Phytochemical Analysis of Tephrosia vogelii across East Africa Reveals Three Chemotypes that Influence Its Use as a Pesticidal Plant
Abstract
1. Introduction
2. Results and Discussion
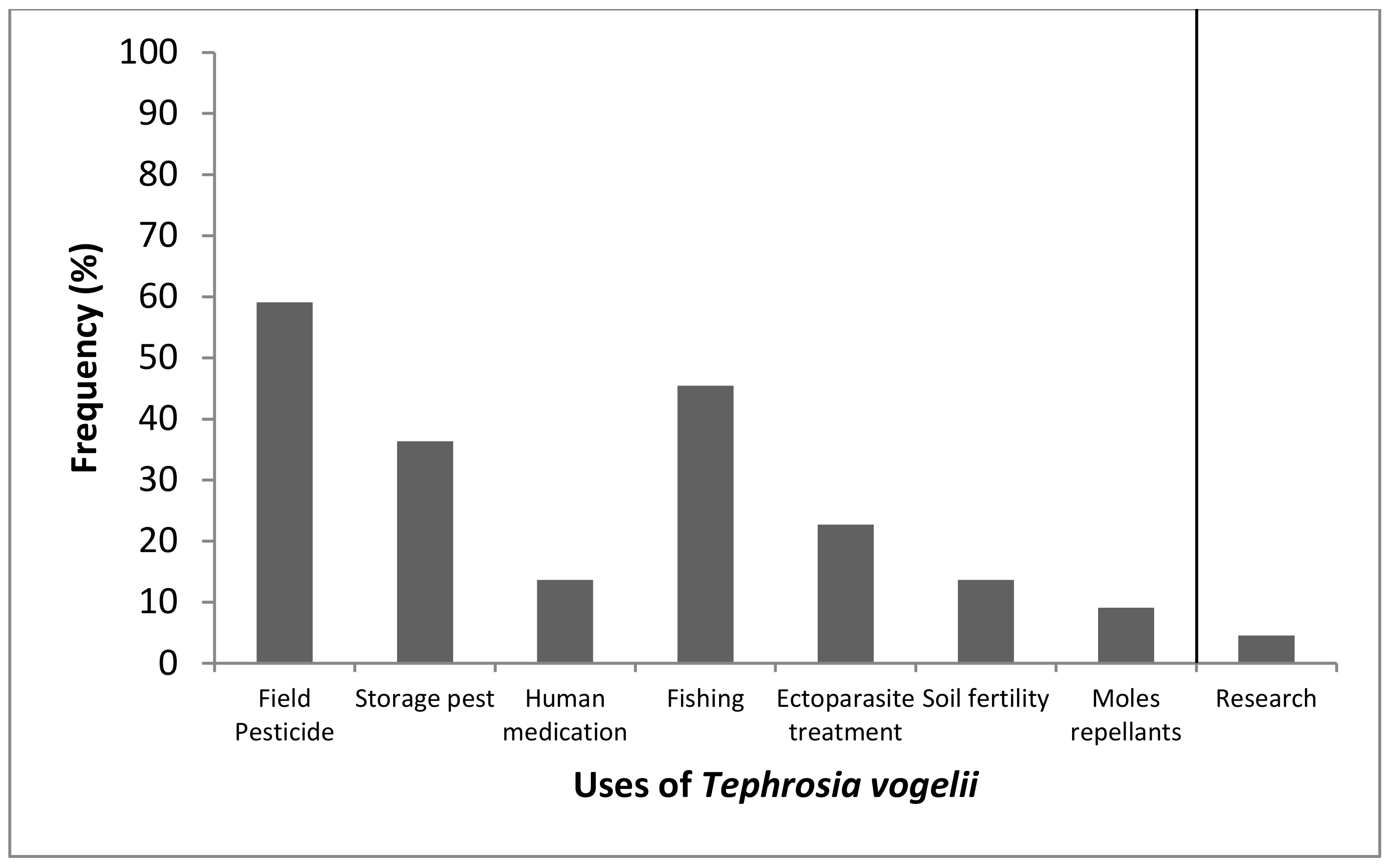
2.1. Status of Use of Tephrosia vogelii by Small Scale Farmers
2.2. Phytochemical Analysis of T. vogelii Leaf Samples
2.3. Frequency of T. vogelii Chemotypes.
2.4. Spatial Distribution of Plants Samples Chemotypes
2.5. Spatial Temporal Variation of Chemotype 1 in T. vogelii
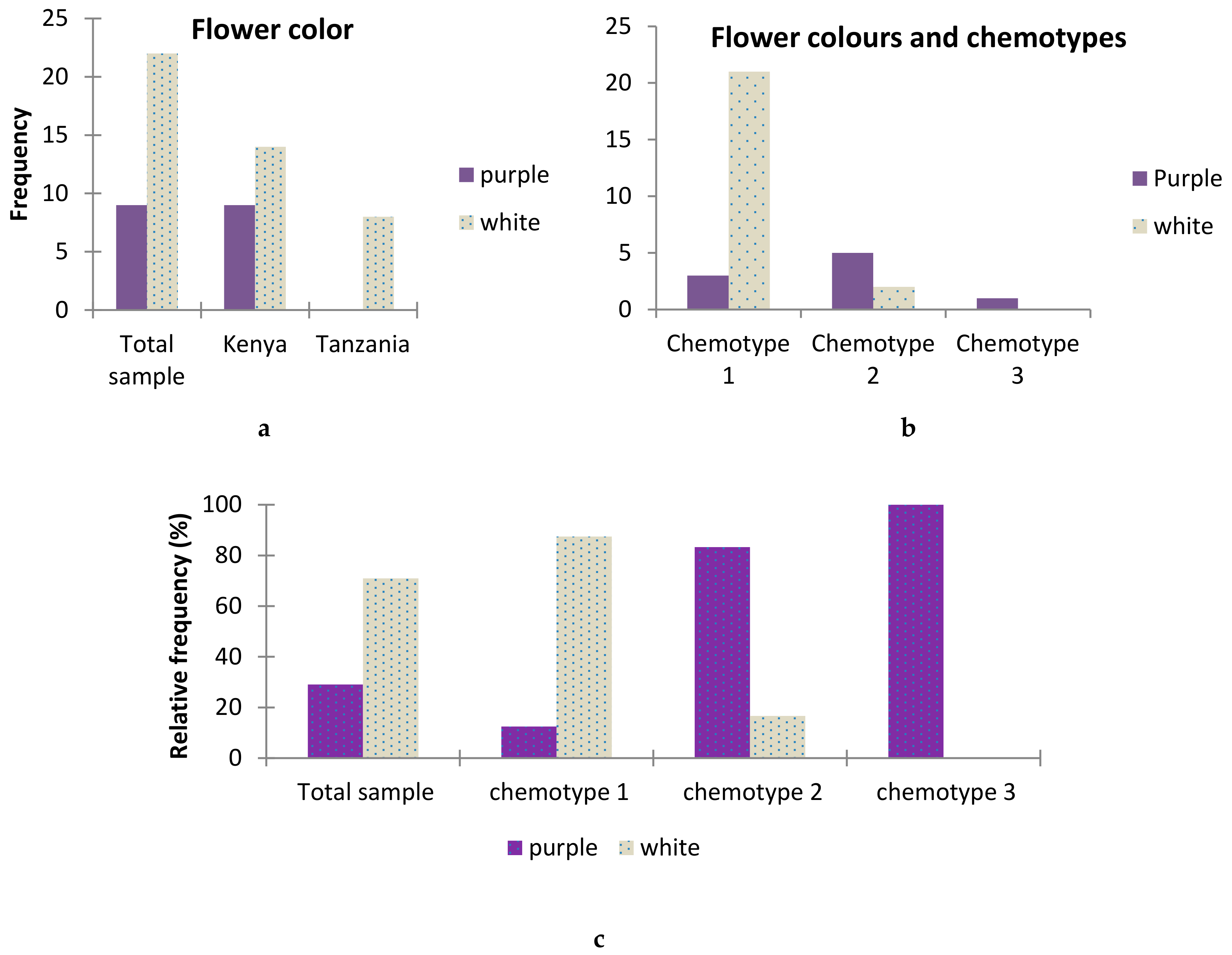
2.6. Association between T.vogelii Flower Color and Chemotypes
2.7. Summary of Indicators for Chemotype Identification
3. Materials and Methods
3.1. Analysis of Tephrosia Vogelii Leaf Samples
3.1.1. Samples Collection
3.1.2. Survey of Farmers Awareness on the Use of T. vogelii
3.1.3. Sample Analysis
3.2. Presentation of Data and Sampling Points
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Burkill, H.M. The Useful Plants of West Tropical Africa. Volume 2: Families EI.; Royal Botanic Gardens: KEW, UK, 1994. [Google Scholar]
- Kamanula, J.; Sileshi, G.W.; Belmain, S.R.; Sola, P.; Mvumi, B.M.; Nyirenda, G.K.; Nyirenda, S.P.; Stevenson, P.C. Farmers’ insect pest management practices and pesticidal plant use in the protection of stored maize and beans in Southern Africa. Int. J. Pest Manag. 2010, 57, 41–49. [Google Scholar] [CrossRef]
- Mafongoya, P.L.; Kuntashula, E. Participatory evaluation of Tephrosia species and provenances for soil fertility improvement and other uses using farmer criteria in eastern Zambia. Exp. Agric. 2005, 41, 69–80. [Google Scholar] [CrossRef]
- Neuwinger, H.D. Plants used for poison fishing in tropical Africa. Toxicon 2004, 44, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Nyirenda, S.P.; Sileshi, G.W.; Belmain, S.R.; Kamanula, J.F.; Mvumi, B.M.; Sola, P.; Nyirenda, G.K.; Stevenson, P.C. Farmers’ ethno-ecological knowledge of vegetable pests and pesticidal plant use in Malawi and Zambia. Afr. J. Agric. Res. 2011, 6, 1525–1537. [Google Scholar]
- Sileshi, G.; Mafongoya, P.L.; Kwesiga, F.; Nkunika, P. Termite damage to maize grown in agroforestry systems, traditional fallows and monoculture on nitrogen-limited soils in eastern Zambia. Agric. For. Entomol. 2005, 7, 61–69. [Google Scholar] [CrossRef]
- Gbadamosi, I.T.; Erinoso, S.M. A review of twenty ethnobotanicals used in the management of breast cancer in Abeokuta, Ogun State, Nigeria. Afr. J. Pharm. Pharmacol. 2016, 10, 546–564. [Google Scholar] [CrossRef]
- Touqeer, S.; Saeed, M.A.; Ajaib, M. A review on the phytochemistry and pharmacology of genus Tephrosia. Phytopharmacology 2013, 4, 598–637. [Google Scholar]
- Varughese, R.S.; Lam, W.S.-T.; Marican, A.A.B.H.; Viganeshwari, S.H.; Bhave, A.S.; Syn, N.L.; Wang, J.; Wong, A.L.-A.; Kumar, A.P.; Lobie, P.E.; et al. Biopharmacological considerations for accelerating drug development of deguelin, a rotenoid with potent chemotherapeutic and chemopreventive potential. Cancer 2019, 125, 1789–1798. [Google Scholar]
- Antonio, A.P.; Perera, L.M.S.; García, J.A.; Rehmana, M.U.; Choudhary, M.I. Anthelminthic Activity Against Sheep Gastrointestinal Nematodes in Chemical Compounds from Tephrosia Vogelii Leaves. J. Anim. Vet. Sci. 2019, 6, 8–17. [Google Scholar]
- Jacques, D.T.; Safiou, A.; Jédirfort, H.; Souaïbou, F. In Vitro effect of the ethanolic extract of Tephrosia vogelii on Rhipicephalus Sanguineus in Abomey-Calavi. Avicenna J. Phytomed. 2015, 5, 247–259. [Google Scholar]
- Kalume, M.K.; Losson, B.; Angenot, L.; Tits, M.; Wauters, J.N.; Frédérich, M.; Saegerman, C. Rotenoid content and in vitro acaricidal activity of Tephrosia vogelii leaf extract on the tick Rhipicephalus appendiculatus. Vet. Parasitol. 2012, 190, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Marango, S.N.; Khayeka-Wandabwa, C.; Makwali, J.A.; Jumba, B.N. Experimental therapeutic assays of Tephrosia vogelii against Leishmania major infection in murine model: In vitro and in vivo. BMC Res. Notes 2017, 10, 698. [Google Scholar] [CrossRef] [PubMed]
- Okitoi, L.O.; Ondwasy, H.O.; Siamba, D.N.; Nkurumah, D. Traditional herbal preparations for indigenous poultry health management in Western Kenya. Livest. Res. Rural Dev. 2007, 19, 72. [Google Scholar]
- Belmain, S.R.; Amoah, B.A.; Nyirenda, S.P.; Kamanula, J.F. Highly variable insect control efficacy of Tephrosia vogelii chemotypes. J. Agric. Food Chem. 2012, 60, 10055. [Google Scholar] [CrossRef]
- Mkenda, P.A.; Stevenson, P.C.; Ndakidemi, P.; Farman, D.I.; Belmain, S.R. Contact and fumigant toxicity of five pesticidal plants against Callosobruchus maculatus (Coleoptera: Chrysomelidae) in stored cowpea (Vigna unguiculata). Int. J. Trop. Insect Sci. 2015, 35, 172–184. [Google Scholar] [CrossRef]
- Tembo, Y.; Mkindi, A.G.; Mkenda, P.A.; Mpumi, N. Pesticidal Plant Extracts Improve Yield and Reduce Insect Pests on Legume Crops Without Harming Beneficial Arthropods. Front. Plant Sci. 2018, 9, 1425. [Google Scholar] [CrossRef]
- Mhango, W.G.; Snapp, S.S.; Phiri, G.Y.K. Opportunities and constraints to legume diversification for sustainable maize production on smallholder farms in Malawi. Renew. Agric. Food Syst. 2013, 28, 234–244. [Google Scholar] [CrossRef]
- Snapp, S.; Kanyama-Phiri, G.; Kamanga, B.; Gilbert, R.; Wellard, K. Farmer and Researcher Partnerships in Malawi: Developing Soil Fertility Technologies for the Near-Term and Far-Term. Available online: /core/journals/experimental-agriculture/article/farmer-and-researcher-partnerships-in-malawi-developing-soil-fertility-technologies-for-the-nearterm-and-farterm/0E20BDD9E4EFDFC4A74F915536410653 (accessed on 6 August 2019).
- Lambert, N.; Trouslot, M.-F.; Nef-Campa, C.; Chrestin, H. Production of rotenoids by heterotrophic and photomixotrophic cell cultures of Tephrosia vogelii. Phytochemistry 1993, 34, 1515–1520. [Google Scholar] [CrossRef]
- Lokhande, K.B.; Nagar, S.; Swamy, K.V. Molecular interaction studies of deguelin and its derivatives with Cyclin D1 and Cyclin E in cancer cell signaling pathway: The computational approach. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Stevenson, P.C.; Kite, G.C.; Lewis, G.P.; Forest, F.; Nyirenda, S.P.; Belmain, S.R.; Sileshi, G.W.; Veitch, N.C. Distinct chemotypes of Tephrosia vogelii and implications for their use in pest control and soil enrichment. Phytochemistry 2012, 78, 135–146. [Google Scholar] [CrossRef]
- Wang, A.; Wang, W.; Chen, Y.; Ma, F.; Wei, X.; Bi, Y. Deguelin induces PUMA-mediated apoptosis and promotes sensitivity of lung cancer cells (LCCs) to doxorubicin (Dox). Mol. Cell. Biochem. 2018, 442, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Dougoud, J.; Toepfer, S.; Bateman, M.; Jenner, W.H. Efficacy of homemade botanical insecticides based on traditional knowledge. A review. Agron. Sustain. Dev. 2019, 39, 37. [Google Scholar] [CrossRef]
- Kamanula, J.F.; Belmain, S.R.; Hall, D.R.; Farman, D.I.; Goyder, D.J.; Mvumi, B.M.; Masumbu, F.F.; Stevenson, P.C. Chemical variation and insecticidal activity of Lippia javanica (Burm. f.) Spreng essential oil against Sitophilus zeamais Motschulsky. Ind. Crops Prod. 2017, 110, 75–82. [Google Scholar] [CrossRef]
- Sarasan, V.; Kite, G.C.; Sileshi, G.W.; Stevenson, P.C. Applications of phytochemical and in vitro techniques for reducing over-harvesting of medicinal and pesticidal plants and generating income for the rural poor. Plant Cell Rep. 2011, 30, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Gadzirayi, C.; Mutandwa, E.; Mwale, M.; Chindundu, T. Utilization of Tephrosia vogelii in controlling ticks in dairy cows by small-scale commercial farmers in Zimbabwe. Afr. J. Biotechnol. 2009, 8, 17. [Google Scholar]
- Mihale, M.J.; Deng, A.L.; Selemani, H.O.; Kamatenesi, M.M.; Kidukuli, A.W.; Ogendo, J.O. Use of indigenous knowledge in the management of field and storage pests around Lake Victoria basin in Tanzania. Afr. J. Environ. Sci. Technol. 2009, 3, 9. [Google Scholar]
- Snapp, S.S.; Rohrbach, D.D.; Simtowe, F.; Freeman, H.A. Sustainable soil management options for Malawi: Can smallholder farmers grow more legumes? Agric. Ecosyst. Environ. 2002, 91, 159–174. [Google Scholar] [CrossRef]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef]
- Mkindi, A.; Mpumi, N.; Tembo, Y.; Stevenson, P.C.; Ndakidemi, P.A.; Mtei, K.; Machunda, R.; Belmain, S.R. Invasive weeds with pesticidal properties as potential new crops. Ind. Crops Prod. 2017, 110, 113–122. [Google Scholar] [CrossRef]
- Irvine, J.E.; Freyre, R.H. Effect of Planting Time and Photoperiod on Tephrosia vogelii. Agron. J. 1966, 58, 49–51. [Google Scholar] [CrossRef]

| Variables | No. of Observations | No. of Missing Values | No. of Categories | Mode | Mode Frequency | Categories | Frequency Per Category | Rel. Frequency Per Category (%) | Proportion Per Category |
|---|---|---|---|---|---|---|---|---|---|
| Overall | 91 | 0 | 3 | Chemotype 1 | 67 | Chemotype 1 | 67 | 74 | 1 |
| Chemotype 2 | 18 | 20 | 0 | ||||||
| Chemotype 3 | 6 | 7 | 0 | ||||||
| Kenya | 57 | 0 | 3 | Chemotype 1 | 44 | Chemotype 1 | 44 | 77 | 1 |
| Chemotype 2 | 10 | 18 | 0 | ||||||
| Chemotype 3 | 3 | 5 | 0 | ||||||
| Malawi | 20 | 0 | 3 | Chemotype 1 | 9 | Chemotype 1 | 9 | 45 | 0 |
| Chemotype 2 | 8 | 40 | 0 | ||||||
| Chemotype 3 | 3 | 15 | 0 | ||||||
| Tanzania | 14 | 0 | 1 | Chemotype 1 | 14 | Chemotype 1 | 14 | 100 | 1 |
| Chemotype 2 | 0 | 0 | 0 | ||||||
| Chemotype 3 | 0 | 0 | 0 |
| Location | Dry Season Deguelin (ppm) | Wet Season Deguelin (ppm) |
|---|---|---|
| Same | 6841 ± 523 a | 8756 ± 197 a |
| Iringa | 5644 ± 1202 a | 4879 ± 132 bc |
| Morogoro | 5423 ± 1621 a | 6229 ± 207 b |
| Kilimanjaro | 5144 ± 682 a | 6377 ± 791 b |
| Mbeya | 5699 ± 314 a | 3385 ± 196 c |
| Arusha | 5339 ± 139 a | 4803 ± 4 bc |
| One way ANOVA F statistics | 0.27 ns | 7.09 ** |
| Option | Results | Reliability for Chemotypes Identification |
|---|---|---|
| Tested Options | ||
| Elevation | No correlation | Not reliable |
| Season | No correlation | Not reliable: Although wet season enhances higher content of bioactive compounds in chemotype 1 |
| Flower Color | Positive correlation | Somewhat reliable: Could be used to decide on the chemotype where white flowers are known to be related with chemotype 1. N.B., a few plants with chemotype 1 had purple flowers. |
| Proposed Options | ||
| Simple assays | Report from Belmain et al., 2012 | Reliable: Test assessment of plant (10% leaf powder in small test container with bruchids), could be a rapid, simple and affordable tool. Pesticidal properties of Tephrosia are fast acting and chemotype could be determined in 48 h. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mkindi, A.G.; Tembo, Y.; Mbega, E.R.; Medvecky, B.; Kendal-Smith, A.; Farrell, I.W.; Ndakidemi, P.A.; Belmain, S.R.; Stevenson, P.C. Phytochemical Analysis of Tephrosia vogelii across East Africa Reveals Three Chemotypes that Influence Its Use as a Pesticidal Plant. Plants 2019, 8, 597. https://doi.org/10.3390/plants8120597
Mkindi AG, Tembo Y, Mbega ER, Medvecky B, Kendal-Smith A, Farrell IW, Ndakidemi PA, Belmain SR, Stevenson PC. Phytochemical Analysis of Tephrosia vogelii across East Africa Reveals Three Chemotypes that Influence Its Use as a Pesticidal Plant. Plants. 2019; 8(12):597. https://doi.org/10.3390/plants8120597
Chicago/Turabian StyleMkindi, Angela G., Yolice Tembo, Ernest R. Mbega, Beth Medvecky, Amy Kendal-Smith, Iain W. Farrell, Patrick A. Ndakidemi, Steven R. Belmain, and Philip C. Stevenson. 2019. "Phytochemical Analysis of Tephrosia vogelii across East Africa Reveals Three Chemotypes that Influence Its Use as a Pesticidal Plant" Plants 8, no. 12: 597. https://doi.org/10.3390/plants8120597
APA StyleMkindi, A. G., Tembo, Y., Mbega, E. R., Medvecky, B., Kendal-Smith, A., Farrell, I. W., Ndakidemi, P. A., Belmain, S. R., & Stevenson, P. C. (2019). Phytochemical Analysis of Tephrosia vogelii across East Africa Reveals Three Chemotypes that Influence Its Use as a Pesticidal Plant. Plants, 8(12), 597. https://doi.org/10.3390/plants8120597







