Polygonum multiflorum Extract Exerts Antioxidative Effects and Increases Life Span and Stress Resistance in the Model Organism Caenorhabditis elegans via DAF-16 and SIR-2.1
Abstract
1. Introduction
2. Results
2.1. PME Increases Life Span, but Not Resistance AGAINST Thermal Stress
2.2. Antioxidative and Radical-Scavenging Effects of PME
2.3. PME Modulates the Intracellular Localization of DAF-16, but Not SKN-1
2.4. Effects of PME in C. elegans Are Partly Dependent on DAF-16 and SIR-2.1
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bounda, G.A.; Feng, Y.U. Review of clinical studies of Polygonum multiflorum Thunb. and its isolated bioactive compounds. Pharmacogn. Res. 2015, 7, 225–236. [Google Scholar]
- Lin, L.; Ni, B.; Lin, H.; Zhang, M.; Li, X.; Yin, X.; Qu, C.; Ni, J. Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum Thunb.: A review. J. Ethnopharmacol. 2015, 159, 158–183. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Xu, J.W. Biological Activities of 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-Glucoside in Antiaging and Antiaging-Related Disease Treatments. Oxid. Med. Cell. Longev. 2016, 2016, 4973239. [Google Scholar] [CrossRef] [PubMed]
- Büchter, C.; Zhao, L.; Havermann, S.; Honnen, S.; Fritz, G.; Proksch, P.; Wätjen, W. TSG (2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-glucoside) from the Chinese Herb Polygonum multiflorum Increases Life Span and Stress Resistance of Caenorhabditis elegans. Oxid. Med. Cell. Longev. 2015, 2015, 124357. [Google Scholar] [CrossRef] [PubMed]
- Tosato, M.; Zamboni, V.; Ferrini, A.; Cesari, M. The aging process and potential interventions to extend life expectancy. J. Clin. Interv. Aging 2007, 2, 401–412. [Google Scholar]
- Harman, D. The free radical theory of aging. Antioxid. Redox Signal. 2003, 5, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.C.; Cheng, F.C.; Wang, M.F. Beneficial effects of different Polygonum multiflorum Thunb. extracts on memory and hippocampus morphology. J. Nutr. Sci. Vitaminol. 2002, 48, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. An experimental study on the anti-senility effects of shou xing bu zhi. Zhong Xi Yi Jie He Za Zhi 1989, 9, 226–227. [Google Scholar] [PubMed]
- Li, X.; Matsumoto, K.; Murakami, Y.; Tezuka, Y.; Wu, Y.; Kadota, S. Neuroprotective effects of Polygonum multiflorum on nigrostriatal dopaminergic degeneration induced by paraquat and maneb in mice. Pharmacol. Biochem. Behav. 2005, 82, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.F.; Durairajan, S.S.; Lu, J.H.; Koo, I.; Li, M. In vitro screening on amyloid precursor protein modulation of plants used in Ayurvedic and traditional Chinese medicine for memory improvement. J. Ethnopharmacol. 2012, 141, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Um, M.Y.; Choi, W.H.; Aan, J.Y.; Kim, S.R.; Ha, T.Y. Protective effect of Polygonum multiflorum Thunb on amyloid beta-peptide 25-35 induced cognitive deficits in mice. J. Ethnopharmacol. 2006, 104, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Steele, M.L.; Truong, J.; Govindaraghavan, S.; Ooi, L.; Sucher, N.J.; Münch, G. Cytoprotective properties of traditional Chinese medicinal herbal extracts in hydrogen peroxide challenged human U373 astroglia cells. Neurochem. Int. 2013, 62, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.C.; Wang, M.F.; Chang, H.C. Polygonum multiflorum extracts improve cognitive performance in senescence accelerated mice. Am. J. Chin. Med. 2003, 31, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Kim, H.N.; Kim, Y.R.; Choi, Y.W.; Choi, Y.H.; Lee, J.H.; Shin, H.K.; Choi, B.T. Hexane extract from Polygonum multiflorum attenuates glutamate-induced apoptosis in primary cultured cortical neurons. J. Ethnopharmacol. 2013, 145, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.X.; Xue, Y.M.; Hui, T.T.; Wang, W.J.; Zhang, Q.L. Studies on the chemical constituents of the leaves of Polygonum multiflorum. Zhong Yao Cai 2009, 32, 891–893. [Google Scholar] [PubMed]
- Senchuk, M.M.; Dues, D.J.; Van Raamsdonk, J.M. Measuring Oxidative Stress in Caenorhabditis elegans: Paraquat and Juglone Sensitivity. Bio-Protocol 2017, 7, e2086. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Li, S.; Rodriguez-Rocha, H.; Burns, M.; Panayiotidis, M.I. Molecular mechanisms of pesticide-induced neurotoxicity: Relevance to Parkinson’s disease. Chem. Biol. Interact. 2010, 188, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Chompoo, J.; Taira, N. Significant longevity-extending effects of Alpinia zerumbet leaf extract on the life span of Caenorhabditis elegans. Biosci. Biotechnol. Biochem. 2013, 77, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Zarse, K.; Schmeisser, S.; Birringer, M. Differential effects of resveratrol and SRT1720 on lifespan of adult Caenorhabditis elegans. Horm. Metab. Res. 2010, 42, 837–839. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Rezaizadehnajafi, L.; Wink, M. Influence of resveratrol on oxidative stress resistance and life span in Caenorhabditis elegans. J. Pharm. Pharmacol. 2013, 65, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Bass, T.M.; Weinkove, D.; Houthoofd, K. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech. Aging Dev. 2007, 128, 546–552. [Google Scholar] [CrossRef] [PubMed]
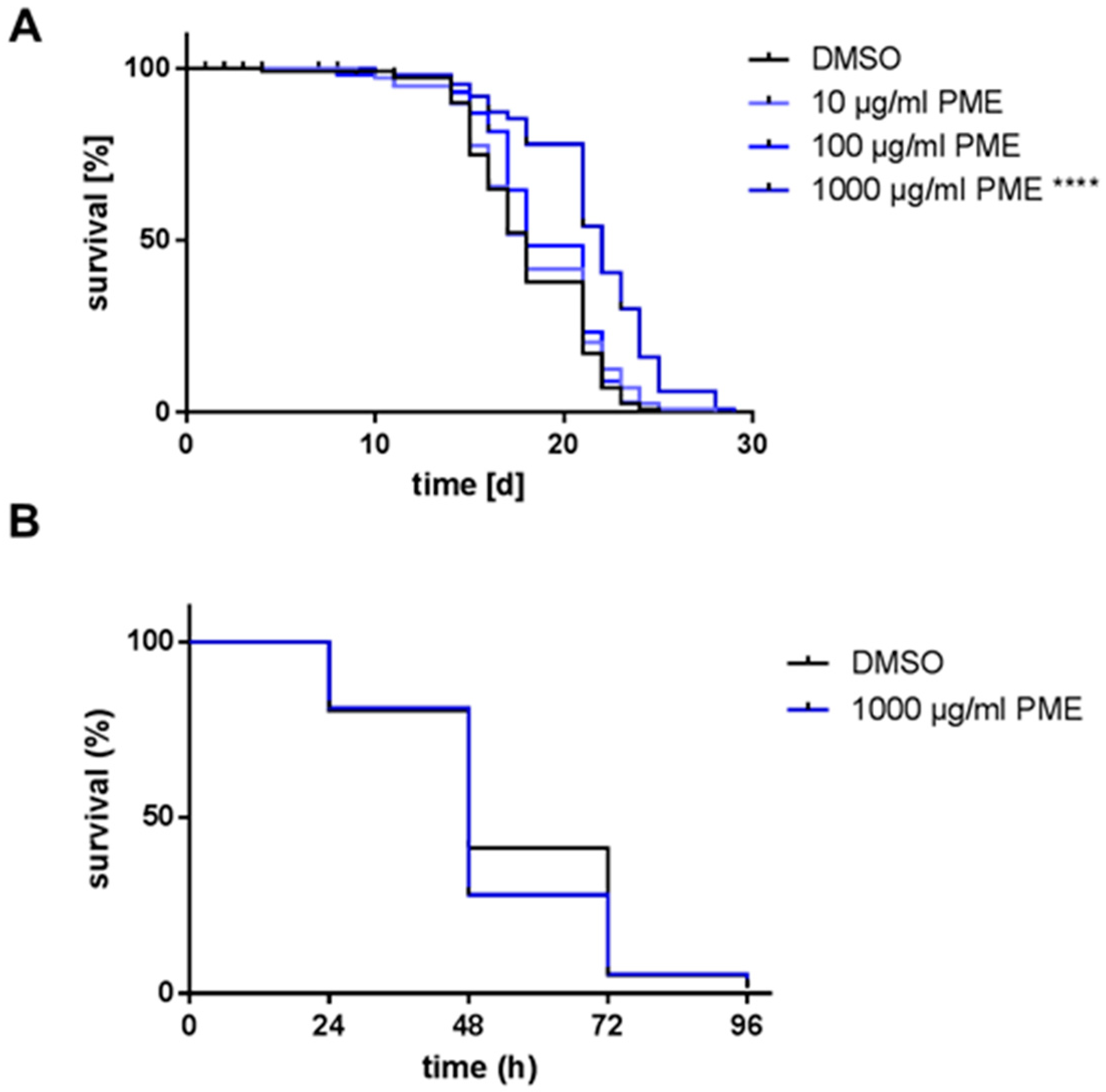
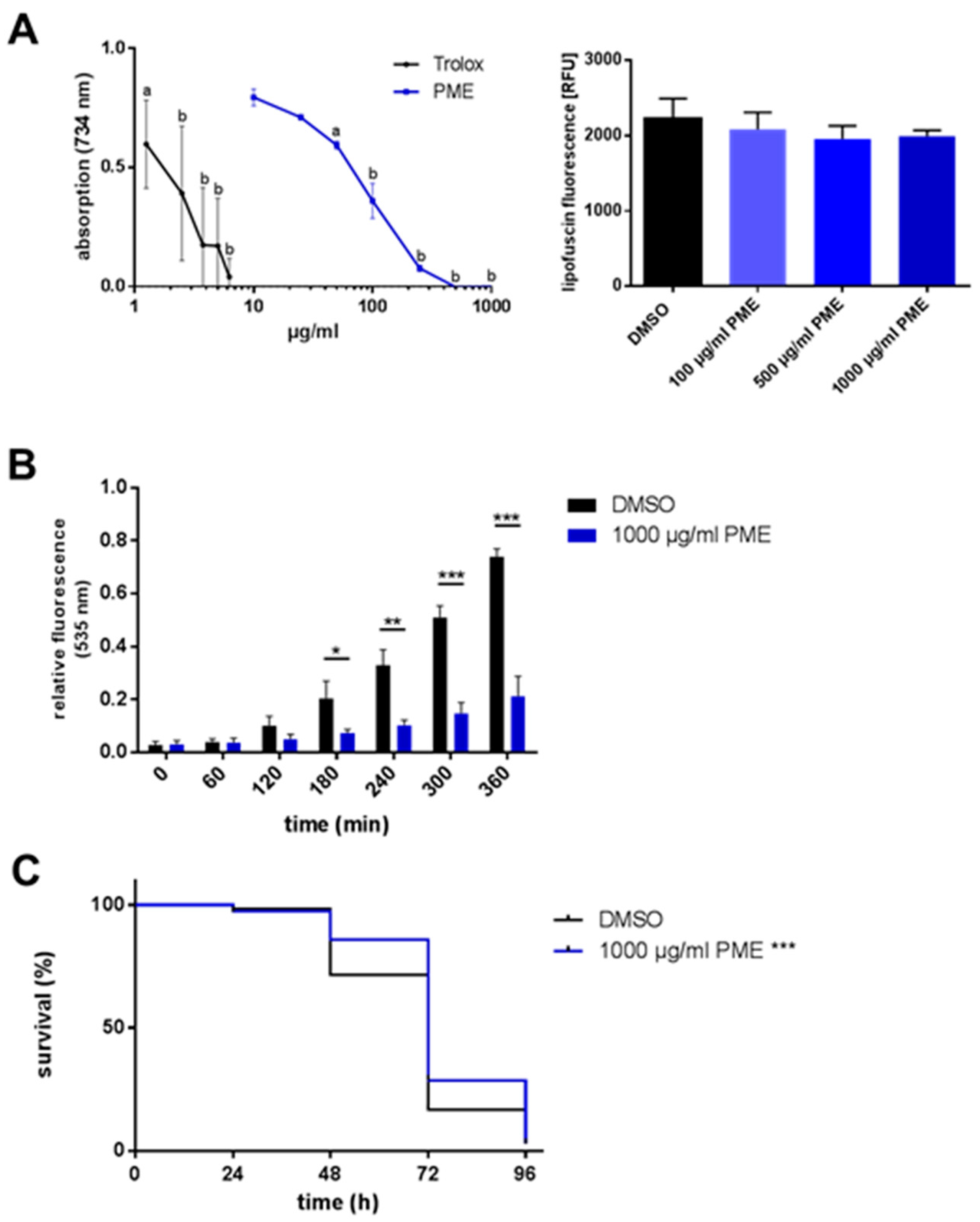
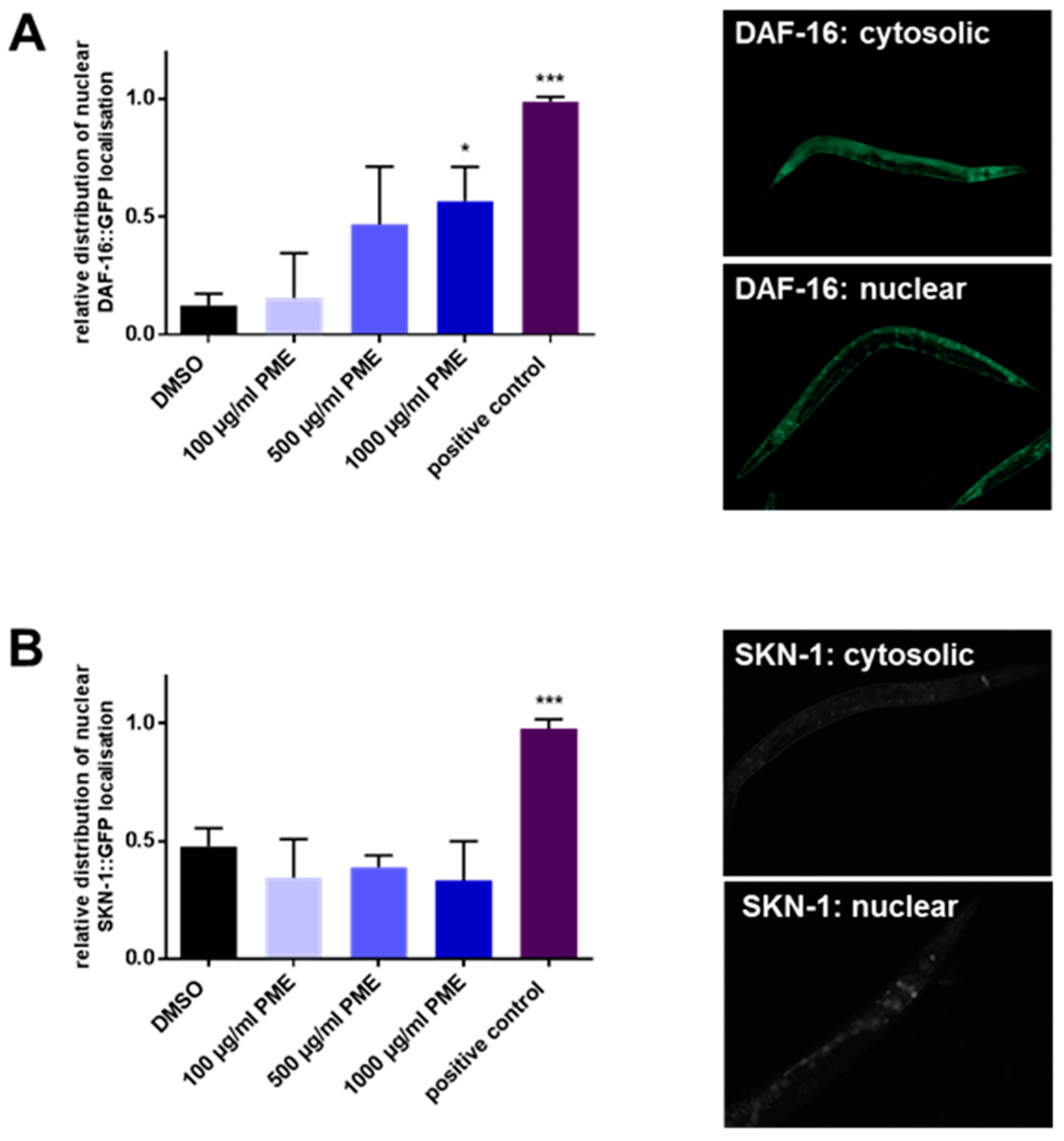
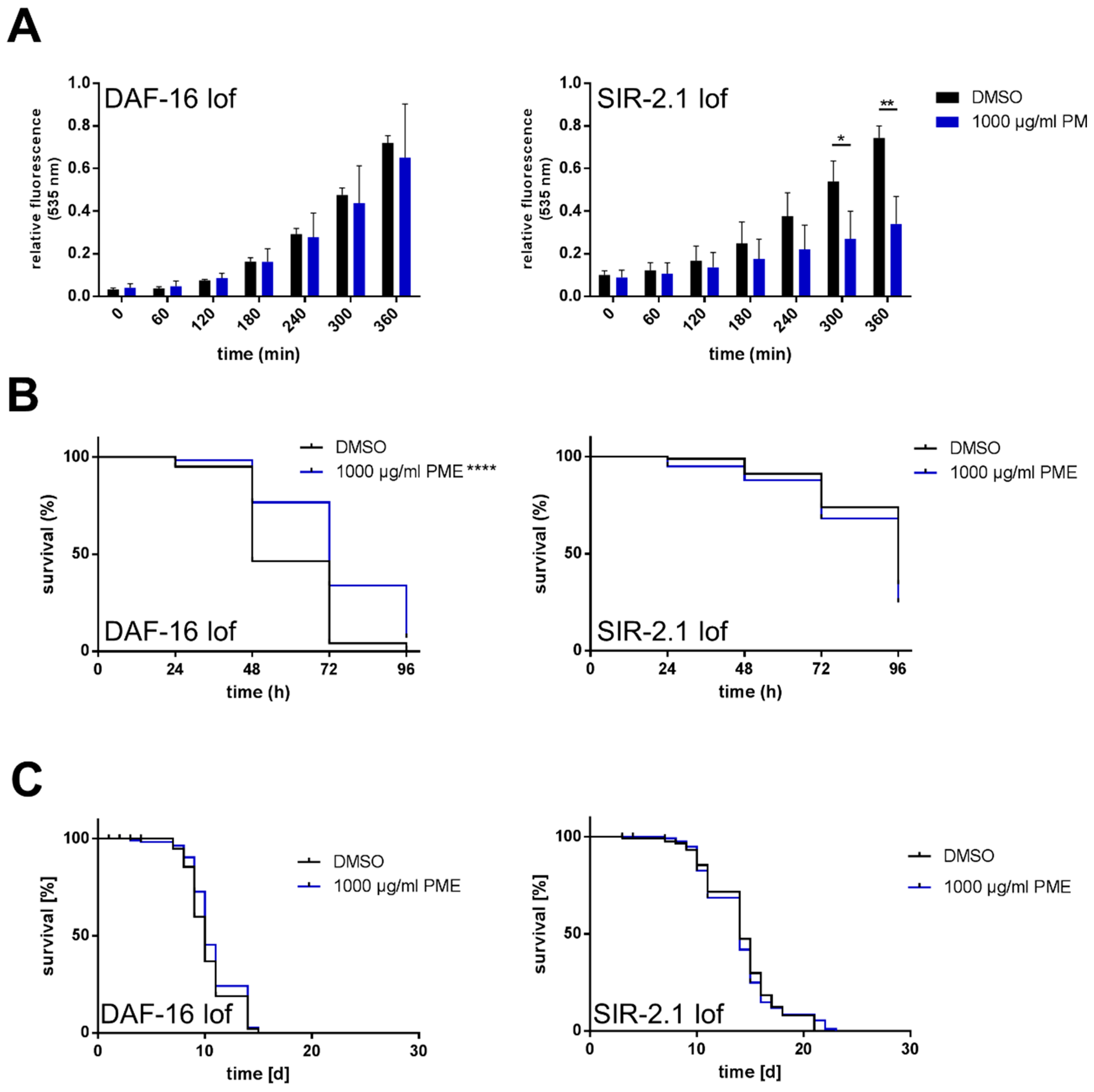
| Genotype | Treatment | Mean [d] ± SD | % Difference to Control | Median [d] ± SD | % Difference to Control | p-Value vs. Control |
|---|---|---|---|---|---|---|
| wild type | DMSO | 18.1 ± 0.319 | 18.0 ± 0.340 | |||
| PME 10 µg/mL | 18.4 ± 0.341 | +1.5 | 18.0 ± 0.387 | ±0 | 0.308 | |
| PME 100 µg/mL | 19.0 ± 0.308 | +4.8 | 18.0 ± 0.457 | ±0 | 0.067 | |
| PME 1000 µg/mL | 21.5 ± 0.348 | +18.6 | 22.0 ± 0.513 | +22.2 | <0.0001 | |
| CF1038 (mu86) [∆daf-16] | DMSO | 10.4 ± 0.199 | 10.0 ± 0.199 | |||
| PME 1000 µg/mL | 10.7 ± 0.214 | +3.7 | 10.0 ± 0.195 | ±0 | 0.073 | |
| VC199 (ok434) [∆sir-2.1] | DMSO | 14.2 ± 0.312 | 14.0 ± 0.444 | |||
| PME 1000 µg/mL | 14.0 ± 0.318 | −0.8 | 14.0 ± 0.521 | ±0 | 0.478 |
| Genotype | Treatment | Mean [d] ± SD | % Difference to Control | Median [d] ± SD | % Difference to Control | p-Value vs. Control |
|---|---|---|---|---|---|---|
| wild type | DMSO | 68.8 ± 1.246 | 72.0 ± 1.212 | |||
| PME 1000 µg/mL | 74.8 ± 1.269 | +8.8 | 72.0 ± 1.419 | ±0 | 0.0004 | |
| wild type | DMSO | 71.8 ± 1.021 | 72.0 ± 1.084 | |||
| PME 1 µg/mL | 72.4 ± 1.192 | +0.9 | 72.0 ± 1.274 | ±0 | 0.373 | |
| PME 5 µg/mL | 70.2 ± 1.198 | −2.2 | 72.0 ± 1.219 | ±0 | 0.405 | |
| PME 10 µg/mL | 72.2 ± 1.139 | +0.6 | 72.0 ± 1.170 | ±0 | 0.461 | |
| PME 50 µg/mL | 71.7 ± 1.144 | ±0.0 | 72.0 ± 1.244 | ±0 | 0.630 | |
| PME 100 µg/mL | 73.0 ± 1.140 | +1.8 | 72.0 ± 1.174 | ±0 | 0.401 | |
| PME 500 µg/mL | 80.8 ± 0.994 | +12.6 | 72.0 ± 1.568 | ±0 | <0.0001 | |
| CF1038 (mu86) [∆daf-16] | DMSO | 58.9 ± 1.184 | 48.0 ± 1.970 | |||
| PME 1000 µg/mL | 74.1 ± 1.419 | +25.8 | 72.0 ± 2.020 | ±50.0 | <0.0001 | |
| VC199 (ok434) [∆sir-2.1] | DMSO | 87.3 ± 1.215 | 96.0 ± 2.168 | |||
| PME 1000 µg/mL | 84.2 ± 1.499 | −3.5 | 96.0 ± 1.812 | ±0 | 0.053 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saier, C.; Büchter, C.; Koch, K.; Wätjen, W. Polygonum multiflorum Extract Exerts Antioxidative Effects and Increases Life Span and Stress Resistance in the Model Organism Caenorhabditis elegans via DAF-16 and SIR-2.1. Plants 2018, 7, 60. https://doi.org/10.3390/plants7030060
Saier C, Büchter C, Koch K, Wätjen W. Polygonum multiflorum Extract Exerts Antioxidative Effects and Increases Life Span and Stress Resistance in the Model Organism Caenorhabditis elegans via DAF-16 and SIR-2.1. Plants. 2018; 7(3):60. https://doi.org/10.3390/plants7030060
Chicago/Turabian StyleSaier, Christina, Christian Büchter, Karoline Koch, and Wim Wätjen. 2018. "Polygonum multiflorum Extract Exerts Antioxidative Effects and Increases Life Span and Stress Resistance in the Model Organism Caenorhabditis elegans via DAF-16 and SIR-2.1" Plants 7, no. 3: 60. https://doi.org/10.3390/plants7030060
APA StyleSaier, C., Büchter, C., Koch, K., & Wätjen, W. (2018). Polygonum multiflorum Extract Exerts Antioxidative Effects and Increases Life Span and Stress Resistance in the Model Organism Caenorhabditis elegans via DAF-16 and SIR-2.1. Plants, 7(3), 60. https://doi.org/10.3390/plants7030060




