Chromatographic Fingerprinting of the Old World Lupins Seed Alkaloids: A Supplemental Tool in Species Discrimination
Abstract
1. Introduction
2. Results
2.1. OWL Qualitative Alkaloid Composition
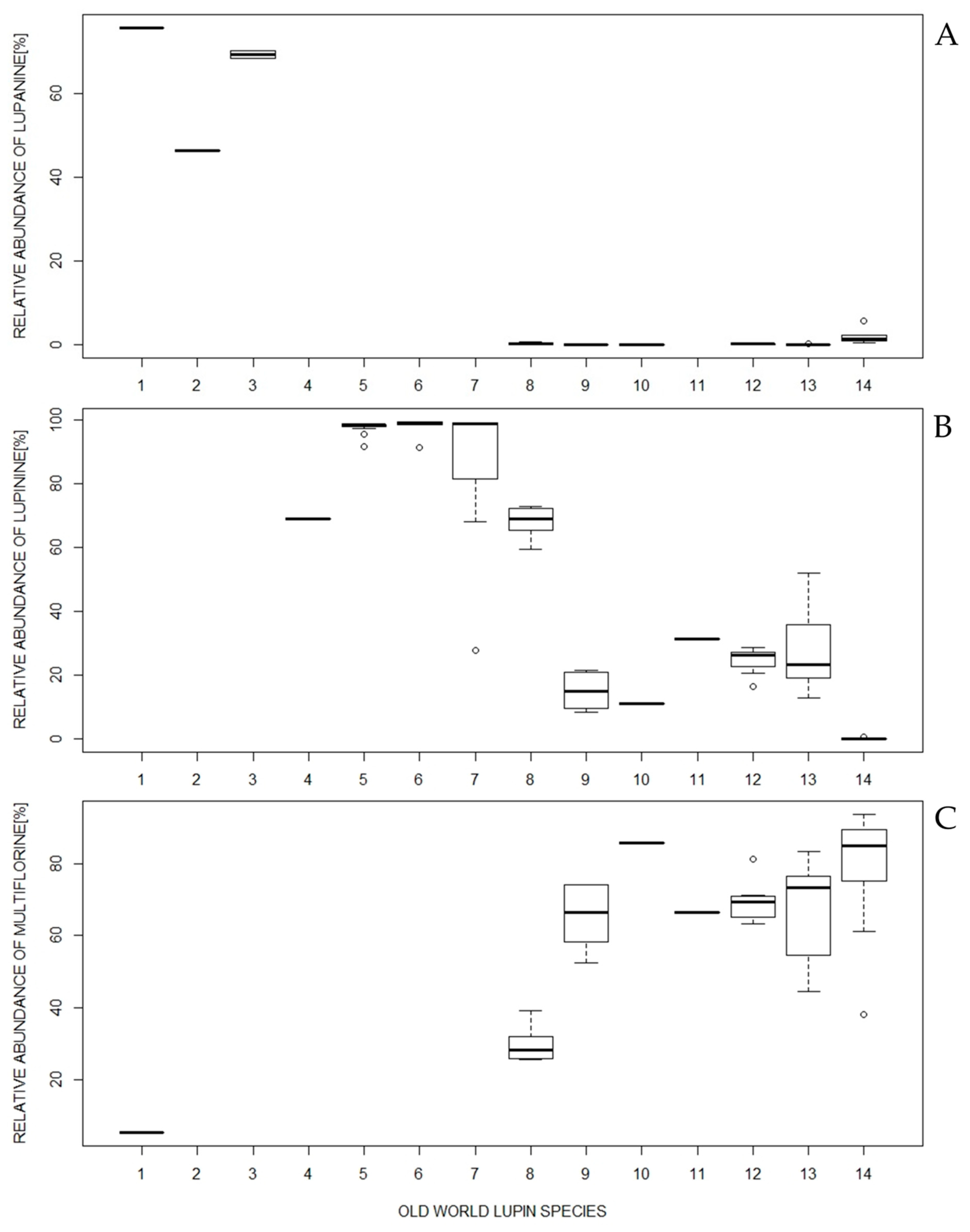
2.1.1. Species Possessing Lupanine as the Most Abundant Major Alkaloid
- L. albus and L. angustifolius, contained two other alkaloids in common, 13-hydroxylupanine and angustifoline. Both species could be distinguished using albine, ammodendrine, and multiflorine (and its derivatives), which were present in L. albus only, while L. angustifolius contained isolupanine as well as greater proportions of 13-hydroxylupanine and angustifoline.
- In L. mariae-josephi, lupanine and its derivatives were predominant. Albine and ammodendrine were not detected, while angustifoline and multiflorine were not major alkaloids for this species.
2.1.2. Species Possessing Lupinine as the Most Abundant Major Alkaloid
- L. luteus contained sparteine as an additional, major alkaloid, and the sparteine content was greatest in this species compared with all the other OWLs. Moreover, ammodendrine is present.
- In both the subspecies of L. hispanicus, lupinine is predominant (~97%). L. hispanicus subsp. hispanicus was characterized as having gramine as the second major alkaloid, while for L. hispanicus subsp. bicolor, gramine was the major alkaloid in only one accession.
- L. × hispanicoluteus had a lupinine content that ranged from 28% to 99%, and gramine comprised 0.5–62% of the total alkaloid content. Moreover, sparteine, as well as ammodendrine and its derivatives, were also occasionally detected. In comparison, in the L. luteus parental lines (Wt96839, Wt98066, and Wt98067), lupinine (58–74%), sparteine (22–41%), ammodendrine (~0.5–2%), and sometimes gramine, were detected, while the L. hispanicus parental line (Wt96383) was distinguished by greater proportions of lupinine (>90%) and gramine as the major alkaloids. Because of recombined arrangements of parental alleles in each of the analyzed L. × hispanicoluteus accession, they may be considered as a separate genotype [6]. The resulting diverse alkaloid compositions make the identification of this hybrid species, although possible, more difficult than those of other OWLs.
2.1.3. Species Possessing Lupinine and Multiflorine as the Two Major Alkaloids with the Greatest Relative Abundances
- In L. atlanticus, the most distinguishing feature was the 2:1 ratio of the relative abundance of lupinine to multiflorine. In addition to the multiflorine derivatives detected in certain accessions, no other major alkaloids were detected within this species.
- L. palaestinus was characterized as having the greatest proportions of ammodendrine and sparteine within the group. The relative abundance of ammodendrine was also the highest among the entire OWL group.
- L. anatolicus was unique, with its multiflorine content being the greatest among the group members and eight times greater than its lupinine content. Moreover, no additional major alkaloids were detected.
- L. digitatus, like L. anatolicus, did not have additional major alkaloids, but the ratio of lupinine to multiflorine was close to 1:2.
- The L. cosentinii and L. pilosus alkaloid profiles were considerably similar, with lupinine to multiflorine ratios of ~1:2. L. cosentinii could be distinguished by the lack of sparteine, while this alkaloid was present in certain L. pilosus accessions.
2.1.4. Species Possessing Multiflorine as the Most Abundant Major Alkaloid
- L. micranthus has a distinct alkaloid composition, with dominating relative abundances of multiflorine and its derivatives (Figure 2C, Table 1, Table S1). Lupanine and angustifoline were also detected in all the accessions, although, in certain cases, they did not exceed 1% of the total alkaloid content. Nonetheless, the relative abundance of angustifoline in L. micranthus was the second greatest compared with the other OWLs. Additionally, in particular accessions, albine could be detected. Other than in this species, it was only found in L. albus.
2.2. OWL Total Alkaloid Contents
2.2.1. Intraspecies Variation
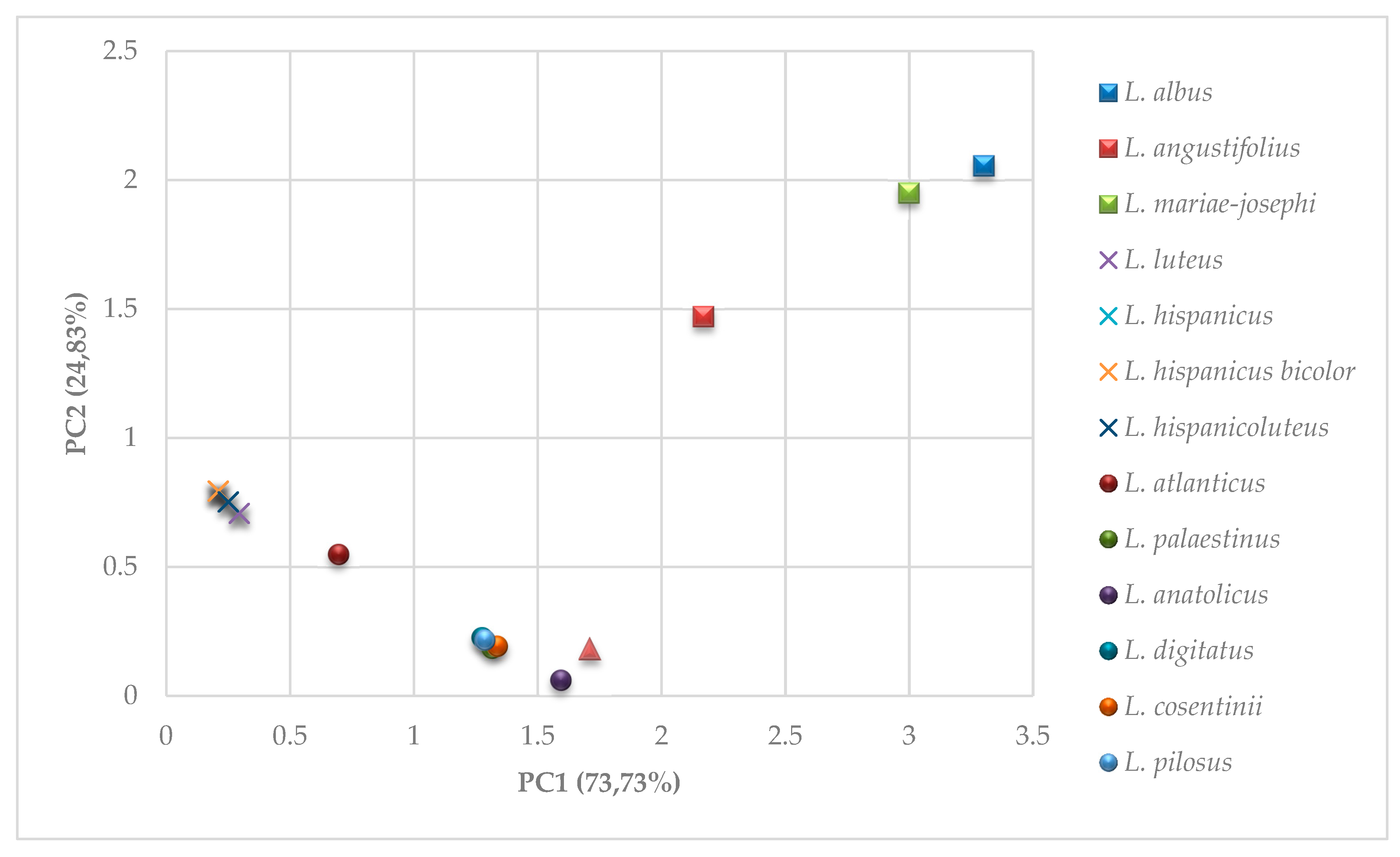
2.2.2. Attempts of Interspecies Analyses
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction Method and Assessments of the Total Contents and Relative Abundances of Alkaloids in OWL Seeds
4.3. Analyses of OWL Species Variability in Terms of Seed Alkaloid Content and Composition
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cowling, W.A.; Buirchell, B.J.; Tapia, M.E. Lupine Lupinus L. Promoting the Conservation and Use of Underutilized and Neglected Crops 23; International Board for Plant Genetic Resources (IBPGR): Rome, Italy, 1998; p. 105. [Google Scholar]
- Aïnouche, A.; Bayer, R.J.; Misset, M.T. Molecular phylogeny, diversification and character evolution in Lupinus (Fabaceae) with special attention to Mediterranean and African lupines. Plant Syst. Evol. 2004, 246, 211–222. [Google Scholar] [CrossRef]
- Drummond, C.S. Diversification of Lupinus (L.) in the western New World: Derived evolution of perennial life history and colonization of montane habitats. Mol. Phylogen. Evol. 2008, 48, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Gladstones, J.S. Lupins of the Mediterranean Region and Africa; Department of Agriculture of Western Australia: South Perth, WA, Australia, 1974; Volume 26, p. 48.
- Święcicki, W.; Święcicki, W.K.; Wolko, B. Lupinus anatolicus—A new lupin species of the Old World. Genet. Resour. Crop Evol. 1996, 43, 109–117. [Google Scholar] [CrossRef]
- Święcicki, W.; Święcicki, W.K.; Nijaki, T. Lupinus xhispanicoluteus—An interspecific hybrid of Old World lupins. Acta Soc. Bot. Pol. 1999, 68, 217–220. [Google Scholar] [CrossRef]
- Pascual, H. Lupinus mariae-josephi (Fabaceae), nueva y sopredente especie descubierta en Espana [Lupinus mariae-josephi (Fabaceae), new and surprising species discovered in Spain]. Anales Jard. Bot. Madrid 2004, 61, 69–72. [Google Scholar] [CrossRef]
- Naganowska, B.; Wolko, B.; Sliwinska, E.; Kaczmarek, Z. Nuclear DNA content variation and species relationships in the genus Lupinus (Fabaceae). Ann. Bot. 2003, 92, 349–355. [Google Scholar] [CrossRef]
- Przybylska, J.; Zimniak-Przybylska, Z. Electrophoretic patterns of seed globulins in the Old-World Lupinus species. Genet. Resour. Crop Evol. 1995, 42, 69–75. [Google Scholar] [CrossRef]
- Gupta, S.; Buirchell, B.J.; Cowling, W.A. Interspecific reproductive barriers and genomic similarity among the rough-seeded Lupinus species. Plant Breed. 1996, 115, 123–127. [Google Scholar] [CrossRef]
- Wolko, B.; Weeden, N.F. Relationships among lupin species as reflected by isozyme phenotype. Genetica Pol. 1990, 31, 189–197. [Google Scholar]
- Käss, E.; Wink, M. Molecular phylogeny and phylogeography of Lupinus (L.) inferred from nucleotide sequences of therbcL gene and ITS 1 + 2 regions of rDNA. Plant Syst. Evol. 1997, 208, 139–167. [Google Scholar] [CrossRef]
- Plitmann, U.; Pazy, B. Cytogeographical distribution of old world Lupinus. Webbia 1984, 38, 531–539. [Google Scholar] [CrossRef]
- Święcicki, W.K.; Wolko, B.; Naganowska, B. Lupinus anatolicus and L. xhispanicoluteus-morphological, biochemical and cytological characterization. In Proceedings of the XVI EUCARPIA Genetic Resources Section Workshop. Broad Variation and Precise Characterization-Limitation for the Future, Poznań, Poland, 16–20 May 2001; pp. 281–284. [Google Scholar]
- Mahé, F.; Pascual, H.; Coriton, O.; Huteau, V.; Navarro Perris, A.; Misset, M.-T.; Aïnouche, A. New data and phylogenetic placement of the enigmatic Old World lupin: Lupinus mariae-josephi H. Pascual. Genet. Resour. Crop Evol. 2011, 58, 101–114. [Google Scholar] [CrossRef]
- Abraham, E.M.; Ganopoulos, I.; Madesis, P.; Mavromatis, A.; Mylona, P.; Nianiou-Obeidat, I.; Parissi, Z.; Polidoros, A.; Tani, E.; Vlachostergios, D. The use of lupin as a source of protein in animal feeding: Genomic tools and breeding approaches. Int. J. Mol. Sci. 2019, 20, 851. [Google Scholar] [CrossRef] [PubMed]
- Bunsupa, S.; Yamazaki, M.; Saito, K. Quinolizidine alkaloid biosynthesis: Recent advances and future prospects. Front. Plant Sci. 2012, 3, 239. [Google Scholar] [CrossRef] [PubMed]
- Wink, M.; Botschen, F.; Gosmann, C.; Schäfer, H.; Waterman, P.G. Chemotaxonomy seen from a phylogenetic perspective and evolution of secondary metabolism. In Annual Plant Reviews. Biochemistry of Plant Secondary Metabolism; Wink, M., Ed.; Wiley: Hoboken, NJ, USA, 2010; Volume 40. [Google Scholar]
- Cowling, W.A.; Huyghe, C.; Święcicki, W. Lupin Breeding. In Lupins as Crop Plants: Biology, Production, and Utilization; Gladstones, J.S., Atkins, C.A., Hamblin, J., Eds.; CAB International: Wallingford, UK, 1998; pp. 93–120. [Google Scholar]
- Kamel, K.A.; Święcicki, W.; Kaczmarek, Z.; Barzyk, P. Quantitative and qualitative content of alkaloids in seeds of a narrow-leafed lupin (Lupinus angustifolius L.) collection. Genet. Resour. Crop Evol. 2016, 63, 711–719. [Google Scholar] [CrossRef]
- Kroc, M.; Rybiński, W.; Wilczura, P.; Kamel, K.; Kaczmarek, Z.; Barzyk, P.; Święcicki, W. Quantitative and qualitative analysis of alkaloids composition in the seeds of a white lupin (Lupinus albus L.) collection. Genet. Resour. Crop Evol. 2017, 64, 1853–1860. [Google Scholar] [CrossRef]
- Wink, M.; Meißner, C.; Witte, L. Patterns of quinolizidine alkaloids in 56 species of the genus Lupinus. Phytochem. 1995, 38, 139–153. [Google Scholar] [CrossRef]
- Leete, E. Biosynthesis and metabolism of gramine in Lupinus hartwegii. Phytochemistry 1975, 14, 471–474. [Google Scholar] [CrossRef]
- Saito, K.; Koike, Y.; Suzuki, H.; Murakoshi, I. Biogenetic implication of lupin alkaloid biosynthesis in bitter and sweet forms of Lupinus luteus and L. albus. Phytochemistry 1993, 34, 1041–1044. [Google Scholar] [CrossRef]
- Hirai, M.Y.; Suzuki, H.; Yamazaki, M.; Saito, K. Biochemical and partial molecular characterization of bitter and sweet forms of Lupinus angustifolius, an experimental model for study of molecular regulation of quinolizidine alkaloid biosynthesis. Chem. Pharm. Bull. 2000, 48, 1458–1461. [Google Scholar] [CrossRef][Green Version]
- Suzuki, H.; Murakoshi, I.; Saito, K. A novel O-tigloyltransferase for alkaloid biosynthesis in plants. Purification, characterization, and distribution in Lupinus plants. J. Biol. Chem. 1994, 269, 15853–15860. [Google Scholar] [PubMed]
- Bunsupa, S.; Katayama, K.; Ikeura, E.; Oikawa, A.; Toyooka, K.; Saito, K.; Yamazaki, M. Lysine decarboxylase catalyzes the first step of quinolizidine alkaloid biosynthesis and coevolved with alkaloid production in leguminosae. Plant Cell 2012, 24, 1202–1216. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Hirai, M.Y.; Suzuki, H.; Yamazaki, M.; Saito, K. Molecular characterization of a novel quinolizidine alkaloid o-tigloyltransferase: cDNA cloning, catalytic activity of recombinant protein and expression analysis in Lupinus plants. Plant Cell Physiol. 2005, 46, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Bunsupa, S.; Okada, T.; Saito, K.; Yamazaki, M. An acyltransferase-like gene obtained by differential gene expression profiles of quinolizidine alkaloid-producing and nonproducing cultivars of Lupinus angustifolius. Plant Biotechnol. 2011, 28, 89–94. [Google Scholar] [CrossRef]
- Yang, T.; Nagy, I.; Mancinotti, D.; Otterbach, S.L.; Andersen, T.B.; Motawia, M.S.; Asp, T.; Geu-Flores, F. Transcript profiling of a bitter variety of narrow-leafed lupin to discover alkaloid biosynthetic genes. J. Exp. Bot. 2017, 68, 5527–5537. [Google Scholar] [CrossRef]
- Frick, K.M.; Foley, R.C.; Kamphuis, L.G.; Siddique, K.H.M.; Garg, G.; Singh, K.B. Characterization of the genetic factors affecting quinolizidine alkaloid biosynthesis and its response to abiotic stress in narrow-leafed lupin (Lupinus angustifolius L.). Plant Cell Environ. 2018, 23, 13172. [Google Scholar] [CrossRef]
- Kroc, M.; Koczyk, G.; Kamel, K.A.; Czepiel, K.; Fedorowicz-Strońska, O.; Krajewski, P.; Kosińska, J.; Podkowiński, J.; Wilczura, P.; Święcicki, W. Transcriptome-derived investigation of biosynthesis of quinolizidine alkaloids in narrow-leafed lupin (Lupinus angustifolius L.) highlights candidate genes linked to iucundus locus. Sci. Rep. 2019, 9, 2231. [Google Scholar] [CrossRef]
- Kroc, M.; Czepiel, K.; Wilczura, P.; Mokrzycka, M.; Święcicki, W. Development and Validation of a Gene-Targeted dCAPS Marker for Marker-Assisted Selection of Low-Alkaloid Content in Seeds of Narrow-Leafed Lupin (Lupinus angustifolius L.). Genes 2019, 10, 428. [Google Scholar] [CrossRef]
- Frick, K.M.; Kamphuis, L.G.; Siddique, K.H.; Singh, K.B.; Foley, R.C. Quinolizidine alkaloid biosynthesis in lupins and prospects for grain quality improvement. Front. Plant Sci. 2017, 8, 87. [Google Scholar] [CrossRef]
- Święcicki, W.; Kroc, M.; Kamel, K. Lupins. In Grain legumes, Handbook of Plant Breeding; De Ron, A.M., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 10, pp. 179–218. [Google Scholar]
- Święcicki, W.K.; Cowling, W.A.; Buirchell, B.J. Databases and genetic resources evaluation in Lupinus collections. In Proceedings of the 9th International Lupin Conference. Lupin, an ancient crop for the new millennium, Klink/Muritz, Germany, 20–24 June 2000; pp. 132–137. [Google Scholar]
- Aïnouche, A.; Bayer, R. Genetic evidence supports the new anatolian lupine accession, Lupinus anatolicus, as an old world “rough-seeded” lupine (section Scabrispermae) related to L. pilosus. Folia Geobot. 2000, 35, 83–95. [Google Scholar] [CrossRef]
- Aïnouche, A.; Greinwald, R.; Witte, L.; Huon, A. Seed alkaloid composition of Lupinus tassilicus maire (Fabaceae: Genisteae) and comparison with its related rough seeded lupin species. Biochem. Syst. Ecol. 1996, 24, 405–414. [Google Scholar] [CrossRef]
- Cook, D.; Lee, S.T.; Pfister, J.A.; Stonecipher, C.A.; Welch, K.D.; Green, B.T.; Panter, K.E. Alkaloid profiling as an approach to differentiate Lupinus garfieldensis, Lupinus sabinianus and Lupinus sericeus. Phytochem. Anal. 2012, 23, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Boschin, G.; Annicchiarico, P.; Resta, D.; D’Agostina, A.; Arnoldi, A. Quinolizidine alkaloids in seeds of lupin genotypes of different origins. J. Agric. Food Chem. 2008, 56, 3657–3663. [Google Scholar] [CrossRef] [PubMed]
- Osorio, C.E.; Amiard, V.S.E.; Aravena-Calvo, J.; Udall, J.A.; Doyle, J.J.; Maureira-Butler, I.J. Chromatographic fingerprinting of Lupinus luteus L. (Leguminosae) main secondary metabolites: A case of domestication affecting crop variability. Genet. Resour. Crop Evol. 2018, 65, 1281–1291. [Google Scholar] [CrossRef]
- Muzquiz, M.; Guillamon, E.; Burbano, C.; Pascual, H.; Cabellos, B.; Cuadrado, C.; Pedrosa, M.M. Chemical composition of a new Lupinus species found in Spain, Lupinus mariae-josephi H. Pascual (Fabaceae). Span. J. Agric. Res. 2011, 9, 1233–1244. [Google Scholar] [CrossRef]
- Ciesiołka, D.; Muzquiz, M.; Burbano, C.; Altares, P.; Pedrosa, M.M.; Wysocki, W.; Folkman, W.; Popenda, M.; Gulewicz, K. An effect of various nitrogen forms used as fertilizer on Lupinus albus L. Yield and protein, alkaloid and α-galactosides content. J. Agron. Crop Sci. 2005, 191, 458–463. [Google Scholar] [CrossRef]
- Jansen, G.; Jürgens, H.U.; Ordon, F. Effects of temperature on the alkaloid content of seeds of Lupinus angustifolius cultivars. J. Agron. Crop Sci. 2009, 195, 172–177. [Google Scholar] [CrossRef]
- Christiansen, J.L.; Jørnsgård, B.; Buskov, S.; Olsen, C.E. Effect of drought stress on content and composition of seed alkaloids in narrow-leafed lupin, Lupinus angustifolius L. Eur. J. Agron. 1997, 7, 307–314. [Google Scholar] [CrossRef]
- Jansen, G.; Jürgens, H.-U.; Schliephake, E.; Ordon, F. Effect of the Soil pH on the alkaloid content of Lupinus angustifolius. Int. J. Agron. 2012, Article ID 269878, 1–5. [Google Scholar] [CrossRef]
- Kurlovich, B.S. Lupins (Geography, Classification, Genetic Resources and Breeding); OY International North Express: St. Petersburg, Russia, 2002; p. 468. [Google Scholar]
- Gawłowska, M.; Święcicki, W.; Lahuta, L.; Kaczmarek, Z. Raffinose family oligosaccharides in seeds of Pisum wild taxa, type lines for seed genes, domesticated and advanced breeding materials. Genet. Resour. Crop Evol. 2017, 64, 569–578. [Google Scholar] [CrossRef][Green Version]
- Islam, S.; Ma, W.; Appels, R.; Yan, G. Mass spectrometric fingerprints of seed protein for defining Lupinus spp. relationships. Genet. Resour. Crop Evol. 2013, 60, 939–952. [Google Scholar] [CrossRef]
- Susek, K.; Bielski, W.K.; Hasterok, R.; Naganowska, B.; Wolko, B. A first glimpse of wild lupin karyotype variation as revealed by comparative cytogenetic mapping. Front. Plant Sci. 2016, 7, 1152. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.A.; Demissie, A.; Harborne, J.B. Flavonoids as taxonomic markers in old world Lupinus species. Biochem. Syst. Ecol. 1983, 11, 221–231. [Google Scholar] [CrossRef]
- Käss, E.; Wink, M. Phylogenetic relationships in the Papilionoideae (Family Leguminosae) based on nucleotide sequences of cpDNA (rbcL) and ncDNA (ITS 1 and 2). Mol. Phylogen. Evol. 1997, 8, 65–88. [Google Scholar] [CrossRef]
- Morrison, D.F. Multivariate Statistical Methods; Mc Graw-Hill Book Company: New York, NY, USA, 1967. [Google Scholar]
- Gabriel, K.R. A procedure for testing the homogeneity of all sets of means in analysis of variance. Biometrics 1964, 20, 459–477. [Google Scholar] [CrossRef]
- Caliński, T.; Czajka, S.; Kaczmarek, Z. Principal component analysis and its applications. Biom. Stat. Algorithms 1975, 4, 159–185. [Google Scholar]

| Species | Origin ## | Lupinine | Epilupinine | Multiflorine | 11.12-secodidehydromultiflorine | 13-tigloyloxymultiflorine | 13-angelolyoxomultiflorine | Lupanine | 13-hydroxylupanine | Isolupanine | 11.12-didehydrolupanine | 5.6-didehydrolupanine | Oxolupanine | 13-benzoyloxylupanine | Sparteine | Angustifoline | Albine | Ammodendrine | N-methylammodendrine | Gramine |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L. angustifolius | W | 46.40 # | 35.60 | 2.50 # | 15.50 # | |||||||||||||||
| L. albus | W | 5.52 # | 1.74 # | 76.06 | 8.23 # | 2.07 # | 4.48 # | 1.04 # | ||||||||||||
| L. luteus | W | 67.46 | 0.33 # | 28.86 | 1.40 # | 1.77 # | ||||||||||||||
| L. anatolicus | W | 11.12 | 85.68 | |||||||||||||||||
| L. palaestinus | W | 15.07 | 65.36 | 1.48 # | 0.96 # | 0.79 # | 1.12 # | 6.22 | 7.22 | 0.59 # | ||||||||||
| L. cosentinii | W | 25.35 | 66.64 | 3.30 | 1.15 # | 0.34 # | 0.76 # | 1.36 # | ||||||||||||
| G | 23.51 | 73.94 | 1.04 # | 1.13 # | ||||||||||||||||
| L. atlanticus | W | 66.68 | 30.35 | 1.35 # | ||||||||||||||||
| G | 72.12 | 26.03 | 0.76 # | |||||||||||||||||
| U | 63.99 | 34.40 | ||||||||||||||||||
| L. pilosus | W | 27.76 | 63.85 | 2.67 | 0.87 # | 0.94 # | 1.31 # | 0.77 # | ||||||||||||
| U | 28.06 | 69.56 | 0.77 # | 0.78 # | 0.51 # | |||||||||||||||
| L. digitatus | U | 31.50 | 66.43 | |||||||||||||||||
| L. micranthus | W | 91.61 | 3.20 | 1.77 | ||||||||||||||||
| G | 81.70 | 11.18 | 2.15 # | 3.20 # | 0.35 # | |||||||||||||||
| L. × hispanicoluteus | W | 83.72 | 3.68 # | 1.81 # | 0.43 # | 10.31 # | ||||||||||||||
| L. hispanicus subsa. bicolor | W | 97.18 | 2.62 # | |||||||||||||||||
| U | 98.29 | |||||||||||||||||||
| L. hispanicus subsp. hispanicus | W | 97.51 | 0.15 # | 2.20 | ||||||||||||||||
| L. mariae-josephi | W | 68.42 | 5.12 | 18.27 | 4.05 | 3.01 | ||||||||||||||
| I | 70.30 | 4.76 | 20.78 | 3.48 |
| Species | Number of Accessions | Total Alkaloid Content (% of the seed DW). Mean Values for Species | Variation Coefficient (%) | F-Statistic for Differences Between Accessions | |
|---|---|---|---|---|---|
| Minimum | Maximum | ||||
| L. palaestinus | 6 | 0.0774 | 0.2308 | 35.48 | 23.266 ** |
| L. cosentinii | 5 | 0.1317 | 0.2509 | 27.90 | 3.259 |
| L. atlanticus | 5 | 0.1263 | 0.2640 | 26.33 | 56.128 ** |
| L. pilosus | 6 | 0.0872 | 0.4517 | 54.23 | 77.281 ** |
| L. × hispanicoluteus | 7 | 0.0087 | 2.1189 | 78.31 | 17.829 ** |
| L. hispanicus subsp. bicolor | 4 | 0.6912 | 1.5982 | 36.75 | 5.302 |
| L. hispanicus subsp. hispanicus | 13 | 0.7743 | 1.9834 | 30.20 | 3.213 * |
| Homogenous Groups | Mean Values of Total Alkaloid Contents | Sum of Squares | Species | |
|---|---|---|---|---|
| Group | Species | |||
| 1 | 5.6611 | L. mariae-josephi | ||
| 2 | 1.0431 | 1.2101 | 0.5706 | L. hispanicus subsp. hispanicus L. hispanicus subsp. bicolor L. × hispanicoluteus L. micranthus |
| 1.0772 | ||||
| 0.9868 | ||||
| 0.8984 | ||||
| 3 | 0.1765 | 0.2588 | 0.0946 | L. pilosus L. atlanticus L. cosentinii L. palaestinus L. anatolicus |
| 0.1827 | ||||
| 0.1799 | ||||
| 0.1436 | ||||
| 0.1177 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Święcicki, W.; Czepiel, K.; Wilczura, P.; Barzyk, P.; Kaczmarek, Z.; Kroc, M. Chromatographic Fingerprinting of the Old World Lupins Seed Alkaloids: A Supplemental Tool in Species Discrimination. Plants 2019, 8, 548. https://doi.org/10.3390/plants8120548
Święcicki W, Czepiel K, Wilczura P, Barzyk P, Kaczmarek Z, Kroc M. Chromatographic Fingerprinting of the Old World Lupins Seed Alkaloids: A Supplemental Tool in Species Discrimination. Plants. 2019; 8(12):548. https://doi.org/10.3390/plants8120548
Chicago/Turabian StyleŚwięcicki, Wojciech, Katarzyna Czepiel, Paulina Wilczura, Paweł Barzyk, Zygmunt Kaczmarek, and Magdalena Kroc. 2019. "Chromatographic Fingerprinting of the Old World Lupins Seed Alkaloids: A Supplemental Tool in Species Discrimination" Plants 8, no. 12: 548. https://doi.org/10.3390/plants8120548
APA StyleŚwięcicki, W., Czepiel, K., Wilczura, P., Barzyk, P., Kaczmarek, Z., & Kroc, M. (2019). Chromatographic Fingerprinting of the Old World Lupins Seed Alkaloids: A Supplemental Tool in Species Discrimination. Plants, 8(12), 548. https://doi.org/10.3390/plants8120548





