Effects of the Number of Seeds per Berry on Fruit Growth Characteristics, Especially on the Duration of Stage II in Blueberry
Abstract
:1. Introduction
2. Results
2.1. Effects of Different Pollen Sources on Berry Characteristics
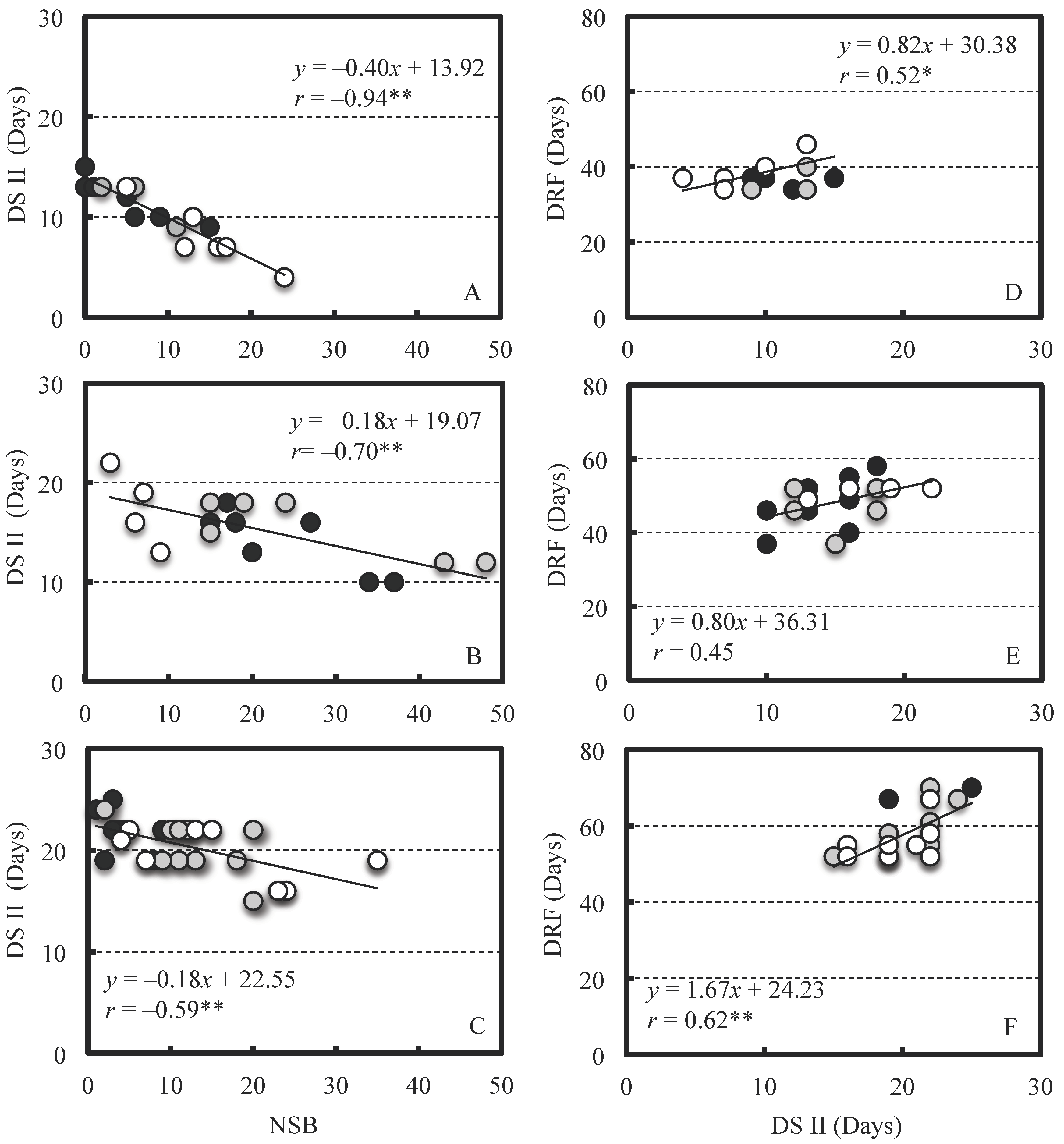
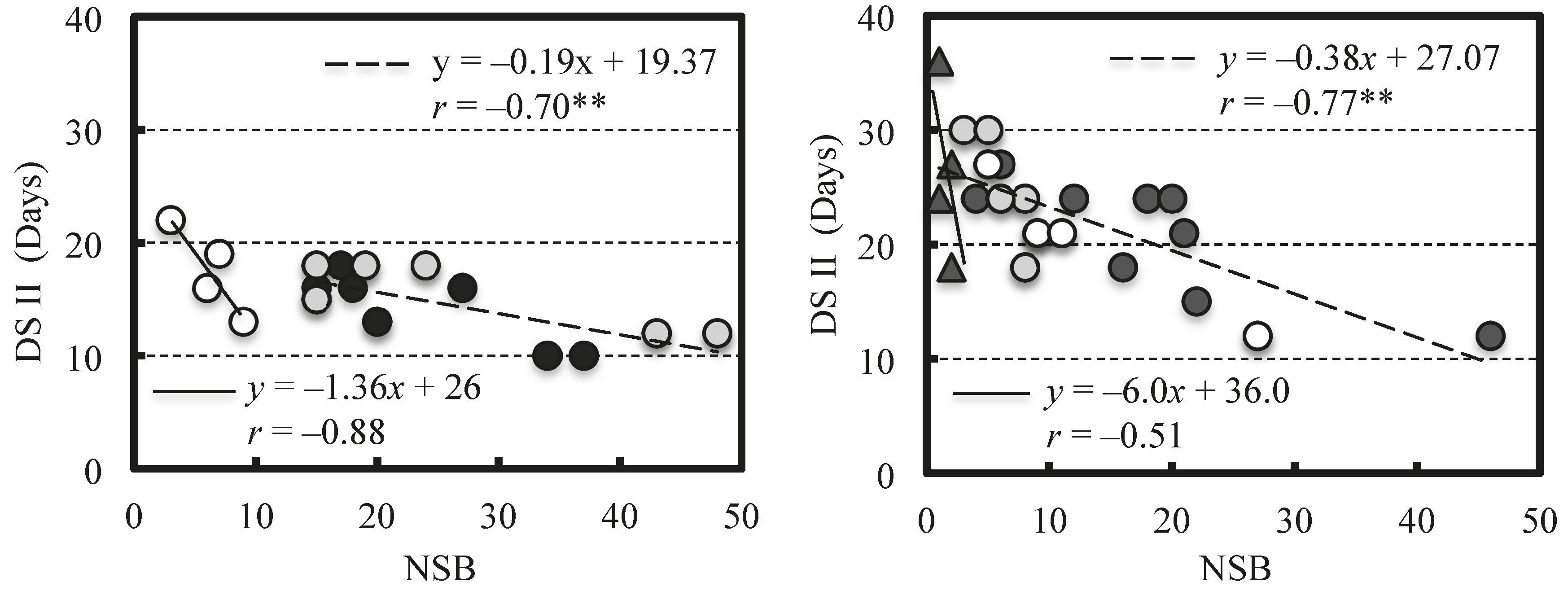
2.2. Effect of NSB on Berry Growth
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Pollination Treatments and Pollen Sources
4.3. Measurements
4.4. Data Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Taira, S.; Matsumoto, N.; Ono, M. Accumulation of soluble and insoluble tannins during fruit development in nonastringent and astringent persimmon. J. Jpn. Soc. Hortic. Sci. 1998, 67, 572–576. [Google Scholar] [CrossRef]
- Zheng, G.; Taira, S.; Yonemori, K.; Sugiura, A. Fruit growth and ripening of Japanese persimmon (Diospyros kaki Thunb.) grown at two locations with different temperature conditions. J. Jpn. Soc. Hortic. Sci. 1990, 59, 471–477. [Google Scholar] [CrossRef]
- Nii, N. Seasonal changes of fruit growth in Japanese persimmon, Diospyros kaki cv. Fuyu, in relation to vascular tissue development in the fruit stalk. J. Jpn. Soc. Hortic. Sci. 1980, 49, 160–170. [Google Scholar] [CrossRef]
- Ohara, H.; Kato, M.; Matsui, H.; Hirata, N.; Takahashi, E. Comparison of the level of endogenous plant growth substances in CPPU-treated and –untreated Kiwifruit. J. Jpn. Soc. Hortic. Sci. 1997, 65, 693–705. [Google Scholar] [CrossRef]
- Tukey, H.B. Growth of the peach embryo in relation to growth of fruit and season of ripening. Proc. Amer. Soc. Hort. Sci. 1933, 30, 209–218. [Google Scholar]
- Shimura, I.; Kobayashi, M.; Ishikawa, S. Characteristics of fruit growth and development in highbush and rabbiteye blueberries (Vaccinium corymbosum L. and V. ashei Reade) and the differences among their cultivars. J. Jpn. Soc. Hortic. Sci. 1986, 55, 46–50. [Google Scholar] [CrossRef]
- Darrow, G.M. Seed number in blueberry fruits. Proc. Amer. Soc. Hort. Sci. 1958, 72, 212–215. [Google Scholar]
- Lang, G.A.; Danka, R.G. Honey-bee-mediated cross-versus self pollination of ‘Sharpblue’ blueberry increase fruit size and hastens ripening. J. Amer. Soc. Hort. Sci. 1991, 116, 770–773. [Google Scholar]
- Suzuki, A.; Shimizu, T.; Aoba, K. Effects of leaf/fruit ratio and pollen density on highbush blueberry fruit quality and maturation. J. Jpn. Soc. Hortic. Sci. 1998, 67, 739–743. [Google Scholar] [CrossRef]
- Suzuki, A.; Kawata, N. Relationship between Anthesis and harvest date in Highbush Blueberry. J. Jpn. Soc. Hortic. Sci. 2001, 70, 60–62. [Google Scholar] [CrossRef]
- Iwasaki, N.; Ono, T.; Sasame, K. Effects of self or cross pollination and seed number per berry on berry maturation in highbush blueberry. Hortic. Res. 2006, 5, 153–156. [Google Scholar] [CrossRef]
- Miller, S.; Alspach, P.; Scalzo, J.; Meekings, J. Pollination of ‘Hortblue Petite’ Blueberry: Evidence of metaxenia in a new ornamental home-garden cultivar. HortScience 2011, 46, 1468–1471. [Google Scholar]
- Denney, O.J. Xenia includes metaxenia. HortScience 1992, 27, 722–728. [Google Scholar]
- Ehlenfeldt, M.K. Self- and cross-fertility in recently released highbush blueberry cultivars. HortScience 2001, 36, 133–135. [Google Scholar]
- Ehlenfeldt, M.K. Investigations of metaxenia in northern highbush blueberry (Vaccinium corymbosum L.) cultivars. J. Amer. Pomol. Soc. 2003, 57, 26–31. [Google Scholar]
- Gupton, C.L. Evidence of xenia in blueberry. Acta Hortic. 1997, 446, 119–123. [Google Scholar] [CrossRef]
- Tukey, H.B. Development of cherry and peach fruits as affected by destruction of the embryo. Bot. Gaz. 1936, 98, 1–24. [Google Scholar] [CrossRef]
- Goto, R.; Watanabe, M.; Murakami, M.; Sagawa, S.; Komori, S.; Suzuki, A. The changesin IAA and ABA concentrations and ethylen evolution during fruit development in blueberry. Hortic. Res. 2013, 12, 165–171. [Google Scholar] [CrossRef]
- Kitajima, A.; Ohshita, Y.; Nakano, M.; Ishida, M. Comparisons of fruit set and quality between seeded and parthenocarpic kaki fruits cv. Fuyu in relation to the location of the fruiting shoot. J. Jpn. Soc. Hortic. Sci. 1993, 62, 505–512. [Google Scholar] [CrossRef]
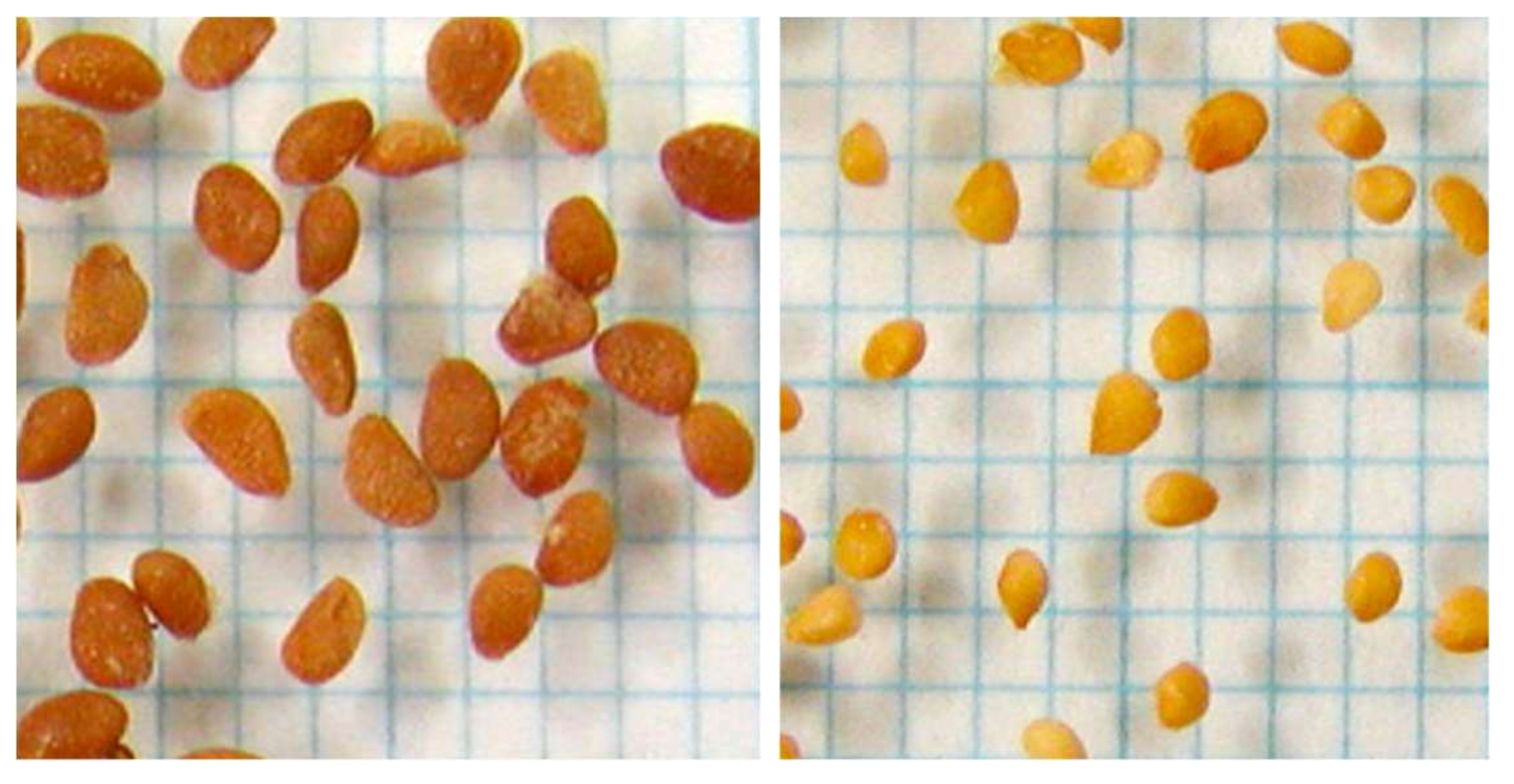
| Seed Parent | Pollen Source | Number of Brownish Seeds (no.) | Days to Ripening from Flowering | Berry Weight (g) | Brix (%) |
|---|---|---|---|---|---|
| 2013 | |||||
| Collins | Open (27) z | 5.3 ± 0.83 b y | 55.2 ± 0.85 a | 1.2 ± 0.05 b | 13.5 ± 0.23 a |
| Bluejay (43) | 19.2 ± 1.99 a | 54.5 ± 0.68 a | 2.1 ± 0.07 a | 13.8 ± 0.18 a | |
| Herbert (45) | 13.8 ± 0.19 b | 55.9 ± 0.69 a | 1.8 ± 0.07 b | 13.8 ± 0.19 a | |
| Bluejay | Open (51) | 12.1 ± 1.79 ab | 60.6 ± 0.87 ab | 1.2 ± 0.05 b | 12.6 ± 0.18 a |
| Bluejay (13) | 8.0 ± 1.21 b | 64.7 ± 2.02 a | 1.1 ± 0.08 b | 12.4 ± 0.30 a | |
| Herbert (22) | 21.2 ± 4.97 a | 58.0 ± 1.09 b | 1.5 ± 0.13 a | 12.4 ± 0.44 a | |
| Darrow | Open (26) | 5.7 ± 1.37 b | 75.5 ± 1.22 a | 1.9 ± 0.12 b | 10.6 ± 0.27 b |
| Bluejay (57) | 13.4 ± 1.21 a | 71.2 ± 0.67 b | 2.3 ± 0.08 a | 11.4 ± 0.13 a | |
| Herbert (41) | 10.0 ± 1.18 ab | 73.3 ± 0.99 ab | 2.1 ± 0.09 ab | 11.1 ± 0.13 ab | |
| 2015 | |||||
| Herbert | Herbert (43) | 2.2 ± 0.18 c | 68.5 ± 0.85 a | 0.94 ± 0.04 c | - |
| Bluejay (75) | 27.4 ± 2.26 a | 59.3 ± 0.67 b | 1.39 ± 0.05 a | - | |
| Darrow (81) | 13.6 ± 1.03 b | 61.4 ± 0.77 b | 1.21 ± 0.05 b | - | |
| Open (87) | 7.8 ± 0.63 c | 60.3 ± 0.61 b | 0.98 ± 0.07 c | - |
| df | Mean Square | F-value | Probability | |
|---|---|---|---|---|
| Collins | ||||
| Pollen source: A | 2 | 0.0690 | 0.6041 | 0.5483 |
| Ln (NSB): B | 1 | 10.0185 | 87.7344 | 6.3 × 10 −16 ** |
| A × B | 2 | 0.0289 | 0.2528 | 0.7770 |
| Residuals | 118 | 0.1142 | ||
| Bluejay | ||||
| Pollen source: A | 2 | 0.2557 | 4.1672 | 0.0191 |
| Ln (NSB): B | 1 | 2.4738 | 40.3156 | 1.4 × 10 −8 ** |
| A × B | 2 | 0.5424 | 8.8400 | 0.0004 ** |
| Residuals | 80 | 0.0614 | ||
| Darrow | ||||
| Pollen source: A | 2 | 0.1823 | 1.0165 | 0.3650 |
| Ln (NSB): B | 1 | 20.8407 | 116.2287 | 2.8 × 10 −19 ** |
| A × B | 2 | 0.1094 | 0.6104 | 0.5448 |
| Residuals | 118 | 0.1793 |
| df | Mean Square | F-value | Probability | |
|---|---|---|---|---|
| Collins | ||||
| Pollen source | 2 | 0.0541 | 0.4797 | 0.6201 |
| Ln (NSB) | 1 | 11.9638 | 106.0910 | 3.3 × 10 −18 ** |
| Residuals | 120 | 0.1128 | ||
| Darrow | ||||
| Pollen source | 2 | 3.3654 | 0.1652 | 0.8479 |
| Ln (NSB) | 1 | 1647.0494 | 80.8417 | 4.3 × 10 −15 ** |
| Residuals | 120 | 20.3738 |
| df | Mean Square | F-value | Probability | |
|---|---|---|---|---|
| With self-pollination | ||||
| Pollen source: A | 3 | 0.7520 | 8.7712 | 0.00001 ** |
| Ln (NSB): B | 1 | 8.4055 | 98.0405 | 5.9 × 10 −20 ** |
| A × B | 3 | 0.2666 | 3.1099 | 0.0269 * |
| Residuals | 274 | 0.0857 | ||
| Without self-pollination | ||||
| Pollen source: A | 2 | 0.0611 | 0.7011 | 0.4971 |
| Ln (NSB): B | 1 | 17.5101 | 200.8371 | 2.7 × 10 −33 ** |
| A × B | 2 | 0.1194 | 1.3691 | 0.2564 |
| Residuals | 233 | 0.0872 |
| df | Mean Square | F-value | Probability | |
|---|---|---|---|---|
| Pollen source | 2 | 0.0396 | 0.4527 | 0.6365 |
| Ln (NSB) | 1 | 17.4503 | 199.5241 | 3.3 × 10 −33 ** |
| Residuals | 235 | 0.0875 |
| df | Mean Square | F-value | Probability | |
|---|---|---|---|---|
| Collins | ||||
| Pollen source (A) | 2 | 0.6444 | 0.5031 | 0.6160 |
| NSB (B) | 1 | 45.1936 | 35.2829 | 4.9 ×10−5 ** |
| A × B | 2 | 0.8747 | 0.6829 | 0.5224 |
| Residuals | 13 | 1.2809 | ||
| Bluejay | ||||
| Pollen source (A) | 2 | 5.5544 | 1.4549 | 0.2718 |
| NSB (B) | 1 | 58.6199 | 15.3541 | 0.0020 ** |
| A × B | 2 | 14.5041 | 3.7990 | 0.0527 |
| Residuals | 12 | 3.8179 | ||
| Darrow | ||||
| Pollen source (A) | 2 | 1.8761 | 0.3661 | 0.6982 |
| NSB (B) | 1 | 19.7509 | 3.8545 | 0.0644 |
| A × B | 2 | 2.1233 | 0.4144 | 0.6666 |
| Residuals | 19 | 5.1241 |
| df | Mean Square | F-value | Probability | |
|---|---|---|---|---|
| Collins | ||||
| Pollen source | 2 | 0.1695 | 0.1382 | 0.8720 |
| NSB | 1 | 71.1657 | 58.0123 | 1.6 ×10−6 ** |
| Residuals | 15 | 1.2267 | ||
| Bluejay | ||||
| Pollen source | 2 | 9.9801 | 1.8674 | 0.1910 |
| NSB | 1 | 75.6774 | 14.1599 | 0.0021 ** |
| Residuals | 14 | 5.3445 | ||
| Darrow | ||||
| Pollen source | 2 | 0.0754 | 0.0156 | 0.9846 |
| NSB | 1 | 35.2276 | 7.2810 | 0.0135 * |
| Residuals | 21 | 4.8383 |
| df | Mean square | F-value | Probability | |
|---|---|---|---|---|
| Pollen source: A | 3 | 20.0138 | 0.2810 | |
| NSB: B | 1 | 87.8476 | 6.1172 | 0.0250 * |
| A × B | 3 | 36.0401 | 2.5096 | 0.0957 |
| Residuals | 16 | 14.3606 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doi, K.; Nozaki, R.; Takahashi, K.; Iwasaki, N. Effects of the Number of Seeds per Berry on Fruit Growth Characteristics, Especially on the Duration of Stage II in Blueberry. Plants 2018, 7, 96. https://doi.org/10.3390/plants7040096
Doi K, Nozaki R, Takahashi K, Iwasaki N. Effects of the Number of Seeds per Berry on Fruit Growth Characteristics, Especially on the Duration of Stage II in Blueberry. Plants. 2018; 7(4):96. https://doi.org/10.3390/plants7040096
Chicago/Turabian StyleDoi, Kenichi, Ryouichi Nozaki, Kouji Takahashi, and Naoto Iwasaki. 2018. "Effects of the Number of Seeds per Berry on Fruit Growth Characteristics, Especially on the Duration of Stage II in Blueberry" Plants 7, no. 4: 96. https://doi.org/10.3390/plants7040096
APA StyleDoi, K., Nozaki, R., Takahashi, K., & Iwasaki, N. (2018). Effects of the Number of Seeds per Berry on Fruit Growth Characteristics, Especially on the Duration of Stage II in Blueberry. Plants, 7(4), 96. https://doi.org/10.3390/plants7040096




