The Quality Assessment of Commercial Lycium Berries Using LC-ESI-MS/MS and Chemometrics
Abstract
1. Introduction
2. Methods
2.1. Instrumentation
2.2. Reagents, Chemicals and Samples
2.3. Sample Extraction and LC Mobile Phase Preparation
2.4. Preparation of Stock Calibration Solution Using Analytical Standards
2.5. Recovery Studies
2.6. LC-ESI-MS/MS Conditions
2.7. LC-UV and GC-MS Methods Used to Obtain Spectra for PCA Analysis
2.8. PCA Analysis
3. Results and Discussion
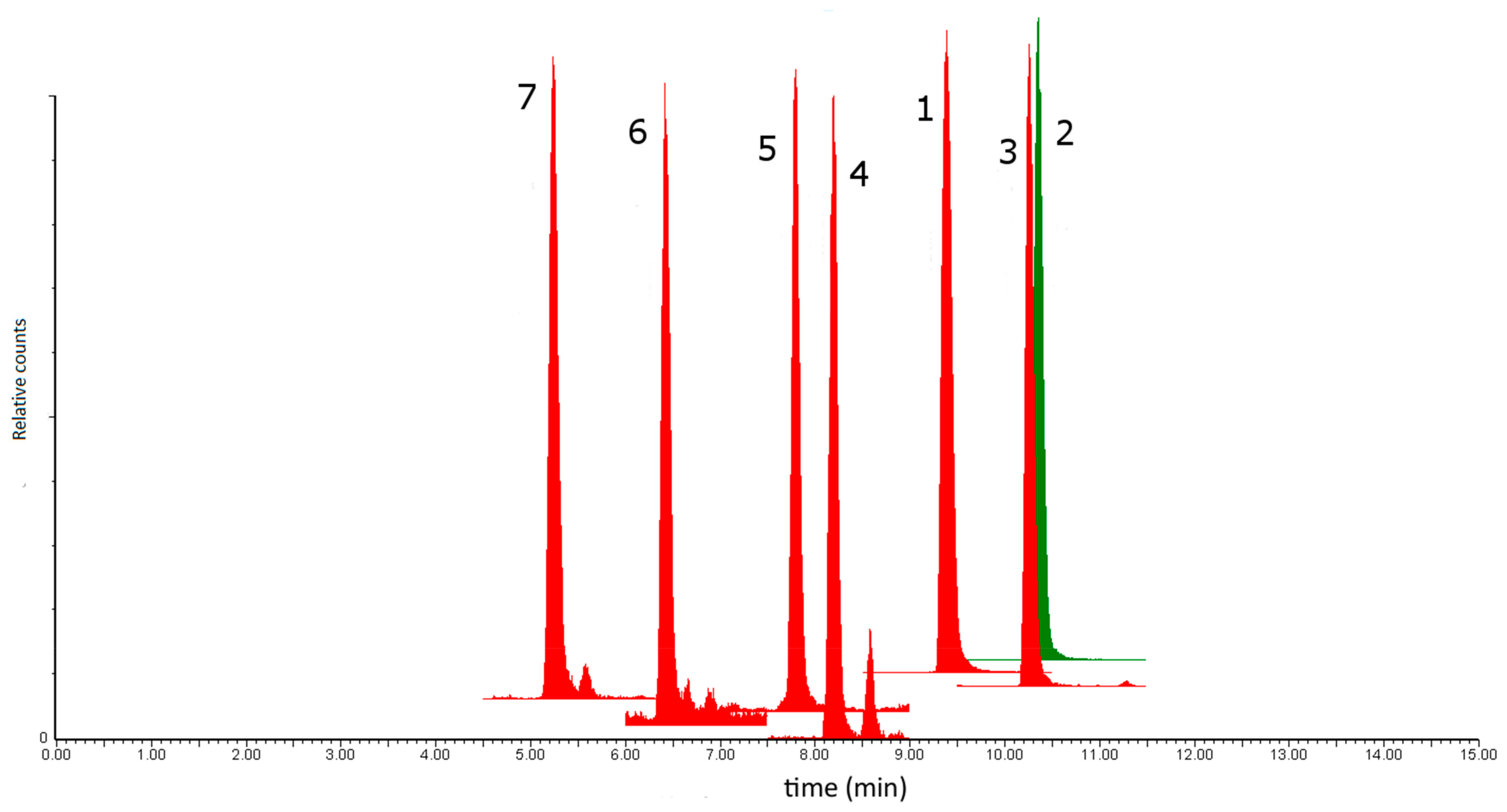
3.1. Chromatographic Data and Recoveries
3.2. Precision and MS Identity Confirmation
3.3. Analyte Concentrations and Fold Variation
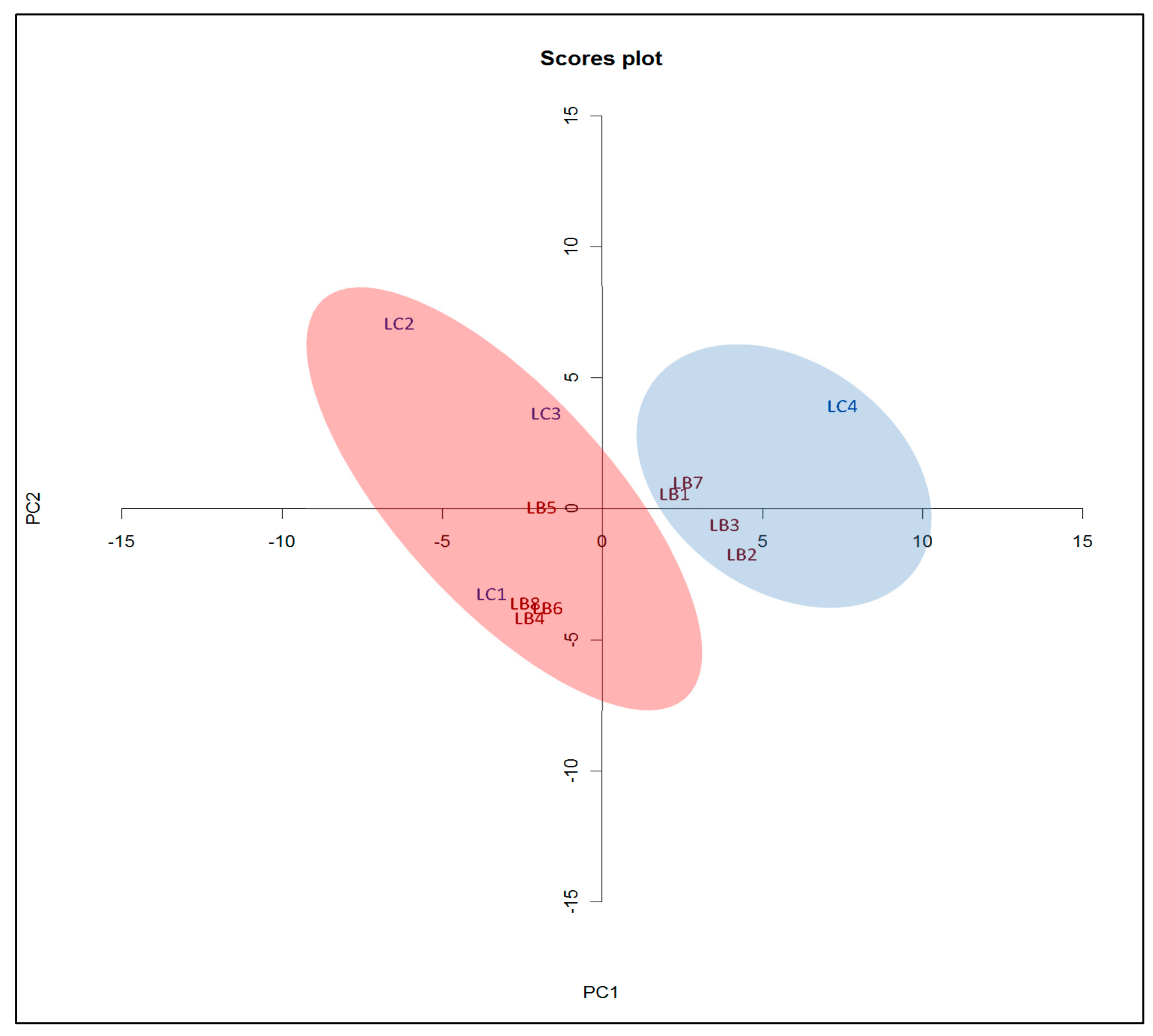
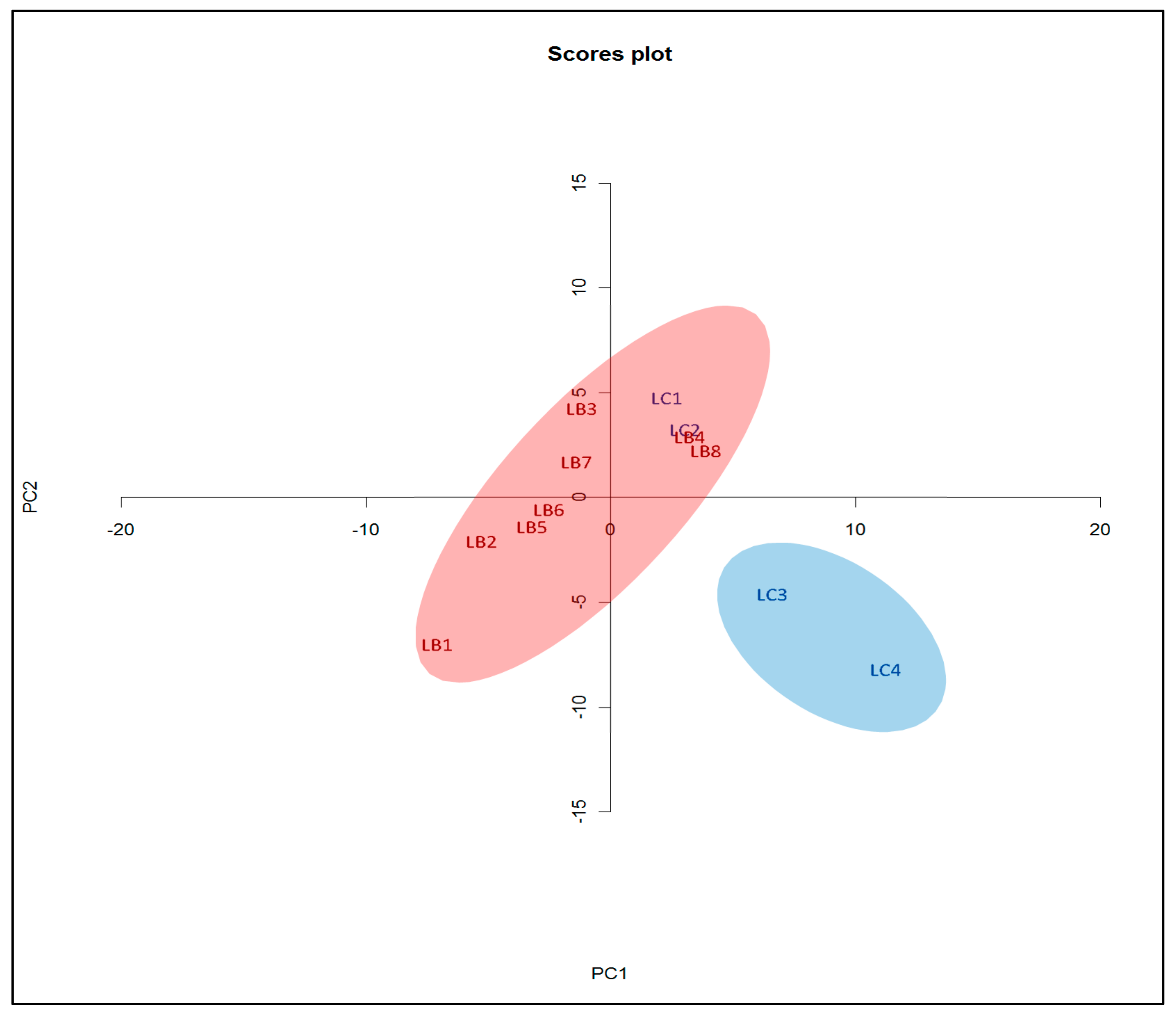
3.4. PCA Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability Statement
References
- Fukuda, T.; Yokoyama, J.; Ohashi, H. Phylogeny and biogeography of the genus Lycium (Solanaceae): Inferences from chloroplast DNA sequences. Mol. Phylogenet Evol. 2001, 19, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Tilburt, J.C.; Kaptchuk, T.J. Herbal medicine research and global health: An ethical analysis. Bull. World Health Organ. 2008, 86, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Amagase, H.; Farnsworth, N.R. A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Res. Int. 2011, 44, 1702–1717. [Google Scholar] [CrossRef]
- Potterat, O. Goji (Lycium barbarum and L. chinense): Phytochemistry, Pharmacology and Safety in the Perspective of Traditional Uses and Recent Popularity. Planta Med. 2010, 76, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Vlase, L.; Vodnar, D.C.; Bischin, C.; Hanganu, D.; Gheldiu, A.M.; Oprean, R.; Silaghi-Dumitrescu, R.; Crișan, G. Polyphenolic Content, Antioxidant and Antimicrobial Activities of Lycium barbarum L. and Lycium chinense Mill Leaves. Molecules 2014, 19, 10056–10073. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.Q.; Zheng, Z.Y.; Xu, X.; Hu, Z.H. Variation in fruit sugar composition of Lycium barbarum L. and Lycium chinense Mill. of different regions and varieties. Biochem. Syst. Ecol. 2010, 38, 275–284. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, Y.H.; Kim, Y.H.; Lee, G.H.; Lee, M.Y. Discrimination of Lycium chinense and Lycium barbarum by taste pattern and betaine analysis. Int. J. Clin. Exp. Med. 2014, 7, 2053–2059. [Google Scholar]
- Zhang, K.Y.B.; Leung, H.W.; Yeung, H.W.; Wong, R.N. Differentiation of Lycium barbarum from its related Lycium species using random amplified polymorphic DNA. Planta Med. 2001, 67, 379–381. [Google Scholar] [CrossRef]
- Sze, S.C.W.; Song, J.X.; Wong, R.N.S.; Feng, Y.B.; Ng, T.B.; Tong, Y.; Zhang, K.Y.B. Application of SCAR (sequence characterized amplified region) analysis to authenticate Lycium barbarum (wolfberry) and its adulterants. Biotechnol. Appl. Biochem. 2008, 5, 15–21. [Google Scholar] [CrossRef]
- Monton, M.R.N.; Soga, T. Metabolome analysis by capillary electrophoresis–mass spectrometry. J. Chromatogr. A. 2007, 1168, 237–246. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant. Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Benchennouf, A.; Grigorakis, S.; Loupassaki, S.; Kokkalou, E. Phytochemical analysis and antioxidant activity of Lycium barbarum (Goji) cultivated in Greece. Pharm. Biol. 2016, 55, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Magiera, S.; Zareba, M. Chromatographic Determination of Phenolic Acids and Flavonoids in Lycium barbarum L. and Evaluation of Antioxidant Activity. Food Anal. Meth. 2015, 8, 2665–2674. [Google Scholar] [CrossRef]
- Li, Y.; Di, R.; Hsu, W.; Huang, Y.Q.; Cheung, H.Y. Quality control of Lycium chinense and Lycium barbarum cortex (Digupi) by HPLC using kukoamines as markers. Chin. Med. 2017, 12, 4. [Google Scholar] [CrossRef]
- Chen, H.; Inbaraj, B.S.; Chen, B. Determination of Phenolic Acids and Flavonoids in Taraxacum formosanum Kitam by Liquid Chromatography Tandem Mass Spectrometry Coupled with a Post-Column Derivatization Technique. Int. J. Mol. Sci. 2012, 13, 260–285. [Google Scholar] [CrossRef]
- Jarouche, M.; Suresh, H.; Low, M.; Lee, S.; Xu, C.; Khoo, C. Quality Control and Variability Assessment of an Eight-Herb Formulation for Hypertension Using Method Validation and Statistical Analysis. Molecules 2019, 24, 1520. [Google Scholar] [CrossRef]
- Therapeutic Goods Administration. Therapeutic Goods Administration Australian regulatory guidelines for complementary medicines (ARGCM) Part B: Listed complementary medicines; Australian Government Department of Health: Canaberra, Australia, 2015. Available online: https://www.tga.gov.au/book/ argcmpart-b-listed-complementary-medicines/ (accessed on 21 March 2018).
- Bensoussan, A.; Lee, S.; Murray, C.; Bourchier, S.; van der Kooy, F.; Pearson, J.L.; Liu, J.; Chang, D.; Khoo, C.S. Choosing chemical markers for quality assurance of complex herbal medicines: Development and application of the herb MaRS criteria. Clin. Pharmacol. Ther. 2015, 97, 628–640. [Google Scholar] [CrossRef]
- Guardia, T.; Rotelli, A.E.; Juarez, A.O.; Pelzer, L.E. Anti-inflammatory properties of plant flavonoids—Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Il Farmaco. 2001, 56, 683–687. [Google Scholar] [CrossRef]
- Qian, J.Y.; Liu, D.; Huang, A.G. The efficiency of flavonoids in polar extracts of Lycium chinense Mill fruits as free radical scavenger. Food Chem. 2004, 87, 283–288. [Google Scholar] [CrossRef]
- Wen, C.C.; Chen, H.M.; Yang, N.S. Developing phytocompounds from medicinal plants as immunomodulators. In Advances in Botanical Research: Recent Trends in Medicinal Plant Research; Academic Press: Cambridge, MA, USA, 2012; Volume 62, pp. 197–272. [Google Scholar] [CrossRef]
- Marles, R.J.; Farnsworth, N.R. Antidiabetic plants and their active constituents. Phytomedicine 1995, 2, 137–189. [Google Scholar] [CrossRef]
- Prince, P.S.M.; Priya, S. Preventive effects of rutin on lysosomal enzymes in isoproterenol induced cardio toxic rats: Biochemical, histological and in vitro evidences. Eur. J. Pharmacol. 2010, 649, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, K.W.; Lee, H.J. Polyphenols suppress and modulate inflammation: Possible roles in health and disease. In Polyphenols in human health and disease, San Diego; Academic Press: Cambridge, MA, USA, 2014; pp. 393–408. [Google Scholar]
- Amagase, H.; Sun, B.; Borek, C. Lycium barbarum (goji) juice improves in vivo antioxidant biomarkers in serum of healthy adults. Nutr. Res. 2009, 29, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ah Kang, K.; Piao, M.J.; Kim, K.C.; Kim, A.D.; Chae, S.; Park, J.S.; Youn, U.J.; Hyun, J.W. Cytoprotective effect of the fruits of Lycium chinense Miller against oxidative stress-induced hepatotoxicity. J. Ethnopharmacol. 2010, 130, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Matkowski, A.; Jamiolkowska-Kozlowska, W.; Nawrot, I. Chinese medicinal herbs as source of antioxidant compounds—Where tradition meets the future. Curr. Med. Chem. 2013, 20, 984–1004. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, H.P.; Huh, H.; Kim, Y.C. Antihepatotoxic zeaxanthins from the fruits of Lycium chinense. Arch. Pharm Res. 1997, 20, 529–532. [Google Scholar] [CrossRef]
- Le, K.; Chiu, F.; Ng, K. Identification and quantification of antioxidants in Fructus lycii. Food Chem. 2007, 105, 353–363. [Google Scholar] [CrossRef]
- Oh, Y.C.; Cho, W.K.; Im, G.Y.; Jeong, Y.H.; Hwang, Y.H.; Liang, C.; Ma, J.Y. Anti-inflammatory effect of Lycium fruit water extract in lipopolysaccharide-stimulated RAW 264.7 macrophage cells. Int. Immunopharmacol. 2012, 13, 181–189. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Yang, H.; Zhu, L. Effects of Lycium barbarum on the behavior, body weight and TNF-alpha level of rat treated with binding. Wei sheng yan jiu—J. Hygiene Res. 2007, 36, 743–745. [Google Scholar]
- Ho, Y.S.; Yu, M.S.; Lai, C.S.W.; So, K.F.; Yuen, W.H.; Chang, R.C.C. Characterizing the neuroprotective effects of alkaline extract of Lycium barbarum on β-amyloid peptide neurotoxicity. Brain Res. 2007, 1158, 123–134. [Google Scholar] [CrossRef]
- Kiso, Y.; Suzuki, Y.; Watanabe, N.; Oshima, Y.; Hikino, H. Antihepatotoxic principles of Curcuma longa rhizomes. Planta Med. 1983, 49, 185–187. [Google Scholar] [CrossRef]
- Zhong, Y.; Shahidi, F.; Naczk, M. Phytochemicals and health benefits of Goji berries. Dried Fruits; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2013; pp. 133–144. [Google Scholar]
- Tang, W.M.; Chan, E.; Kwok, C.Y.; Lee, Y.K.; Wu, J.H.; Wan, C.W.; Chan, R.Y.K.; Yu, P.H.F.; Chan, W.W. A review of the anticancer and immunomodulatory effects of Lycium barbarum fruit. Inflammopharmacology 2012, 20, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 16. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.B.; Hung, D.K.; Chang, F.W.; Ong, E.T.; Chen, B.H. Anti-inflammatory and anti-angiogenic effects of flavonoids isolated from Lycium barbarum Linnaeus on human umbilical vein endothelial cells. Food Funct. 2012, 3, 1068–1081. [Google Scholar] [CrossRef] [PubMed]
- Okutan, H.; Ozcelik, N.; Yilmaz, H.R.; Uz, E. Effects of caffeic acid phenethyl ester on lipid peroxidation and antioxidant enzymes in diabetic rat heart. Clin. Biochem. 2005, 38, 191–196. [Google Scholar] [CrossRef]
- Luo, Q.; Cai, Y.; Yan, J.; Sun, M.; Corke, H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004, 76, 137–149. [Google Scholar] [CrossRef]
- Yao, X.; Peng, Y.; Xu, L.J.; Li, L.; Wu, Q.L.; Xiao, P.G. Phytochemical and biological studies of Lycium medicinal plants. Chem Biodivers. 2011, 8, 976–1010. [Google Scholar] [CrossRef]
- Marques, V.; Farah, A. Chlorogenic acids and related compounds in medicinal plants and infusions. Food Chem. 2009, 113, 1370–1376. [Google Scholar] [CrossRef]
- Chang, R.C.C.; So, K.F. Use of anti-aging herbal medicine, Lycium barbarum, against aging-associated diseases. What do we know so far? Cell Mol. Neurobiol. 2008, 28, 643–652. [Google Scholar] [CrossRef]
- European Commission Directorate. European Commission Directorate for Agriculture Commission decision of August 12, 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. EUR-Lex. 2002. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32002D0657/ (accessed on 14 September 2018).
- R Project for Statistical Computing; Technische Universität, Wien: Wien, Austria, 2001.
- R Development Core Team. R Foundation for Statistical Computing 2009; The R Project for Statistical Computing: Union County, NJ, USA, 2009. [Google Scholar]
- Jarouche, M. Study of the marketplace variation in the chemical profile of Qi Ju Di Huang Wan (Lycium, Chrysanthemum and Rehmannia Formula). Ph.D. Thesis, Western Sydney University, Sydney, Australia, 2014. Available online: http://handle.uws.edu.au:8081/1959.7/uws:30079 (accessed on 12 December 2019).
| Compound | Chemical Structure |
|---|---|
| Rutin |  |
| Narcissin | 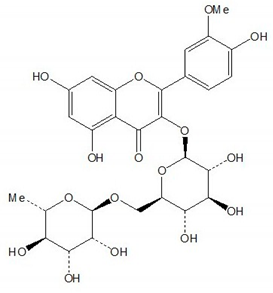 |
| Nictoflorin | 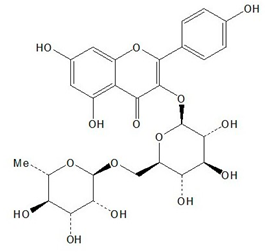 |
| Coumaric acid | 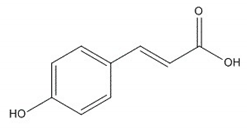 |
| Scopoletin | 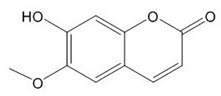 |
| Caffeic acid | 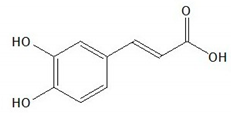 |
| Chlorogenic acid | 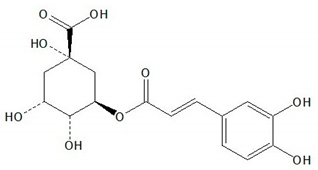 |
| Analyte [References] | Reported Activity | Herb Mars Ranking a,b |
|---|---|---|
| Rutin [3,19,20,21,22,23] | Anti-hepatotoxic, anti-oxidant, cAMP-phosphodiesterase-inhibitor, vasopressor, vasodilator, anti-inflammatory, cytoprotective | 5 |
| Narcissin [24,25,26,27] | Anti-inflammatory, anti-oxidant, hepatoprotective | 4 |
| Nictoflorin [28,29,30,31,32] | Anti-hepatotoxic, anti-oxidant, iNOS-Inhibitor, cAMP-phosphodiesterase-inhibitor, TNF-alpha-inhibitor, neuroprotective | 4 |
| Coumaric acid [33,34] | Anti-hepatotoxic, anti-oxidant | 3 |
| Scopoletin [35,36,37] | Anti-hepatotoxic, anti-oxidant | 3 |
| Caffeic acid [38,39,40] | Anti-oxidant, anti-inflammatory, anti-hepatotoxic, hepatotropic | 2 |
| Chlorogenic acid [41,42] | Hepatotropic, anti-inflammatory | 2 |
| Time (min) | Water (with 0.1 % v/v Formic Acid) % | Acetonitrile % |
|---|---|---|
| Initial | 80 | 20 |
| 1.0 | 75 | 25 |
| 6.0 | 55 | 45 |
| 11.0 | 35 | 65 |
| 13.0 | 25 | 75 |
| 15.0 | 80 | 20 |
| 15.5 | 80 | 20 |
| Analyte | ESI Polarity | Precursor m/z | Product m/z | Respective Voltages (V) | Dwell Time (s) |
|---|---|---|---|---|---|
| Rutin | - | [M-H]− = 609 | 255, 271, 300 | 50, 62, 40 | 0.016 |
| Narcissin | - | [M-H]− = 623 | 299, 315 | 48, 30 | 0.017 |
| Nictoflorin | - | [M-H]− = 593 | 255, 284 | 54, 34 | 0.017 |
| Coumaric acid | - | [M-H]− = 164 | 93, 120 | 12, 26 | 0.016 |
| Scopoletin | - | [M-H]− = 191 | 103, 176 | 24, 16 | 0.016 |
| Caffeic acid * | - | [M-H]− = 179 | 135 | 15 | 0.195 |
| Chlorogenic acid | - | [M-H]− = 353 | 93, 191 | 50, 50 | 0.095 |
| Analyte | Spike Levels a,c,d | Cumulative Results | ||||||
|---|---|---|---|---|---|---|---|---|
| 50% | 100% | 200% | ||||||
| Recovery % | RSD % | Recovery % | RSD % | Recovery % | RSD % | Average Recovery b % | RSD % | |
| Rutin | 93.1 | 3.7 | 90.2 | 7.9 | 89.1 | 8.6 | 90.8 | 6.7 |
| Narcissin | 105.6 | 3.4 | 101.7 | 1.7 | 100.5 | 2.5 | 102.6 | 2.8 |
| Nictoflorin | 92.2 | 3.8 | 94.5 | 2.9 | 95.2 | 2.5 | 93.9 | 3.1 |
| Coumaric acid | 98.4 | 7.5 | 88.6 | 9.3 | 102.0 | 3.8 | 96.3 | 6.8 |
| Scopoletin | 89.6 | 7.0 | 95.3 | 5.5 | 91.2 | 3.3 | 92.0 | 5.3 |
| Caffeic acid | 89.9 | 7.1 | 110.8 | 9.5 | 113.7 | 4.2 | 104.8 | 1.9 |
| Chlorogenic acid | 101 | 9.2 | 112.8 | 8.2 | 114.3 | 7.4 | 109.4 | 4.5 |
| Analyte | Linearity (R2) | Linear Range (µg/mL) | Precision a | LOD (mg/g) b | LOQ (mg/g) c | |
|---|---|---|---|---|---|---|
| Amount (mg/g) (± % RSD) | RT (min) (± % RSD) | |||||
| Rutin | 0.9992 | 57.0-1140.0 | 34.8 (4.3) | 9.37 (0.02) | 0.42 | 1.4 |
| Narcissin | 0.9995 | 3.0-60.0 | 1.65 (2.8) | 10.30 (0.01) | 0.13 | 0.4 |
| Nictoflorin | 0.9994 | 0.6-11.6 | 0.77 (5.9) | 10.20 (0.01) | 0.14 | 0.47 |
| Coumaric acid | 0.9991 | 4.5-89.4 | 10.3 (6.9) | 8.30 (0.02) | 0.25 | 0.84 |
| Scopoletin | 0.9995 | 0.9-17.4 | 1.68 (4.3) | 7.75 (0.03) | 0.06 | 0.20 |
| Caffeic acid | 0.9995 | 0.1-1.6 | 0.11 (9.3) | 6.35 (0.01) | 0.04 | 0.12 |
| Chlorogenic acid | 0.9991 | 2.0-39.6 | 4.19 (5.9) | 5.21 (0.01) | 0.74 | 2.47 |
| Analyte | Relative Intensity | Tolerances | ||||
|---|---|---|---|---|---|---|
| m/z | Standard | Sample | Relative Difference (± %) a | Permitted Tolerance (± %) b | Pass/Fail | |
| Rutin | 300 | 100 | 100 | - | 20 | Pass |
| 271 | 61 | 60 | 1.6 | 20 | Pass | |
| 255 | 31 | 30 | 3.2 | 25 | Pass | |
| Narcissin | 315 | 100 | 100 | - | 20 | Pass |
| 299 | 61 | 53 | 14 | 20 | Pass | |
| Nictoflorin | 284 | 100 | 100 | - | 25 | Pass |
| 255 | 86 | 73 | 16 | 25 | Pass | |
| Coumaric acid | 120 | 100 | 100 | - | 25 | Pass |
| 93 | 29 | 28 | 3.5 | 25 | Pass | |
| Scopoletin | 176 | 100 | 100 | - | 25 | Pass |
| 103 | 40 | 36 | 10 | 25 | Pass | |
| Caffeic acid | 135 | 100 | 100 | - | 25 | Pass |
| Chlorogenic acid | 191 | 100 | 100 | - | 25 | Pass |
| 93 | 50 | 37 | 26 | 25 | Fail | |
| Analyte Concentrations in Sample (mg/g) (± % RSD) a | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample d | Rutin | Narcissin | Nictoflorin | Coumaric Acid | Scopoletin | Caffeic Acid | Chlorogenic Acid | Total Concentration |
| LB1 | 16.1 (9.3) | 0.37 (3.0) | 0.37 (8.5) | 6.84 (4.5) | 0.77 (6.7) | 0.18 (3.8) | 3.71 (5.4) | 28.4 |
| LB2 | 43.1 (8.5) | 0.56 (4.8) | 0.26 (1.9) | 10.3 (7.0) | 0.33 (9.0) | <LOD | <LOD | 54.6 |
| LB3 | 19.5 (3.3) | 0.94 (7.5) | 0.43 (4.9) | 10.6 (4.1) | 1.59 (3.8) | 0.08 (9.4) | 1.44 (6.6) | 34.4 |
| LB4 | 19.1 (3.8) | 1.23 (6.8) | 0.58 (7.8) | 10.2 (5.3) | 1.32 (6.9) | <LOD | 1.11 (5.4) | 33.5 |
| LB5 | 48.2 (6.2) | 1.46 (5.2) | 0.7 (7.8) | 12.1 (5.7) | 0.78 (6.7) | 0.15 (7.1) | 3.71 (5.3) | 67.3 |
| LB6 | 23.1 (2.8) | 0.62 (6.5) | 0.41 (5.8) | 11.2 (4.1) | 1.19 (6.0) | 0.24 (7.0) | 4.12 (5.8) | 40.7 |
| LB7 c | 34.8 (4.3) | 1.65 (2.8) | 0.77 (5.9) | 10.3 (6.9) | 1.68 (4.3) | 0.11 (9.3) | 4.19 (5.9) | 53.4 |
| LB8 | 49.2 (3.3) | 1.43 (6.6) | 0.67 (4.6) | 12.2 (5.9) | 2.17 (6.9) | 0.15 (7.0) | 7.15 (9.0) | 72.9 |
| LC1 | 25.3 (2.5) | 1.23 (4.4) | 0.43 (8.0) | 9.78 (5.8) | 2.59 (6.4) | 0.09 (6.1) | 2.91 (8.3) | 42.1 |
| LC2 | 21.5 (3.6) | 0.98 (2.6) | 0.51 (3.7) | 10.2 (5.6) | 1.60 (5.5) | 0.16 (4.3) | 4.24 (7.2) | 39.2 |
| LC3 | 35.1 (6.7) | 1.11 (3.5) | 0.44 (3.6) | 8.61 (7.1) | 2.36 (5.1) | 0.32 (6.4) | 9.12 (7.3) | 57.0 |
| LC4 | 33.1 (4.0) | 1.25 (3.4) | 0.63 (4.9) | 10.2 (7.0) | 2.61 (4.7) | 0.18 (6.7) | 5.17 (5.3) | 53.1 |
| Mean (mg/g) | 30.7 | 1.07 | 0.52 | 10.2 | 1.58 | 0.17 | 4.26 | 48.1 |
| Fold variation b | 3.1 | 3.9 | 6.0 | 1.8 | 7.8 | 4.3 | 7.3 | N/A |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarouche, M.; Suresh, H.; Hennell, J.; Sullivan, S.; Lee, S.; Singh, S.; Power, D.; Xu, C.; Khoo, C. The Quality Assessment of Commercial Lycium Berries Using LC-ESI-MS/MS and Chemometrics. Plants 2019, 8, 604. https://doi.org/10.3390/plants8120604
Jarouche M, Suresh H, Hennell J, Sullivan S, Lee S, Singh S, Power D, Xu C, Khoo C. The Quality Assessment of Commercial Lycium Berries Using LC-ESI-MS/MS and Chemometrics. Plants. 2019; 8(12):604. https://doi.org/10.3390/plants8120604
Chicago/Turabian StyleJarouche, Mariam, Harsha Suresh, James Hennell, Shaun Sullivan, Samiuela Lee, Swastika Singh, Declan Power, Cindy Xu, and Cheang Khoo. 2019. "The Quality Assessment of Commercial Lycium Berries Using LC-ESI-MS/MS and Chemometrics" Plants 8, no. 12: 604. https://doi.org/10.3390/plants8120604
APA StyleJarouche, M., Suresh, H., Hennell, J., Sullivan, S., Lee, S., Singh, S., Power, D., Xu, C., & Khoo, C. (2019). The Quality Assessment of Commercial Lycium Berries Using LC-ESI-MS/MS and Chemometrics. Plants, 8(12), 604. https://doi.org/10.3390/plants8120604




