Should I Eat or Should I Go? Acridid Grasshoppers and Their Novel Host Plants: Potential for Biotic Resistance
Abstract
1. Introduction
2. Results
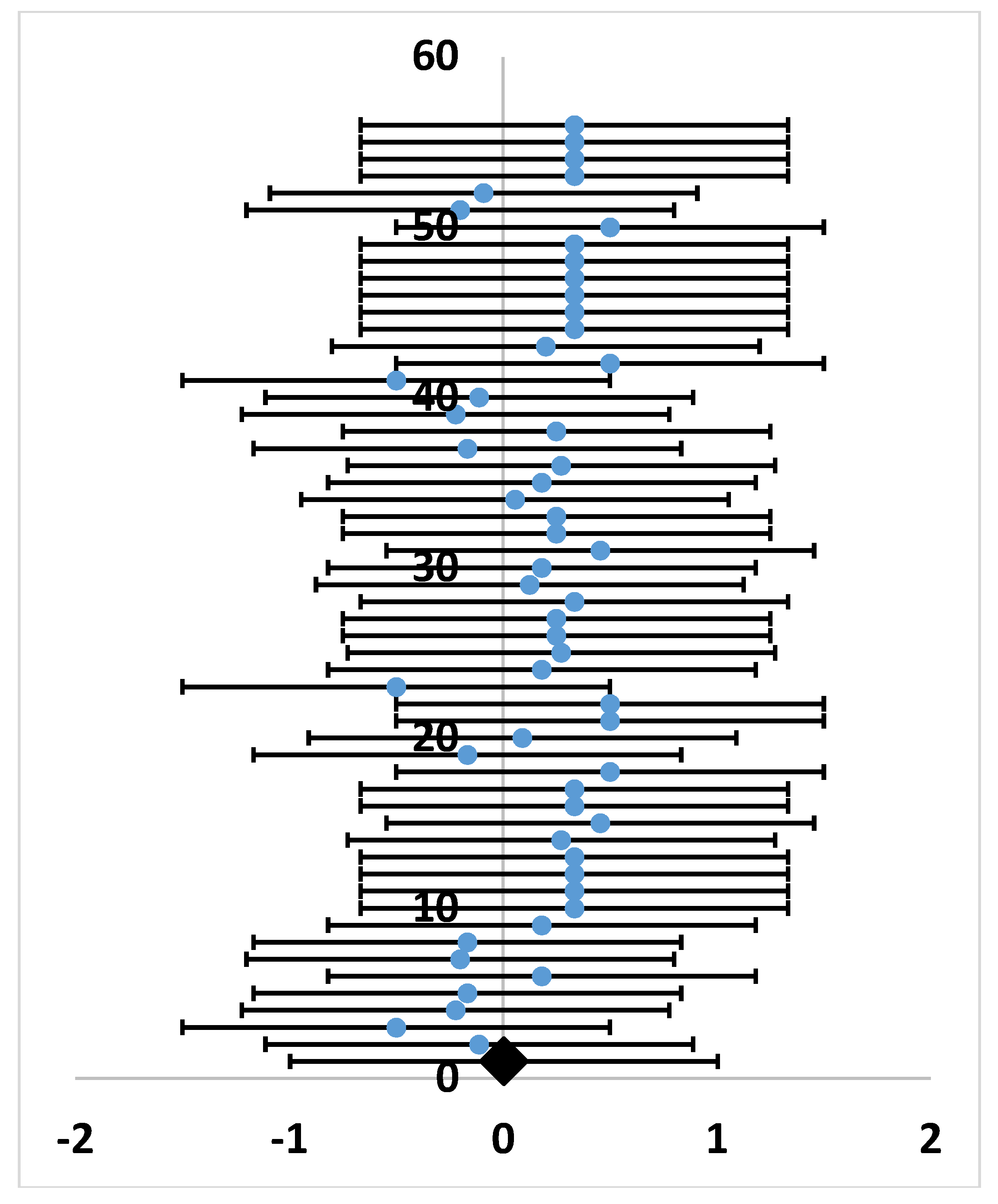
2.1. Studies on Grasshopper Feeding Preferences over the Past 50 Years
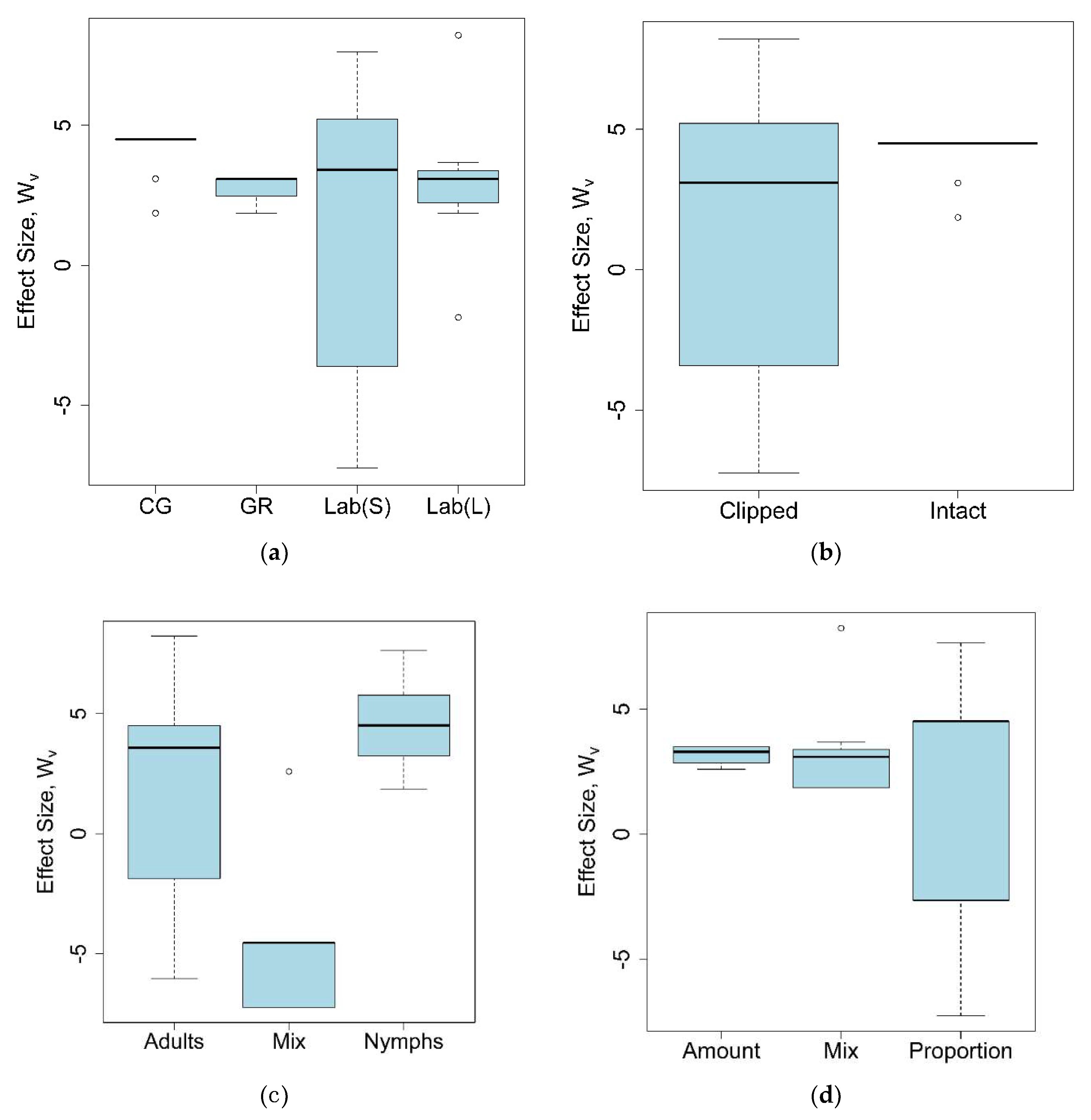
2.2. Grasshopper Feeding Preferences for Native Versus Introduced Plants
2.3. Invasive Potential of Preferred Introduced Host Plants
3. Discussion
3.1. Potential Implications for Biotic Resistance
3.2. Methodological Recommendations
3.2.1. Using a Combination of Choice and No-choice Feeding Trials
3.2.2. Using Grasshopper Activity Measurements in Feeding Trials
3.2.3. Using Standardized Measurements
3.3. Future Directions
3.3.1. Acridid Grasshoppers as a Study Object in Plant Invasion Ecology
3.3.2. Combined Effect of the Plant Origin and Other Factors on Grasshopper Feeding Choice
3.3.3. Time Since Introduction and Plant Resistant Traits
4. Materials and Methods
4.1. Literature Search
4.2. Inclusion Criteria and Data Extraction
- Use at least one plant that is native to North America and one plant that is exotic to North America (could be collected within or outside of North America);
- Report grasshopper preference data for either each plant species or for a group of native plants versus exotic plants (studies reporting grasshopper feeding on plant mixtures versus a single diet, as well as on field sites dominated by a certain plant species, were excluded);
- Report grasshopper preference for plant species growing at the same environmental conditions (studies comparing feeding, for example, at different elevations or temperatures were excluded);
- Report grasshopper preference rather than acceptance of different plants; and
- Use “direct” grasshopper feeding trials on different plant species without previous conditional feeding on a certain plant.
4.3. Data Sources
- JSTOR: https://www.jstor.org/
- ScienceDirect: https://www.sciencedirect.com/
- SpringerLink: https://link.springer.com/
- Web of Science: https://clarivate.com/products/web-of-science/
- IngentaConnect: https://www.ingentaconnect.com/
- Agricola: https://agricola.nal.usda.gov/
- Journal of Orthoptera Research: https://jor.pensoft.net/
- The USDA PLANT database: https://plants.usda.gov/java/
- The Global Biodiversity Information Facility: https://www.gbif.org/
- The NatureServe database: http://www.natureserve.org/
- Center for Invasive Species and Ecosystem Health database: https://www.bugwood.org/
- The Invasive Plant Atlas: https://www.invasiveplantatlas.org/
4.4. Data Synthesis and Analysis
4.4.1. Data Synthesis
4.4.2. Meta-analysis
5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Brockerhoff, E.G.; Garrett, L.J.; Parke, J.L.; Britton, K.O. Live plant imports: the major pathway for forest insect and pathogen invasions of the US. Front. Ecol. Environ. 2012, 10, 135–143. [Google Scholar] [CrossRef]
- Alpert, P. The advantages and disadvantages of being introduced. Biol. Invas. 2006, 8, 1523–1534. [Google Scholar] [CrossRef]
- Pyšek, P.; Jarošík, V.; Pergl, J. Alien plants introduced by different pathways differ in invasion success: unintentional introductions as a threat to natural areas. PLoS ONE 2011, 6, e24890. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D.; McNair, S.; Janecka, J.; Wightman, J.; Simmonds, C.; O’connell, C.; Wong, E.; Russel, L.; Zern, J.; Aquino, T.; et al. Economic and environmental threats of alien plant, animal, and microbe invasions. Agric. Ecosyst. Environ. 2001, 84, 1–20. [Google Scholar] [CrossRef]
- Catford, J.A.; Jansson, R.; Nilsson, C. Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Divers. Distrib. 2009, 15, 22–40. [Google Scholar] [CrossRef]
- Keane, R.M.; Crawley, M.J. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 2002, 17, 164–170. [Google Scholar] [CrossRef]
- Blossey, B.; Notzold, R. Evolution of increased competitive ability in invasive nonindigenous plants: A hypothesis. J. Ecol. 1995, 83, 887–889. [Google Scholar] [CrossRef]
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 436–443. [Google Scholar] [CrossRef]
- Müller-Schärer, H.; Schaffner, U.; Steinger, T. Evolution in invasive plants: implications for biological control. Trends Ecol. Evol. 2004, 19, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Siemann, E.; Rogers, W.E. Reduced resistance of invasive varieties of the alien tree Sapium sebiferum to a generalist herbivore. Oecologia 2003, 135, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Harmoney, K.R.; Hickman, K.R. Comparative morphology of Caucasian old world bluestem and native grasses. Agron. J. 2004, 96, 1540–1544. [Google Scholar] [CrossRef]
- Jogesh, T.; Carpenter, D.; Cappuccino, N. Herbivory on invasive exotic plants and their non-invasive relatives. Biol. Invas. 2008, 10, 797–804. [Google Scholar] [CrossRef]
- Schmidt, C.D.; Hickman, K.R.; Channell, R.; Harmoney, K.; Stark, W. Competitive abilities of native grasses and non-native (Bothriochloa spp.) grasses. Plant Ecol. 2008, 197, 69–80. [Google Scholar] [CrossRef]
- Beaton, L.L.; Van Zandt, P.A.; Esselman, E.J.; Knight, T.M. Comparison of the herbivore defense and competitive ability of ancestral and modern genotypes of an invasive plant, Lespedeza cuneata. Oikos 2011, 120, 1413–1419. [Google Scholar] [CrossRef]
- Elton, C.S. The Ecology of Invasions by Animals and Plants; University of Chicago Press: Chicago, IL, USA, 2000. [Google Scholar]
- Simberloff, D.; Von Holle, B. Positive interactions of nonindigenous species: Invasional meltdown? Biol. Invas. 1999, 1, 21–32. [Google Scholar] [CrossRef]
- Maron, J.L.; Vilà, M. When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos 2001, 95, 361–373. [Google Scholar] [CrossRef]
- Parker, J.D.; Hay, M.E. Biotic resistance to plant invasions? Native herbivores prefer non-native plants. Ecol. Lett. 2005, 8, 959–967. [Google Scholar] [CrossRef]
- Levine, J.M.; Adler, P.B.; Yelenik, S.G. A meta-analysis of biotic resistance to exotic plant invasions. Ecol. Lett. 2004, 7, 975–989. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Kotanen, P.M. Herbivores and the success of exotic plants: a phylogenetically controlled experiment. Ecol. Lett. 2003, 6, 712–715. [Google Scholar] [CrossRef]
- Parker, J.D.; Burkepile, D.E.; Hay, M.E. Opposing effects of native and exotic herbivores on plant invasions. Science 2006, 311, 1459–1461. [Google Scholar] [CrossRef] [PubMed]
- Hull-Sanders, H.M.; Clare, R.; Johnson, R.H.; Meyer, G.A. Evaluation of the evolution of increased competitive ability (EICA) hypothesis: Loss of defense against generalist but not specialist herbivores. J. Chem. Ecol. 2007, 33, 781–799. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Siemann, E.; Rogers, W.E.; DeWalt, S.J. Decreased resistance and increased tolerance to native herbivores of the invasive plant Sapium sebiferum. Ecography 2008, 31, 663–671. [Google Scholar] [CrossRef]
- Fielding, D.J.; Conn, J.S. Feeding preference for and impact on an invasive weed (Crepis tectorum) by a native, generalist insect herbivore, Melanoplus borealis (Orthoptera: Acrididae). Ann. Entomol. Soc. Am. 2011, 104, 1303–1308. [Google Scholar] [CrossRef]
- Fan, S.; Yu, D.; Liu, C. The invasive plant Alternanthera philoxeroides was suppressed more intensively than its native congener by a native generalist: implications for the biotic resistance hypothesis. PLoS ONE 2013, 8, e83619. [Google Scholar] [CrossRef] [PubMed]
- Avanesyan, A.; Culley, T.M. Herbivory of native and exotic North-American prairie grasses by nymph Melanoplus grasshoppers. Plant Ecol. 2015, 216, 451–464. [Google Scholar] [CrossRef]
- Avanesyan, A.; Culley, T.M. Feeding preferences of Melanoplus femurrubrum grasshoppers on native and exotic grasses: behavioral and molecular approaches. Entomol. Exp. Appl. 2015, 157, 152–163. [Google Scholar] [CrossRef]
- Morrison, W.E.; Hay, M.E. Herbivore preference for native vs. exotic plants: Generalist herbivores from multiple continents prefer exotic plants that are evolutionarily naïve. PLoS ONE 2011, 6, e17227. [Google Scholar] [CrossRef] [PubMed]
- Capinera, J.L.; Scott, R.D.; Walker, T.J. Field Guide to Grasshoppers, Crickets, and Katydids of the United States; Cornell University Press: Ithaca, NY, USA, 2004. [Google Scholar]
- Saul, W.C.; Jeschke, J.M. Eco-evolutionary experience in novel species interactions. Ecol. Lett. 2015, 18, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Avanesyan, A.; Culley, T.M. Tolerance of native and exotic prairie grasses to herbivory by Melanoplus grasshoppers: Application of a nondestructive method for estimating plant biomass changes as a response to herbivory. J. Torr. Bot. Soc. 2017, 144, 15–25. [Google Scholar] [CrossRef]
- Cumberland, C.; Jonas, J.L.; Paschke, M.W. Impact of grasshoppers and an invasive grass on establishment and initial growth of restoration plant species. Restoration Ecol. 2017, 25, 385–395. [Google Scholar] [CrossRef]
- Schaffner, U.; Ridenour, W.M.; Wolf, V.C.; Bassett, T.; Müller, C.; Müller-Schärer, H.; Sutherland, S.; Lortie, C.J.; Callaway, R.M. Plant invasions, generalist herbivores, and novel defense weapons. Ecology 2011, 92, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.L.; Joern, A. Dietary selection and nutritional regulation in a common mixed-feeding insect herbivore. Entomol. Exp. Appl. 2013, 148, 20–26. [Google Scholar] [CrossRef]
- Lankau, R.A.; Rogers, W.E.; Siemann, E. Constraints on the utilisation of the invasive Chinese tallow tree Sapium sebiferum by generalist native herbivores in coastal prairies. Ecol. Entomol. 2004, 29, 66–75. [Google Scholar] [CrossRef]
- Whipple, S.D.; Brust, M.L.; Hoback, W.W.; Farnsworth-Hoback, K.M. The grasshoppers Arphia xanthoptera and Dichromorpha viridis prefer introduced smooth brome over other grasses. Great Plains Res. 2009, 19, 179–186. [Google Scholar]
- Neyeloff, J.L.; Fuchs, S.C.; Moreira, L.B. Meta-analyses and Forest plots using a Microsoft excel spreadsheet: Step-by-step guide focusing on descriptive data analysis. BMC Res. Notes 2012, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Begna, S.H.; Fielding, D.J. Damage potential of grasshoppers (Orthoptera: Acrididae) on early growth stages of small-grains and canola under subarctic conditions. J. Econ. Entomol. 2003, 96, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Joern, A.; Behmer, S.T. Impact of diet quality on demographic attributes in adult grasshoppers and the nitrogen limitation hypothesis. Ecol. Entomol. 1998, 23, 174–184. [Google Scholar] [CrossRef]
- Belovsky, G.E.; Slade, J.B. Grasshoppers affect grassland ecosystem functioning: Spatial and temporal variation. Basic Appl. Ecol. 2018, 26, 24–34. [Google Scholar] [CrossRef]
- Otte, D. Plant preference and plant succession: A consideration of evolution and plant preference in Schistocerca. Oecologia 1975, 18, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Litt, A.R.; Cord, E.E.; Fulbright, T.E.; Schuster, G.L. Effects of invasive plants on arthropods. Conserv. Biol. 2014, 28, 1532–1549. [Google Scholar] [CrossRef] [PubMed]
- Bezemer, T.M.; Harvey, J.A.; Cronin, J.T. Response of native insect communities to invasive plants. Annu. Rev. Entomol. 2014, 59, 119–141. [Google Scholar] [CrossRef] [PubMed]
- Dillemuth, F.P.; Rietschier, E.A.; Cronin, J.T. Patch dynamics of a native grass in relation to the spread of invasive smooth brome (Bromus inermis). Biol. Invas. 2009, 11, 1381–1391. [Google Scholar] [CrossRef]
- Chu, I.W.; Knutson, H. Preferences of eight grasshopper among eleven species of cultivated grasses. J. Kans. Entomol. Soc. 1970, 43, 20–31. [Google Scholar]
- Hewitt, G.B.; Blickenstaff, C.C. Evaluation of methods for screening grasses for resistance to grasshopper feeding. J. Range Manage. 1974, 27, 285–287. [Google Scholar] [CrossRef]
- Branson, D.H.; Sword, G.A. Grasshopper herbivory affects native plant diversity and abundance in a grassland dominated by the exotic grass Agropyron cristatum. Restoration Ecol. 2009, 17, 89–96. [Google Scholar] [CrossRef]
- Jones, C.G.; Coleman, J.S. Leaf disc size and insect feeding preference: implications for assays and studies on induction of plant defense. Entomol. Exp. Appl. 1988, 47, 167–172. [Google Scholar] [CrossRef]
- Peterson, C.H.; Renaud, P.E. Analysis of feeding preference experiments. Oecologia 1989, 80, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Avanesyan, A. Plant DNA detection from grasshopper guts: A step-by-step protocol, from tissue preparation to obtaining plant DNA sequences. Appl. Plant Sci. 2014, 2, 1300082. [Google Scholar] [CrossRef] [PubMed]
- McClenaghan, B.; Gibson, J.F.; Shokralla, S.; Hajibabaei, M. Discrimination of grasshopper (Orthoptera: Acrididae) diet and niche overlap using next-generation sequencing of gut contents. Ecol. Evol. 2015, 5, 3046–3055. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, H.; McNeill, M.R.; Qin, X.; Ma, J.; Tu, X.; Cao, G.; Wang, G.; Nong, X.; Zhang, Z. Quantitative analysis of diet structure by real-time PCR, reveals different feeding patterns by two dominant grasshopper species. Sci. Rep. 2016, 6, 32166. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; McNeill, M.R.; Ma, J.; Qin, X.; Tu, X.; Cao, G.; Wang, G.; Nong, X.; Zhang, Z. Gut transcriptome analysis shows different food utilization efficiency by the grasshopper Oedaleous asiaticus (Orthoptera: Acrididae). J. Econ. Entomol. 2017, 110, 1831–1840. [Google Scholar] [CrossRef] [PubMed]
- Sunny, A.; Diwakar, S.; Sharma, G.P. Native insects and invasive plants encounters. Arthropod-Plant Int. 2015, 9, 323–331. [Google Scholar] [CrossRef]
- Joern, A. Insect herbivory in the transition to California annual grasslands: Did grasshoppers deliver the coup de grass? In Grassland Structure and Function; Springer: Dordrecht, The Netherlands, 1989; pp. 117–134. [Google Scholar]
- Stromberg, M.R.; Corbin, J.D.; D’Antonio, C.M. California grasslands: ecology and management. California grassland restoration. In Ecology and Management of California Grasslands; University of California Press: Berkeley, CA, USA, 2007; pp. 254–280. [Google Scholar]
- Lambdon, P.W.; Hulme, P.E. How strongly do interactions with closely-related native species influence plant invasions? Darwin’s naturalization hypothesis assessed on Mediterranean islands. J. Biogeography 2006, 33, 1116–1125. [Google Scholar] [CrossRef]
- Boys, H.A. Food selection by Oedaleus senegalensis (Acrididae: Orthoptera) in grassland and millet fields. Entomol. Exp. Appl. 1978, 24, 278–286. [Google Scholar] [CrossRef]
- deJonge, R.B.; Bourchier, R.S.; Smith, S.M. Initial response by a native beetle, Chrysochus auratus (Coleoptera: Chrysomelidae), to a novel introduced host-plant, Vincetoxicum rossicum (Gentianales: Apocynaceae). Environ. Entomol. 2017, 46, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Flanders, K.L.; Hawkes, J.G.; Radcliffe, E.B.; Lauer, F.I. Insect resistance in potatoes: Sources, evolutionary relationships, morphological and chemical defenses, and ecogeographical associations. Euphytica 1992, 61, 83–111. [Google Scholar] [CrossRef]
- Stout, J.C.; Tiedeken, E.J. Direct interactions between invasive plants and native pollinators: Evidence, impacts and approaches. Funct. Ecol. 2017, 31, 38–46. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. Royal Stat. Soc. Series B 1995, 57, 289–300. [Google Scholar]
- Hedges, L.V.; Gurevitch, J.; Curtis, P.S. The meta-analysis of response ratios in experimental ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- R Core Team (2013). R: A language and environment for statistical computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: http://www.R-project.org/. (accessed on 4 October 2018).
- Richardson, D.M.; Pyšek, P. Fifty years of invasion ecology–the legacy of Charles Elton. Divers. Distrib. 2008, 14, 161–168. [Google Scholar] [CrossRef]
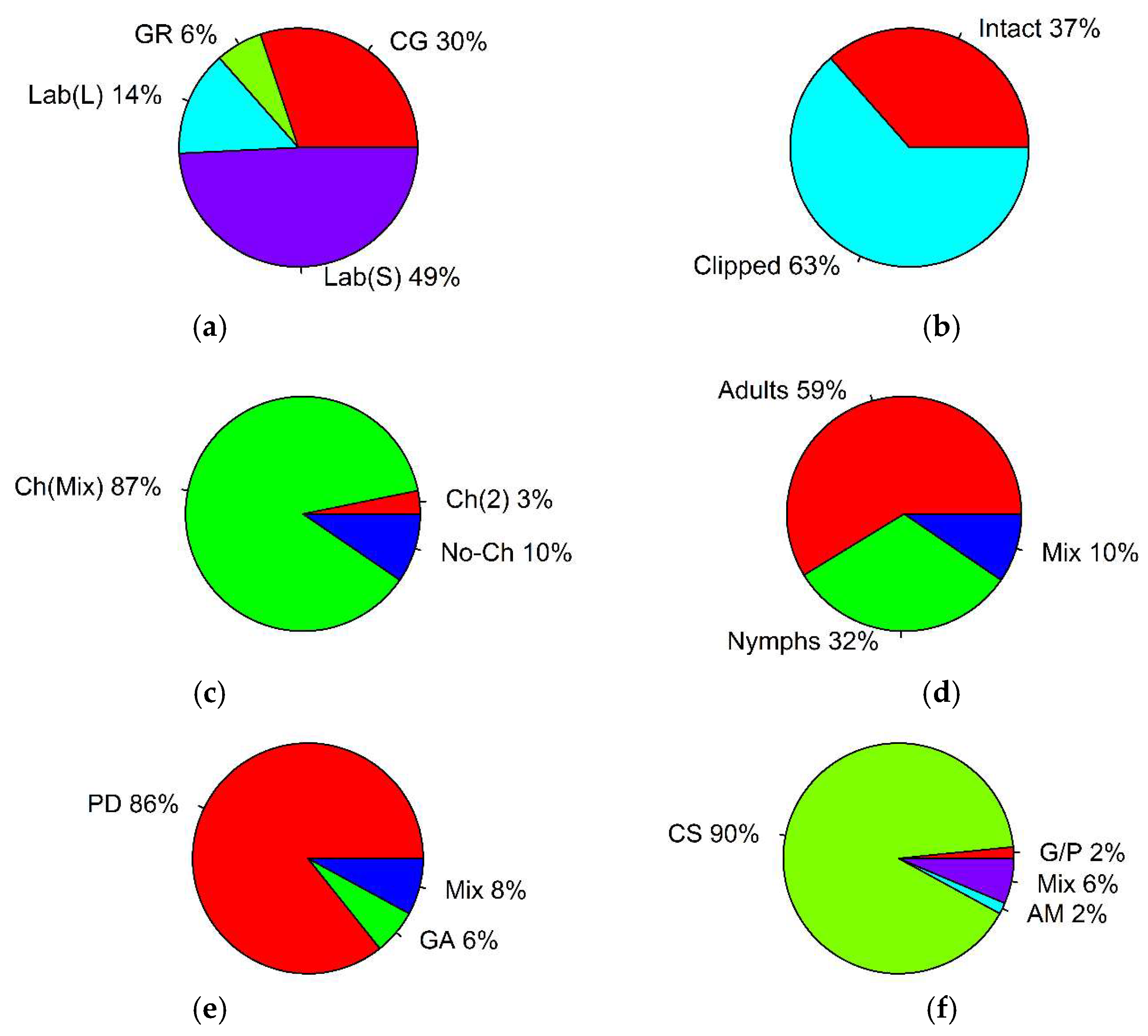
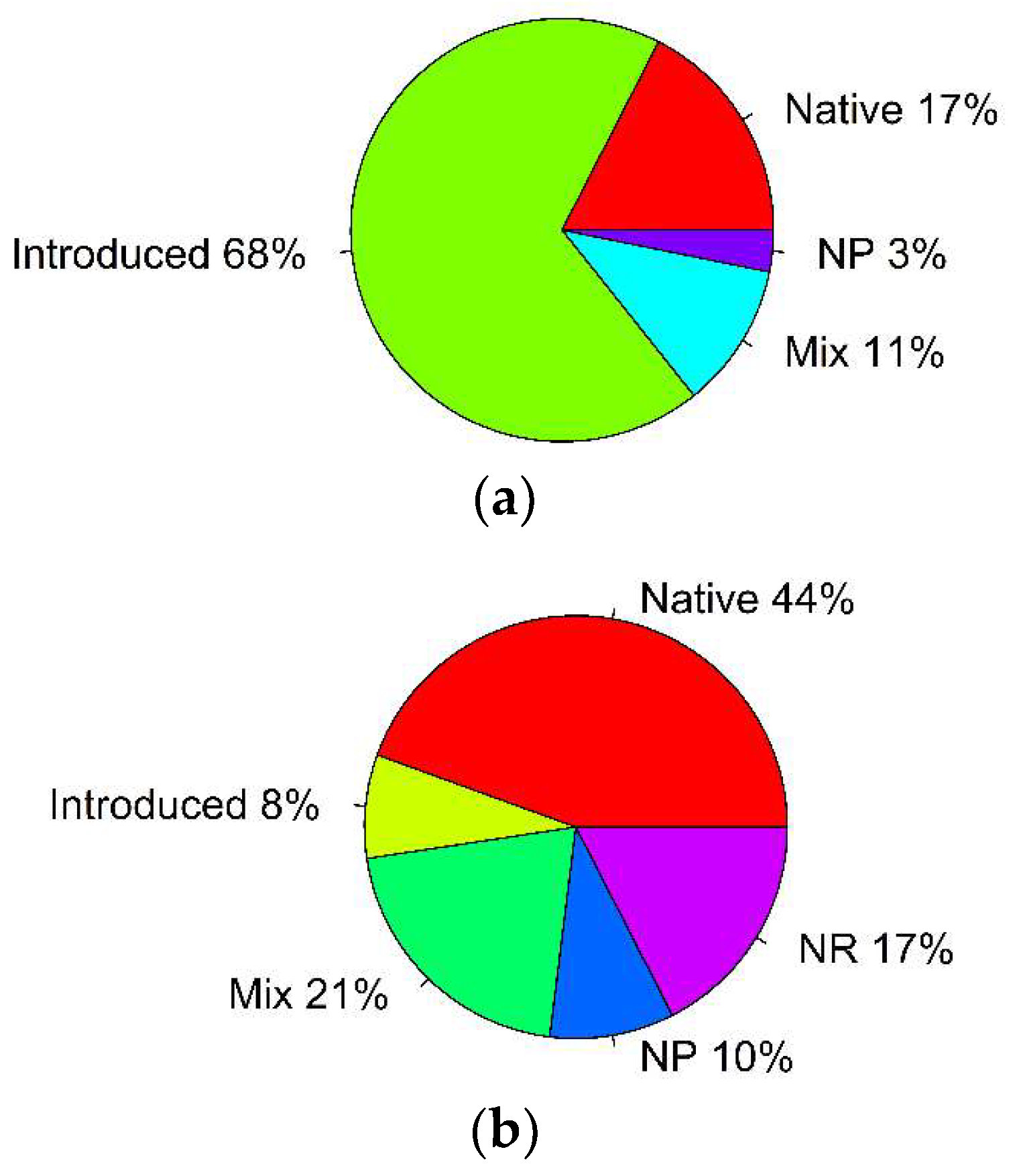
| Parameter | Type | Number of Records | % of Total | p-Value, Binomial Test | (i/m) * Q |
|---|---|---|---|---|---|
| experimental environment | common garden | 19 | 30 | p = 0.382 | 0.0375 |
| greenhouse | 4 | 6 | p < 0.001 * | 0.0125 | |
| laboratory (clipped plant leaves) | 9 | 14 | p = 0.057 | 0.0025 | |
| laboratory (clipped plant stems with leaves) | 31 | 49 | p < 0.001 * | 0.0125 | |
| plant material | intact plants | 23 | 37 | p = 0.04 | 0.025 |
| clipped plants | 40 | 63 | p = 0.04 | 0.025 | |
| preference trial | choice (two plant species) | 2 | 3 | p < 0.001 * | 0.016 |
| choice (plant mixture) | 55 | 87 | p < 0.001 * | 0.016 | |
| no-choice | 6 | 10 | p < 0.001 * | 0.016 | |
| grasshopper life stage | adults | 37 | 59 | p < 0.001 * | 0.016 |
| nymphs | 20 | 32 | p = 0.894 | 0.033 | |
| mixed | 6 | 10 | p < 0.001 * | 0.016 | |
| general preference measurements | plant damage | 54 | 86 | p < 0.001 * | 0.016 |
| grasshopper activity | 4 | 6 | p < 0.001 * | 0.016 | |
| mixed | 5 | 8 | p < 0.001 * | 0.016 | |
| grasshopper activity measurements | growth and performance | 1 | 2 | p < 0.001 * | 0.0125 |
| consumption | 57 | 90 | p < 0.001 * | 0.0125 | |
| assimilation | 1 | 2 | p < 0.001 * | 0.0125 | |
| mixed | 4 | 6 | p < 0.001 * | 0.0125 | |
| grasshopper most preferred host plants | native plants | 11 | 17 | p < 0.001 * | 0.0125 |
| introduced plants | 43 | 68 | p = 0.005 * | 0.025 | |
| mixed | 7 | 11 | p < 0.001 * | 0.0125 | |
| no preferences | 2 | 3 | p < 0.001 * | 0.0125 | |
| grasshopper least preferred host plants | native plants | 28 | 44 | p = 0.45 | 0.025 |
| introduced plants | 5 | 8 | p < 0.001 * | 0.008 | |
| mixed | 13 | 21 | p < 0.001 * | 0.008 | |
| no preferences | 6 | 10 | p < 0.001 * | 0.008 | |
| not reported | 11 | 17 | p < 0.001 * | 0.008 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avanesyan, A. Should I Eat or Should I Go? Acridid Grasshoppers and Their Novel Host Plants: Potential for Biotic Resistance. Plants 2018, 7, 83. https://doi.org/10.3390/plants7040083
Avanesyan A. Should I Eat or Should I Go? Acridid Grasshoppers and Their Novel Host Plants: Potential for Biotic Resistance. Plants. 2018; 7(4):83. https://doi.org/10.3390/plants7040083
Chicago/Turabian StyleAvanesyan, Alina. 2018. "Should I Eat or Should I Go? Acridid Grasshoppers and Their Novel Host Plants: Potential for Biotic Resistance" Plants 7, no. 4: 83. https://doi.org/10.3390/plants7040083
APA StyleAvanesyan, A. (2018). Should I Eat or Should I Go? Acridid Grasshoppers and Their Novel Host Plants: Potential for Biotic Resistance. Plants, 7(4), 83. https://doi.org/10.3390/plants7040083






