Elemental Profiles in Cycas micronesica Stems
Abstract
1. Introduction
2. Materials and Methods
3. Results
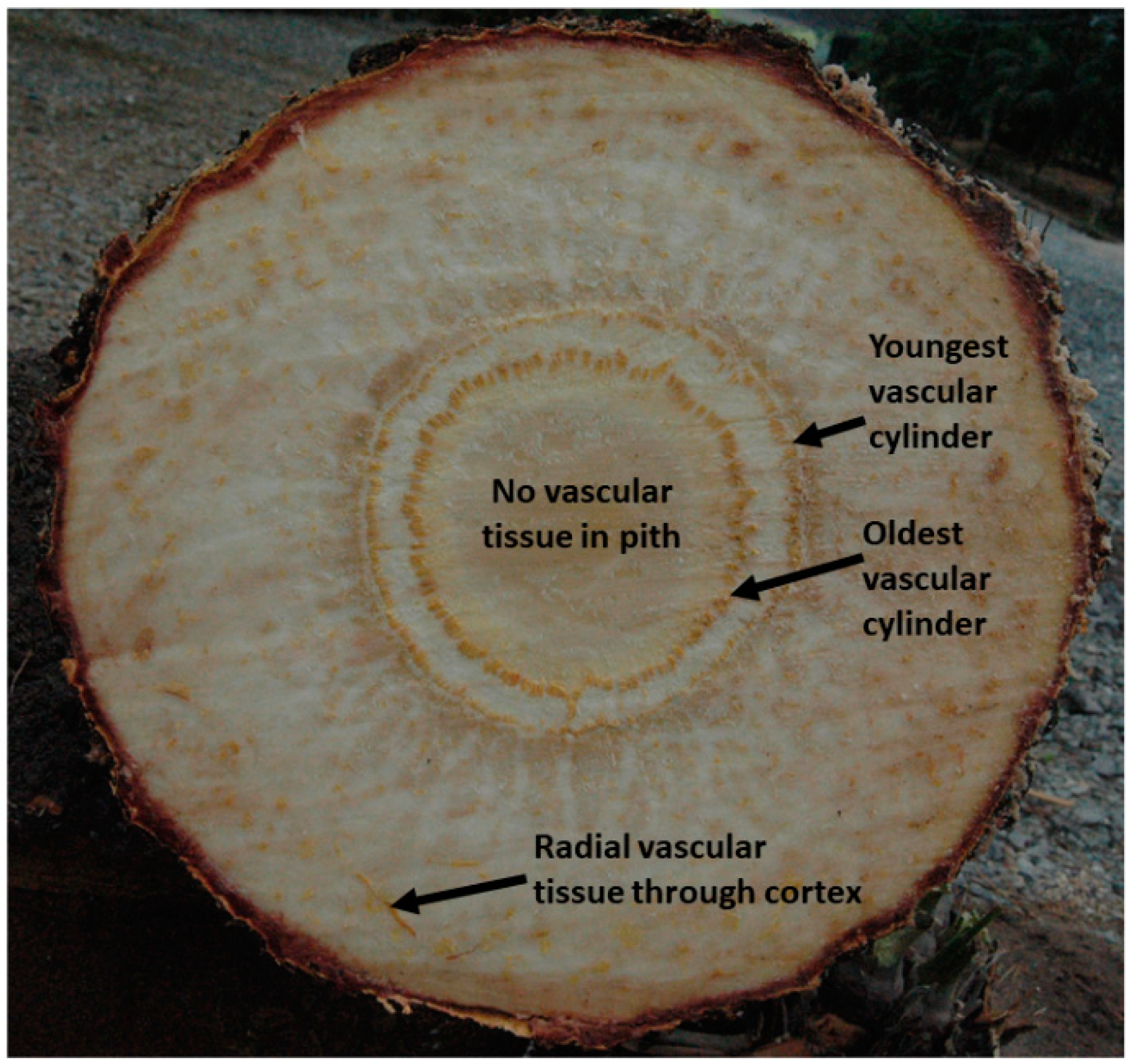
3.1. Radial Differences in Resource Allocation
3.2. Interactions among Axial and Radial Stem Sections
3.3. Axial Differences in Element Concentrations
3.4. Unaffected Elements
3.5. Leaf Tissue
3.6. Derived Traits
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Aerts, R.; Chapin, F.S., III. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv. Ecol. Res. 2000, 30, 1–67. [Google Scholar]
- Lambers, H.; Chapin, F.S., III; Pons, T.L. Plant Physiological Ecology; Springer: Berlin, Germany, 1998; ISBN 9780387783406. [Google Scholar]
- Schlesinger, W.H. Biogeochemistry. An Analysis of Global Change; Academic Press: San Diego, CA, USA, 1997; ISBN 9780126251555. [Google Scholar]
- Salt, D.E.; Baxter, I.; Lahner, B. Ionomics and the study of the plant ionome. Annu. Rev. Plant Biol. 2008, 59, 709–733. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Brown, P.H. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, K.; Broadley, M.R.; El-Serehy, H.A.; George, T.S.; McNicol, J.W.; Moraes, M.F.; White, P.J. Variation in the angiosperm ionome. Physiol. Plant. 2018, 163, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Kerkhoff, A.J.; Fagan, W.F.; Elser, J.J.; Enquist, B.J. Phylogenetic and growth form variation in the scaling of nitrogen and phosphorus in the seed plants. Am. Nat. 2006, 168, E103–E122. [Google Scholar] [CrossRef] [PubMed]
- Minden, V.; Kleyer, M. Internal and external regulation of plant organ stoichiometry. Plant Biol. 2014, 16, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Fortunel, C.; Fine, P.V.; Baraloto, C. Leaf, stem and root tissue strategies across 758 Neotropical tree species. Funct. Ecol. 2012, 26, 1153–1161. [Google Scholar] [CrossRef]
- Yan, Z.; Li, P.; Chen, Y.; Han, W.; Fang, J. Nutrient allocation strategies of woody plants: An approach from the scaling of nitrogen and phosphorus between twig stems and leaves. Sci. Rep. 2016, 6, 20099. [Google Scholar] [CrossRef] [PubMed]
- Meerts, P. Mineral nutrient concentrations in sapwood and heartwood: A literature review. Ann. For. Sci. 2002, 59, 713–722. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Kerkhoff, A.J.; Swenson, N.G.; Enquist, B.J. Biological stoichiometry of plant production: Metabolism, scaling and ecological response to global change. New Phytol. 2010, 186, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Heineman, K.D.; Turner, B.L.; Dalling, J.W. Variation in wood nutrients along a tropical soil fertility gradient. New Phytol. 2016, 211, 440–454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiong, G.; Li, J.; Lu, Z.; Li, Y.; Xu, W.; Wang, Y.; Zhao, C.; Tang, Z.; Xie, Z. Nitrogen and phosphorus concentrations and allocation strategies among shrub organs: The effects of plant growth forms and nitrogen-fixation types. Plant Soil 2018, 427, 305–319. [Google Scholar] [CrossRef]
- Calonje, M.; Stevenson, D.W.; Stanberg, L. The World List of Cycads. 2013–2018. Available online: http://www.cycadlist.org (accessed on 19 May 2018).
- Brummitt, N.A.; Bachman, S.P.; Griffiths-Lee, J.; Lutz, M.; Moat, J.F.; Farjon, A.; Donaldson, J.S.; Hilton-Taylor, C.; Meagher, T.R.; Albuquerque, S.; et al. Green Plants in the Red: A Baseline Global Assessment for the IUCN Sampled Red List Index for Plants. PLoS ONE 2015, 10, e0135152. [Google Scholar] [CrossRef] [PubMed]
- Fragniere, Y.; Bétrisey, S.; Cardinaux, L.; Stoffel, M.; Kozlowski, G. Fighting their last stand? A global analysis of the distribution and conservation status of gymnosperms. J. Biogeogr. 2015, 42, 809–820. [Google Scholar] [CrossRef]
- Norstog, K.J.; Nicholls, T.J. The Biology of the Cycads; Cornell University Press: Ithaca, NY, USA, 1997; ISBN 978-0-8014-3033-6. [Google Scholar]
- Mankga, L.T.; Yessoufou, K. Factors driving the global decline of cycad diversity. AoB Plants 2017, 9, plx022. [Google Scholar] [CrossRef] [PubMed]
- Yessoufou, K.; Daru, B.H.; Tafirei, R.; Elansary, H.O.; Rampedi, I. Integrating biogeography, threat and evolutionary data to explore extinction crisis in the taxonomic group of cycads. Ecol. Evol. 2017, 7, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Escalona, H.E.; Li, L.; Yin, Z.; Huang, D.; Engel, M.S. Beetle pollination of cycads in the Mesozoic. Curr. Biol. 2018, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.D. The Cycas rumphii complex (Cycadaceae) in New Guinea and the Western Pacific. Aust. Syst. Bot. 1994, 7, 543–567. [Google Scholar] [CrossRef]
- Marler, T.; Haynes, J.; Lindström, A. 2010 Cycas micronesica. In IUCN 2012. IUCN Red List of Threatened Species. Version 2012.1. Available online: www.iucnredlist.org (accessed on 22 August 2018).
- United States Fish & Wildlife Service [UFWS]. Endangered and threatened wildlife and plants; endangered status for 16 species and threatened status for 7 species in Micronesia. Fed. Regist. 2015, 80, 59424–59497. [Google Scholar]
- Hou, X.; Jones, B.T. Inductively coupled plasma/optical emission spectrometry. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons: Chichester, UK, 2000; pp. 9468–9485. [Google Scholar]
- Marler, T.E. Axial and radial spatial patterns of non-structural carbohydrates in Cycas micronesica stems. Plants 2018, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E.; Lindström, A.; Fisher, J.B. Stem tissue dimensions correlate with ease of horticultural management for six Cycas species. HortScience 2010, 45, 1293–1296. [Google Scholar]
- Fisher, J.B.; Lindström, A.; Marler, T. Tissue responses and solution movement after stem wounding in six Cycas species. HortScience 2009, 44, 848–851. [Google Scholar]
- Niklas, K.J.; Cobb, E.D.; Marler, T. A comparison between the record height-to-stem diameter allometries of pachycaulis and leptocaulis species. Ann. Bot. 2006, 97, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Niklas, K.J.; Marler, T.E. Sex and population differences in the allometry of an endangered cycad species, Cycas micronesica (Cycadales). Int. J. Plant Sci. 2008, 169, 659–665. [Google Scholar] [CrossRef]
- Fisher, J.B.; Marler, T.E. Eccentric growth but no compression wood in a horizontal stem of Cycas micronesica (Cycadales). IAWA J. 2006, 27, 377–382. [Google Scholar] [CrossRef]
- Altaner, C.M.; Jarvis, M.C.; Fisher, J.B.; Marler, T.E. Molecular xylem cell wall structure of an inclined Cycas micronesica stem, a tropical gymnosperm. IAWA J. 2010, 31, 3–11. [Google Scholar] [CrossRef]
- Marler, T.E. Stem carbohydrates and adventitious root formation of Cycas micronesica following Aulacaspis yasumatsui infestation. HortScience 2018, 53. [Google Scholar] [CrossRef]
- Marler, T.E. Bi-directional acclimation of Cycas micronesica leaves to abrupt changes in incident light in understory and open habitats. Photosynthetica 2018, 56, 776–785. [Google Scholar] [CrossRef]
- Grove, T.S.; O’Connell, A.M.; Malajczuk, N. Effects of fire on the growth, nutrient content and rate of nitrogen fixation of the cycad Macrozamia riedlei. Aust. J. Bot. 1980, 28, 271–281. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, C.; Wang, X.; Quan, X. Carbon concentration variability of 10 Chinese temperate tree species. For. Ecol. Manag. 2009, 258, 722–727. [Google Scholar] [CrossRef]
- Thomas, S.C.; Martin, A.R. Carbon content of tree tissues: A synthesis. Forests 2012, 3, 332–352. [Google Scholar] [CrossRef]
- Kinerson, R.S.; Ralston, C.W.; Wells, C.G. Carbon cycling in a loblolly pine plantation. Oecologia 1977, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kauppi, P.E.; Tomppo, E.; Ferm, A. C and N storage in living trees within Finland since 1950s. Plant Soil 1995, 168–169, 633–638. [Google Scholar] [CrossRef]
- Elias, M.; Potvin, C. Assessing inter- and intra-specific variation in trunk carbon concentration for 32 neotropical tree species. Can. J. For. Res. 2003, 33, 1039–1045. [Google Scholar] [CrossRef]
- Chave, J.; Riera, B.; Dubois, M.-A. Estimation of biomass in a neotropical forest of French Guina: Spatial and temporal variability. J. Trop. Ecol. 2001, 17, 79–96. [Google Scholar] [CrossRef]
- Ketterings, Q.M.; Coe, R.; van Noordwijk, M.; Ambagau, Y.; Palm, C.A. Reducing uncertainty in the use of allometric biomass equations for predicting above-ground tree biomass in mixed secondary forests. For. Ecol. Manag. 2001, 146, 199–209. [Google Scholar] [CrossRef]
- Nascimento, H.E.M.; Laurance, W.F. Total aboveground biomass in Central Amazonian rainforests: A landscape-scale study. For. Ecol. Manag. 2002, 168, 311–321. [Google Scholar] [CrossRef]
- Pettersen, R.C. The chemical composition of wood. In The Chemistry of Wood. Advances in Chemistry Series 207; Rowel, R.M., Ed.; American Chemical Society: Washington, DC, USA, 1984; pp. 57–126. [Google Scholar]
- Kozlowski, T.T. Carbohydrate sources and sinks in woody plants. Bot. Rev. 1992, 58, 107–222. [Google Scholar] [CrossRef]
- Lamlon, S.H.; Savidge, R.A. A reassessment of carbon content in wood: Variation within and between 41 North American species. Biomass Energy 2003, 25, 381–388. [Google Scholar] [CrossRef]
- Penninckx, V.; Glineur, S.; Gruber, W.; Herbauts, J.; Meerts, P. Radial variations in wood mineral element concentrations: A comparison of beech and pedunculate oak from the Belgian Ardennes. Ann. For. Sci. 2001, 58, 253–260. [Google Scholar] [CrossRef]
| Variable | Pith | Vascular | Cortex | Significance |
|---|---|---|---|---|
| Carbon (mg·g−1) | 379.92 ± 3.44 a 1 | 403.09 ± 2.75 b | 381.18 ± 3.02 a | <0.0001 |
| Phosphorus (mg·g−1) | 1.69 ± 0.28 a | 2.46 ± 0.26 ab | 3.62 ± 0.36 b | 0.0191 |
| Iron (μg·g−1) | 46.70 ± 3.42 a | 81.51 ± 5.95 b | 58.59 ± 5.20 a | <0.0001 |
| Zinc (μg·g−1) | 21.10 ± 5.57 b | 16.24 ± 3.08 ab | 8.07 ± 0.97 a | 0.0414 |
| Arsenic (μg·g−1) | 0.34 ± 0.08 b | 0.14 ± 0.05 a | 0.13 ± 0.04 a | 0.0021 |
| Lead (μg·g−1) | 2.69 ± 0.36 b | 0.56 ± 0.07 a | 4.22 ± 0.40 c | <0.0001 |
| Variable | Apex Pith | Apex Vascular | Apex Cortex | Base Pith | Base Vascular | Base Cortex | Significance |
|---|---|---|---|---|---|---|---|
| Nitrogen (mg·g−1) | 11.33 ± 1.33 a1 | 11.25 ± 0.77 a | 16.62 ± 1.94 b | 10.46 ± 0.93 a | 10.53 ± 0.73 a | 10.21 ± 0.73 a | 0.0332 |
| Potassium (mg·g−1) | 6.54 ± 0.91 a | 16.27 ± 3.90 b | 9.56 ± 2.24 ab | 5.58 ± 1.49 a | 5.26 ± 0.73 a | 8.10 ± 1.61 ab | 0.0329 |
| Magnesium (mg·g−1) | 2.56 ± 0.38 ab | 4.94 ± 0.99 c | 3.50 ± 0.39 bc | 1.67 ± 0.11 a | 0.98 ± 0.06 a | 1.91 ± 0.15 a | 0.0158 |
| Sodium (mg·g−1) | 0.98 ± 0.28 a | 2.34 ± 0.57 b | 1.65 ± 0.31 a | 1.92 ± 0.31 ab | 0.75 ± 0.15 a | 2.30 ± 0.30 b | 0.0088 |
| Boron (μg·g−1) | 9.00 ± 1.46 a | 20.50 ± 3.73 b | 13.37 ± 1.49 a | 7.61 ± 1.64 a | 9.01 ± 0.31 a | 13.14 ± 0.83 a | 0.0080 |
| Cobalt (μg·g−1) | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.05 ± 0.02 b | 0.01 ± 0.00 a | 0.06 ± 0.01 b | 0.04 ± 0.01 b | 0.0245 |
| Chromium (μg·g−1) | 0.25 ± 0.02 a | 0.27 ± 0.03 a | 0.49 ± 0.09 b | 0.25 ± 0.03 a | 0.44 ± 0.03 b | 0.30 ± 0.03 a | 0.0004 |
| Nickel (μg·g−1) | 0.01 ± 0.00 a | 0.08 ± 0.02 b | 0.01 ± 0.00 a | 0.11 ± 0.04 b | 0.09 ± 0.01 b | 0.01 ± 0.00 a | 0.0478 |
| Variable | Apical | Basal | Significance |
|---|---|---|---|
| Manganese (μg·g−1) | 21.96 ± 3.39 | 12.40 ± 1.16 | 0.0071 |
| Copper (μg·g−1) | 1.99 ± 0.14 | 0.92 ± 0.10 | <0.0001 |
| Arsenic (μg·g−1) | 0.37 ± 0.06 | 0.03 ± 0.01 | <0.0001 |
| Element | Concentration | Element | Concentration |
|---|---|---|---|
| Carbon (mg·g−1) | 478.9 ± 18.8 | Copper (μg·g−1) | 4.2 ± 0.2 |
| Nitrogen (mg·g−1) | 25.1 ± 2.5 | Boron (μg·g−1) | 13.6 ± 0.1 |
| Potassium (mg·g−1) | 15.3 ± 0.6 | Arsenic (μg·g−1) | 0.08 ± 0.03 |
| Phosphorus (mg·g−1) | 2.9 ± 0.1 | Cadmium (μg·g−1) | 0.04 ± 0.01 |
| Calcium (mg·g−1) | 2.8 ± 0.1 | Cobalt (μg·g−1) | ND z |
| Magnesium (mg·g−1) | 2.3 ± 0.1 | Chromium (μg·g−1) | 0.28 ± 0.01 |
| Sodium (mg·g−1) | 0.5 ± 0.03 | Nickel (μg·g−1) | 0.04 ± 0.03 |
| Manganese (μg·g−1) | 23.8 ± 2.3 | Lead (μg·g−1) | 0.60 ± 0.04 |
| Iron (μg·g−1) | 43.5 ± 0.6 | Selenium (μg·g−1) | 0.58 ± 0.25 |
| Zinc (μg·g−1) | 19.0 ± 0.9 |
| Variable | Leaf:Stem Ratio | Radial Discrepancy (%) |
|---|---|---|
| Nitrogen | 2.13 | 19.08 |
| Carbon | 1.23 | 5.75 |
| Phosphorus | 1.10 | 53.31 |
| Potassium | 1.79 | 40.35 |
| Calcium | 0.18 | 30.23 |
| Magnesium | 0.88 | 25.35 |
| Sodium | 0.31 | 24.75 |
| Manganese | 1.39 | 33.98 |
| Iron | 0.70 | 42.71 |
| Zinc | 1.26 | 61.76 |
| Copper | 2.87 | 16.13 |
| Boron | 1.12 | 40.00 |
| Arsenic | 0.38 | 61.76 |
| Cadmium | 2.06 | 9.09 |
| Chromium | 0.85 | 37.50 |
| Nickel | 0.80 | 87.50 |
| Lead | 0.24 | 86.73 |
| Selenium | 0.80 | 37.50 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marler, T.E. Elemental Profiles in Cycas micronesica Stems. Plants 2018, 7, 94. https://doi.org/10.3390/plants7040094
Marler TE. Elemental Profiles in Cycas micronesica Stems. Plants. 2018; 7(4):94. https://doi.org/10.3390/plants7040094
Chicago/Turabian StyleMarler, Thomas E. 2018. "Elemental Profiles in Cycas micronesica Stems" Plants 7, no. 4: 94. https://doi.org/10.3390/plants7040094
APA StyleMarler, T. E. (2018). Elemental Profiles in Cycas micronesica Stems. Plants, 7(4), 94. https://doi.org/10.3390/plants7040094





