultrasound Assisted Extraction of trans Rosmarinic Acid from Plectranthus scutellarioides (L.) R.Br. Leaves. Plants. 2019; 8(3):50. https://doi.org/10.3390/plants8030050
Chicago/Turabian StyleTungmunnithum, Duangjai, Laurine Garros, Samantha Drouet, Sullivan Renouard, Eric Lainé, and Christophe Hano. 2019. "Green Ultrasound Assisted Extraction of trans Rosmarinic Acid from Plectranthus scutellarioides (L.) R.Br. Leaves" Plants 8, no. 3: 50. https://doi.org/10.3390/plants8030050
APA StyleTungmunnithum, D., Garros, L., Drouet, S., Renouard, S., Lainé, E., & Hano, C. (2019). Green Ultrasound Assisted Extraction of trans Rosmarinic Acid from Plectranthus scutellarioides (L.) R.Br. Leaves. Plants, 8(3), 50. https://doi.org/10.3390/plants8030050