Measuring the Mechanical Properties of Plant Cell Walls
Abstract
:1. Why Should We Study Cytomechanics?
2. The Mechanical Cell Wall Properties Are Defined by Its Composition
3. Measuring Turgor Pressure
4. Measuring Mechanical Cell Wall Properties
| Force | Position | |||||||
|---|---|---|---|---|---|---|---|---|
| Method | Resolution | Range | Resolution | Range | Specimen aspect ratio | High throughput | Non- destructive | |
| Magnetic or | ||||||||
| Optical tweezers | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | |
| Extensometer | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | |
| Microfluidic chip | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | |
| AFM | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ | ✓ | |
| CFM | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | |
| RT-CFM | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
5. Cellular Force Microscopy
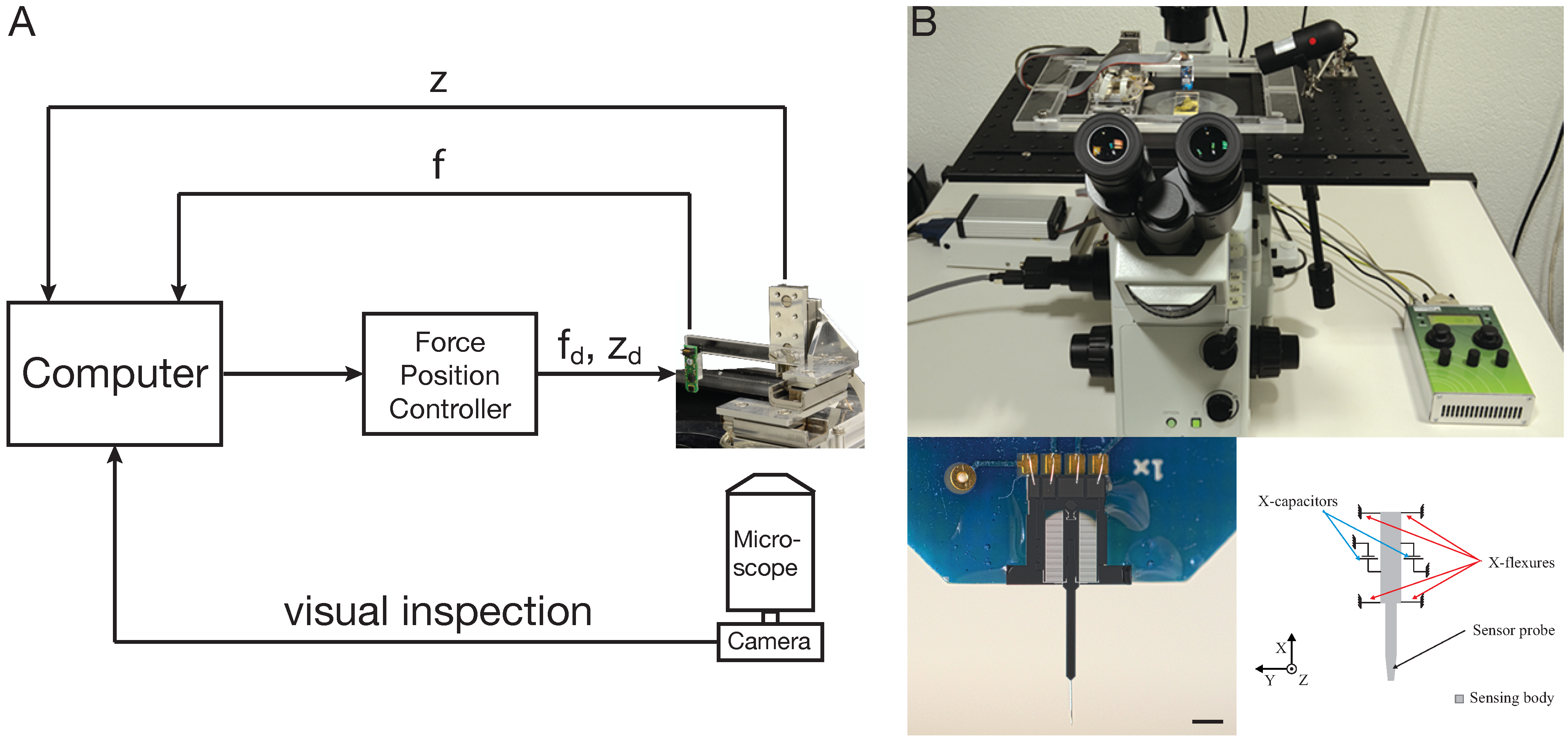
6. Real-Time Cellular Force Microscopy

7. Outlook
Acknowledgments
Conflicts of Interest
References
- Kasper, G.; Glaeser, J.; Geissler, S.; Ode, A.; Tuischer, J.; Matziolis, G.; Perka, C.; Duda, G. Matrix metalloprotease activity is an essential link between mechanical stimulus and mesenchymal stem cell behavior. Stem Cells 2007, 25, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Khani, M.; Tafazzoli-Shadpour, M.; Rostami, M.; Peirovi, H.; Janmaleki, M. Evaluation of mechanical properties of human mesenchymal stem cells during differentiation to smooth muscle cells. Ann. Biomed. Eng. 2014, 42, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Morsi, Y.; Manasseh, R. From mechanical stimulation to biological pathways in the regulation of stem cell fate. Cell Biochem. Funct. 2014, 32, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Strauss, S.; Dudziak, S.; Hagemann, R.; Barcikowski, S.; Fliess, M.; Israelowitz, M.; Kracht, D.; Kuhbier, J.; Radtke, C.; Reimers, K.; et al. Induction of osteogenic differentiation of adipose derived stem cells by microstructured nitinol actuator-mediated mechanical stress. PLoS One 2012, 7, e51264. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Sanchez, M.; Serman, F.; Ahmadi, P.; Farge, E. Mechanical induction in embryonic development and tumor growth integrative cues through molecular to multicellular interplay and evolutionary perspectives. Methods Cell Biol. 2010, 98, 295–321. [Google Scholar] [PubMed]
- Piccolo, S. Mechanics in the embryo. Nature 2013, 504, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Pouille, P.A.; Ahmadi, P.; Brunet, A.C.; Farge, E. Mechanical signals trigger myosin II redistribution and mesoderm invagination in Drosophila embryos. Sci. Signal. 2009, 2, ra16. [Google Scholar] [CrossRef] [PubMed]
- Lokody, I. Tumour-promoting tissue mechanics. Nat. Rev. Cancer 2014, 14, 296. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Beningo, K. Cancer cell invasion is enhanced by applied mechanical stimulation. PLoS One 2011, 6, e17277. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007, 3, 413–438. [Google Scholar] [CrossRef] [PubMed]
- Burgert, I.; Keplinger, T. Plant micro- and nanomechanics: Experimental techniques for plant cell-wall analysis. J. Exp. Bot. 2013, 64, 4635–4649. [Google Scholar] [CrossRef] [PubMed]
- Geitmann, A. Experimental approaches used to quantify physical parameters at cellular and subcellular levels. Am. J. Bot. 2006, 93, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Milani, P.; Braybrook, S.; Boudaoud, A. Shrinking the hammer: Micromechanical approaches to morphogenesis. J. Exp. Bot. 2013, 64, 4651–4662. [Google Scholar] [CrossRef] [PubMed]
- Moulia, B. Plant biomechanics and mechanobiology are convergent paths to flourishing interdisciplinary research. J. Exp. Bot. 2013, 64, 4617–4633. [Google Scholar] [CrossRef] [PubMed]
- Routier-Kierzkowska, A.; Smith, R. Measuring the mechanics of morphogenesis. Curr. Opin. Plant Biol. 2013, 16, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Berry, P.; Sylvester-Bradley, R.; Berry, S. Ideotype design for lodging-resistant wheat. Euphytica 2007, 154, 165–179. [Google Scholar] [CrossRef]
- Flintham, J.E.; Börner, A.; Worland, A.J.; Gale, M.D. Optimizing wheat grain yield: Effects of Rht (gibberellin-insensitive) dwarfing genes. J. Agric. Sci. 1997, 128, 11–25. [Google Scholar] [CrossRef]
- Crook, M.J.; Ennos, A.R. The effect of nitrogen and growth regulators on stem and root characteristics associated with lodging in two cultivars of winter wheat. J. Exp. Bot. 1995, 46, 931–938. [Google Scholar] [CrossRef]
- Onoda, Y.; Westoby, M.; Adler, P.; Choong, A.; Clissold, F.; Cornelissen, J.; Diaz, S.; Dominy, N.; Elgart, A.; Enrico, L.; et al. Global patterns of leaf mechanical properties. Ecol. Lett. 2011, 14, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Whitney, H.; Federle, W. Biomechanics of plant-insect interactions. Curr. Opin. Plant Biol. 2013, 16, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Dimarco, R.; Nice, C.; Fordyce, J. Family matters: Effect of host plant variation in chemical and mechanical defenses on a sequestering specialist herbivore. Oecologia 2012, 170, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Goriely, A.; Tabor, M. Estimates of biomechanical forces in Magnaporthe grisea. Mycol. Res. 2006, 110, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Barrière, Y.; Argillier, O. Brown-midrib genes of maize: A review. Agronomie 1993, 13, 865–876. [Google Scholar] [CrossRef]
- Jorgenson, L. Brown midrib in maize and its linkage relations. Agron. J. 1931, 549–557. [Google Scholar] [CrossRef]
- Mechin, V.; Laluc, A.; Legee, F.; Cezard, L.; Denoue, D.; Barriere, Y.; Lapierre, C. Impact of the brown-midrib bm5 mutation on maize lignins. J. Agric. Food Chem. 2014, 62, 5102–5107. [Google Scholar] [CrossRef] [PubMed]
- Sattler, S.E.; Funnell-Harris, D.L.; Pedersen, J.F. Brown midrib mutations and their importance to the utilization of maize, sorghum, and pearl millet lignocellulosic tissues. Plant Sci. 2010, 178, 229–238. [Google Scholar] [CrossRef]
- Pauly, M.; Keegstra, K. Cell-wall carbohydrates and their modification as a resource for biofuels. Plant J. 2008, 54, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Pauly, M.; Albersheim, P.; Darvill, A.; York, W. Molecular domains of the cellulose/xyloglucan network in the cell walls of higher plants. Plant J. 1999, 20, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Cosgrove, D. A revised architecture of primary cell walls based on biomechanical changes induced by substrate-specific endoglucanases. Plant Physiol. 2012, 158, 1933–1943. [Google Scholar] [CrossRef] [PubMed]
- Schopfer, P. Biomechanics of plant growth. Am. J. Bot. 2006, 93, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Carroll, A.; Akhmetova, L.; Somerville, C. Real-time imaging of cellulose reorientation during cell wall expansion in Arabidopsis roots. Plant Physiol. 2010, 152, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Preston, R. The case for multinet growth in growing walls of plant cells. Planta 1982, 155, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.R.; Barnes, W.S.; Bedinger, P. 2,6-Dichlorobenzonitrile, a cellulose biosynthesis inhibitor, affects morphology and structural integrity of petunia and lily pollen tubes. J. Plant Physiol. 2002, 159, 61–67. [Google Scholar] [CrossRef]
- Aouar, L.; Chebli, Y.; Geitmann, A. Morphogenesis of complex plant cell shapes: The mechanical role of crystalline cellulose in growing pollen tubes. Sex. Plant Reprod. 2010, 23, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Castle, E.S. Membrane tension and orientation of structure in the plant cell wall. J. Cell. Comp. Physiol. 1937, 10, 113–121. [Google Scholar] [CrossRef]
- Draeger, C.; Vogler, H.; Ndinyanka, T.; Weber, A.; Knox, J.; Felekis, D.; Smith, R.; Nelson, B.; Grossniklaus, U.; Ringli, C. Synergistic influence of cell wall xyloglucan and extensins on the growth and mechanical properties of Arabidopsis pollen tubes. Submitted for publication. 2015. [Google Scholar]
- Miedes, E.; Suslov, D.; Vandenbussche, F.; Kenobi, K.; Ivakov, A.; van der Straeten, D.; Lorences, E.; Mellerowicz, E.; Verbelen, J.; Vissenberg, K. Xyloglucan endotransglucosylase/hydrolase (XTH) overexpression affects growth and cell wall mechanics in etiolated Arabidopsis hypocotyls. J. Exp. Bot. 2013, 64, 2481–2497. [Google Scholar] [CrossRef] [PubMed]
- Verhertbruggen, Y.; Marcus, S.; Chen, J.; Knox, J. Cell wall pectic arabinans influence the mechanical properties of Arabidopsis thaliana inflorescence stems and their response to mechanical stress. Plant Cell Physiol. 2013, 54, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.; Wu, H. THESEUS 1, FERONIA and relatives: A family of cell wall-sensing receptor kinases? Curr. Opin. Plant Biol. 2011, 14, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Lindner, H.; Muller, L.; Boisson-Dernier, A.; Grossniklaus, U. CrRLK1L receptor-like kinases: Not just another brick in the wall. Curr. Opin. Plant Biol. 2012, 15, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Hofte, H. Growth control: A saga of cell walls, ROS, and peptide receptors. Plant Cell 2014, 26, 1848–1856. [Google Scholar] [CrossRef] [PubMed]
- Beck, W.A. Determining the osmotic value at incipient plasmolysis. Trans. Am. Microsc. Soc. 1929, 48, 204–208. [Google Scholar] [CrossRef]
- De Vries, H. Eine Methode zur Analyse der Turgorkraft; Bernstein: Berlin, Germany, 1884. [Google Scholar]
- Green, P. Growth physics in Nitella: A method for continuous in vivo analysis of extensibility based on a micro-manometer technique for turgor pressure. Plant Physiol. 1968, 43, 1169–1184. [Google Scholar] [CrossRef] [PubMed]
- Tomos, A.; Leigh, R. The pressure probe: A versatile tool in plant cell physiology. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 447–472. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, U.; Steudle, E. Physical Aspects of Water Relations of Plant Cells; Woolhouse, H., Ed.; Elsevier: London, UK, 1979; Volume 6, pp. 45–117. [Google Scholar]
- Franks, P. Use of the pressure probe in studies of stomatal function. J. Exp. Bot. 2003, 54, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Husken, D.; Steudle, E.; Zimmermann, U. Pressure probe technique for measuring water relations of cells in higher plants. Plant Physiol. 1978, 61, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.; Buckley, T.; Shope, J.; Mott, K. Guard cell volume and pressure measured concurrently by confocal microscopy and the cell pressure probe. Plant Physiol. 2001, 125, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Hukin, D.; Doering-Saad, C.; Thomas, C.; Pritchard, J. Sensitivity of cell hydraulic conductivity to mercury is coincident with symplasmic isolation and expression of plasmalemma aquaporin genes in growing maize roots. Planta 2002, 215, 1047–1056. [Google Scholar] [PubMed]
- Proseus, T.; Ortega, J.; Boyer, J. Separating growth from elastic deformation during cell enlargement. Plant Physiol. 1999, 119, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Lintilhac, P.; Wei, C.; Tanguay, J.; Outwater, J. Ball tonometry: A rapid, nondestructive method for measuring cell turgor pressure in thin-walled plant cells. J. Plant Growth Regul. 2000, 19, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Wei, C. An insight into cell elasticity and load-bearing ability. Measurement and theory. Plant Physiol. 2001, 126, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Chanliaud, E.; Gidley, M.J. In vitro synthesis and properties of pectin/Acetobacter xylinus cellulose composites. Plant J. 1999, 20, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D. Wall extensibility: Its nature, measurement and relationship to plant cell growth. New Phytol. 1993, 124, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.; Steele, D.; Chen, A.; Tobyn, M.; Staniforth, J. The mechanical properties of compacts of microcrystalline cellulose and silicified microcrystalline cellulose. Int. J. Pharm. 2000, 200, 67–72. [Google Scholar] [CrossRef]
- Kutschera, U. Cessation of cell elongation in rye coleoptiles is accompanied by a loss of cell-wall plasticity. J. Exp. Bot. 1996, 47, 1387–1394. [Google Scholar] [CrossRef]
- Wei, C.; Lintilhac, L.; Lintilhac, P. Loss of stability, pH, and the anisotropic extensibility of Chara cell walls. Planta 2006, 223, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, C.G.; Sanati, Nezhad, A.; Ghanbari, M.; Naghavi, M.; Packirisamy, M.; Geitmann, A. TipChip: A modular, MEMS-based platform for experimentation and phenotyping of tip-growing cells. Plant J. 2013, 73, 1057–1068. [Google Scholar]
- Sanati Nezhad, A.; Naghavi, M.; Packirisamy, M.; Bhat, R.; Geitmann, A. Quantification of the Young’s modulus of the primary plant cell wall using Bending-Lab-On-Chip (BLOC). Lab Chip 2013, 13, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Sanati Nezhad, A.; Naghavi, M.; Packirisamy, M.; Bhat, R.; Geitmann, A. Quantification of cellular penetrative forces using lab-on-a-chip technology and finite element modeling. Proc. Natl. Acad. Sci. USA 2013, 110, 8093–8098. [Google Scholar] [CrossRef] [PubMed]
- Hawes, C.; Osterrieder, A.; Sparkes, I.; Ketelaar, T. Optical tweezers for the micromanipulation of plant cytoplasm and organelles. Curr. Opin. Plant Biol. 2010, 13, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Wang, C.F.; Mehta, D.S.; Chiou, A. Optical tweezers as sub-pico-newton force transducers. Opt. Commun. 2001, 195, 41–48. [Google Scholar] [CrossRef]
- Zlatanova, J.; Leuba, S. Magnetic tweezers: A sensitive tool to study DNA and chromatin at the single-molecule level. Biochem. Cell Biol. 2003, 81, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, J.; Lewis, L.; Aubin, C.; Geitmann, A. Finite-element analysis of geometrical factors in micro-indentation of pollen tubes. Biomech. Model. Mechanobiol. 2006, 5, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Routier-Kierzkowska, A.; Weber, A.; Kochova, P.; Felekis, D.; Nelson, B.; Kuhlemeier, C.; Smith, R. Cellular force microscopy for in vivo measurements of plant tissue mechanics. Plant Physiol. 2012, 158, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Vogler, H.; Draeger, C.; Weber, A.; Felekis, D.; Eichenberger, C.; Routier-Kierzkowska, A.; Boisson-Dernier, A.; Ringli, C.; Nelson, B.; Smith, R.; et al. The pollen tube: A soft shell with a hard core. Plant J. 2013, 73, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Milani, P.; Gholamirad, M.; Traas, J.; Arneodo, A.; Boudaoud, A.; Argoul, F.; Hamant, O. In vivo analysis of local wall stiffness at the shoot apical meristem in Arabidopsis using atomic force microscopy. Plant J. 2011, 67, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Peaucelle, A.; Braybrook, S.; Le Guillou, L.; Bron, E.; Kuhlemeier, C.; Höfte, H. Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Curr. Biol. 2011, 21, 1720–1726. [Google Scholar] [CrossRef] [PubMed]
- Forouzesh, E.; Goel, A.; Mackenzie, S.; Turner, J. In vivo extraction of Arabidopsis cell turgor pressure using nanoindentation in conjunction with finite element modeling. Plant J. 2013, 73, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wan, K.; Roberts, K.; Bischof, J.; Nelson, B. Mechanical property characterization of mouse zona pellucida. IEEE Trans. Nanobiosci. 2003, 2, 279–286. [Google Scholar] [CrossRef]
- Felekis, D.; Muntwyler, S.; Vogler, H.; Beyeler, F.; Grossniklaus, U.; Nelson, B. Quantifying growth mechanics of living, growing plant cells in situ using microrobotics. IET Micro Nano Lett. 2011, 6, 311–316. [Google Scholar] [CrossRef] [Green Version]
- Boisson-Dernier, A.; Lituiev, D.; Nestorova, A.; Franck, C.; Thirugnanarajah, S.; Grossniklaus, U. ANXUR receptor-like kinases coordinate cell wall integrity with growth at the pollen tube tip via NADPH oxidases. PLoS Biol. 2013, 11, e1001719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassig, R.; Gutermuth, T.; Bey, T.; Konrad, K.; Romeis, T. Pollen tube NAD(P)H oxidases act as a speed control to dampen growth rate oscillations during polarized cell growth. Plant J. 2014, 78, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Franck, C.; Vogler, H.; Grossniklaus, U.; University of Zurich, Zürich, Switzerland. Unpublished data. 2014.
- Felekis, D.; Vogler, H.; Mecja, G.; Muntwyler, S.; Nestorova, A.; Huang, T.; Sakar, M.; Grossniklaus, U.; Nelson, B. Real-time, automated characterization of 3D morphology and mechanics of developing plant cells. Int.l J. Rob. Res. 2015. [Google Scholar] [CrossRef]
- Felekis, D.; Vogler, H.; Mecja, G.; Muntwyler, S.; Sakar, M.S.; Grossniklaus, U.; Nelson, B.J. High-throughput analysis of the morphology and mechanics of tip growing cells using a microrobotic platform. In Proceedings of the 2014 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS 2014), Chicago, IL, USA, 14–18 September 2014.
- Geitmann, A.; Parre, E. The local cytomechanical properties of growing pollen tubes correspond to the axial distribution of structural cellular elements. Sex. Plant Reprod. 2004, 17, 9–16. [Google Scholar] [CrossRef]
- Beyeler, F.; Muntwyler, S.; Nagy, Z.; Graetzel, C.; Moser, M.; Nelson, B. Design and calibration of a MEMS sensor for measuring the force and torque acting on a magnetic microrobot. J. Micromech. Microeng. 2008, 18, ARTN 025004. [Google Scholar] [CrossRef]
- Beyeler, F.; Muntwyler, S.; Nelson, B. A six-axis MEMS force-torque sensor with micro-Newton and nano-Newtonmeter resolution. J. Microelectromech. Syst. 2009, 18, 433–441. [Google Scholar] [CrossRef]
- Garcia, R.; Herruzo, E. The emergence of multifrequency force microscopy. Nat. Nanotechnol. 2012, 7, 217–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pukhova, V.; Banfi, F.; Ferrini, G. Energy dissipation in multifrequency atomic force microscopy. Beilstein J. Nanotechnol. 2014, 5, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Shamsudhin, N.; Rothuizen, H.; Drechsler, U.; Koelmans, W.; Bhaskaran, H.; Quenzer, H.; Wagner, B.; Despont, M. Note: Micro-cantilevers with AlN actuators and PtSi tips for multi-frequency atomic force microscopy. Rev. Sci. Instrum. 2012, 83, 096107. [Google Scholar] [CrossRef] [PubMed]
- Shamsudhin, N.; Rothuizen, H.; Despont, M.; Lygeros, J.; Sebastian, A. Micro-cantilever design and modeling framework for quantitative multi-frequency AFM. In Proceedings of the 2012 12th IEEE Conference on Nanotechnology (IEEE-NANO), Birmingham, UK, 20–23 August 2012.
- Gao, G.; Yang, S.; Xing, D. Viscoelasticity imaging of biological tissues with phase-resolved photoacoustic measurement. Opt. Lett. 2011, 36, 3341–3343. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, S.; Chen, C.; Xing, D. Simultaneous optical absorption and viscoelasticity imaging based on photoacoustic lock-in measurement. Opt. Lett. 2014, 39, 2565–2568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Maslov, K.; Wang, L. Subwavelength-resolution label-free photoacoustic microscopy of optical absorption in vivo. Opt. Lett. 2010, 35, 3195–3197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Maslov, K.; Hu, S.; Chen, R.; Zhou, Q.; Shung, K.; Wang, L. Reflection-mode submicron-resolution in vivo photoacoustic microscopy. J. Biomed. Opt. 2012, 17, 020501. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogler, H.; Felekis, D.; Nelson, B.J.; Grossniklaus, U. Measuring the Mechanical Properties of Plant Cell Walls. Plants 2015, 4, 167-182. https://doi.org/10.3390/plants4020167
Vogler H, Felekis D, Nelson BJ, Grossniklaus U. Measuring the Mechanical Properties of Plant Cell Walls. Plants. 2015; 4(2):167-182. https://doi.org/10.3390/plants4020167
Chicago/Turabian StyleVogler, Hannes, Dimitrios Felekis, Bradley J. Nelson, and Ueli Grossniklaus. 2015. "Measuring the Mechanical Properties of Plant Cell Walls" Plants 4, no. 2: 167-182. https://doi.org/10.3390/plants4020167
APA StyleVogler, H., Felekis, D., Nelson, B. J., & Grossniklaus, U. (2015). Measuring the Mechanical Properties of Plant Cell Walls. Plants, 4(2), 167-182. https://doi.org/10.3390/plants4020167






