Outstanding Characteristics of Thai Non-GM Bred Waxy Cassava Starches Compared with Normal Cassava Starch, Waxy Cereal Starches and Stabilized Cassava Starches
Abstract
:1. Introduction
2. Results and Discussion
2.1. Amylose Contents
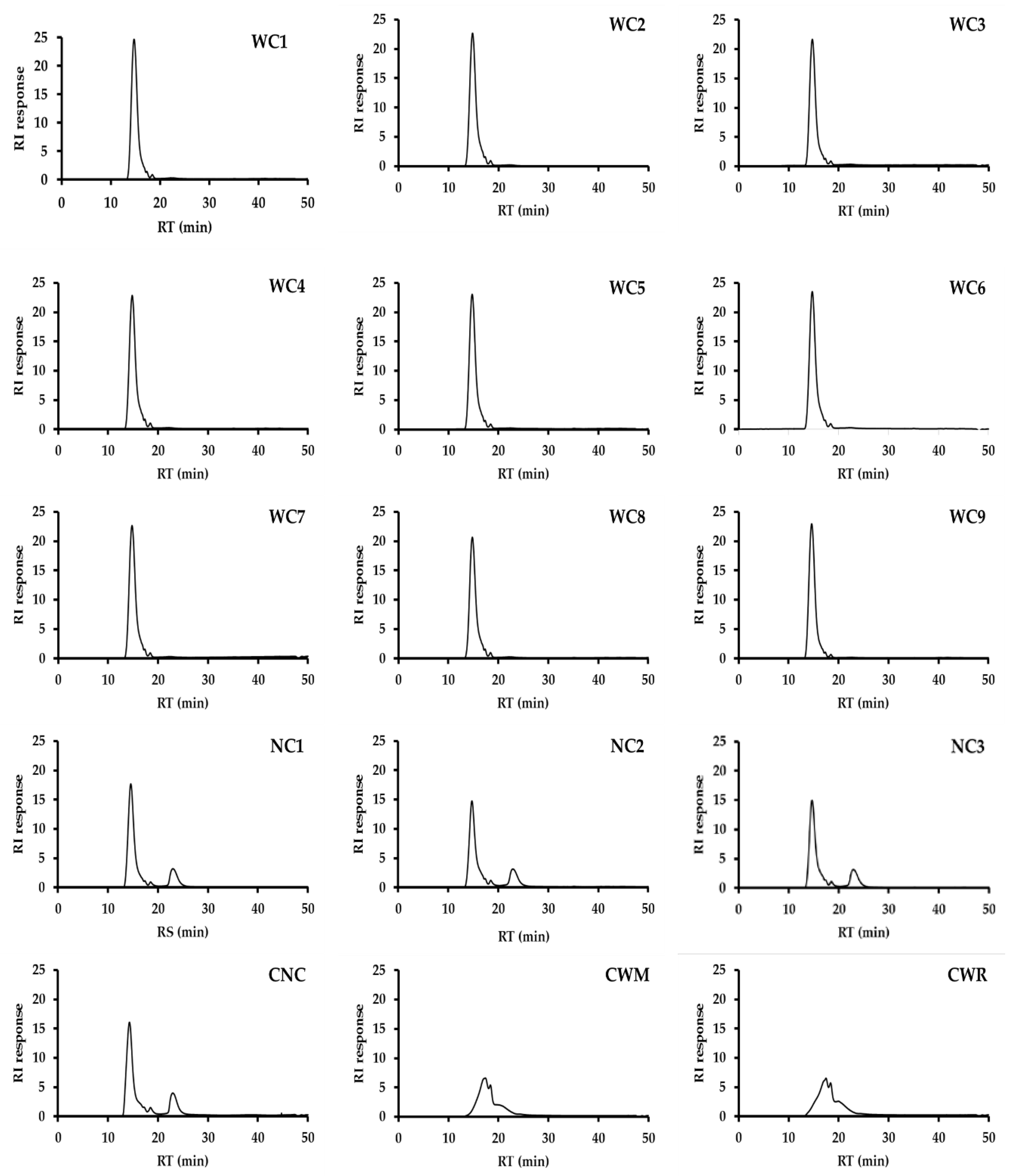
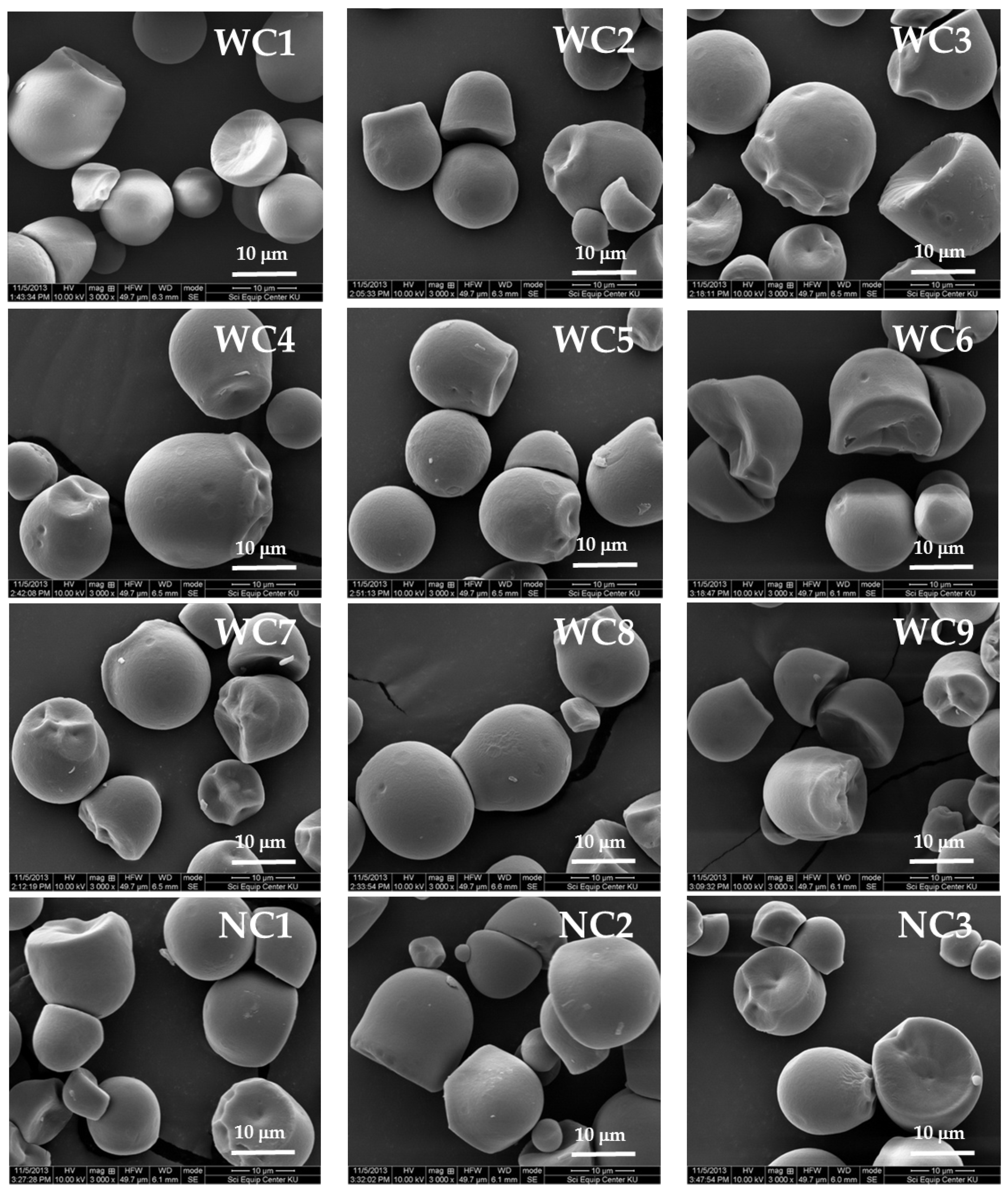
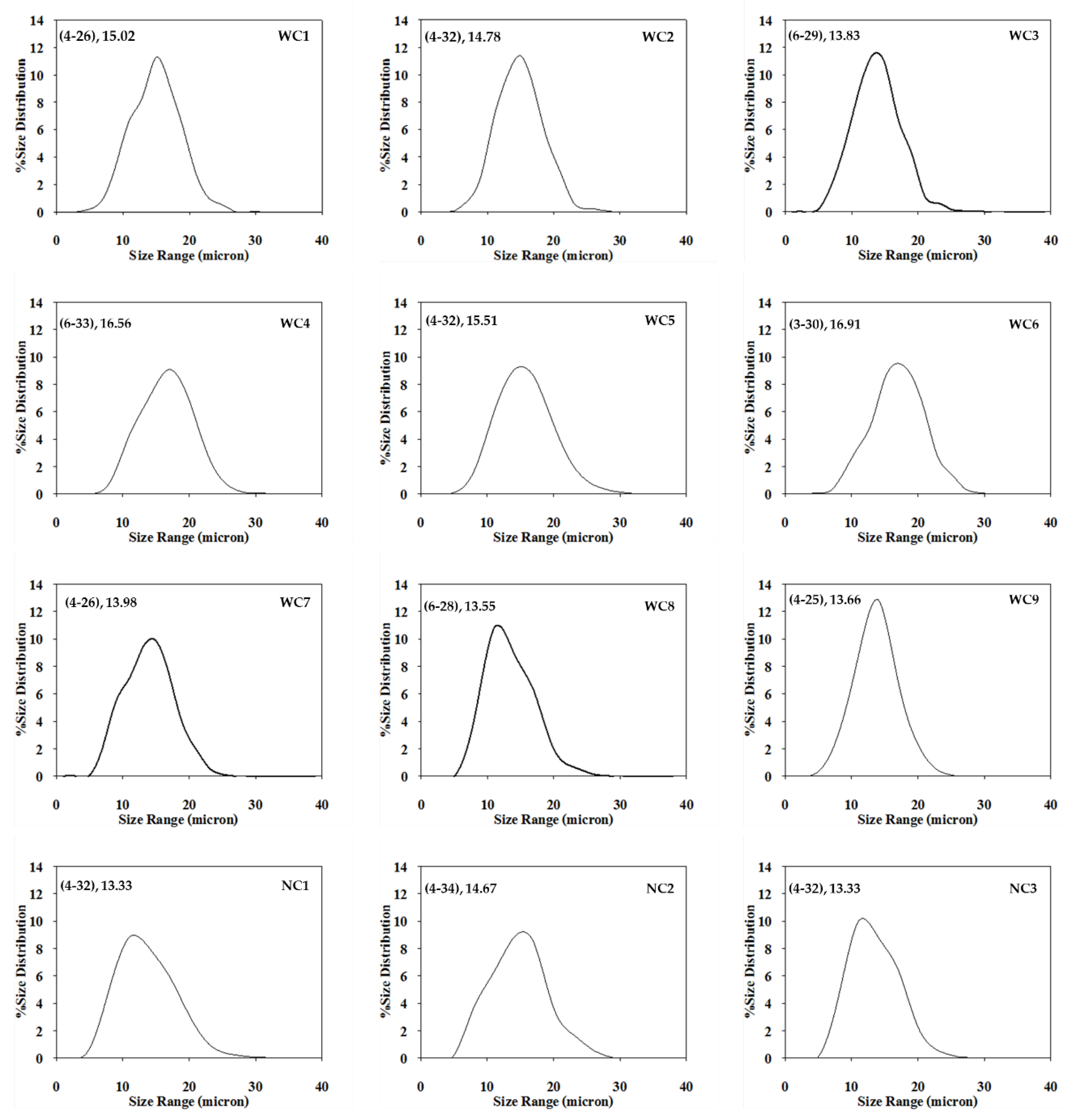
2.2. Morphology and Size Distribution
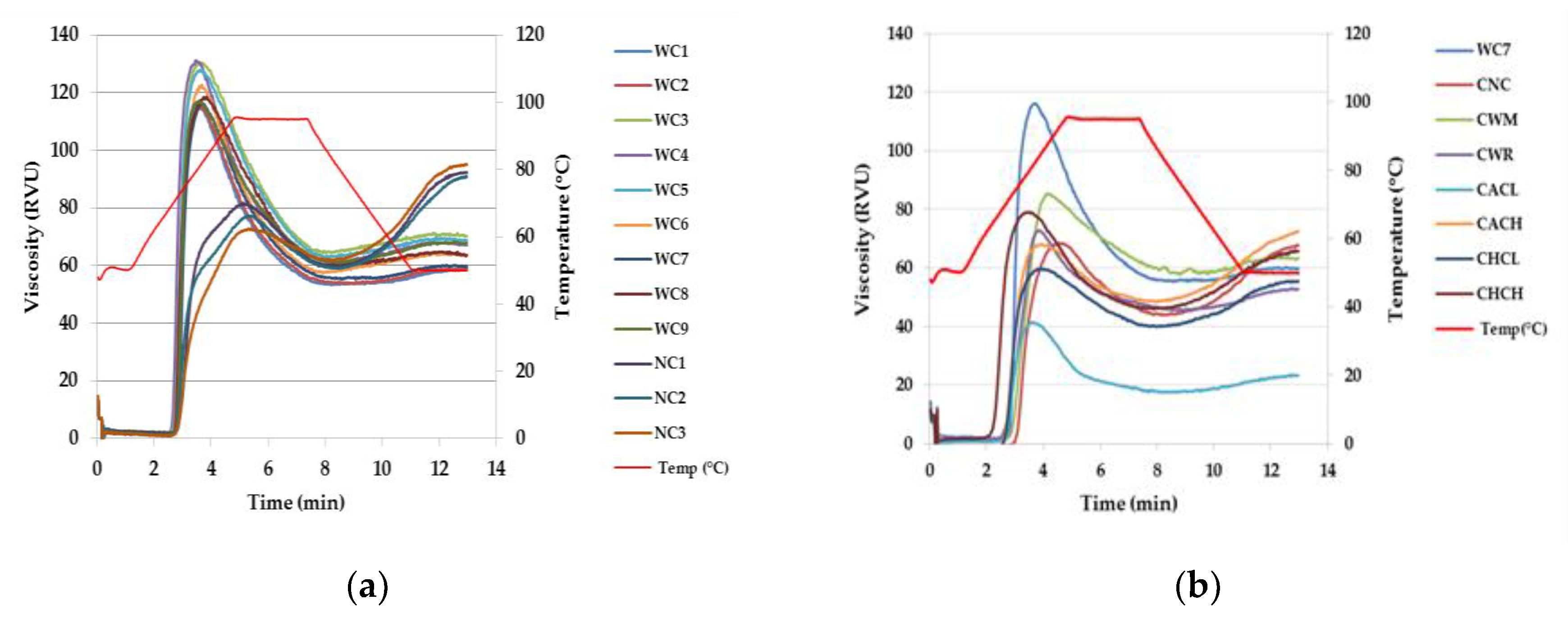
2.3. Starch Paste Behavior
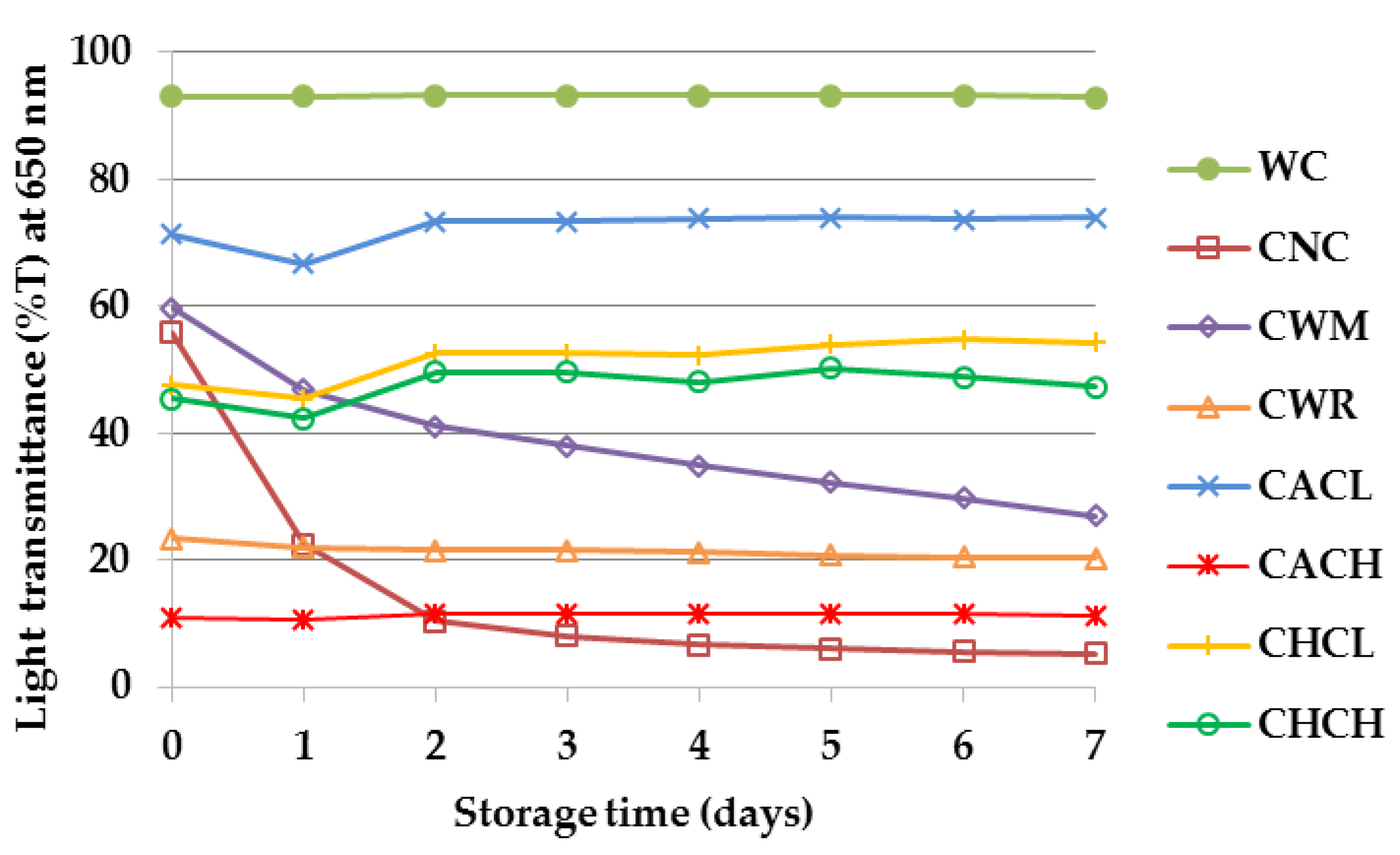
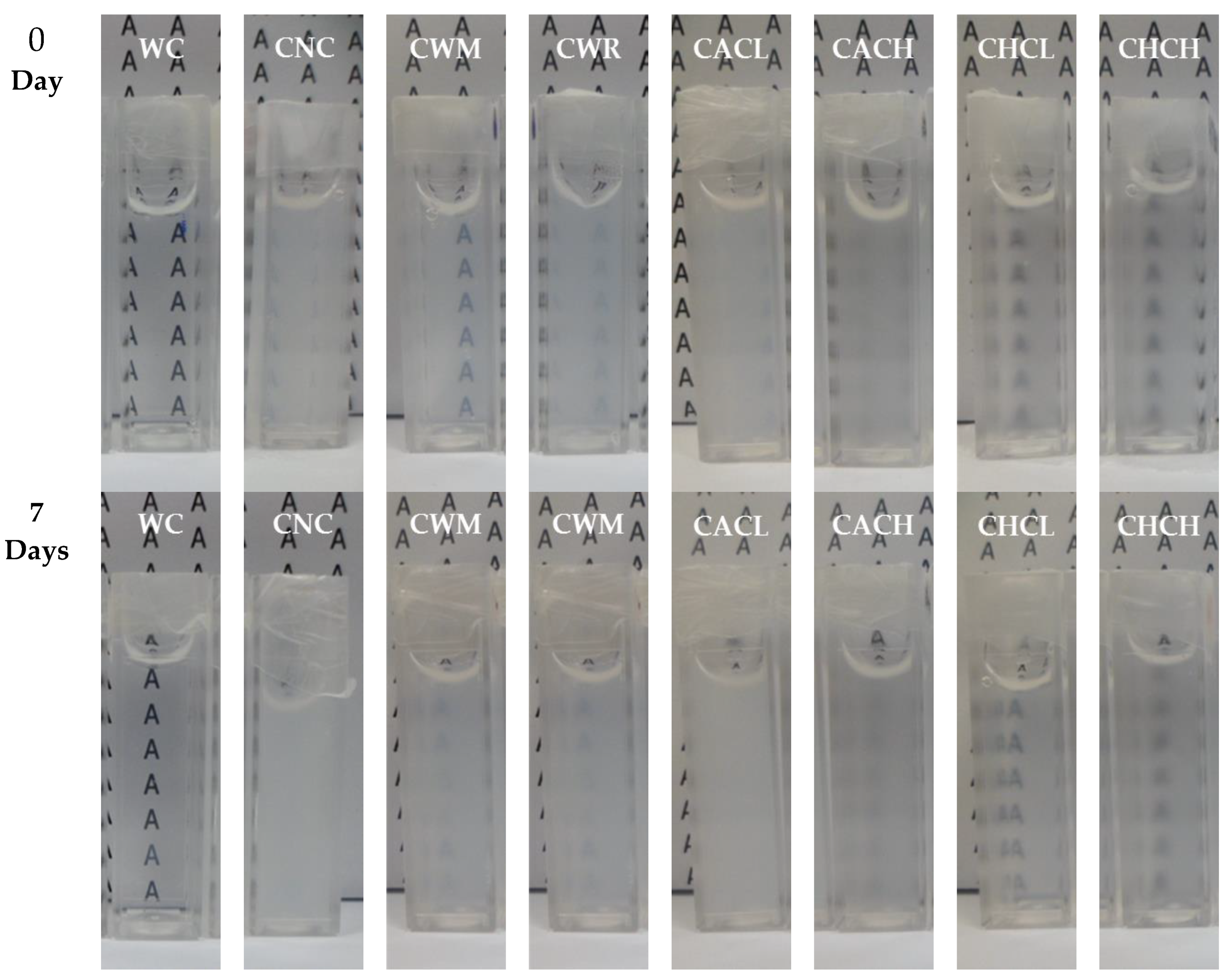
2.4. Paste Clarity
2.5. Swelling Power, Solubility and Close Packing Concentration
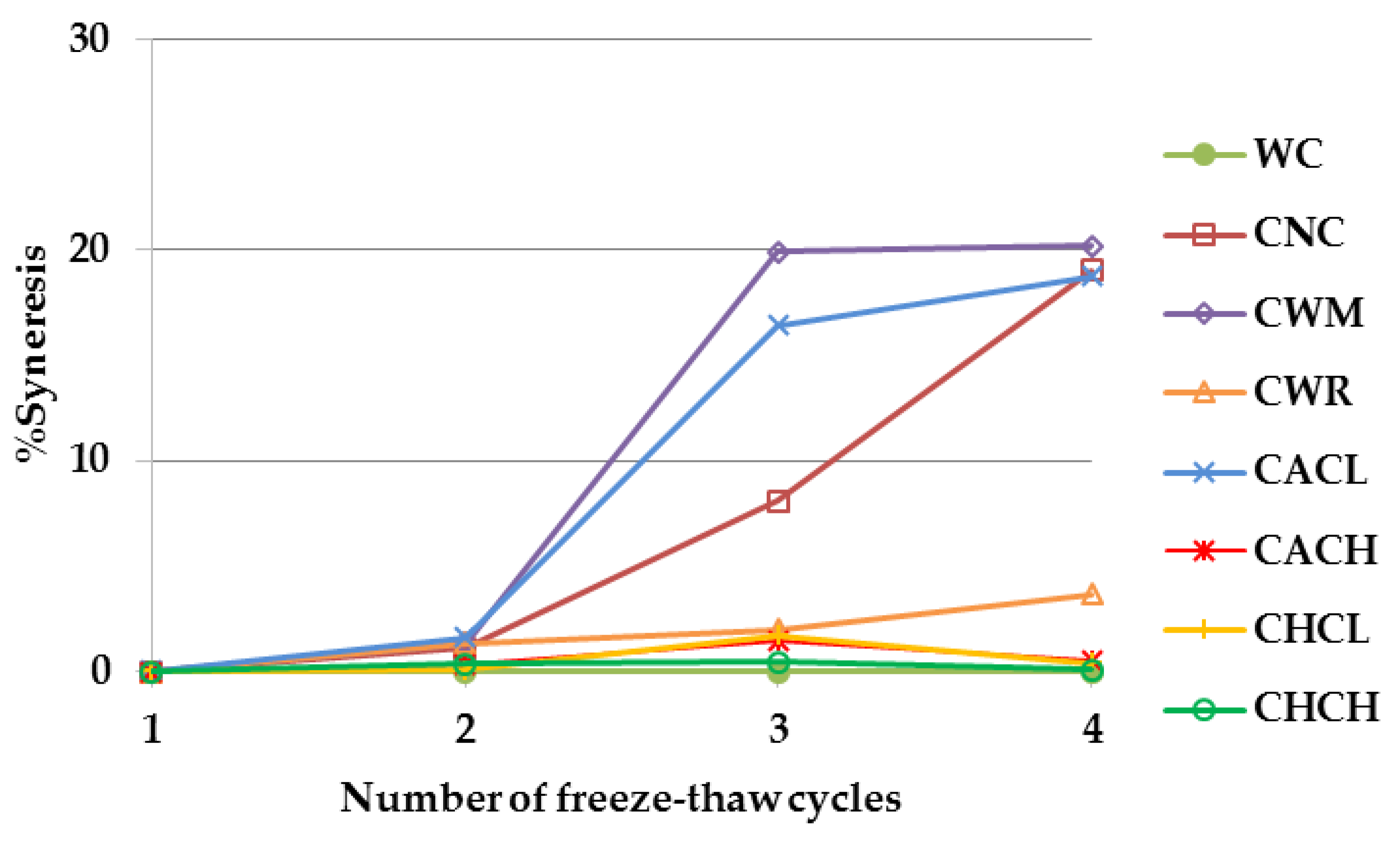
2.6. Syneresis after Freeze-Thaw
3. Materials and Methods
3.1. Materials
3.2. Starch Isolation from Fresh Roots
3.3. Amylose Content Determination
3.4. Scanning Electron Microscopy (SEM)
3.5. Granule Size Distribution
3.6. Pasting Properties
3.7. Paste Clarity
3.8. Swelling Power, Solubility and Close Packing Concentration Measurement
3.9. Freeze-Thaw Stability
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rolland-Sabaté, A.; Sánchez, T.; Buléon, A.; Colonna, P.; Ceballos, H.; Zhao, S.S.; Zhang, P.; Dufour, D. Molecular and supra-molecular structure of waxy starches developed from cassava (Manihot esculenta Crantz). Carbohydr. Polym. 2013, 92, 1451–1462. [Google Scholar] [CrossRef]
- Sriroth, K.; Santisopasri, V.; Pechalanuwat, C.; Kurotjanawong, K.; Piyachomkwan, K.; Oates, C.G. Cassava starch granule structure-function properties: Influence of time and conditions at harvest on four cultivars of cassava starch. Carbohydr. Polym. 1999, 38, 161–170. [Google Scholar] [CrossRef]
- Sánchez, T.; Salcedo, E.; Ceballos, H.; Dufour, D.; Mafla, G.; Morante, N.; Calle, F.; Pérez, J.C.; Debouck, D.; Jaramillo, G.; et al. Screening of starch quality traits in cassava (Manihot esculenta Crantz). Starch/Stärke 2009, 61, 12–19. [Google Scholar] [CrossRef]
- John, J.K.; Raja, K.C.M. Properties of cassava starch-dicarboxylic acid complexes. Carbohydr. Polym. 1999, 39, 181–186. [Google Scholar] [CrossRef]
- Zhu, F. Composition, structure, physicochemical properties, and modifications of cassava starch – Review. Carbohydr. Polym. 2015, 122, 456–480. [Google Scholar] [CrossRef]
- Hsieh, C.F.; Liu, W.; Whaley, J.K.; Shi, Y.C. Structure, properties, and potential applications of waxy tapioca starch – A review. Trends Food Sci. Technol. 2019, 83, 225–234. [Google Scholar] [CrossRef]
- Bull, S.E.; Seung, D.; Chanez, C.; Mehta, D.; Kuon, J.E.; Truernit, E.; Hochmuth, A.; Zurkirchen, I.; Zeeman, S.C.; Gruissem, W.; et al. Accelerated ex situ breeding of GBSS- and PTST1-edited cassava for modified starch. Sci. Adv. 2018, 4, 1–12. [Google Scholar] [CrossRef]
- Ceballos, H.; Sánchez, T.; Morante, N.; Fregene, M.; Dufour, D.; Smith, A.M.; Denyer, K.; Pérez, J.C.; Calle, F.; Mestres, C. Discovery of an amylose-free starch mutant in cassava (Manihot esculenta Crantz). J. Agric. Food Chem. 2007, 55, 7469–7476. [Google Scholar] [CrossRef]
- Sánchez, T.; Dufour, D.; Moreno, I.X.; Ceballos, H. Comparison of pasting and gel stabilities of waxy and normal starches from potato, maize, and rice with those of a novel waxy cassava starch under thermal, chemical, and mechanical stress. J. Agric. Food Chem. 2010, 58, 5093–5099. [Google Scholar] [CrossRef]
- Aiemnaka, P.; Wongkaew, A.; Chanthaworn, J.; Nagashima, S.K.; Boonma, S.; Authapun, J.; Jenweerawat, S.; Kongsila, P.; Kittipadakul, P.; Nakasathien, S.; et al. Molecular characterization of a spontaneous waxy starch mutation in cassava. Crop. Sci. 2012, 52, 2121–2130. [Google Scholar] [CrossRef]
- Rojanaridpiched, C.; Vichukit, V.; Phumichai, C. Process for improving waxy-starch cassava variety having improved qualifications and low cyanide. WO2016/118091 A2; World Intellectual Property Organization, 2016. Available online: https://patents.google.com/patent/WO2016118091A2/ru (accessed on 11 September 2019).
- Rolland-Sabaté, A.; Sánchez, T.; Buléon, A.; Colonna, P.; Jaillais, B.; Ceballos, H.; Dufour, D. Structural characterization of novel cassava starches with low and high-amylose contents in comparison with other commercial sources. Food Hydrocoll. 2012, 27, 161–174. [Google Scholar] [CrossRef]
- Karlström, A.; Calle, F.; Salazar, S.; Morante, N.; Dufour, D.; Ceballose, H. Biological implications in cassava for the production of amylose-free starch: Impact on root yield and related traits. Front. Plant Sci. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Morante, N.; Ceballos, H.; Sánchez, T.; Rolland-Sabaté, A.; Calle, F.; Hershey, C.; Gibert, O.; Dufour, D. Discovery of new Spontaneous sources of amylose-free cassava starch and analysis of their structure and techno-functional properties. Food Hydrocoll. 2016, 56, 383–395. [Google Scholar] [CrossRef]
- Pulido Diaz, A.; Lourdin, D.; Della Valle, G.; Fernández Quintero, A.; Ceballos, H.; Tran, T.; Dufour, D. Thermomechanical characterization of an amylose-free starch extracted from cassava (Manihot esculenta Crantz). Carbohydr. Polym. 2017, 157, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, K.; Nishinari, K. Effects of concentration dependence of retrogradation behaviour of dispersions for native and chemically modified potato starch. Food Hydrocoll. 2000, 14, 395–401. [Google Scholar] [CrossRef]
- Lawal, O.S. Succinyl and acetyl starch derivatives of a hybrid maize: Physicochemical characteristics and retrogradation properties monitored by differential scanning calorimetry. Carbohydr. Res. 2004, 339, 2673–2682. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Piyachomkwan, K.; Sriroth, K. Gelatinization and thermal properties of modified cassava starches. Starch/Stärke 2007, 59, 46–55. [Google Scholar] [CrossRef]
- Thomas, D.J.; Atwell, W.A. Starch modifications. In Starches; Thomas, D.J., Atwell, W.A., Eds.; American Association of Cereal Chemists (AACC): St. Paul, MN, USA, 1988; pp. 43–48. [Google Scholar]
- Light, J.M. Modified food starches; Why, What, Where, and How. Cereal Foods World, USA. 1990; 20. [Google Scholar]
- Chuenkamol, B.; Puttanlek, C.; Rungsardthong, V.; Uttapap, D. Characterization of low-substituted hydroxypropylated canna starch. Food Hydrocoll. 2007, 21, 1123–1132. [Google Scholar] [CrossRef]
- Jyothi, A.N.; Moorthy, S.N.; Rajasekharan, K.N. Studies on the synthesis and properties of hydroxypropyl derivatives of cassava (Manihot esculenta Crantz) starch. J. Sci. Food Agric. 2007, 87, 1964–1972. [Google Scholar] [CrossRef]
- Gomand, S.V.; Lamberts, L.; Visser, R.G.F.; Delcour, J.A. Physicochemical properties of potato and cassava starches and their mutants in relation to their structural properties. Food Hydrocoll. 2010, 24, 424–433. [Google Scholar] [CrossRef]
- Govindasamy, S.; Oates, C.G.; Wong, H.A. Characterization of changes of sago starch components during hydrolysis by a thermostable alpha-amylase. Carbohydr. Polym. 1992, 18, 89–100. [Google Scholar] [CrossRef]
- Santisopasri, V.; Kurotjanawong, K.; Chotineeranat, S.; Piyachomkwan, K.; Sriroth, K.; Oates, C.G. Impact of water stress on yield and quality of cassava starch. Ind. Crops Prod. 2001, 13, 115–129. [Google Scholar] [CrossRef]
- Newport Scientific. Operation Manual of the Series 4 Rapid Visco Analyzer. Australia. 1995; 93. [Google Scholar]
- Jacobson, M.R.; Obanni, M.; BeMiller, J.N. Retrogradation of starches from different botanical sources. Cereal Chem. 1997, 74, 511–518. [Google Scholar] [CrossRef]
- Eerlingen, R.C.; Jacobs, H.; Block, K.; Delcour, J.A. Effects of hydrothermal treatments on the rheological properties of potato starch. Carbohydr. Res. 1997, 297, 347–356. [Google Scholar] [CrossRef]
| Sample name | Starch variety | Amylose content (%) |
|---|---|---|
| WC1 | HBwx 09-562-19 Thai non-GM bred waxy cassava | 0.00 ± 0.00 d |
| WC2 | HBwx 09-754-16 Thai non-GM bred waxy cassava | 0.00 ± 0.00 d |
| WC3 | HBwx 09-612-18 Thai non-GM bred waxy cassava | 0.00 ± 0.00 d |
| WC4 | HBwx 09-1041-6 Thai non-GM bred waxy cassava | 0.00 ± 0.00 d |
| WC5 | HBwx 09-826-2 Thai non-GM bred waxy cassava | 0.00 ± 0.00 d |
| WC6 | HBwx 09-317-6 Thai non-GM bred waxy cassava | 0.00 ± 0.00 d |
| WC7 | HBwx 09-19-2 Thai non-GM bred waxy cassava | 0.00 ± 0.00 d |
| WC8 | HBwx 09-635-4 Thai non-GM bred waxy cassava | 0.00 ± 0.00 d |
| WC9 | HBwx 09-989-9 Thai non-GM bred waxy cassava | 0.00 ± 0.00 d |
| NC1 | KU50 wild-type normal cassava | 18.15 ± 0.68 c |
| NC2 | HB80 wild-type normal cassava | 19.59 ± 0.30 b |
| NC3 | R1 wild-type normal cassava | 19.05 ± 0.68 b |
| CNC | Commercial normal cassava | 20.97 ± 0.33 a |
| CWM | Commercial waxy maize | 0.00 ± 0.00 d |
| CWR | Commercial waxy rice | 0.00 ± 0.00 d |
| Sample | PV (RVU) | TV (RVU) | BD (RVU) | SB (RVU) | PT (℃) |
|---|---|---|---|---|---|
| WC1 | 119 ± 0 d | 55 ± 1 fg | 64 ± 1 de | 7 ± 1 g | 72 ± 0 bcd |
| WC2 | 122 ± 0 c | 56 ± 1 ef | 67 ± 1 bc | 7 ± 1 g | 71 ± 0 efgh |
| WC3 | 126 ± 1 b | 58 ± 1 bcd | 68 ± 0 ab | 6 ± 1 g | 71 ± 0 fghi |
| WC4 | 131 ± 0 a | 61 ± 1 b | 70 ± 0 a | 6 ± 0 g | 70 ± 0 k |
| WC5 | 126 ± 0 b | 57 ± 0 cde | 68 ± 0 ab | 7 ± 1 g | 71 ± 0 hij |
| WC6 | 116 ± 1 e | 56 ± 0 ef | 60 ± 0 f | 6 ± 0 g | 70 ± 0 ijk |
| WC7 | 126 ± 1 b | 56 ± 0 def | 70 ± 1 a | 7 ± 0 g | 72 ± 1 bc |
| WC8 | 120 ± 0 d | 58 ± 0 cde | 63 ± 0 e | 7 ± 1 g | 71 ± 0 ghi |
| WC9 | 119 ± 1 d | 54 ± 1 g | 66 ± 0 cd | 10 ± 1 f | 72 ± 0 cde |
| NC1 | 94 ± 1 f | 64 ± 0 a | 30 ± 1 hi | 37 ± 2 a | 73 ± 0 b |
| NC2 | 89 ± 0 g | 58 ± 0 cde | 32 ± 0 gh | 31 ± 0 b | 71 ± 0 defgh |
| NC3 | 80 ± 0 g | 59 ± 0 bc | 29 ± 0 i | 32 ± 1 b | 71 ± 0 cdefg |
| CNC | 69 ± 1 k | 44 ± 1 i | 25 ± 0 j | 24 ± 1 c | 74 ± 1 a |
| CWM | 85 ± 0 h | 57 ± 0 cde | 28 ± 0 i | 6 ± 0 g | 72 ± 0 cdef |
| CWR | 73 ± 1 j | 45 ± 0 i | 28 ± 1 i | 8 ± 0 g | 67 ± 0 l |
| CACL | 42 ± 1 m | 17 ± 1 k | 24 ± 0 j | 6 ± 0 g | 70 ± 0 jk |
| CACH | 68 ± 0 k | 48 ± 0 h | 20 ± 0 k | 24 ± 0 c | 68 ± 0 l |
| CHCL | 60 ± 1 l | 40 ± 1 j | 20 ± 0 k | 16 ± 2 e | 69 ± 1 l |
| CHCH | 79 ± 1 i | 46 ± 1 i | 33 ± 0 g | 20 ± 1 d | 64 ± 1 m |
| Sample | Light transmittance (%T) at 650 nm | Δ%T | |
|---|---|---|---|
| 0 day | 7 day | ||
| WC1 | 94.28 ± 0.49 a | 94.70 ± 0.47 a | 0 |
| WC2 | 94.80 ± 1.05 a | 94.83 ± 1.19 a | 0 |
| WC3 | 93.53 ± 0.86 ab | 93.78 ± 0.90 a | 0 |
| WC4 | 91.35 ± 0.56 b | 91.63 ± 0.15 a | 0 |
| WC5 | 92.90 ± 2.03 ab | 92.93 ± 1.82 a | 0 |
| WC6 | 93.75 ± 1.10 ab | 94.05 ± 1.33 a | 0 |
| WC7 | 92.55 ± 0.99 ab | 92.95 ± 0.55 a | 0 |
| WC8 | 92.45 ± 0.75 ab | 92.85 ± 0.87 a | 0 |
| WC9 | 93.33 ± 0.68 ab | 93.95 ± 1.11 a | 0 |
| NC1 | 56.18 ± 3.49 c | 36.90 ± 3.24 b | 34.3 |
| NC2 | 53.10 ± 2.17 d | 27.73 ± 4.10 c | 47.8 |
| NC3 | 53.13 ± 1.90 d | 29.83 ± 5.24 c | 43.9 |
| Sample | Swelling power (g/g) | %Solubility | C* |
|---|---|---|---|
| WC | 77.65 ± 7.68 a | 6.40 ± 0.14 f | 1.38 ± 0.14 e |
| CNC | 59.44 ± 2.64 cd | 28.29 ± 1.67 b | 2.35 ± 0.05 bc |
| CWM | 50.50 ± 1.16 ef | 13.10 ± 0.20 e | 2.28 ± 0.06 bc |
| CWR | 57.56 ± 0.77 de | 12.64 ± 0.47 e | 1.99 ± 0.02 d |
| CACL | 68.43 ± 1.38 de | 38.06 ± 0.24 a | 2.36 ± 0.04 b |
| CACH | 46.77 ± 0.86 f | 20.16 ± 0.10 d | 2.68 ± 0.05 a |
| CHCL | 59.02 ± 1.06 d | 23.30 ± 0.24 c | 2.21 ± 0.03 c |
| CHCH | 66.26 ± 2.23 bc | 23.90 ± 1.43 c | 1.98 ± 0.03 d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toae, R.; Sriroth, K.; Rojanaridpiched, C.; Vichukit, V.; Chotineeranat, S.; Wansuksri, R.; Chatakanonda, P.; Piyachomkwan, K. Outstanding Characteristics of Thai Non-GM Bred Waxy Cassava Starches Compared with Normal Cassava Starch, Waxy Cereal Starches and Stabilized Cassava Starches. Plants 2019, 8, 447. https://doi.org/10.3390/plants8110447
Toae R, Sriroth K, Rojanaridpiched C, Vichukit V, Chotineeranat S, Wansuksri R, Chatakanonda P, Piyachomkwan K. Outstanding Characteristics of Thai Non-GM Bred Waxy Cassava Starches Compared with Normal Cassava Starch, Waxy Cereal Starches and Stabilized Cassava Starches. Plants. 2019; 8(11):447. https://doi.org/10.3390/plants8110447
Chicago/Turabian StyleToae, Roselawatee, Klanarong Sriroth, Chareinsuk Rojanaridpiched, Vichan Vichukit, Sunee Chotineeranat, Rungtiva Wansuksri, Pathama Chatakanonda, and Kuakoon Piyachomkwan. 2019. "Outstanding Characteristics of Thai Non-GM Bred Waxy Cassava Starches Compared with Normal Cassava Starch, Waxy Cereal Starches and Stabilized Cassava Starches" Plants 8, no. 11: 447. https://doi.org/10.3390/plants8110447
APA StyleToae, R., Sriroth, K., Rojanaridpiched, C., Vichukit, V., Chotineeranat, S., Wansuksri, R., Chatakanonda, P., & Piyachomkwan, K. (2019). Outstanding Characteristics of Thai Non-GM Bred Waxy Cassava Starches Compared with Normal Cassava Starch, Waxy Cereal Starches and Stabilized Cassava Starches. Plants, 8(11), 447. https://doi.org/10.3390/plants8110447




