Evaluation of Switchgrass Genotypes for Cold-Tolerant Seed Germination from Native Populations in the Northeast USA
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Switchgrass Genotypes
4.2. Seed Germination Tests
4.3. Tetrazolium Test
4.4. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clifton-Brown, J.; Harfouche, A.; Casler, M.D.; Dylan Jones, H.; Macalpine, W.J.; Murphy Bokern, D.; Bastien, C. Breeding progress and preparedness for mass-scale deployment of perennial lignocellulosic biomass crops switchgrass, miscanthus, willow and poplar. Gcb Bioenergy 2018, 11, 118–151. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, S.B.; Kszos, L.A. Development of switchgrass (Panicum virgatum) as a bioenergy feedstock in the United States. Biomass Bioenergy 2005, 28, 515–535. [Google Scholar] [CrossRef]
- Moser, L.E.; Vogel, K.P. Switchgrass, big bluestem, and indiangrass. In Forages, An Introduction to Grassland Agriculture, 5th ed.; Barnes, R.F., Miller, D.A., Nelson, C.J., Eds.; University Press: Ames, IA, USA, 1995; pp. 409–420. [Google Scholar]
- Stoof, C.R.; Richards, B.K.; Woodbury, P.B.; Fabio, E.S.; Brumbach, A.R.; Cherney, J.; Mayton, H. Untapped potential: Opportunities and challenges for sustainable bioenergy production from marginal lands in the Northeast USA. Bioenergy Res. 2015, 8, 482–501. [Google Scholar] [CrossRef]
- Lu, F.; Lipka, A.E.; Glaubitz, J.; Elshire, R.; Cherney, J.H.; Casler, M.D.; Buckler, E.S.; Costich, D.E. Switchgrass genomic diversity, ploidy, and evolution: Novel insights from a network-based SNP discovery protocol. PLoS Genet. 2013, 9, e1003215. [Google Scholar] [CrossRef] [PubMed]
- Casler, M.D.; Stendal, C.A.; Kapich, L.; Vogel, K.P. Genetic diversity, plant adaptation regions, and gene pools for switchgrass. Crop Sci. 2007, 47, 2261–2273. [Google Scholar] [CrossRef]
- Weaver, J.E. Prairie Plants and Their Environment; A Fifty Year Study in the Midwest; University of Nebraska Press: Lincoln, NE, USA, 1968. [Google Scholar]
- Casler, M.D. Switchgrass breeding, genetics, and genomics. In Switchgrass; Springer: London, UK, 2012; pp. 29–53. [Google Scholar]
- Casler, M.D.; Vogel, K.P.; Taliaferro, C.M.; Ehlke, N.J.; Berdahl, J.D.; Brummer, E.C.; Kallenbach, R.; West, C.P.; Mitchell, R.B. Latitudinal and longitudinal adaptation of switchgrass populations. Crop Sci. 2007, 47, 2249–2260. [Google Scholar] [CrossRef]
- Porter, C.L., Jr. An analysis of variation between upland and lowland switchgrass, Panicum virgatum L., in central Oklahoma. Ecology 1966, 47, 980–992. [Google Scholar] [CrossRef]
- Seepaul, R.; Macoon, B.; Reddy, K.R.; Baldwin, B. Switchgrass (Panicum virgatum L.) intraspecific variation and thermotolerance classification using in vitro seed germination assay. Am. J. Plant Sci. 2011, 2. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. N. Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Duclos, D.V.; Ray, D.T.; Johnson, D.J.; Taylor, A.G. Investigating seed dormancy in switchgrass (Panicum virgatum L.): Understanding the physiology and mechanisms of coat-imposed seed dormancy. Ind. Crop. Prod. 2013, 45, 377–387. [Google Scholar] [CrossRef]
- Duclos, D.V.; Altobello, C.O.; Taylor, A.G. Investigating seed dormancy in switchgrass (Panicum virgatum L.): Elucidating the effect of temperature regimes and plant hormones on embryo dormancy. Ind. Crop. Prod. 2014, 58, 148–159. [Google Scholar] [CrossRef]
- Wright, L.; Turhollow, A. Switchgrass selection as a “model” bioenergy crop: A history of the process. Biomass Bioenergy 2010, 34, 851–868. [Google Scholar] [CrossRef]
- Casler, M.D.; Sosa, S.; Hoffman, L.; Mayton, H.; Ernst, C.; Adler, P.R.; Bonos, S.A. Biomass yield of switchgrass cultivars under high-versus low-input conditions. Crop Sci. 2017, 57, 821–832. [Google Scholar] [CrossRef][Green Version]
- Aiken, G.E.; Springer, T.L. Seed size distribution, germination, and emergence of 6 switchgrass cultivars. J. Range Manag. 1995, 48, 455–458. [Google Scholar] [CrossRef]
- Mitchell, R.; Vogel, K.P.; Sarath, G. Managing and enhancing switchgrass as a bioenergy feedstock. Biofuels Bioprod. Biorefin. 2008, 2, 530–539. [Google Scholar] [CrossRef]
- Hanson, J.D.; Johnson, H.A. Germination of switchgrass under various temperature and pH regimes. Seed Technol. 2005, 27, 203–210. [Google Scholar]
- Eagles, H.A.; Hardacre, A.K. Genetic variation in maize for early seedling growth in a low temperature environment. N. Z. J. Agric. Res. 1979, 22, 553–559. [Google Scholar] [CrossRef]
- Burow, G.; Burke, J.J.; Xin, Z.; Franks, C.D. Genetic dissection of early-season cold tolerance in sorghum (Sorghum bicolor (L.) Moench). Mol. Breed. 2011, 28, 391–402. [Google Scholar] [CrossRef]
- Kollipara, K.; Saab, I.N.; Wych, R.D.; Lauer, M.J.; Singletary, G.W. Expression profiling of reciprocal maize hybrids divergent for cold germination and desiccation tolerance. Plant Phys. 2002, 129, 974–992. [Google Scholar] [CrossRef]
- Jha, U.C.; Bohra, A.; Jha, R. Breeding approaches and genomics technologies to increase crop yield under low-temperature stress. Plant Cell Rep. 2017, 36, 1–35. [Google Scholar] [CrossRef]
- Xing, X.; Liu, Y.; Kong, X.; Liu, Y.; Li, D. Overexpression of a maize dehydrin gene, ZmDHN2b, in tobacco enhances tolerance to low temperature. Plant Growth Reg. 2011, 65, 109–118. [Google Scholar] [CrossRef]
- Bahri, B.A.; Daverdin, G.; Xu, X.; Cheng, J.F.; Barry, K.W.; Brummer, E.C.; Devos, K.M. Natural variation in genes potentially involved in plant architecture and adaptation in switchgrass (Panicum virgatum L.). BMC Evol. Biol. 2018, 18, 91. [Google Scholar] [CrossRef] [PubMed]
- Serba, D.; Wu, L.; Daverdin, G.; Bahri, B.A.; Wang, X.; Kilian, A.; Bouton, J.H.; Brummer, E.C.; Saha, M.C.; Devos, K.M. Linkage maps of lowland and upland tetraploid switchgrass ecotypes. Bioenergy Res. 2018, 6, 953–965. [Google Scholar] [CrossRef][Green Version]
- Casler, M.D.; Vogel, K.P.; Harrison, M. Switchgrass germplasm resources. Crop Sci. 2015, 55, 463–2478. [Google Scholar] [CrossRef]
- AOSA. Rules for testing seeds. Principles and procedures. Assn. Off. Seed Anal. 2014, 1, 6–25. [Google Scholar]
- Maguire, J.D. Speed of germination-aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Coolbear, P.; Francis, A.; Grierson, D. The effect of low temperature pre-sowing treatment on the germination performance and membrane integrity of artificially aged tomato seeds. J. Exp. Bot. 1984, 35, 1609–1617. [Google Scholar] [CrossRef]
- Miller, A.A.; Peters, J. AOSA/SCST tetrazolium testing handbook. Collins Co Tetrazolium Subcomm. Assoc. Off. Seed Anal. Soc. Commer. Seed Technol. 2010, 39–40. [Google Scholar]
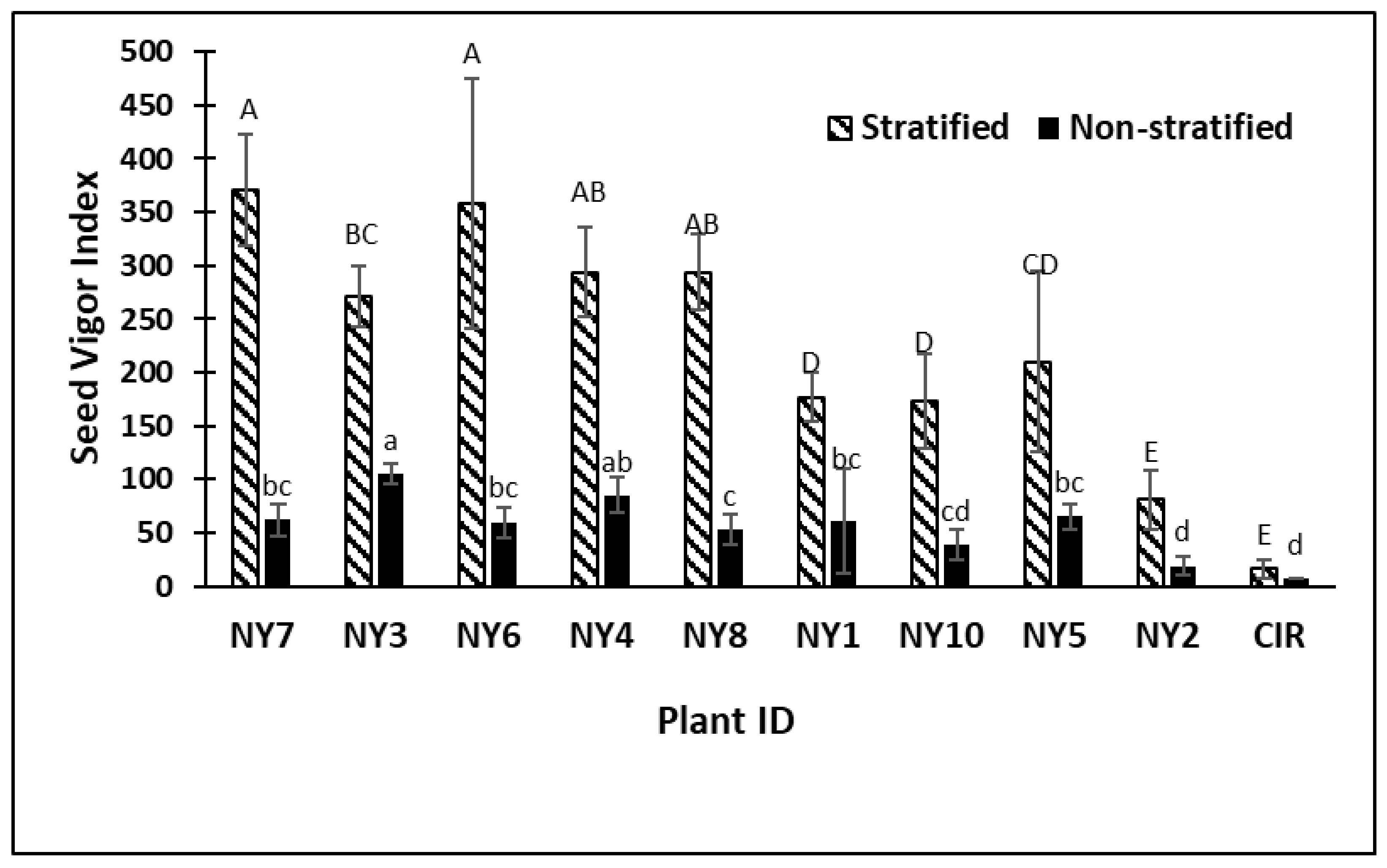
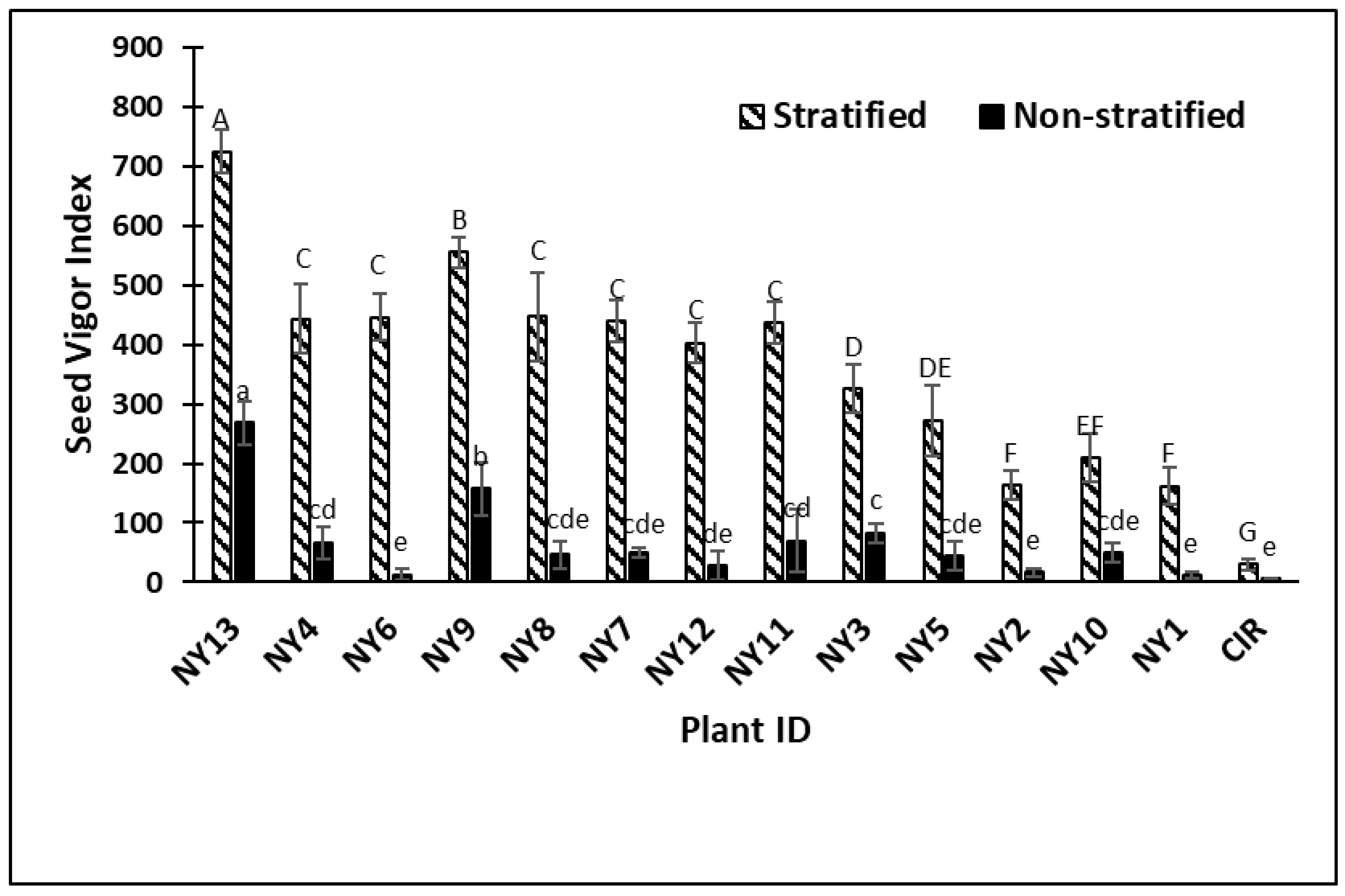
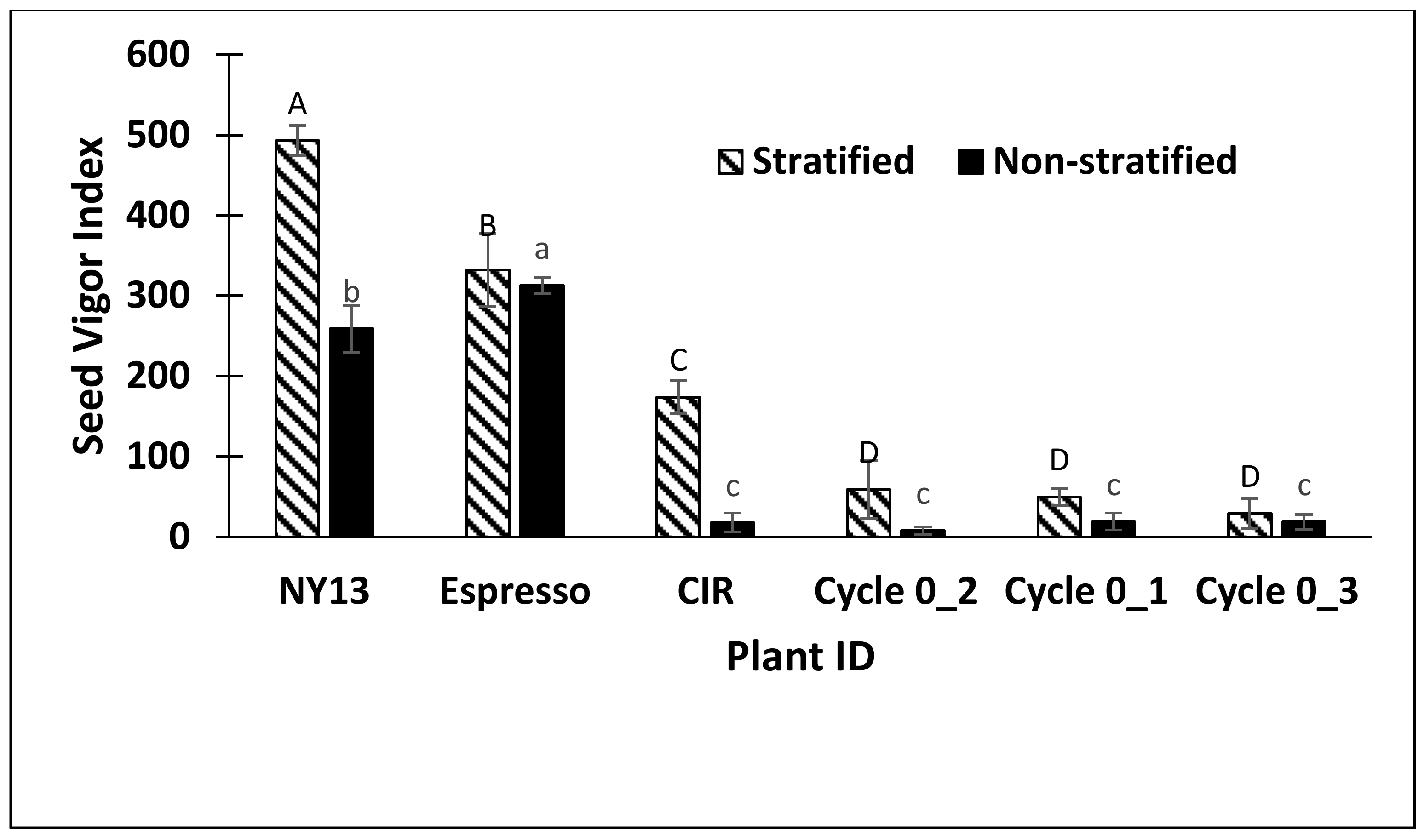
| ID | Experiments | Plant Vigor | Plant Height | Parent Location | Parent GRIN Plant Name |
|---|---|---|---|---|---|
| NY1 | 1, 2 | 5 | 114 | Orleans Co, NY | 9106194 |
| NY2 | 1, 2 | 5 | 127 | Orleans Co, NY | 9106194 |
| NY3 | 1, 2 | 5 | 107 | Niagara Co, NY | 9106191 |
| NY4 | 1, 2 | 3 | 109 | Genesee Co, NY | 9106195 |
| NY5 | 1, 2 | 4 | 117 | Genesee Co, NY | 9106195 |
| NY6 | 1, 2 | 5 | 129 | Genesee Co, NY | 9106195 |
| NY7 | 1, 2 | 3 | 124 | Steuben Co, NY | 9106189 |
| NY8 | 1, 2 | 4 | 96 | Genesee Co, NY | 9106195 |
| NY9 | 2 | 4 | 107 | Steuben Co, NY | 9106189 |
| NY10 | 2 | 3 | 117 | Hudson Co, NY | 9086098 |
| NY11 | 2 | 4 | 129 | Steuben Co, NY | 9106189 |
| NY12 | 2 | 3 | 129 | Genesee Co, NY | 9106195 |
| NY13 | 1, 2, 3 | 4 | 112 | Genesee Co, NY | 9106195 |
| CIR | 1, 2, 3 | - | - | Ernst Seed, PA * | Cave-in-Rock |
| Espresso | 3 | - | - | Starkville, MS ** | |
| Cycle 0 | 3 | - | - | Multiple locations |
| ID | MPG % | T50 | ||
|---|---|---|---|---|
| Stratified | Non-Stratified | Stratified | Non-Stratified | |
| NY7 | 55 A | 13 BC | 12 CD | 19 ABC |
| NY3 | 44 B | 18 AB | 14 AB | 13 BC |
| NY6 | 40 BC | 9 C | 10 D | 11 C |
| NY4 | 40 BC | 20 A | 12 CD | 24 A |
| NY8 | 39 BC | 11 C | 11 CD | 19 ABC |
| NY1 | 33 CD | 13 BC | 16 A | 23 AB |
| NY10 | 28 D | 8 CD | 12 BC | 17 ABC |
| NY5 | 26 D | 13 BC | 10 CD | 17 ABC |
| NY2 | 15 E | 3 DE | 15 A | 10 C |
| CIR | 3 F | 1 E | 12 CD | 11 C |
| P value < | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| LSD | 10.0 | 6.1 | 2.2 | 9.1 |
| ID | MPG % | T50 | ||
|---|---|---|---|---|
| Stratified | Non-Stratified | Stratified | Non-Stratified | |
| NY13 | 67 A | 28 A | 7 E | 9 E |
| NY4 | 59 AB | 11 CDE | 11 BCDE | 13 CDE |
| NY6 | 58 AB | 3 EF | 11 BCDE | 21 BC |
| NY9 | 57 AB | 21 AB | 8 GH | 11 DE |
| NY8 | 57 AB | 8 DEF | 11 BCDE | 20 BCD |
| NY7 | 55 B | 9 DEF | 11 BCDE | 13 CDE |
| NY12 | 52 BC | 5 EF | 11 BCDE | 18 BCD |
| NY11 | 50 BC | 14 BCD | 10 EFG | 20 BCD |
| NY3 | 45 C | 18 BC | 11 BCDE | 19 BCD |
| NY5 | 32 D | 9 DEF | 9 EFD | 19 BCD |
| NY2 | 32 D | 5 EF | 17 A | 33 A |
| NY10 | 32 D | 11 CDE | 13 BC | 25 AB |
| NY1 | 27 D | 3 EF | 13 BC | 26 AB |
| CIR | 4 E | 2 F | 14 B | 32 A |
| P value < | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| LSD | 10.4 | 8.6 | 2.6 | 9.1 |
| ID | MPG % | T50 | ||
|---|---|---|---|---|
| Stratified | Non-Stratified | Stratified | Non-Stratified | |
| NY13 | 89 A | 78 A | 6 C | 11 CD |
| Espresso | 68 B | 83 A | 8 BC | 11 D |
| CIR | 51 C | 5 B | 12 AB | 17 AB |
| Cycle 0_2 | 16 D | 4 B | 11 AB | 20 A |
| Cycle 0_1 | 13 D | 8 B | 12 AB | 17 AB |
| Cycle 0_3 | 8 D | 7 B | 15 A | 14 BC |
| P value ≤ | 0.0001 | 0.0001 | 0.004 | 0.0002 |
| LSD | 11.7 | 6.7 | 3.9 | 3.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayton, H.; Amirkhani, M.; Loos, M.; Crawford, J.; Crawford, R.; Hansen, J.; Viands, D.; Salon, P.; Taylor, A. Evaluation of Switchgrass Genotypes for Cold-Tolerant Seed Germination from Native Populations in the Northeast USA. Plants 2019, 8, 394. https://doi.org/10.3390/plants8100394
Mayton H, Amirkhani M, Loos M, Crawford J, Crawford R, Hansen J, Viands D, Salon P, Taylor A. Evaluation of Switchgrass Genotypes for Cold-Tolerant Seed Germination from Native Populations in the Northeast USA. Plants. 2019; 8(10):394. https://doi.org/10.3390/plants8100394
Chicago/Turabian StyleMayton, Hilary, Masoume Amirkhani, Michael Loos, Jamie Crawford, Ryan Crawford, Julie Hansen, Donald Viands, Paul Salon, and Alan Taylor. 2019. "Evaluation of Switchgrass Genotypes for Cold-Tolerant Seed Germination from Native Populations in the Northeast USA" Plants 8, no. 10: 394. https://doi.org/10.3390/plants8100394
APA StyleMayton, H., Amirkhani, M., Loos, M., Crawford, J., Crawford, R., Hansen, J., Viands, D., Salon, P., & Taylor, A. (2019). Evaluation of Switchgrass Genotypes for Cold-Tolerant Seed Germination from Native Populations in the Northeast USA. Plants, 8(10), 394. https://doi.org/10.3390/plants8100394






