Molecular Mechanisms and Experimental Strategies for Understanding Plant Drought Response
Abstract
1. Introduction
2. Sensing and Signaling of Water Stress
2.1. Local Sensing and Signaling Pathways
2.2. Propagation of Rapid Systemic Signals—Communication Between the Root and the Shoot
2.3. Phytohormonal Regulation of Drought Signaling
3. Key Transcription Factors in Drought Response
3.1. ABA-Dependent Pathway
3.2. ABA-Independent Pathway
3.3. Integration of ABA-Dependent and ABA-Independent Signals
4. Accumulation of Osmolytes and Cellular Protectants
4.1. Ammonium Compounds
4.2. Sugars and Polyols
4.3. Amino Acids
4.4. Dehydrins—Cellular Protectants
4.5. Potassium Ions
5. ROS Generation and Effects of Oxidative Stress
5.1. Sites of ROS Production in Plants
5.2. ROS Signaling and Antioxidant Systems
5.2.1. Enzymatic Antioxidant System
5.2.2. Non-Enzymatic Antioxidant System
5.3. Methods for Studying Oxidative Stress in Plants
5.3.1. Indirect Methods
5.3.2. Direct Methods
6. Mechanisms of Cell Wall Remodeling and Growth Regulation
6.1. Cell Wall Mechanics in Expansion Growth
6.2. Remodeling of CW Architecture
6.2.1. Expansins
6.2.2. Xyloglucan Endotransglucosylases/Hydrolases
6.2.3. Pectins and Pectin Methylesterases
6.3. ABA and Auxin Interplay
6.4. Methodological Frameworks in Plant Water Status
6.4.1. Water Potential—Golden Standard Tools
6.4.2. A New Era of Measuring Water Potential
6.4.3. Osmotic Potential and Turgor Dynamics
7. Water Use Efficiency and Stomatal Regulation
7.1. Mechanisms Regulating Stomatal Aperture
7.2. Photosynthetic Limitations Caused by Closed Stomata
7.3. Evaluation of the Stress-Imposed Damage to the Photosynthetic Apparatus
7.3.1. Assessment of PSII Efficiency and Photoprotection Using PAM Fluorometry
7.3.2. Quantification of Photosynthetic Pigments and Anthocyanins
8. Integrating Multi-Omics and High-Throughput Phenotyping in Drought Research
9. Conclusions and Future Perspectives
- Accurately quantifying and standardizing drought severity on plants (e.g., by measuring soil water content or Ψw) to reflect realistic deficits, thereby ensuring translational validity of laboratory results. An alternative could be to use a liquid medium containing PolyEthylene Glycol (PEG); however, this method may not fully reflect the water stress that plants experience in nature.
- To enable valid comparisons across independent studies, the research framework should include a minimum set of crucial physiological parameters, such as leaf water potential, photosynthetic efficiency, oxidative stress parameters, and pigment analysis. This standardized baseline is essential for distinguishing the actual physiological status of a stressed plant.
- Categorizing the studied species or cultivars within established drought resistance strategies would enable the translation of research data into practical, useful information for other scientists and breeders.
- Functional redundancy within transcription factor families (e.g., WRKY, NAC, DREB) often masks the impact of single-gene modifications. Future research must incorporate multi-omics approach to map regulatory hubs, enabling simultaneous editing of multi-genes or trait stacking. Manipulating entire gene clusters is necessary to bypass redundancy and engineer robust drought resilience.
- In nature, drought stress rarely occurs in isolation. Therefore, experimental designs should consider realistic combinations of various factors, such as water scarcity, high irradiance, heat waves, and elevated atmospheric CO2 levels, projected over the coming decades, providing a comprehensive picture of the climatic relationships relevant to plant survival in future agroecosystems.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of Extreme Weather Disasters on Global Crop Production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef] [PubMed]
- IPCC. 2023: Climate Change 2023: Synthesis Report, Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Romanello, M.; Walawender, M.; Hsu, S.C.; Moskeland, A.; Palmeiro-Silva, Y.; Scamman, D.; Ali, Z.; Ameli, N.; Angelova, D.; Ayeb-Karlsson, S.; et al. The 2024 Report of the Lancet Countdown on Health and Climate Change: Facing Record-Breaking Threats from Delayed Action. Lancet 2024, 404, 1847–1896. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, R.; Schipper, E.L.F.; Ospina, D.; Mirazo, P.; Alencar, A.; Anvari, M.; Artaxo, P.; Biresselioglu, M.E.; Blome, T.; Boeckmann, M.; et al. Ten New Insights in Climate Science 2024. One Earth 2025, 8, 101285. [Google Scholar] [CrossRef] [PubMed]
- 2025 Set to Be Second or Third Warmest Year on Record, Continuing Exceptionally High Warming Trend. Available online: https://wmo.int/media/news/2025-set-be-second-or-third-warmest-year-record-continuing-exceptionally-high-warming-trend (accessed on 15 December 2025).
- Guastello, P.; Smith, K.H.; Knutson, C.; Svoboda, M.; Tsegai, D.; Diallo, B.L.; Acebal, J. Drought Hotspots Around the World 2023–2025; United Nations Convention to Combat Desertification: Bonn, Germany, 2025. [Google Scholar]
- Khan, A.A.; Wang, Y.F.; Akbar, R.; Alhoqail, W.A. Mechanistic Insights and Future Perspectives of Drought Stress Management in Staple Crops. Front. Plant Sci. 2025, 16, 1547452. [Google Scholar] [CrossRef]
- Ajayi, A.T.; Momoh, M.E.; Oladipo, O.E.; Dada, O.O.; Amoo, A.A. Ethidium Bromide-Induced Genetic Variability and Drought Tolerance in Cowpea (Vigna unguiculata L. Walp.) Under Field Conditions. J. Soil Plant Environ. 2025, 4, 18–44. [Google Scholar] [CrossRef]
- Balting, D.F.; AghaKouchak, A.; Lohmann, G.; Ionita, M. Northern Hemisphere Drought Risk in a Warming Climate. npj Clim. Atmos. Sci. 2021, 4, 61. [Google Scholar] [CrossRef]
- Gebrechorkos, S.H.; Sheffield, J.; Vicente-Serrano, S.M.; Funk, C.; Miralles, D.G.; Peng, J.; Dyer, E.; Talib, J.; Beck, H.E.; Singer, M.B.; et al. Warming Accelerates Global Drought Severity. Nature 2025, 642, 628–635. [Google Scholar] [CrossRef]
- Haghpanah, M.; Hashemipetroudi, S.; Arzani, A.; Araniti, F. Drought Tolerance in Plants: Physiological and Molecular Responses. Plants 2024, 13, 2962. [Google Scholar] [CrossRef]
- Shavrukov, Y.; Kurishbayev, A.; Jatayev, S.; Shvidchenko, V.; Zotova, L.; Koekemoer, F.; De Groot, S.; Soole, K.; Langridge, P. Early Flowering as a Drought Escape Mechanism in Plants: How Can It Aid Wheat Production? Front. Plant Sci. 2017, 8, 302418. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The Physiology of Plant Responses to Drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Lu, W.; Wu, B.; Wang, L.; Gao, Y. Multi-Scale Drought Resilience in Terrestrial Plants: From Molecular Mechanisms to Ecosystem Sustainability. Water 2025, 17, 2516. [Google Scholar] [CrossRef]
- Takahashi, F.; Kuromori, T.; Urano, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Drought Stress Responses and Resistance in Plants: From Cellular Responses to Long-Distance Intercellular Communication. Front. Plant Sci. 2020, 11, 556972. [Google Scholar] [CrossRef] [PubMed]
- Ravi, B.; Foyer, C.H.; Pandey, G.K. The Integration of Reactive Oxygen Species (ROS) and Calcium Signalling in Abiotic Stress Responses. Plant Cell Environ. 2023, 46, 1985–2006. [Google Scholar] [CrossRef] [PubMed]
- Kollist, H.; Zandalinas, S.I.; Sengupta, S.; Nuhkat, M.; Kangasjärvi, J.; Mittler, R. Rapid Responses to Abiotic Stress: Priming the Landscape for the Signal Transduction Network. Trends Plant Sci. 2019, 24, 25–37. [Google Scholar] [CrossRef]
- Salvi, P.; Manna, M.; Kaur, H.; Thakur, T.; Gandass, N.; Bhatt, D.; Muthamilarasan, M. Phytohormone Signaling and Crosstalk in Regulating Drought Stress Response in Plants. Plant Cell Rep. 2021, 40, 1305–1329. [Google Scholar] [CrossRef]
- Christmann, A.; Grill, E.; Huang, J. Hydraulic Signals in Long-Distance Signaling. Curr. Opin. Plant Biol. 2013, 16, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Møller, I.M.; Murphy, A.; Zeiger, E. Plant Physiology and Development; Oxford Univesity Press: Oxford, UK, 2023. [Google Scholar] [CrossRef]
- Juenger, T.E.; Verslues, P.E. Time for a Drought Experiment: Do You Know Your Plants’ Water Status? Plant Cell 2023, 35, 10–23. [Google Scholar] [CrossRef]
- De Swaef, T.; Pieters, O.; Appeltans, S.; Borra-Serrano, I.; Coudron, W.; Couvreur, V.; Garre, S.; Lootens, P.; Nicolaï, B.; Pols, L.; et al. On the Pivotal Role of Water Potential to Model Plant Physiological Processes. Silico Plants 2022, 4, diab038. [Google Scholar] [CrossRef]
- Soil Water Dynamics|Learn Science at Scitable. Available online: https://www.nature.com/scitable/knowledge/library/soil-water-dynamics-103089121/ (accessed on 15 December 2025).
- Prieto, I.; Armas, C.; Pugnaire, F.I. Water Release through Plant Roots: New Insights into Its Consequences at the Plant and Ecosystem Level. New Phytol. 2012, 193, 830–841. [Google Scholar] [CrossRef]
- Xiong, Y.; Song, X.; Mehra, P.; Yu, S.; Li, Q.; Tashenmaimaiti, D.; Bennett, M.; Kong, X.; Bhosale, R.; Huang, G. ABA-Auxin Cascade Regulates Crop Root Angle in Response to Drought. Curr. Biol. 2025, 35, 542–553.e4. [Google Scholar] [CrossRef] [PubMed]
- Kohorn, B.D.; Kobayashi, M.; Johansen, S.; Riese, J.; Huang, L.F.; Koch, K.; Fu, S.; Dotson, A.; Byers, N. An Arabidopsis Cell Wall-Associated Kinase Required for Invertase Activity and Cell Growth. Plant J. 2006, 46, 307–316. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Katagiri, T.; Shinozaki, K.; Qi, Z.; Tatsumi, H.; Furuichi, T.; Kishigami, A.; Sokabe, M.; Kojima, I.; Sato, S.; et al. Arabidopsis Plasma Membrane Protein Crucial for Ca2+ Influx and Touch Sensing in Roots. Proc. Natl. Acad. Sci. USA 2007, 104, 3639–3644. [Google Scholar] [CrossRef]
- Haswell, E.S.; Peyronnet, R.; Barbier-Brygoo, H.; Meyerowitz, E.M.; Frachisse, J.M. Two MscS Homologs Provide Mechanosensitive Channel Activities in the Arabidopsis Root. Curr. Biol. 2008, 18, 730–734. [Google Scholar] [CrossRef]
- Lee, J.S.; Wilson, M.E.; Richardson, R.A.; Haswell, E.S. Genetic and Physical Interactions between the Organellar Mechanosensitive Ion Channel Homologs MSL1, MSL2, and MSL3 Reveal a Role for Inter-organellar Communication in Plant Development. Plant Direct 2019, 3, e00124. [Google Scholar] [CrossRef]
- Yamanaka, T.; Nakagawa, Y.; Mori, K.; Nakano, M.; Imamura, T.; Kataoka, H.; Terashima, A.; Iida, K.; Kojima, I.; Katagiri, T.; et al. MCA1 and MCA2 That Mediate Ca2+ Uptake Have Distinct and Overlapping Roles in Arabidopsis. Plant Physiol. 2010, 152, 1284–1296. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Ali, S.; Park, S.; Bae, H. Deciphering the Role of Mechanosensitive Channels in Plant Root Biology: Perception, Signaling, and Adaptive Responses. Planta 2023, 258, 105. [Google Scholar] [CrossRef]
- Yuan, F.; Yang, H.; Xue, Y.; Kong, D.; Ye, R.; Li, C.; Zhang, J.; Theprungsirikul, L.; Shrift, T.; Krichilsky, B.; et al. OSCA1 Mediates Osmotic-Stress-Evoked Ca2+ Increases Vital for Osmosensing in Arabidopsis. Nature 2014, 514, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhang, P.; Lu, X.; Wang, G.; Wang, Z.; Zhang, Q.; Zhang, X.; Wei, X.; Mei, F.; Wei, L.; et al. Systematic Analysis of the Maize OSCA Genes Revealing ZmOSCA Family Members Involved in Osmotic Stress and ZmOSCA2.4 Confers Enhanced Drought Tolerance in Transgenic Arabidopsis. Int. J. Mol. Sci. 2020, 21, 351. [Google Scholar] [CrossRef]
- Toriyama, T.; Shinozawa, A.; Yasumura, Y.; Saruhashi, M.; Hiraide, M.; Ito, S.; Matsuura, H.; Kuwata, K.; Yoshida, M.; Baba, T.; et al. Sensor Histidine Kinases Mediate ABA and Osmostress Signaling in the Moss Physcomitrium patens. Curr. Biol. 2022, 32, 164–175.e8. [Google Scholar] [CrossRef]
- Monshausen, G.B.; Bibikova, T.N.; Weisenseel, M.H.; Gilroy, S. Ca2+ Regulates Reactive Oxygen Species Production and PH during Mechanosensing in Arabidopsis Roots. Plant Cell 2009, 21, 2341–2356. [Google Scholar] [CrossRef]
- Jubany-Mari, T.; Alegre-Batlle, L.; Jiang, K.; Feldman, L.J. Use of a Redox-Sensing GFP (c-RoGFP1) for Real-Time Monitoring of Cytosol Redox Status in Arabidopsis Thaliana Water-Stressed Plants. FEBS Lett. 2010, 584, 889–897. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Intracellular Redox Compartmentation and ROS-Related Communication in Regulation and Signaling. Plant Physiol. 2016, 171, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive Oxygen Species, Abiotic Stress and Stress Combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ohura, I.; Kawakita, K.; Yokota, N.; Fujiwara, M.; Shimamoto, K.; Doke, N.; Yoshioka, H. Calcium-Dependent Protein Kinases Regulate the Production of Reactive Oxygen Species by Potato NADPH Oxidase. Plant Cell 2007, 19, 1065–1080. [Google Scholar] [CrossRef]
- Suzuki, N.; Miller, G.; Morales, J.; Shulaev, V.; Torres, M.A.; Mittler, R. Respiratory Burst Oxidases: The Engines of ROS Signaling. Curr. Opin. Plant Biol. 2011, 14, 691–699. [Google Scholar] [CrossRef]
- Zou, J.J.; Wei, F.J.; Wang, C.; Wu, J.J.; Ratnasekera, D.; Liu, W.X.; Wu, W.H. Arabidopsis Calcium-Dependent Protein Kinase CPK10 Functions in Abscisic Acid- and Ca2+-Mediated Stomatal Regulation in Response to Drought Stress. Plant Physiol. 2010, 154, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Li, S.; Asim, M.; Mao, J.; Xu, D.; Ullah, Z.; Liu, G.; Wang, Q.; Liu, H. The Arabidopsis Calcium-Dependent Protein Kinases (CDPKs) and Their Roles in Plant Growth Regulation and Abiotic Stress Responses. Int. J. Mol. Sci. 2018, 19, 1900. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Seth, C.S.; Roychoudhury, A. The Molecular Paradigm of Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) with Different Phytohormone Signaling Pathways during Drought Stress in Plants. Plant Physiol. Biochem. 2024, 206, 108259. [Google Scholar] [CrossRef]
- Christmann, A.; Weiler, E.W.; Steudle, E.; Grill, E. A Hydraulic Signal in Root-to-Shoot Signalling of Water Shortage. Plant J. 2007, 52, 167–174. [Google Scholar] [CrossRef]
- Wu, F.; Chi, Y.; Jiang, Z.; Xu, Y.; Xie, L.; Huang, F.; Wan, D.; Ni, J.; Yuan, F.; Wu, X.; et al. Hydrogen Peroxide Sensor HPCA1 Is an LRR Receptor Kinase in Arabidopsis. Nature 2020, 578, 577–581. [Google Scholar] [CrossRef]
- Fichman, Y.; Zandalinas, S.I.; Peck, S.; Luan, S.; Mittler, R. HPCA1 Is Required for Systemic Reactive Oxygen Species and Calcium Cell-to-Cell Signaling and Plant Acclimation to Stress. Plant Cell 2022, 34, 4453–4471. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Cao, J.; Ge, K.; Li, L. The Site of Water Stress Governs the Pattern of ABA Synthesis and Transport in Peanut. Sci. Rep. 2016, 6, 32143. [Google Scholar] [CrossRef]
- Endo, A.; Sawada, Y.; Takahashi, H.; Okamoto, M.; Ikegami, K.; Koiwai, H.; Seo, M.; Toyomasu, T.; Mitsuhashi, W.; Shinozaki, K.; et al. Drought Induction of Arabidopsis 9-Cis-Epoxycarotenoid Dioxygenase Occurs in Vascular Parenchyma Cells. Plant Physiol. 2008, 147, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Takasaki, H.; Takahashi, F.; Suzuki, T.; Iuchi, S.; Mitsuda, N.; Ohme-Takagi, M.; Ikeda, M.; Seo, M.; Yamaguchi-Shinozaki, K.; et al. Arabidopsis thaliana NGATHA1 Transcription Factor Induces ABA Biosynthesis by Activating NCED3 Gene during Dehydration Stress. Proc. Natl. Acad. Sci. USA 2018, 115, E11178–E11187. [Google Scholar] [CrossRef]
- Tombesi, S.; Nardini, A.; Frioni, T.; Soccolini, M.; Zadra, C.; Farinelli, D.; Poni, S.; Palliotti, A. Stomatal Closure Is Induced by Hydraulic Signals and Maintained by ABA in Drought-Stressed Grapevine. Sci. Rep. 2015, 5, 12449. [Google Scholar] [CrossRef]
- Manzi, M.; Lado, J.; Rodrigo, M.J.; Zacariás, L.; Arbona, V.; Gómez-Cadenas, A. Root ABA Accumulation in Long-Term Water-Stressed Plants Is Sustained by Hormone Transport from Aerial Organs. Plant Cell Physiol. 2015, 56, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, F. Exogenous Abscisic Acid Priming Modulates Water Relation Responses of Two Tomato Genotypes With Contrasting Endogenous Abscisic Acid Levels to Progressive Soil Drying Under Elevated CO2. Front. Plant Sci. 2021, 12, 733658. [Google Scholar] [CrossRef]
- Teng, Z.; Lyu, J.; Chen, Y.; Zhang, J.; Ye, N. Effects of Stress-Induced ABA on Root Architecture Development: Positive and Negative Actions. Crop. J. 2023, 11, 1072–1079. [Google Scholar] [CrossRef]
- Santiago, J.; Dupeux, F.; Betz, K.; Antoni, R.; Gonzalez-Guzman, M.; Rodriguez, L.; Márquez, J.A.; Rodriguez, P.L. Structural Insights into PYR/PYL/RCAR ABA Receptors and PP2Cs. Plant Sci. 2012, 182, 3–11. [Google Scholar] [CrossRef]
- Miyakawa, T.; Fujita, Y.; Yamaguchi-Shinozaki, K.; Tanokura, M. Structure and Function of Abscisic Acid Receptors. Trends Plant Sci. 2013, 18, 259–266. [Google Scholar] [CrossRef]
- Ghanizadeh, H.; Qamer, Z.; Zhang, Y.; Wang, A. The Multifaceted Roles of PP2C Phosphatases in Plant Growth, Signaling, and Responses to Abiotic and Biotic Stresses. Plant Commun. 2025, 6, 101457. [Google Scholar] [CrossRef]
- Umezawa, T.; Sugiyama, N.; Takahashi, F.; Anderson, J.C.; Ishihama, Y.; Peck, S.C.; Shinozaki, K. Genetics and Phosphoproteomics Reveal a Protein Phosphorylation Network in the Abscisic Acid Signaling Pathway in Arabidopsis thaliana. Sci. Signal. 2013, 6, rs8. [Google Scholar] [CrossRef]
- Liu, H.; Song, S.; Zhang, H.; Li, Y.; Niu, L.; Zhang, J.; Wang, W. Signaling Transduction of ABA, ROS, and Ca2+ in Plant Stomatal Closure in Response to Drought. Int. J. Mol. Sci. 2022, 23, 14824. [Google Scholar] [CrossRef]
- Hsu, P.K.; Dubeaux, G.; Takahashi, Y.; Schroeder, J.I. Signaling Mechanisms in Abscisic Acid-Mediated Stomatal Closure. Plant J. 2021, 105, 307–321. [Google Scholar] [CrossRef]
- Imes, D.; Mumm, P.; Böhm, J.; Al-Rasheid, K.A.S.; Marten, I.; Geiger, D.; Hedrich, R. Open Stomata 1 (OST1) Kinase Controls R-Type Anion Channel QUAC1 in Arabidopsis Guard Cells. Plant J. 2013, 74, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Malcheska, F.; Ahmad, A.; Batool, S.; Müller, H.M.; Ludwig-Müller, J.; Kreuzwieser, J.; Randewig, D.; Hänsch, R.; Mendel, R.R.; Hell, R.; et al. Drought-Enhanced Xylem Sap Sulfate Closes Stomata by Affecting ALMT12 and Guard Cell ABA Synthesis. Plant Physiol. 2017, 174, 798–814. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, M.; Shen, J.; Chen, D.; Zheng, Y.; Zhang, W. ZmOST1 Mediates Abscisic Acid Regulation of Guard Cell Ion Channels and Drought Stress Responses. J. Integr. Plant Biol. 2019, 61, 478–491. [Google Scholar] [CrossRef]
- Fujita, Y.; Nakashima, K.; Yoshida, T.; Katagiri, T.; Kidokoro, S.; Kanamori, N.; Umezawa, T.; Fujita, M.; Maruyama, K.; Ishiyama, K.; et al. Three SnRK2 Protein Kinases Are the Main Positive Regulators of Abscisic Acid Signaling in Response to Water Stress in Arabidopsis. Plant Cell Physiol. 2009, 50, 2123–2132. [Google Scholar] [CrossRef]
- Yoshida, T.; Fujita, Y.; Maruyama, K.; Mogami, J.; Todaka, D.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Four Arabidopsis AREB/ABF Transcription Factors Function Predominantly in Gene Expression Downstream of SnRK2 Kinases in Abscisic Acid Signalling in Response to Osmotic Stress. Plant Cell Environ. 2015, 38, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Suzuki, T.; Osakabe, Y.; Betsuyaku, S.; Kondo, Y.; Dohmae, N.; Fukuda, H.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A Small Peptide Modulates Stomatal Control via Abscisic Acid in Long-Distance Signalling. Nature 2018, 556, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhu, Q.; Qin, P.; Yu, L.; Li, W.; Sun, H. The Peptide-Encoding CLE25 Gene Modulates Drought Response in Cotton. Agriculture 2025, 15, 1226. [Google Scholar] [CrossRef]
- Zhao, W.; Sun, Y.; Li, J.; Wu, Y.; Tian, Y.; Wei, J.; Tian, Y. Dual Roles of SmCLE25 Peptide in Regulating Stresses Response and Leaf Senescence. BMC Plant Biol. 2025, 25, 1474. [Google Scholar] [CrossRef]
- Zhang, F.P.; Sussmilch, F.; Nichols, D.S.; Cardoso, A.A.; Brodribb, T.J.; McAdam, S.A.M. Leaves, Not Roots or Floral Tissue, Are the Main Site of Rapid, External Pressure-Induced ABA Biosynthesis in Angiosperms. J. Exp. Bot. 2018, 69, 1261–1267. [Google Scholar] [CrossRef]
- McLachlan, D.H.; Pridgeon, A.J.; Hetherington, A.M. How Arabidopsis Talks to Itself about Its Water Supply. Mol. Cell 2018, 70, 991–992. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; He, Q.; Li, H.; Zhang, X.; Zhang, F. Comparative Proteomics Illustrates the Complexity of Drought Resistance Mechanisms in Two Wheat (Triticum aestivum L.) Cultivars under Dehydration and Rehydration. BMC Plant Biol. 2016, 16, 188. [Google Scholar] [CrossRef]
- Jogawat, A.; Yadav, B.; Chhaya; Lakra, N.; Singh, A.K.; Narayan, O.P. Crosstalk between Phytohormones and Secondary Metabolites in the Drought Stress Tolerance of Crop Plants: A Review. Physiol. Plant 2021, 172, 1106–1132. [Google Scholar] [CrossRef]
- Huang, X.; Hou, L.; Meng, J.; You, H.; Li, Z.; Gong, Z.; Yang, S.; Shi, Y. The Antagonistic Action of Abscisic Acid and Cytokinin Signaling Mediates Drought Stress Response in Arabidopsis. Mol. Plant 2018, 11, 970–982. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, A.; Ramakrishnan, M.; Van Ha, C.; Zheng, B.; Bhardwaj, M.; Tran, L.S.P. Roles of Abscisic Acid and Auxin in Plants during Drought: A Molecular Point of View. Plant Physiol. Biochem. 2023, 204, 108129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bao, Z.; Smoljan, A.; Liu, Y.; Wang, H.; Friml, J. Foraging for Water by MIZ1-Mediated Antagonism between Root Gravitropism and Hydrotropism. Proc. Natl. Acad. Sci. USA 2025, 122, e2427315122. [Google Scholar] [CrossRef]
- Gui, J.; Zheng, S.; Liu, C.; Shen, J.; Li, J.; Li, L. OsREM4.1 Interacts with OsSERK1 to Coordinate the Interlinking between Abscisic Acid and Brassinosteroid Signaling in Rice. Dev. Cell 2016, 38, 201–213. [Google Scholar] [CrossRef]
- Ha, Y.; Shang, Y.; Nam, K.H. Brassinosteroids Modulate ABA-Induced Stomatal Closure in Arabidopsis. J. Exp. Bot. 2016, 67, 6297–6308. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.M.; Shang, Y.; Yang, D.; Nam, K.H. Brassinosteroid Reduces ABA Accumulation Leading to the Inhibition of ABA-Induced Stomatal Closure. Biochem. Biophys. Res. Commun. 2018, 504, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Khosravifar, F.; Mohammadi, M.; Eghlima, G. Brassinosteroids Improve Drought Resistance in Zinnia by Regulating Antioxidant Activity and Hormonal Interactions with ABA and Salicylic Acid. Plant Growth Regul. 2025, 105, 2259–2273. [Google Scholar] [CrossRef]
- Savchenko, T.; Kolla, V.A.; Wang, C.Q.; Nasafi, Z.; Hicks, D.R.; Phadungchob, B.; Chehab, W.E.; Brandizzi, F.; Froehlich, J.; Dehesh, K. Functional Convergence of Oxylipin and Abscisic Acid Pathways Controls Stomatal Closure in Response to Drought. Plant Physiol. 2014, 164, 1151–1160. [Google Scholar] [CrossRef]
- Rao, S.; Tian, Y.; Zhang, C.; Qin, Y.; Liu, M.; Niu, S.; Li, Y.; Chen, J. The JASMONATE ZIM-Domain-OPEN STOMATA1 Cascade Integrates Jasmonic Acid and Abscisic Acid Signaling to Regulate Drought Tolerance by Mediating Stomatal Closure in Poplar. J. Exp. Bot. 2023, 74, 443–457. [Google Scholar] [CrossRef]
- de Ollas, C.; Arbona, V.; Gómez-Cadenas, A. Jasmonoyl Isoleucine Accumulation Is Needed for Abscisic Acid Build-up in Roots of Arabidopsis under Water Stress Conditions. Plant Cell Environ. 2015, 38, 2157–2170. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Xie, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Abscisic Acid and Jasmonic Acid Are Involved in Drought Priming-Induced Tolerance to Drought in Wheat. Crop. J. 2021, 9, 120–132. [Google Scholar] [CrossRef]
- Visentin, I.; Vitali, M.; Ferrero, M.; Zhang, Y.; Ruyter-Spira, C.; Novák, O.; Strnad, M.; Lovisolo, C.; Schubert, A.; Cardinale, F. Low Levels of Strigolactones in Roots as a Component of the Systemic Signal of Drought Stress in Tomato. New Phytol. 2016, 212, 954–963. [Google Scholar] [CrossRef]
- Van Ha, C.; Leyva-Gonzalez, M.A.; Osakabe, Y.; Tran, U.T.; Nishiyama, R.; Watanabe, Y.; Tanaka, M.; Seki, M.; Yamaguchi, S.; Dong, N.V.; et al. Positive Regulatory Role of Strigolactone in Plant Responses to Drought and Salt Stress. Proc. Natl. Acad. Sci. USA 2014, 111, 851–856. [Google Scholar] [CrossRef]
- Li, C.; Tan, D.X.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin Mediates the Regulation of ABA Metabolism, Free-Radical Scavenging, and Stomatal Behaviour in Two Malus Species under Drought Stress. J. Exp. Bot. 2015, 66, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin Regulates the Functional Components of Photosynthesis, Antioxidant System, Gene Expression, and Metabolic Pathways to Induce Drought Resistance in Grafted Carya Cathayensis Plants. Sci. Total Environ. 2020, 713, 136675. [Google Scholar] [CrossRef] [PubMed]
- Bandurska, H. Drought Stress Responses: Coping Strategy and Resistance. Plants 2022, 11, 922. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, T.; Muller, B. The Physiological Basis of Drought Tolerance in Crop Plants: A Scenario-Dependent Probabilistic Approach. Annu. Rev. Plant Biol. 2018, 69, 733–759. [Google Scholar] [CrossRef] [PubMed]
- Gallé, Á.; Csiszár, J.; Benyó, D.; Laskay, G.; Leviczky, T.; Erdei, L.; Tari, I. Isohydric and Anisohydric Strategies of Wheat Genotypes under Osmotic Stress: Biosynthesis and Function of ABA in Stress Responses. J. Plant Physiol. 2013, 170, 1389–1399. [Google Scholar] [CrossRef]
- Álvarez-Maldini, C.; Acevedo, M.; Estay, D.; Aros, F.; Dumroese, R.K.; Sandoval, S.; Pinto, M. Examining Physiological, Water Relations, and Hydraulic Vulnerability Traits to Determine Anisohydric and Isohydric Behavior in Almond (Prunus dulcis) Cultivars: Implications for Selecting Agronomic Cultivars under Changing Climate. Front. Plant Sci. 2022, 13, 974050. [Google Scholar] [CrossRef]
- Onyemaobi, O.; Sangma, H.; Garg, G.; Wallace, X.; Kleven, S.; Suwanchaikasem, P.; Roessner, U.; Dolferus, R. Reproductive Stage Drought Tolerance in Wheat: Importance of Stomatal Conductance and Plant Growth Regulators. Genes 2021, 12, 1742. [Google Scholar] [CrossRef]
- Wagh, K.; Stavreva, D.A.; Upadhyaya, A.; Hager, G.L. Transcription Factor Dynamics: One Molecule at a Time. Annu. Rev. Cell Dev. Biol. 2023, 39, 277–305. [Google Scholar] [CrossRef]
- Jin, J.; Tian, F.; Yang, D.C.; Meng, Y.Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a Central Hub for Transcription Factors and Regulatory Interactions in Plants. Nucleic Acids Res. 2017, 45, D1040–D1045. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, X.; Shen, X. Regulatory Network Established by Transcription Factors Transmits Drought Stress Signals in Plant. Stress Biol. 2022, 2, 26. [Google Scholar] [CrossRef]
- Yoshida, T.; Fujita, Y.; Sayama, H.; Kidokoro, S.; Maruyama, K.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1, AREB2, and ABF3 Are Master Transcription Factors That Cooperatively Regulate ABRE-Dependent ABA Signaling Involved in Drought Stress Tolerance and Require ABA for Full Activation. Plant J. 2010, 61, 672–685. [Google Scholar] [CrossRef]
- Collin, A.; Daszkowska-Golec, A.; Szarejko, I. Updates on the Role of ABSCISIC ACID INSENSITIVE 5 (ABI5) and ABSCISIC ACID-RESPONSIVE ELEMENT BINDING FACTORs (ABFs) in ABA Signaling in Different Developmental Stages in Plants. Cells 2021, 10, 1996. [Google Scholar] [CrossRef]
- Yu, Y.; Qian, Y.; Jiang, M.; Xu, J.; Yang, J.; Zhang, T.; Gou, L.; Pi, E. Regulation Mechanisms of Plant Basic Leucine Zippers to Various Abiotic Stresses. Front. Plant Sci. 2020, 11, 561913. [Google Scholar] [CrossRef] [PubMed]
- Kimotho, R.N.; Baillo, E.H.; Zhang, Z. Transcription Factors Involved in Abiotic Stress Responses in Maize (Zea mays L.) and Their Roles in Enhanced Productivity in the Post Genomics Era. PeerJ 2019, 7, e7211. [Google Scholar] [CrossRef]
- Hsieh, T.H.; Li, C.W.; Su, R.C.; Cheng, C.P.; Sanjaya; Tsai, Y.C.; Chan, M.T. A Tomato BZIP Transcription Factor, SlAREB, Is Involved in Water Deficit and Salt Stress Response. Planta 2010, 231, 1459–1473. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Meng, X.; Cai, J.; Li, G.; Dong, T.; Li, Z. Basic Leucine Zipper Transcription Factor SlbZIP1 Mediates Salt and Drought Stress Tolerance in Tomato. BMC Plant Biol. 2018, 18, 83. [Google Scholar] [CrossRef]
- Tu, M.; Wang, X.; Zhu, Y.; Wang, D.; Zhang, X.; Cui, Y.; Li, Y.; Gao, M.; Li, Z.; Wang, Y.; et al. VlbZIP30 of Grapevine Functions in Dehydration Tolerance via the Abscisic Acid Core Signaling Pathway. Hortic. Res. 2018, 5, 49. [Google Scholar] [CrossRef]
- Tu, M.; Wang, X.; Feng, T.; Sun, X.; Wang, Y.; Huang, L.; Gao, M.; Wang, Y.; Wang, X. Expression of a Grape (Vitis vinifera) BZIP Transcription Factor, VlbZIP36, in Arabidopsis thaliana Confers Tolerance of Drought Stress during Seed Germination and Seedling Establishment. Plant Sci. 2016, 252, 311–323. [Google Scholar] [CrossRef]
- Liu, J.; Chu, J.; Ma, C.; Jiang, Y.; Ma, Y.; Xiong, J.; Cheng, Z.M. Overexpression of an ABA-Dependent Grapevine BZIP Transcription Factor, VvABF2, Enhances Osmotic Stress in Arabidopsis. Plant Cell Rep. 2019, 38, 587–596. [Google Scholar] [CrossRef]
- Baldoni, E.; Genga, A.; Cominelli, E. Plant MYB Transcription Factors: Their Role in Drought Response Mechanisms. Int. J. Mol. Sci. 2015, 16, 15811. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, H.; Wang, K.; Tang, X.; Li, S.; Zhang, N.; Si, H. MYB Transcription Factors: Acting as Molecular Switches to Regulate Different Signaling Pathways to Modulate Plant Responses to Drought Stress. Ind. Crops Prod. 2025, 226, 120676. [Google Scholar] [CrossRef]
- Ding, Z.; Li, S.; An, X.; Liu, X.; Qin, H.; Wang, D. Transgenic Expression of MYB15 Confers Enhanced Sensitivity to Abscisic Acid and Improved Drought Tolerance in Arabidopsis thaliana. J. Genet. Genom. 2009, 36, 17–29. [Google Scholar] [CrossRef]
- Ma, Y.; Tang, M.; Wang, M.; Yu, Y.; Ruan, B. Advances in Understanding Drought Stress Responses in Rice: Molecular Mechanisms of ABA Signaling and Breeding Prospects. Genes 2024, 15, 1529. [Google Scholar] [CrossRef]
- Li, B.; Liu, R.; Liu, J.; Zhang, H.; Tian, Y.; Chen, T.; Li, J.; Jiao, F.; Jia, T.; Li, Y.; et al. ZmMYB56 Regulates Stomatal Closure and Drought Tolerance in Maize Seedlings through the Transcriptional Regulation of ZmTOM7. New Crops 2024, 1, 100012. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, M.; Tian, Y.; He, W.; Han, L.; Xia, G. Over-Expression of TaMYB33 Encoding a Novel Wheat MYB Transcription Factor Increases Salt and Drought Tolerance in Arabidopsis. Mol. Biol. Rep. 2012, 39, 7183–7192. [Google Scholar] [CrossRef]
- Wei, Q.; Luo, Q.; Wang, R.; Zhang, F.; He, Y.; Zhang, Y.; Qiu, D.; Li, K.; Chang, J.; Yang, G.; et al. A Wheat R2R3-Type MYB Transcription Factor TaODORANT1 Positively Regulates Drought and Salt Stress Responses in Transgenic Tobacco Plants. Front. Plant Sci. 2017, 8, 260621. [Google Scholar] [CrossRef]
- Peng, D.; Li, L.; Wei, A.; Zhou, L.; Wang, B.; Liu, M.; Lei, Y.; Xie, Y.; Li, X. TaMYB44-5A Reduces Drought Tolerance by Repressing Transcription of TaRD22-3A in the Abscisic Acid Signaling Pathway. Planta 2024, 260, 52. [Google Scholar] [CrossRef] [PubMed]
- Conti, V.; Parrotta, L.; Romi, M.; Del Duca, S.; Cai, G. Tomato Biodiversity and Drought Tolerance: A Multilevel Review. Int. J. Mol. Sci. 2023, 24, 10044. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, J.; Li, S.; Song, Q.; Miao, M.; Fan, T.; Tang, X. The 1R-MYB Transcription Factor SlMYB1L Modulates Drought Tolerance via an ABA-Dependent Pathway in Tomato. Plant Physiol. Biochem. 2025, 222, 109721. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, P.; Gao, Z.; Long, T.; Xing, C.; Li, J.; Xue, J.; Chen, G.; Xie, Q.; Hu, Z. Silencing of SlMYB78-like Reduces the Tolerance to Drought and Salt Stress via the ABA Pathway in Tomato. Int. J. Mol. Sci. 2024, 25, 11449. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Quan, R.; Chen, G.; Yu, G.; Li, X.; Han, Z.; Xu, W.; Li, G.; Shi, J.; Li, B. An R2R3-MYB Transcription Factor VyMYB24, Isolated from Wild Grape Vitis yanshanesis J. X. Chen., Regulates the Plant Development and Confers the Tolerance to Drought. Front. Plant Sci. 2022, 13, 966641. [Google Scholar] [CrossRef]
- Fang, L.; Wang, Z.; Su, L.; Gong, L.; Xin, H. Vitis Myb14 Confer Cold and Drought Tolerance by Activating Lipid Transfer Protein Genes Expression and Reactive Oxygen Species Scavenge. Gene 2024, 890, 147792. [Google Scholar] [CrossRef]
- Singh, D.; Laxmi, A. Transcriptional Regulation of Drought Response: A Tortuous Network of Transcriptional Factors. Front. Plant Sci. 2015, 6, 165462. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, P. The DREB Transcription Factor, a Biomacromolecule, Responds to Abiotic Stress by Regulating the Expression of Stress-Related Genes. Int. J. Biol. Macromol. 2023, 243, 125231. [Google Scholar] [CrossRef]
- Xie, Z.; Nolan, T.; Jiang, H.; Tang, B.; Zhang, M.; Li, Z.; Yin, Y. The AP2/ERF Transcription Factor TINY Modulates Brassinosteroid-Regulated Plant Growth and Drought Responses in Arabidopsis. Plant Cell 2019, 31, 1788. [Google Scholar] [CrossRef]
- Ravikumar, G.; Manimaran, P.; Voleti, S.R.; Subrahmanyam, D.; Sundaram, R.M.; Bansal, K.C.; Viraktamath, B.C.; Balachandran, S.M. Stress-Inducible Expression of AtDREB1A Transcription Factor Greatly Improves Drought Stress Tolerance in Transgenic Indica Rice. Transgenic Res. 2014, 23, 421. [Google Scholar] [CrossRef] [PubMed]
- Bhanbhro, N.; Wang, H.J.; Yang, H.; Xu, X.J.; Jakhar, A.M.; Shalmani, A.; Zhang, R.X.; Bakhsh, Q.; Akbar, G.; Jakhro, M.I.; et al. Revisiting the Molecular Mechanisms and Adaptive Strategies Associated with Drought Stress Tolerance in Common Wheat (Triticum aestivum L.). Plant Stress 2024, 11, 100298. [Google Scholar] [CrossRef]
- Maqsood, H.; Munir, F.; Amir, R.; Gul, A. Genome-Wide Identification, Comprehensive Characterization of Transcription Factors, Cis-Regulatory Elements, Protein Homology, and Protein Interaction Network of DREB Gene Family in Solanum lycopersicum. Front. Plant Sci. 2022, 13, 1031679. [Google Scholar] [CrossRef]
- Xiong, H.; He, H.; Chang, Y.; Miao, B.; Liu, Z.; Wang, Q.; Dong, F.; Xiong, L. Multiple Roles of NAC Transcription Factors in Plant Development and Stress Responses. J. Integr. Plant Biol. 2025, 67, 510–538. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.S.P.; Nakashima, K.; Sakuma, Y.; Simpson, S.D.; Fujita, Y.; Maruyama, K.; Fujita, M.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Isolation and Functional Analysis of Arabidopsis Stress-Inducible NAC Transcription Factors That Bind to a Drought-Responsive Cis-Element in the Early Responsive to Dehydration Stress 1 Promoter. Plant Cell 2004, 16, 2481. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Liu, S.; Tang, B.; Chen, J.; Xie, Z.; Nolan, T.M.; Jiang, H.; Guo, H.; Lin, H.Y.; Li, L.; et al. RD26 Mediates Crosstalk between Drought and Brassinosteroid Signalling Pathways. Nat. Commun. 2017, 8, 14573. [Google Scholar] [CrossRef]
- Jensen, M.K.; Lindemose, S.; de Masi, F.; Reimer, J.J.; Nielsen, M.; Perera, V.; Workman, C.T.; Turck, F.; Grant, M.R.; Mundy, J.; et al. ATAF1 Transcription Factor Directly Regulates Abscisic Acid Biosynthetic Gene NCED3 in Arabidopsis thaliana. FEBS Open Bio 2013, 3, 321–327. [Google Scholar] [CrossRef]
- Joshi, R.; Wani, S.H.; Singh, B.; Bohra, A.; Dar, Z.A.; Lone, A.A.; Pareek, A.; Singla-Pareek, S.L. Transcription Factors and Plants Response to Drought Stress: Current Understanding and Future Directions. Front. Plant Sci. 2016, 7, 204078. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Chung, P.J.; Jeong, J.S.; Jang, G.; Bang, S.W.; Jung, H.; Kim, Y.S.; Ha, S.H.; Choi, Y.D.; Kim, J.K. The Rice OsNAC6 Transcription Factor Orchestrates Multiple Molecular Mechanisms Involving Root Structural Adaptions and Nicotianamine Biosynthesis for Drought Tolerance. Plant Biotechnol. J. 2017, 15, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Wang, H.; Liu, S.; Li, Z.; Yang, X.; Yan, J.; Li, J.; Tran, L.S.P.; Qin, F. A Transposable Element in a NAC Gene Is Associated with Drought Tolerance in Maize Seedlings. Nat. Commun. 2015, 6, 8326. [Google Scholar] [CrossRef]
- Liu, H.; Song, S.; Liu, M.; Mu, Y.; Li, Y.; Xuan, Y.; Niu, L.; Zhang, H.; Wang, W. Transcription Factor ZmNAC20 Improves Drought Resistance by Promoting Stomatal Closure and Activating Expression of Stress-Responsive Genes in Maize. Int. J. Mol. Sci. 2023, 24, 4712. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Li, X.; Feng, Y.-J.; Han, D.-J.; Zheng, W.-J.; Kang, Z.-S. Functional Characterization of TaNAC6-3B: A Key Regulator of Drought Tolerance in Wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2025, 229, 110578. [Google Scholar] [CrossRef]
- Chen, N.; Shao, Q.; Lu, Q.; Li, X.; Gao, Y.; Xiao, Q. Research Progress on Function of NAC Transcription Factors in Tomato (Solanum lycopersicum L.). Euphytica 2023, 219, 22. [Google Scholar] [CrossRef]
- Jian, W.; Zheng, Y.; Yu, T.; Cao, H.; Chen, Y.; Cui, Q.; Xu, C.; Li, Z. SlNAC6, A NAC Transcription Factor, Is Involved in Drought Stress Response and Reproductive Process in Tomato. J. Plant Physiol. 2021, 264, 153483. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, C.; Shao, X.; Hu, Z.; Li, J.; Wang, P.; Wang, A.; Yu, J.; Shi, K. Transcriptomic and Genetic Approaches Reveal an Essential Role of the NAC Transcription Factor SlNAP1 in the Growth and Defense Response of Tomato. Hortic. Res. 2020, 7, 209. [Google Scholar] [CrossRef]
- Liu, B.; Ouyang, Z.; Zhang, Y.; Li, X.; Hong, Y.; Huang, L.; Liu, S.; Zhang, H.; Li, D.; Song, F. Tomato NAC Transcription Factor SlSRN1 Positively Regulates Defense Response against Biotic Stress but Negatively Regulates Abiotic Stress Response. PLoS ONE 2014, 9, e102067. [Google Scholar] [CrossRef]
- Jin, Z.-L.; Wang, W.-N.; Nan, Q.; Liu, J.-W.; Ju, Y.-L.; Fang, Y.-L. VvNAC17, a Grape NAC Transcription Factor, Regulates Plant Response to Drought-Tolerance and Anthocyanin Synthesis. Plant Physiol. Biochem. 2025, 219, 109379. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, S.; Zhou, X.; Ma, X.; Ayiguzeli, M.; Zhong, H.; Zhang, F.; Zhang, C.; Yadav, V.; Wu, X.; et al. VvNAC33 Functions as a Key Regulator of Drought Tolerance in Grapevine by Modulating Reactive Oxygen Species Production. Plant Physiol. Biochem. 2025, 224, 109971. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liang, G.; Yu, D. Activated Expression of WRKY57 Confers Drought Tolerance in Arabidopsis. Mol. Plant 2012, 5, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yu, D. Activated Expression of AtWRKY53 Negatively Regulates Drought Tolerance by Mediating Stomatal Movement. Plant Cell Rep. 2015, 34, 1295–1306. [Google Scholar] [CrossRef]
- Ren, X.; Chen, Z.; Liu, Y.; Zhang, H.; Zhang, M.; Liu, Q.; Hong, X.; Zhu, J.K.; Gong, Z. ABO3, a WRKY Transcription Factor, Mediates Plant Responses to Abscisic Acid and Drought Tolerance in Arabidopsis. Plant J. 2010, 63, 417. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, S.; Ye, N.; Jiang, M.; Cao, J.; Zhang, J. WRKY Transcription Factors in Plant Responses to Stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef]
- Khoso, M.A.; Hussain, A.; Ritonga, F.N.; Ali, Q.; Channa, M.M.; Alshegaihi, R.M.; Meng, Q.; Ali, M.; Zaman, W.; Brohi, R.D.; et al. WRKY Transcription Factors (TFs): Molecular Switches to Regulate Drought, Temperature, and Salinity Stresses in Plants. Front. Plant Sci. 2022, 13, 1039329. [Google Scholar] [CrossRef]
- Raineri, J.; Wang, S.; Peleg, Z.; Blumwald, E.; Chan, R.L. The Rice Transcription Factor OsWRKY47 Is a Positive Regulator of the Response to Water Deficit Stress. Plant Mol. Biol. 2015, 88, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Wu, T.; Ma, Z.; Li, Z.; Chen, H.; Zhang, M.; Bian, M.; Bai, H.; Jiang, W.; Du, X. Rice Transcription Factor Oswrky55 Is Involved in the Drought Response and Regulation of Plant Growth. Int. J. Mol. Sci. 2021, 22, 4337. [Google Scholar] [CrossRef]
- Leng, P.; Zhao, J. Transcription Factors as Molecular Switches to Regulate Drought Adaptation in Maize. Theor. Appl. Genet. 2020, 133, 1455–1465. [Google Scholar] [CrossRef]
- He, G.H.; Xu, J.Y.; Wang, Y.X.; Liu, J.M.; Li, P.S.; Chen, M.; Ma, Y.Z.; Xu, Z.S. Drought-Responsive WRKY Transcription Factor Genes TaWRKY1 and TaWRKY33 from Wheat Confer Drought and/or Heat Resistance in Arabidopsis. BMC Plant Biol. 2016, 16, 116. [Google Scholar] [CrossRef]
- Ye, H.; Qiao, L.; Guo, H.; Guo, L.; Ren, F.; Bai, J.; Wang, Y. Genome-Wide Identification of Wheat WRKY Gene Family Reveals That TaWRKY75-A Is Referred to Drought and Salt Resistances. Front. Plant Sci. 2021, 12, 663118. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, J.; Li, Y.; Rong, X.; Sun, J.; Sun, T.; Li, M.; Wang, L.; Feng, Y.; Chai, R.; et al. Expression of TaWRKY44, a Wheat WRKY Gene, in Transgenic Tobacco Confers Multiple Abiotic Stress Tolerances. Front. Plant Sci. 2015, 6, 615. [Google Scholar] [CrossRef]
- Yu, Y.; Song, T.; Wang, Y.; Zhang, M.; Li, N.; Yu, M.; Zhang, S.; Zhou, H.; Guo, S.; Bu, Y.; et al. The Wheat WRKY Transcription Factor TaWRKY1-2D Confers Drought Resistance in Transgenic Arabidopsis and Wheat (Triticum aestivum L.). Int. J. Biol. Macromol. 2023, 226, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Luo, W.; Ge, M.; Guan, Y.; Tang, Y.; Chen, W.; Lv, J. A Group I WRKY Gene, TaWRKY133, Negatively Regulates Drought Resistance in Transgenic Plants. Int. J. Mol. Sci. 2022, 23, 12026. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Lv, L.; Duan, L.; Wu, J.; Shao, Q.; Li, X.; Lu, Q. A WRKY Transcription Factor, SlWRKY75, Positively Regulates Tomato (Solanum lycopersicum L.) Resistance to Ralstonia Solanacearum. Front. Plant Sci. 2025, 16, 1704937. [Google Scholar] [CrossRef]
- Zhang, W.; Fu, Y.; Li, M.; Zhang, S.; Zhang, Y.; Liu, X.; Huang, L.; Liang, X.; Shen, Q. SlWRKY75 Functions as a Differential Regulator to Enhance Drought Tolerance in Tomato (Solanum lycopersicum L.). Plant Physiol. Biochem. 2025, 227, 110189. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.F.; Liu, J.K.; Yang, F.M.; Zhang, G.Y.; Wang, D.; Zhang, L.; Ou, Y.B.; Yao, Y.A. The WRKY Transcription Factor WRKY8 Promotes Resistance to Pathogen Infection and Mediates Drought and Salt Stress Tolerance in Solanum lycopersicum. Physiol. Plant 2020, 168, 98–117. [Google Scholar] [CrossRef]
- Li, W.; Li, D.H.; Li, H.Y.; Wang, M.C.; Wang, Z.; Liu, J.H. The Tomato WRKY Transcription Factor SlWRKY17 Positively Regulates Drought Stress Tolerance in Transgenic Tobacco Plants. Russ. J. Plant Physiol. 2022, 69, 154. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X.; Yang, Y.; Liu, C.; Zhou, G.; Wan, H.; Cheng, Y. Tomato WRKY81 Acts as a Negative Regulator for Drought Tolerance by Modulating Guard Cell H2O2–Mediated Stomatal Closure. Environ. Exp. Bot. 2020, 171, 103960. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Guo, R.; Wang, Y.; Guo, C.; Li, Z.; Chen, Z.; Gao, H.; Wang, X. Over-Expression of a Grape WRKY Transcription Factor Gene, VlWRKY48, in Arabidopsis Thaliana Increases Disease Resistance and Drought Stress Tolerance. Plant Cell Tissue Organ Cult. 2018, 132, 359–370. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, R.; Ye, X.; Zheng, X.; Tan, B.; Wang, W.; Li, Z.; Li, J.; Cheng, J.; Feng, J. Overexpressing VvWRKY18 from Grapevine Reduces the Drought Tolerance in Arabidopsis by Increasing Leaf Stomatal Density. J. Plant Physiol. 2022, 275, 153741. [Google Scholar] [CrossRef]
- Hou, L.; Fan, X.; Hao, J.; Liu, G.; Zhang, Z.; Liu, X. Negative Regulation by Transcription Factor VvWRKY13 in Drought Stress of Vitis vinifera L. Plant Physiol. Biochem. 2020, 148, 114–121. [Google Scholar] [CrossRef]
- Takahashi, F.; Kuromori, T.; Sato, H.; Shinozaki, K. Regulatory Gene Networks in Drought Stress Responses and Resistance in Plants. Adv. Exp. Med. Biol. 2018, 1081, 189–214. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Yoshida, T.; Yamaguchi-Shinozaki, K. Pivotal Role of the AREB/ABF-SnRK2 Pathway in ABRE-Mediated Transcription in Response to Osmotic Stress in Plants. Physiol. Plant 2013, 147, 15–27. [Google Scholar] [CrossRef]
- Ma, Z.; Hu, L.; Zhong, Y. Structure, Evolution, and Roles of MYB Transcription Factors Proteins in Secondary Metabolite Biosynthetic Pathways and Abiotic Stresses Responses in Plants: A Comprehensive Review. Front. Plant Sci. 2025, 16, 1626844. [Google Scholar] [CrossRef]
- Wang, R.S.; Pandey, S.; Li, S.; Gookin, T.E.; Zhao, Z.; Albert, R.; Assmann, S.M. Common and Unique Elements of the ABA-Regulated Transcriptome of Arabidopsis Guard Cells. BMC Genom. 2011, 12, 216. [Google Scholar] [CrossRef]
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two Transcription Factors, DREB1 and DREB2, with an EREBP/AP2 DNA Binding Domain Separate Two Cellular Signal Transduction Pathways in Drought- and Low-Temperature-Responsive Gene Expression, Respectively, in Arabidopsis. Plant Cell 1998, 10, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Mizoi, J.; Kidokoro, S.; Maruyama, K.; Nakajima, J.; Nakashima, K.; Mitsuda, N.; Takiguchi, Y.; Ohme-Takagi, M.; Kondou, Y.; et al. Arabidopsis GROWTH-REGULATING FACTOR7 Functions as a Transcriptional Repressor of Abscisic Acid– and Osmotic Stress–Responsive Genes, Including DREB2A. Plant Cell 2012, 24, 3393–3405. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Sakuma, Y.; Tran, L.S.P.; Maruyama, K.; Kidokoro, S.; Fujita, Y.; Fujita, M.; Umezawa, T.; Sawano, Y.; Miyazono, K.I.; et al. Arabidopsis DREB2A-Interacting Proteins Function as RING E3 Ligases and Negatively Regulate Plant Drought Stress–Responsive Gene Expression. Plant Cell 2008, 20, 1693–1707. [Google Scholar] [CrossRef]
- Kim, J.S.; Mizoi, J.; Yoshida, T.; Fujita, Y.; Nakajima, J.; Ohori, T.; Todaka, D.; Nakashima, K.; Hirayama, T.; Shinozaki, K.; et al. An ABRE Promoter Sequence Is Involved in Osmotic Stress-Responsive Expression of the DREB2A Gene, Which Encodes a Transcription Factor Regulating Drought-Inducible Genes in Arabidopsis. Plant Cell Physiol. 2011, 52, 2136–2146. [Google Scholar] [CrossRef]
- Chen, Y.; Xia, P. NAC Transcription Factors as Biological Macromolecules Responded to Abiotic Stress: A Comprehensive Review. Int. J. Biol. Macromol. 2025, 308, 142400. [Google Scholar] [CrossRef]
- Uçarlı, C. Drought Stress and the Role of NAC Transcription Factors in Drought Response. In Drought Stress: Review and Recommendation; Springer Nature: Cham, Switzerland, 2025; pp. 295–320. [Google Scholar] [CrossRef]
- Tang, Y.; Xia, P. WRKY Transcription Factors: Key Regulators in Plant Drought Tolerance. Plant Sci. 2025, 359, 112647. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Ma, C.; Wang, Z.; Shi, X.; Duan, W.; Fu, X.; Liu, J.; Guo, C.; Xiao, K. Transcription Factor Gene TaWRKY76 Confers Plants Improved Drought and Salt Tolerance through Modulating Stress Defensive-Associated Processes in Triticum aestivum L. Plant Physiol. Biochem. 2024, 216, 109147. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Wang, H.; Tang, X. NAC Transcription Factors in Plant Multiple Abiotic Stress Responses: Progress and Prospects. Front. Plant Sci. 2015, 6, 156056. [Google Scholar] [CrossRef]
- Sosa-Valencia, G.; Palomar, M.; Covarrubias, A.A.; Reyes, J.L. The Legume MiR1514a Modulates a NAC Transcription Factor Transcript to Trigger PhasiRNA Formation in Response to Drought. J. Exp. Bot. 2017, 68, 2013–2026. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Zhou, L.; Chen, W.; Ye, N.; Xia, J.; Zhuang, C. Overexpression of a MicroRNA-Targeted NAC Transcription Factor Improves Drought and Salt Tolerance in Rice via ABA-Mediated Pathways. Rice 2019, 12, 76. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of Osmoprotectants in Improving Salinity and Drought Tolerance in Plants: A Review. Rev. Environ. Sci. Biotechnol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones Regulate Accumulation of Osmolytes Under Abiotic Stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in Plants under Abiotic Stresses: New Insights into a Classical Phenomenon. Planta 2019, 251, 3. [Google Scholar] [CrossRef]
- Huang, S.; Zuo, T.; Ni, W. Important Roles of Glycinebetaine in Stabilizing the Structure and Function of the Photosystem II Complex under Abiotic Stresses. Planta 2020, 251, 36. [Google Scholar] [CrossRef]
- Jarin, A.; Ghosh, U.K.; Hossain, M.d.S.; Mahmud, A.; Khan, M.d.A.R. Glycine Betaine in Plant Responses and Tolerance to Abiotic Stresses. Discov. Agric. 2024, 2, 127. [Google Scholar] [CrossRef]
- Basit, F.; Alyafei, M.; Hayat, F.; Al-Zayadneh, W.; El-Keblawy, A.; Sulieman, S.; Sheteiwy, M.S. Deciphering the Role of Glycine Betaine in Enhancing Plant Performance and Defense Mechanisms against Environmental Stresses. Front. Plant Sci. 2025, 16, 1582332. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Khan, M.A.R. Understanding the Roles of Osmolytes for Acclimatizing Plants to Changing Environment: A Review of Potential Mechanism. Plant Signal. Behav. 2021, 16, 1913306. [Google Scholar] [CrossRef]
- Van Den Ende, W.; Valluru, R. Sucrose, Sucrosyl Oligosaccharides, and Oxidative Stress: Scavenging and Salvaging? J. Exp. Bot. 2009, 60, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of Soluble Sugars in Reactive Oxygen Species Balance and Responses to Oxidative Stress in Plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef]
- Koch, K.E. Carbohydrate-Modulated Gene Expression in Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 509–540. [Google Scholar] [CrossRef]
- Hincha, D.K.; Zuther, E.; Hellwege, E.M.; Heyer, A.G. Specific Effects of Fructo- and Gluco-Oligosaccharides in the Preservation of Liposomes during Drying. Glycobiology 2002, 12, 103–110. [Google Scholar] [CrossRef]
- Crowe, J.H. Trehalose As a “Chemical Chaperone”. Adv. Exp. Med. Biol. 2007, 594, 143–158. [Google Scholar] [CrossRef]
- Sengupta, S.; Mukherjee, S.; Goswami, L.; Sangma, S.; Mukherjee, A.; Mukherjee, R.; Roy, N.; Basak, P.; Majumder, A.L. Manipulation of Inositol Metabolism for Improved Plant Survival under Stress: A “Network Engineering Approach”. J. Plant Biochem. Biotechnol. 2012, 21, 15–23. [Google Scholar] [CrossRef]
- Koyro, H.W.; Ahmad, P.; Geissler, N. Abiotic Stress Responses in Plants: An Overview. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Springer: New York, NY, USA, 2012; pp. 1–28. [Google Scholar] [CrossRef]
- Ben Rejeb, K.; Lefebvre-De Vos, D.; Le Disquet, I.; Leprince, A.S.; Bordenave, M.; Maldiney, R.; Jdey, A.; Abdelly, C.; Savouré, A. Hydrogen Peroxide Produced by NADPH Oxidases Increases Proline Accumulation during Salt or Mannitol Stress in Arabidopsis Thaliana. New Phytol. 2015, 208, 1138–1148. [Google Scholar] [CrossRef]
- Szlachtowska, Z.; Rurek, M. Plant Dehydrins and Dehydrin-like Proteins: Characterization and Participation in Abiotic Stress Response. Front. Plant Sci. 2023, 14, 1213188. [Google Scholar] [CrossRef]
- Hsiao, A.S. Protein Disorder in Plant Stress Adaptation: From Late Embryogenesis Abundant to Other Intrinsically Disordered Proteins. Int. J. Mol. Sci. 2024, 25, 1178. [Google Scholar] [CrossRef]
- Graether, S.P.; Boddington, K.F. Disorder and Function: A Review of the Dehydrin Protein Family. Front. Plant Sci. 2014, 5, 576. [Google Scholar] [CrossRef] [PubMed]
- Tompa, P.; Szász, C.; Buday, L. Structural Disorder Throws New Light on Moonlighting. Trends Biochem. Sci. 2005, 30, 484–489. [Google Scholar] [CrossRef]
- Sun, Z.; Li, S.; Chen, W.; Zhang, J.; Zhang, L.; Sun, W.; Wang, Z. Plant Dehydrins: Expression, Regulatory Networks, and Protective Roles in Plants Challenged by Abiotic Stress. Int. J. Mol. Sci. 2021, 22, 12619. [Google Scholar] [CrossRef] [PubMed]
- Riyazuddin, R.; Nisha, N.; Singh, K.; Verma, R.; Gupta, R. Involvement of Dehydrin Proteins in Mitigating the Negative Effects of Drought Stress in Plants. Plant Cell Rep. 2021, 41, 519–533. [Google Scholar] [CrossRef]
- Mulet, J.M.; Porcel, R.; Yenush, L. Modulation of Potassium Transport to Increase Abiotic Stress Tolerance in Plants. J. Exp. Bot. 2023, 74, 5989–6005. [Google Scholar] [CrossRef] [PubMed]
- Pyo, Y.J.; Gierth, M.; Schroeder, J.I.; Cho, M.H. High-Affinity K(+) Transport in Arabidopsis: AtHAK5 and AKT1 Are Vital for Seedling Establishment and Postgermination Growth under Low-Potassium Conditions. Plant Physiol. 2010, 153, 863–875. [Google Scholar] [CrossRef]
- Yang, L.; Liu, H.; Fu, S.M.; Ge, H.M.; Tang, R.J.; Yang, Y.; Wang, H.H.; Zhang, H.X. Na+/H+ and K+/H+ Antiporters AtNHX1 and AtNHX3 from Arabidopsis Improve Salt and Drought Tolerance in Transgenic Poplar. Biol. Plant 2017, 61, 641–650. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, Z.; Liu, C.; Shao, Z.; Zhang, N.; Suo, M.; Liu, Y.; Wang, L. Genome-Wide Identification and Drought Stress-Induced Expression Analysis of the NHX Gene Family in Potato. Front. Genet. 2024, 15, 1396375. [Google Scholar] [CrossRef]
- Grzebisz, W.; Gransee, A.; Szczepaniak, W.; Diatta, J. The Effects of Potassium Fertilization on Water-Use Efficiency in Crop Plants. J. Plant Nutr. Soil Sci. 2013, 176, 355–374. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D. Potassium Supply for Improvement of Cereals Growth under Drought: A Review. Agron. J. 2024, 116, 3368–3382. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Al Mahmud, J.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Cakmak, I.; Rengel, Z. Humboldt Review: Potassium May Mitigate Drought Stress by Increasing Stem Carbohydrates and Their Mobilization into Grains. J. Plant Physiol. 2024, 303, 154325. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Rahman, M.M.; Ghosh, T.K.; Kabir, A.H.; Abdelrahman, M.; Rahman Khan, M.A.; Mochida, K.; Tran, L.S.P. Potassium in Plant Physiological Adaptation to Abiotic Stresses. Plant Physiol. Biochem. 2022, 186, 279–289. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic Life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Fathi, A.; Shiade, S.R.G.; Saleem, A.; Shohani, F.; Fazeli, A.; Riaz, A.; Zulfiqar, U.; Shabaan, M.; Ahmed, I.; Rahimi, M. Reactive Oxygen Species (ROS) and Antioxidant Systems in Enhancing Plant Resilience Against Abiotic Stress. Int. J. Agron. 2025, 2025, 8834883. [Google Scholar] [CrossRef]
- Janku, M.; Luhová, L.; Petrivalský, M. On the Origin and Fate of Reactive Oxygen Species in Plant Cell Compartments. Antioxidants 2019, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. Production and Scavenging of Reactive Oxygen Species in Chloroplasts and Their Functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.B.; Hideg, É.; Krieger-Liszkay, A. Production, Detection, and Signaling of Singlet Oxygen in Photosynthetic Organisms. Antioxid. Redox Signal. 2013, 18, 2145–2162. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The Roles of Reactive Oxygen Metabolism in Drought: Not So Cut and Dried. Plant Physiol. 2014, 164, 1636. [Google Scholar] [CrossRef]
- Huang, S.; Van Aken, O.; Schwarzländer, M.; Belt, K.; Millar, A.H. The Roles of Mitochondrial Reactive Oxygen Species in Cellular Signaling and Stress Response in Plants. Plant Physiol. 2016, 171, 1551–1559. [Google Scholar] [CrossRef]
- Vanlerberghe, G.C. Alternative Oxidase: A Mitochondrial Respiratory Pathway to Maintain Metabolic and Signaling Homeostasis during Abiotic and Biotic Stress in Plants. Int. J. Mol. Sci. 2013, 14, 6805–6847. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive Oxygen Species Homeostasis and Signalling during Drought and Salinity Stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, W.; Li, B.; Wang, Y.; Li, K.; Sodmergen, T.; Han, C.; Zhang, Y.; Li, X. An Endoplasmic Reticulum Response Pathway Mediates Programmed Cell Death of Root Tip Induced by Water Stress in Arabidopsis. New Phytol. 2010, 186, 681–695. [Google Scholar] [CrossRef]
- Zheng, C.; Chen, J.P.; Wang, X.W.; Li, P. Reactive Oxygen Species in Plants: Metabolism, Signaling, and Oxidative Modifications. Antioxidants 2025, 14, 617. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Daudi, A.; Butt, V.S.; Bolwell, G.P. Reactive Oxygen Species and Their Role in Plant Defence and Cell Wall Metabolism. Planta 2012, 236, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, W.C.; Han, C.; Wang, S.; Bai, M.Y.; Song, C.P. Reactive Oxygen Species: Multidimensional Regulators of Plant Adaptation to Abiotic Stress and Development. J. Integr. Plant Biol. 2024, 66, 330–367. [Google Scholar] [CrossRef]
- Bienert, G.P.; Møller, A.L.B.; Kristiansen, K.A.; Schulz, A.; Møller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific Aquaporins Facilitate the Diffusion of Hydrogen Peroxide across Membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Schoebel, S.; Schmitz, F.; Dong, H.; Hedfalk, K. Characterization of Aquaporin-Driven Hydrogen Peroxide Transport. Biochim. Biophys. Acta (BBA) Biomembr. 2020, 1862, 183065. [Google Scholar] [CrossRef] [PubMed]
- Černý, M.; Habánová, H.; Berka, M.; Luklová, M.; Brzobohatý, B. Hydrogen Peroxide: Its Role in Plant Biology and Crosstalk with Signalling Networks. Int. J. Mol. Sci. 2018, 19, 2812. [Google Scholar] [CrossRef]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaev, V.; Dangl, J.L.; Mittler, R. The Plant NADPH Oxidase RBOHD Mediates Rapid Systemic Signaling in Response to Diverse Stimuli. Sci. Signal. 2009, 2, ra45. [Google Scholar] [CrossRef]
- Dubiella, U.; Seybold, H.; Durian, G.; Komander, E.; Lassig, R.; Witte, C.P.; Schulze, W.X.; Romeis, T. Calcium-Dependent Protein Kinase/NADPH Oxidase Activation Circuit Is Required for Rapid Defense Signal Propagation. Proc. Natl. Acad. Sci. USA 2013, 110, 8744–8749. [Google Scholar] [CrossRef]
- Gilroy, S.; Suzuki, N.; Miller, G.; Choi, W.G.; Toyota, M.; Devireddy, A.R.; Mittler, R. A Tidal Wave of Signals: Calcium and ROS at the Forefront of Rapid Systemic Signaling. Trends Plant Sci. 2014, 19, 623–630. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Gill, R.; Yadav, S.; Hasanuzzaman, M.; Fujita, M.; Mishra, P.; Sabat, S.C.; Tuteja, N. Superoxide Dismutase—Mentor of Abiotic Stress Tolerance in Crop Plants. Environ. Sci. Pollut. Res. 2015, 22, 10375–10394. [Google Scholar] [CrossRef]
- Cruz De Carvalho, M.H. Drought Stress and Reactive Oxygen Species. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- Mishra, N.; Jiang, C.; Chen, L.; Paul, A.; Chatterjee, A.; Shen, G. Achieving Abiotic Stress Tolerance in Plants through Antioxidative Defense Mechanisms. Front. Plant Sci. 2023, 14, 1110622. [Google Scholar] [CrossRef]
- Mohagheghian, B.; Saeidi, G.; Arzani, A. Phenolic Compounds, Antioxidant Enzymes, and Oxidative Stress in Barley (Hordeum vulgare L.) Genotypes under Field Drought-Stress Conditions. BMC Plant Biol. 2025, 25, 709. [Google Scholar] [CrossRef]
- Jurado-Mañogil, C.; Martínez-Melgarejo, P.A.; Martínez-García, P.; Rubio, M.; Hernández, J.A.; Barba-Espín, G.; Diaz-Vivancos, P.; Martínez-García, P.J. Comprehensive Study of the Hormonal, Enzymatic and Osmoregulatory Response to Drought in Prunus Species. Sci. Hortic. 2024, 326, 112786. [Google Scholar] [CrossRef]
- Hou, P.; Wang, F.; Luo, B.; Li, A.; Wang, C.; Shabala, L.; Ahmed, H.A.I.; Deng, S.; Zhang, H.; Song, P.; et al. Antioxidant Enzymatic Activity and Osmotic Adjustment as Components of the Drought Tolerance Mechanism in Carex duriuscula. Plants 2021, 10, 436. [Google Scholar] [CrossRef]
- Seminario, A.; Song, L.; Zulet, A.; Nguyen, H.T.; González, E.M.; Larrainzar, E. Drought Stress Causes a Reduction in the Biosynthesis of Ascorbic Acid in Soybean Plants. Front. Plant Sci. 2017, 8, 264600. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Munné-Bosch, S. Isoprenoids: An Evolutionary Pool for Photoprotection. Trends Plant Sci. 2005, 10, 166–169. [Google Scholar] [CrossRef]
- Hix, L.M.; Lockwood, S.F.; Bertram, J.S. Bioactive Carotenoids: Potent Antioxidants and Regulators of Gene Expression. Redox Rep. 2004, 9, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants in Plants: Location and Functional Significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Gouda, M.H.B.; Cordella, C.B.Y.; Duarte-Sierra, A. Advances in Reactive Oxygen Species Detection across Biological Systems with Relevance to Postharvest Research. Sci. Hortic. 2025, 353, 114485. [Google Scholar] [CrossRef]
- Akter, S.; Khan, M.S.; Smith, E.N.; Flashman, E. Measuring ROS and Redox Markers in Plant Cells. RSC Chem. Biol. 2021, 2, 1384–1401. [Google Scholar] [CrossRef]
- Martin, R.E.; Postiglione, A.E.; Muday, G.K. Reactive Oxygen Species Function as Signaling Molecules in Controlling Plant Development and Hormonal Responses. Curr. Opin. Plant Biol. 2022, 69, 102293. [Google Scholar] [CrossRef] [PubMed]
- Venkidasamy, B.; Karthikeyan, M.; Ramalingam, S. Methods/Protocols for Determination of Oxidative Stress in Crop Plants. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 421–435. [Google Scholar] [CrossRef]
- Yamada, M.; Morishita, H.; Urano, K.; Shiozaki, N.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Yoshiba, Y. Effects of Free Proline Accumulation in Petunias under Drought Stress. J. Exp. Bot. 2005, 56, 1975–1981. [Google Scholar] [CrossRef]
- Wang, G.P.; Zhang, X.Y.; Li, F.; Luo, Y.; Wang, W. Overaccumulation of Glycine Betaine Enhances Tolerance to Drought and Heat Stress in Wheat Leaves in the Protection of Photosynthesis. Photosynthetica 2010, 48, 117–126. [Google Scholar] [CrossRef]
- Khan, P.; Abdelbacki, A.M.M.; Albaqami, M.; Jan, R.; Kim, K.M. Proline Promotes Drought Tolerance in Maize. Biology 2025, 14, 41. [Google Scholar] [CrossRef]
- Iskandar, H.M.; Casu, R.E.; Fletcher, A.T.; Schmidt, S.; Xu, J.; Maclean, D.J.; Manners, J.M.; Bonnett, G.D. Identification of Drought-Response Genes and a Study of Their Expression during Sucrose Accumulation and Water Deficit in Sugarcane Culms. BMC Plant Biol. 2011, 11, 12. [Google Scholar] [CrossRef]
- Kim, Y.N.; Khan, M.A.; Kang, S.M.; Hamayun, M.; Lee, I.J. Enhancement of Drought-Stress Tolerance of Brassica oleracea Var. italica L. by Newly Isolated Variovorax sp. YNA59. J. Microbiol. Biotechnol. 2020, 30, 1500–1509. [Google Scholar] [CrossRef]
- Gowtham, H.G.; Singh, B.; Murali, M.; Shilpa, N.; Prasad, M.; Aiyaz, M.; Amruthesh, K.N.; Niranjana, S.R. Induction of Drought Tolerance in Tomato upon the Application of ACC Deaminase Producing Plant Growth Promoting Rhizobacterium Bacillus subtilis Rhizo SF 48. Microbiol. Res. 2020, 234, 126422. [Google Scholar] [CrossRef]
- Chun, H.J.; Lim, L.H.; Cheong, M.S.; Baek, D.; Park, M.S.; Cho, H.M.; Lee, S.H.; Jin, B.J.; No, D.H.; Cha, Y.J.; et al. Arabidopsis Ccoaomt1 Plays a Role in Drought Stress Response via Ros- and Aba-dependent Manners. Plants 2021, 10, 831. [Google Scholar] [CrossRef]
- Zhang, H.X.; Zhang, Y.; Zhang, B.W. Pepper SBP-Box Transcription Factor, CaSBP13, Plays a Negatively Role in Drought Response. Front. Plant Sci. 2024, 15, 1412685. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Lee, Y.J.; Seo, H.U.; Kim, J.H.; Jang, C.S. Physio-Biochemical and Molecular Characterization of a Rice Drought-Insensitive TILLING Line 1 (Ditl1) Mutant. Physiol. Plant 2022, 174, e13718. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Wu, T.; Pei, T.; Wang, Z.; Yang, H.; Jiang, J.; Zhang, H.; Chen, X.; Li, J.; Xu, X. Overexpression of SlGATA17 Promotes Drought Tolerance in Transgenic Tomato Plants by Enhancing Activation of the Phenylpropanoid Biosynthetic Pathway. Front. Plant Sci. 2021, 12, 634888. [Google Scholar] [CrossRef]
- Tee, E.E.; Fairweather, S.J.; Vo, H.M.; Zhao, C.; Breakspear, A.; Kimura, S.; Carmody, M.; Wrzaczek, M.; Bröer, S.; Faulkner, C.; et al. SAL1-PAP Retrograde Signaling Orchestrates Photosynthetic and Extracellular Reactive Oxygen Species for Stress Responses. Plant J. 2025, 122, e70271. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Fluhr, R. Singlet Oxygen Plays an Essential Role in the Root’s Response to Osmotic Stress. Plant Physiol. 2018, 177, 1717–1727. [Google Scholar] [CrossRef]
- Schwarzländer, M.; Fricker, M.D.; Sweetlove, L.J. Monitoring the in Vivo Redox State of Plant Mitochondria: Effect of Respiratory Inhibitors, Abiotic Stress and Assessment of Recovery from Oxidative Challenge. Biochim. Biophys. Acta (BBA) Bioenerg. 2009, 1787, 468–475. [Google Scholar] [CrossRef]
- Ortega-Villasante, C.; Burén, S.; Blázquez-Castro, A.; Barón-Sola, Á.; Hernández, L.E. Fluorescent in Vivo Imaging of Reactive Oxygen Species and Redox Potential in Plants. Free Radic. Biol. Med. 2018, 122, 202–220. [Google Scholar] [CrossRef]
- Nietzel, T.; Elsässer, M.; Ruberti, C.; Steinbeck, J.; Ugalde, J.M.; Fuchs, P.; Wagner, S.; Ostermann, L.; Moseler, A.; Lemke, P.; et al. The Fluorescent Protein Sensor RoGFP2-Orp1 Monitors in Vivo H2O2 and Thiol Redox Integration and Elucidates Intracellular H2O2 Dynamics during Elicitor-Induced Oxidative Burst in Arabidopsis. New Phytol. 2019, 221, 1649–1664. [Google Scholar] [CrossRef] [PubMed]
- Dopp, I.J.; Kalac, K.; Mackenzie, S.A. Hydrogen Peroxide Sensor HyPer7 Illuminates Tissue-Specific Plastid Redox Dynamics. Plant Physiol. 2023, 193, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Exposito-Rodriguez, M.; Laissue, P.P.; Yvon-Durocher, G.; Smirnoff, N.; Mullineaux, P.M. Photosynthesis-Dependent H2O2 Transfer from Chloroplasts to Nuclei Provides a High-Light Signalling Mechanism. Nat. Commun. 2017, 8, 49. [Google Scholar] [CrossRef]
- Costa, A.; Drago, I.; Behera, S.; Zottini, M.; Pizzo, P.; Schroeder, J.I.; Pozzan, T.; Schiavo, F.L. H2O2 in Plant Peroxisomes: An in Vivo Analysis Uncovers a Ca2+-Dependent Scavenging System. Plant J. 2010, 62, 760–772. [Google Scholar] [CrossRef]
- Rodrigues, O.; Reshetnyak, G.; Grondin, A.; Saijo, Y.; Leonhardt, N.; Maurel, C.; Verdoucq, L. Aquaporins Facilitate Hydrogen Peroxide Entry into Guard Cells to Mediate ABA- and Pathogen-Triggered Stomatal Closure. Proc. Natl. Acad. Sci. USA 2017, 114, 9200–9205. [Google Scholar] [CrossRef]
- Haber, Z.; Lampl, N.; Meyer, A.J.; Zelinger, E.; Hipsch, M.; Rosenwasser, S. Resolving Diurnal Dynamics of the Chloroplastic Glutathione Redox State in Arabidopsis Reveals Its Photosynthetically Derived Oxidation. Plant Cell 2021, 33, 1828–1844. [Google Scholar] [CrossRef]
- Fichman, Y.; Mittler, R. A Systemic Whole-Plant Change in Redox Levels Accompanies the Rapid Systemic Response to Wounding. Plant Physiol. 2021, 186, 4–8. [Google Scholar] [CrossRef]
- Hipsch, M.; Lampl, N.; Zelinger, E.; Barda, O.; Waiger, D.; Rosenwasser, S. Sensing Stress Responses in Potato with Whole-Plant Redox Imaging. Plant Physiol. 2021, 187, 618–631. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Plant Cell Wall Loosening by Expansins. Annu. Rev. Cell Dev. Biol. 2024, 40, 329–352. [Google Scholar] [CrossRef]
- Ali, O.; Cheddadi, I.; Landrein, B.; Long, Y. Revisiting the Relationship between Turgor Pressure and Plant Cell Growth. New Phytol. 2023, 238, 62–69. [Google Scholar] [CrossRef]
- Peters, R.L.; Steppe, K.; Cuny, H.E.; De Pauw, D.J.W.; Frank, D.C.; Schaub, M.; Rathgeber, C.B.K.; Cabon, A.; Fonti, P. Turgor—A Limiting Factor for Radial Growth in Mature Conifers along an Elevational Gradient. New Phytol. 2021, 229, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Zhou, Z.; Cui, S.; Zhang, X.; Zhu, S.; Wang, Y.; Zenda, T.; Wenjing, L. Advanced Imaging-Enabled Understanding of Cell Wall Remodeling Mechanisms Mediating Plant Drought Stress Tolerance. Front. Plant Sci. 2025, 16, 1635078. [Google Scholar] [CrossRef]
- Voothuluru, P.; Wu, Y.; Sharp, R.E. Not so Hidden Anymore: Advances and Challenges in Understanding Root Growth under Water Deficits. Plant Cell 2024, 36, 1377. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, F.S.; Rodrigues, J.M.; Lima, L.L.; Mesquita, R.O.; Carpinetti, P.A.; Machado, J.P.B.; Vital, C.E.; Vidigal, P.M.; Ramos, M.E.S.; Maximiano, M.R.; et al. Remodeling of the Cell Wall as a Drought-Tolerance Mechanism of a Soybean Genotype Revealed by Global Gene Expression Analysis. aBIOTECH 2021, 2, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J.; Sorek, N. Plant Cell Wall Extensibility: Connecting Plant Cell Growth with Cell Wall Structure, Mechanics, and the Action of Wall-Modifying Enzymes. J. Exp. Bot. 2016, 67, 463–476. [Google Scholar] [CrossRef]
- Gao, Y.; Lynch, J.P. Reduced Crown Root Number Improves Water Acquisition under Water Deficit Stress in Maize (Zea mays L.). J. Exp. Bot. 2016, 67, 4545–4557. [Google Scholar] [CrossRef]
- Kang, J.; Peng, Y.; Xu, W. Crop Root Responses to Drought Stress: Molecular Mechanisms, Nutrient Regulations, and Interactions with Microorganisms in the Rhizosphere. Int. J. Mol. Sci. 2022, 23, 9310. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.; Chen, P.; Shen, X.; Zhang, Y.; Li, X.; Jiang, L.; Xie, Y.; Niu, C.; Zhang, J.; Huang, X.; et al. MdMYB88 and MdMYB124 Enhance Drought Tolerance by Modulating Root Vessels and Cell Walls in Apple. Plant Physiol. 2018, 178, 1296–1309. [Google Scholar] [CrossRef] [PubMed]
- Cantó-Pastor, A.; Kajala, K.; Shaar-Moshe, L.; Manzano, C.; Timilsena, P.; De Bellis, D.; Gray, S.; Holbein, J.; Yang, H.; Mohammad, S.; et al. A Suberized Exodermis Is Required for Tomato Drought Tolerance. Nat. Plants 2024, 10, 118–130. [Google Scholar] [CrossRef]
- Thompson, D.S.; Islam, A. Plant Cell Wall Hydration and Plant Physiology: An Exploration of the Consequences of Direct Effects of Water Deficit on the Plant Cell Wall. Plants 2021, 10, 1263. [Google Scholar] [CrossRef]
- Bacete, L.; Schulz, J.; Engelsdorf, T.; Bartosova, Z.; Vaahtera, L.; Yan, G.; Gerhold, J.M.; Ticha, T.; Øvstebø, C.; Gigli-Bisceglia, N.; et al. THESEUS1 Modulates Cell Wall Stiffness and Abscisic Acid Production in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2022, 119, e2119258119. [Google Scholar] [CrossRef]
- Cuadrado-Pedetti, M.B.; Rauschert, I.; Sainz, M.M.; Amorim-Silva, V.; Botella, M.A.; Borsani, O.; Sotelo-Silveira, M. The Arabidopsis TETRATRICOPEPTIDE THIOREDOXIN-LIKE 1 Gene Is Involved in Anisotropic Root Growth during Osmotic Stress Adaptation. Genes 2021, 12, 236. [Google Scholar] [CrossRef]
- Pietruszka, M. Solutions for a Local Equation of Anisotropic Plant Cell Growth: An Analytical Study of Expansin Activity. J. R. Soc. Interface 2011, 8, 975–987. [Google Scholar] [CrossRef]
- Daher, F.B.; Chen, Y.; Bozorg, B.; Clough, J.; Jönsson, H.; Braybrook, S.A. Anisotropic Growth Is Achieved through the Additive Mechanical Effect of Material Anisotropy and Elastic Asymmetry. eLife 2018, 7, e38161. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Plant Expansins: Diversity and Interactions with Plant Cell Walls. Curr. Opin. Plant Biol. 2015, 25, 162–172. [Google Scholar] [CrossRef]
- Chen, Y.; Han, Y.; Meng, Z.; Zhou, S.; Xiangzhu, K.; Wei, W. Overexpression of the Wheat Expansin Gene TaEXPA2 Improved Seed Production and Drought Tolerance in Transgenic Tobacco Plants. PLoS ONE 2016, 11, e0153494. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Hao, W.; Zhang, L.; Liu, Y.; Chen, L. Expression of Two α-Type Expansins from Ammopiptanthus nanus in Arabidopsis thaliana Enhance Tolerance to Cold and Drought Stresses. Int. J. Mol. Sci. 2019, 20, 5255. [Google Scholar] [CrossRef]
- Ashwin Narayan, J.; Chakravarthi, M.; Nerkar, G.; Manoj, V.M.; Dharshini, S.; Subramonian, N.; Premachandran, M.N.; Arun Kumar, R.; Krishna Surendar, K.; Hemaprabha, G.; et al. Overexpression of Expansin EaEXPA1, a Cell Wall Loosening Protein Enhances Drought Tolerance in Sugarcane. Ind. Crops Prod. 2021, 159, 113035. [Google Scholar] [CrossRef]
- Chebli, Y.; Geitmann, A. Cellular Growth in Plants Requires Regulation of Cell Wall Biochemistry. Curr. Opin. Cell Biol. 2017, 44, 28–35. [Google Scholar] [CrossRef]
- Stratilová, B.; Kozmon, S.; Stratilová, E.; Hrmova, M. Plant Xyloglucan Xyloglucosyl Transferases and the Cell Wall Structure: Subtle but Significant. Molecules 2020, 25, 5619. [Google Scholar] [CrossRef]
- Eklöf, J.M.; Brumer, H. The XTH Gene Family: An Update on Enzyme Structure, Function, and Phylogeny in Xyloglucan Remodeling. Plant Physiol. 2010, 153, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.Z.; Zhu, X.F.; Miller, J.G.; Gregson, T.; Zheng, S.J.; Fry, S.C. Distinct Catalytic Capacities of Two Aluminium-Repressed Arabidopsis thaliana Xyloglucan Endotransglucosylase/Hydrolases, XTH15 and XTH31, Heterologously Produced in Pichia. Phytochemistry 2015, 112, 160–169. [Google Scholar] [CrossRef]
- Franková, L.; Fry, S.C. Biochemistry and Physiological Roles of Enzymes That ‘Cut and Paste’ Plant Cell-Wall Polysaccharides. J. Exp. Bot. 2013, 64, 3519–3550. [Google Scholar] [CrossRef]
- Hrmova, M.; Farkas, V.; Lahnstein, J.; Fincher, G.B. A Barley Xyloglucan Xyloglucosyl Transferase Covalently Links Xyloglucan, Cellulosic Substrates, and (1,3;1,4)-β-D-Glucans. J. Biol. Chem. 2007, 282, 12951–12962. [Google Scholar] [CrossRef] [PubMed]
- Iurlaro, A.; De Caroli, M.; Sabella, E.; De Pascali, M.; Rampino, P.; De Bellis, L.; Perrotta, C.; Dalessandro, G.; Piro, G.; Fry, S.C.; et al. Drought and Heat Differentially Affect XTH Expression and XET Activity and Action in 3-Day-Old Seedlings of Durum Wheat Cultivars with Different Stress Susceptibility. Front. Plant Sci. 2016, 7, 1686. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Yao, F.; Liu, Y.; Xiao, H.; Sun, S.; Jiang, C.; An, Y.; Chen, N.; Huang, L.; Lu, M.; et al. Identification of the Xyloglucan Endotransglycosylase/Hydrolase Genes and the Role of PagXTH12 in Drought Resistance in Poplar. For. Res. 2024, 4, e039. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Chen, Y.; Liu, M.; Guo, X.; Zhang, R.; Luo, K.; Chen, Y. The XTH Gene Family in Cassava: Genomic Characterization, Evolutionary Dynamics, and Functional Roles in Abiotic Stress and Hormonal Response. Agronomy 2025, 15, 2194. [Google Scholar] [CrossRef]
- Braybrook, S.A.; Hofte, H.; Peaucelle, A. Probing the Mechanical Contributions of the Pectin Matrix: Insights for Cell Growth. Plant Signal. Behav. 2012, 7, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Hocq, L.; Pelloux, J.; Lefebvre, V. Connecting Homogalacturonan-Type Pectin Remodeling to Acid Growth. Trends Plant Sci. 2017, 22, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Voxeur, A.; Höfte, H. Cell Wall Integrity Signaling in Plants: “To Grow or Not to Grow That’s the Question”. Glycobiology 2016, 26, 950–960. [Google Scholar] [CrossRef]
- Sénéchal, F.; Wattier, C.; Rustérucci, C.; Pelloux, J. Homogalacturonan-Modifying Enzymes: Structure, Expression, and Roles in Plants. J. Exp. Bot. 2014, 65, 5125–5160. [Google Scholar] [CrossRef]
- Sénéchal, F.; Mareck, A.; Marcelo, P.; Lerouge, P.; Pelloux, J. Arabidopsis PME17 Activity Can Be Controlled by Pectin Methylesterase Inhibitor4. Plant Signal. Behav. 2015, 10, e983351. [Google Scholar] [CrossRef] [PubMed]
- Gallemí, M.; Montesinos, J.C.; Zarevski, N.; Pribyl, J.; Skládal, P.; Hannezo, E.; Benková, E. Dual Role of Pectin Methyl Esterase Activity in the Regulation of Plant Cell Wall Biophysical Properties. Front. Plant Sci. 2025, 16, 1612366. [Google Scholar] [CrossRef]
- Obomighie, I.; Prentice, I.J.; Lewin-Jones, P.; Bachtiger, F.; Ramsay, N.; Kishi-Itakura, C.; Goldberg, M.W.; Hawkins, T.J.; Sprittles, J.E.; Knight, H.; et al. Understanding Pectin Cross-Linking in Plant Cell Walls. Commun. Biol. 2025, 8. [Google Scholar] [CrossRef]
- Jung, N.U.; Giarola, V.; Chen, P.; Knox, J.P.; Bartels, D. Craterostigma plantagineum Cell Wall Composition Is Remodelled during Desiccation and the Glycine-Rich Protein CpGRP1 Interacts with Pectins through Clustered Arginines. Plant J. 2019, 100, 661–676. [Google Scholar] [CrossRef]
- Phyo, P.; Gu, Y.; Hong, M. Impact of Acidic PH on Plant Cell Wall Polysaccharide Structure and Dynamics: Insights into the Mechanism of Acid Growth in Plants from Solid-State NMR. Cellulose 2018, 26, 291–304. [Google Scholar] [CrossRef]
- Wormit, A.; Usadel, B. The Multifaceted Role of Pectin Methylesterase Inhibitors (PMEIs). Int. J. Mol. Sci. 2018, 19, 2878. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, K.; Lathe, R.S.; Kierzkowski, D.; Routier-Kierzkowska, A.L.; Hamant, O.; Bhalerao, R.P. Mechanochemical Feedback Mediates Tissue Bending Required for Seedling Emergence. Curr. Biol. 2021, 31, 1154–1164.e3. [Google Scholar] [CrossRef] [PubMed]
- Peaucelle, A.; Wightman, R.; Höfte, H. The Control of Growth Symmetry Breaking in the Arabidopsis Hypocotyl. Curr. Biol. 2015, 25, 1746–1752. [Google Scholar] [CrossRef]
- Leucci, M.R.; Lenucci, M.S.; Piro, G.; Dalessandro, G. Water Stress and Cell Wall Polysaccharides in the Apical Root Zone of Wheat Cultivars Varying in Drought Tolerance. J. Plant Physiol. 2008, 165, 1168–1180. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.; Liu, H.; Qiu, R.; Gao, Y.; Duan, A. Role of Hydraulic Signal and ABA in Decrease of Leaf Stomatal and Mesophyll Conductance in Soil Drought-Stressed Tomato. Front. Plant Sci. 2021, 12, 653186. [Google Scholar] [CrossRef]
- Forand, A.D.; Finfrock, Y.Z.; Lavier, M.; Stobbs, J.; Qin, L.; Wang, S.; Karunakaran, C.; Wei, Y.; Ghosh, S.; Tanino, K.K. With a Little Help from My Cell Wall: Structural Modifications in Pectin May Play a Role to Overcome Both Dehydration Stress and Fungal Pathogens. Plants 2022, 11, 385. [Google Scholar] [CrossRef]
- Hongo, S.; Sato, K.; Yokoyama, R.; Nishitani, K. Demethylesterification of the Primary Wall by PECTIN METHYLESTERASE35 Provides Mechanical Support to the Arabidopsis Stem. Plant Cell 2012, 24, 2624–2634. [Google Scholar] [CrossRef]
- Yang, W.; Ruan, M.; Xiang, M.; Deng, A.; Du, J.; Xiao, C. Overexpression of a Pectin Methylesterase Gene PtoPME35 from Populus Tomentosa Influences Stomatal Function and Drought Tolerance in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2020, 523, 416–422. [Google Scholar] [CrossRef]
- Arsuffi, G.; Braybrook, S.A. Acid Growth: An Ongoing Trip. J. Exp. Bot. 2018, 69, 137–146. [Google Scholar] [CrossRef]
- Barbez, E.; Dünser, K.; Gaidora, A.; Lendl, T.; Busch, W. Auxin Steers Root Cell Expansion via Apoplastic PH Regulation in Arabidopsis Thaliana. Proc. Natl. Acad. Sci. USA 2017, 114, E4884–E4893. [Google Scholar] [CrossRef]
- Pacifici, E.; Mambro, R.D.; Dello Ioio, R.; Costantino, P.; Sabatini, S. Acidic Cell Elongation Drives Cell Differentiation in the Arabidopsis Root. EMBO J. 2018, 37, e99134. [Google Scholar] [CrossRef]
- Du, M.; Spalding, E.P.; Gray, W.M. Rapid Auxin-Mediated Cell Expansion. Annu. Rev. Plant Biol. 2020, 71, 379–402. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Lindner, H.; Robbins, N.E.; Dinneny, J.R. Growing Out of Stress: The Role of Cell- and Organ-Scale Growth Control in Plant Water-Stress Responses. Plant Cell 2016, 28, 1769–1782. [Google Scholar] [CrossRef]
- Młodzińska-Michta, E.; Swiezewska, E.; Hoffman-Sommer, M.; Piłka, N.; Radkiewicz, M.; Jarzembowski, P. Adaptation of the Maize Seedling Seminal Roots to Drought: Essential Role of Plasma Membrane H+-ATPases Activity. Acta Soc. Bot. Pol. 2023, 92, 1–15. [Google Scholar] [CrossRef]
- Katsuhama, N.; Sakoda, K.; Kimura, H.; Shimizu, Y.; Sakai, Y.; Nagata, K.; Abe, M.; Terashima, I.; Yamori, W. PROTON ATPASE TRANSLOCATION CONTROL 1-Mediated H+-ATPase Translocation Boosts Plant Growth under Drought by Optimizing Root and Leaf Functions. PNAS Nexus 2025, 4, pgaf151. [Google Scholar] [CrossRef] [PubMed]
- Pei, D.; Hua, D.; Deng, J.; Wang, Z.; Song, C.; Wang, Y.; Wang, Y.; Qi, J.; Kollist, H.; Yang, S.; et al. Phosphorylation of the Plasma Membrane H+-ATPase AHA2 by BAK1 Is Required for ABA-Induced Stomatal Closure in Arabidopsis. Plant Cell 2022, 34, 2708–2729. [Google Scholar] [CrossRef] [PubMed]
- Geilfus, C.M. The PH of the Apoplast: Dynamic Factor with Functional Impact Under Stress. Mol. Plant 2017, 10, 1371–1386. [Google Scholar] [CrossRef]
- Miao, R.; Yuan, W.; Wang, Y.; Garcia-Maquilon, I.; Dang, X.; Li, Y.; Zhang, J.; Zhu, Y.; Rodriguez, P.L.; Xu, W. Low ABA Concentration Promotes Root Growth and Hydrotropism through Relief of ABA INSENSITIVE 1-Mediated Inhibition of Plasma Membrane H+-ATPase 2. Sci. Adv. 2021, 7, eabd4113. [Google Scholar] [CrossRef]
- Sharma, A.; Choudhary, P.; Chakdar, H.; Shukla, P. Molecular Insights and Omics-Based Understanding of Plant-Microbe Interactions under Drought Stress. World J. Microbiol. Biotechnol. 2023, 40, 42. [Google Scholar] [CrossRef]
- Takahashi, K.; Hayashi, K.I.; Kinoshita, T. Auxin Activates the Plasma Membrane H+-ATPase by Phosphorylation during Hypocotyl Elongation in Arabidopsis. Plant Physiol. 2012, 159, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.; Klejchová, M.; Snipes, S.A.; Nagpal, P.; Bak, G.; Wang, B.; Dunlap, S.; Park, M.Y.; Kunkel, E.N.; Trinidad, B.; et al. SAUR Proteins and PP2C.D Phosphatases Regulate H+-ATPases and K+ Channels to Control Stomatal Movements. Plant Physiol. 2021, 185, 256–273. [Google Scholar] [CrossRef]
- Xue, Y.; Yang, Y.; Yang, Z.; Wang, X.; Guo, Y. VAMP711 Is Required for Abscisic Acid-Mediated Inhibition of Plasma Membrane H+-ATPase Activity. Plant Physiol 2018, 178, 1332–1343. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Li, M.; Lamin-Samu, A.T.; Yang, D.; Yu, X.; Izhar, M.; Jan, I.; Ali, M.; Lu, G. The Arabidopsis SMALL AUXIN UP RNA32 Protein Regulates ABA-Mediated Responses to Drought Stress. Front. Plant Sci. 2021, 12, 625493. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, W.; Wang, Q.; Cao, Y.; Xu, F.; Dodd, I.C.; Xu, W. ABA Regulation of Root Growth during Soil Drying and Recovery Can Involve Auxin Response. Plant Cell Environ. 2022, 45, 871–883. [Google Scholar] [CrossRef]
- Claeys, H.; Inzé, D. The Agony of Choice: How Plants Balance Growth and Survival under Water-Limiting Conditions. Plant Physiol. 2013, 162, 1768. [Google Scholar] [CrossRef] [PubMed]
- Nardini, A. Hard and Tough: The Coordination between Leaf Mechanical Resistance and Drought Tolerance. Flora 2022, 288, 152023. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.-Z.; Fu, J.-Y.; Du, Y.-Y.; Qu, J.; Song, Y.; Wang, P.-W. The GmXTH1 Gene Improves Drought Stress Resistance of Soybean Seedlings. Mol. Breed. 2021, 42, 3. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wang, X.; Ye, X.; Liu, C.; Han, J. ZmEXPB7, a β-Expansin Gene, Contributes to Drought Tolerance in Arabidopsis. Front. Genet. 2025, 16, 1688658. [Google Scholar] [CrossRef]
- DIetrich, D.; Pang, L.; Kobayashi, A.; Fozard, J.A.; Boudolf, V.; Bhosale, R.; Antoni, R.; Nguyen, T.; Hiratsuka, S.; Fujii, N.; et al. Root Hydrotropism Is Controlled via a Cortex-Specific Growth Mechanism. Nat. Plants 2017, 3. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, S.; Chen, Y.; Yao, Z.; Jiang, S.; Wang, L.; Zhu, X.; Xu, W.; Zhang, J.; Li, Y. Amyloplast Is Involved in the MIZ1-Modulated Root Hydrotropism. J. Plant Physiol. 2024, 296, 154224. [Google Scholar] [CrossRef] [PubMed]
- Shkolnik, D.; Krieger, G.; Nuriel, R.; Fromm, H. Hydrotropism: Root Bending Does Not Require Auxin Redistribution. Mol. Plant 2016, 9, 757–759. [Google Scholar] [CrossRef]
- Akita, K.; Miyazawa, Y. Auxin Biosynthesis, Transport, and Response Directly Attenuate Hydrotropism in the Latter Stages to Fine-Tune Root Growth Direction in Arabidopsis. Physiol. Plant 2023, 175, e14051. [Google Scholar] [CrossRef]
- Cochard, H.; Badel, E.; Herbette, S.; Delzon, S.; Choat, B.; Jansen, S. Methods for Measuring Plant Vulnerability to Cavitation: A Critical Review. J. Exp. Bot. 2013, 64, 4779–4791. [Google Scholar] [CrossRef] [PubMed]
- Mucchiani, C.; Karydis, K. Development of an Automated and Artificial Intelligence Assisted Pressure Chamber for Stem Water Potential Determination. Comput. Electron. Agric. 2024, 222, 109016. [Google Scholar] [CrossRef]
- Rodriguez-Dominguez, C.M.; Forner, A.; Martorell, S.; Choat, B.; Lopez, R.; Peters, J.M.R.; Pfautsch, S.; Mayr, S.; Carins-Murphy, M.R.; McAdam, S.A.M.; et al. Leaf Water Potential Measurements Using the Pressure Chamber: Synthetic Testing of Assumptions towards Best Practices for Precision and Accuracy. Plant Cell Environ. 2022, 45, 2037–2061. [Google Scholar] [CrossRef]
- Carella, A.; Fischer, B.; Massenti, P.T.; Lo Bianco, R.; Carella, A.; Tomas, P.; Massenti, R.; Lo Bianco, R. Continuous Plant-Based and Remote Sensing for Determination of Fruit Tree Water Status. Horticulturae 2024, 10, 516. [Google Scholar] [CrossRef]
- Mucchiani, C.; Zaccaria, D.; Karydis, K. Assessing the Potential of Integrating Automation and Artificial Intelligence across Sample-Destructive Methods to Determine Plant Water Status: A Review and Score-Based Evaluation. Comput. Electron. Agric. 2024, 224, 108992. [Google Scholar] [CrossRef]
- Dainese, R.; Lopes, B.d.C.F.L.; Tedeschi, G.; Lamarque, L.J.; Delzon, S.; Fourcaud, T.; Tarantino, A. Cross-Validation of the High-Capacity Tensiometer and Thermocouple Psychrometer for Continuous Monitoring of Xylem Water Potential in Saplings. J. Exp. Bot. 2022, 73, 400–412. [Google Scholar] [CrossRef]
- Dainese, R.; Tarantino, A. Measurement of Plant Xylem Water Pressure Using the High-Capacity Tensiometer and Implications for the Modelling of Soil–Atmosphere Interaction. Géotechnique 2021, 71, 441–454. [Google Scholar] [CrossRef]
- Black, W.L.; Santiago, M.; Zhu, S.; Stroock, A.D. Ex Situ and In Situ Measurement of Water Activity with a MEMS Tensiometer. Anal. Chem. 2020, 92, 716–723. [Google Scholar] [CrossRef]
- Kisekka, I.; Peddinti, S.R.; Savchik, P.; Yang, L.; Culumber, M.; Bali, K.; Milliron, L.; Edwards, E.; Nocco, M.; Reyes, C.A.; et al. Multisite Evaluation of Microtensiometer and Osmotic Cell Stem Water Potential Sensors in Almond Orchards. Comput. Electron. Agric. 2024, 227, 109547. [Google Scholar] [CrossRef]
- Blanco, V.; Kalcsits, L. Microtensiometers Accurately Measure Stem Water Potential in Woody Perennials. Plants 2021, 10, 2780. [Google Scholar] [CrossRef]
- Conesa, M.R.; Conejero, W.; Vera, J.; Ruiz-Sánchez, M.C. Assessment of Trunk Microtensiometer as a Novel Biosensor to Continuously Monitor Plant Water Status in Nectarine Trees. Front. Plant Sci. 2023, 14, 1123045. [Google Scholar] [CrossRef]
- Vaccaro, G.; Fusco, M.; Alagna, V.; Franco, L.; Motisi, A.; Iovino, M. Assessing Microtensiometers for Monitoring Stem Water Potential in Mandarin (Citrus reticulata Blanco) Orchard under Different Irrigation Regimes. Agric. Water Manag. 2025, 320, 109873. [Google Scholar] [CrossRef]
- Jain, P.; Liu, W.; Zhu, S.; Chang, C.Y.Y.; Melkonian, J.; Rockwell, F.E.; Pauli, D.; Sun, Y.; Zipfel, W.R.; Michele Holbrook, N.; et al. A Minimally Disruptive Method for Measuring Water Potential in Planta Using Hydrogel Nanoreporters. Proc. Natl. Acad. Sci. USA 2021, 118, e2008276118. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Villadangos, S. Cheap, Cost-Effective, and Quick Stress Biomarkers for Drought Stress Detection and Monitoring in Plants. Trends Plant Sci. 2023, 28, 527–536. [Google Scholar] [CrossRef]
- Jain, P.; Sen, S.; Rockwell, F.E.; Twohey, R.J.; Huber, A.E.; Desai, S.A.; Wu, I.F.; De Swaef, T.; Ilman, M.M.; Studer, A.J.; et al. Loss of Conductance between Mesophyll Symplasm and Intercellular Air Spaces Explains Nonstomatal Control of Transpiration. Proc. Natl. Acad. Sci. USA 2025, 122, e2504862122. [Google Scholar] [CrossRef]
- Turner, N.C. Turgor Maintenance by Osmotic Adjustment: 40 Years of Progress. J. Exp. Bot. 2018, 69, 3223–3233. [Google Scholar] [CrossRef] [PubMed]
- Beauzamy, L.; Nakayama, N.; Boudaoud, A. Flowers under Pressure: Ins and Outs of Turgor Regulation in Development. Ann. Bot. 2014, 114, 1517–1533. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, M.K.; Scoffoni, C.; Ardy, R.; Zhang, Y.; Sun, S.; Cao, K.; Sack, L. Rapid Determination of Comparative Drought Tolerance Traits: Using an Osmometer to Predict Turgor Loss Point. Methods Ecol. Evol. 2012, 3, 880–888. [Google Scholar] [CrossRef]
- Ball, R.A.; Oosterhuis, D.M. Measurement of Root and Leaf Osmotic Potential Using the Vapor-Pressure Osmometer. Environ. Exp. Bot. 2005, 53, 77–84. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Scoffoni, C.; Sack, L. The Determinants of Leaf Turgor Loss Point and Prediction of Drought Tolerance of Species and Biomes: A Global Meta-Analysis. Ecol. Lett. 2012, 15, 393–405. [Google Scholar] [CrossRef]
- Banks, J.M.; Hirons, A.D. Alternative Methods of Estimating the Water Potential at Turgor Loss Point in Acer Genotypes. Plant Methods 2019, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Bristow, S.T.; Knipfer, T. Extension of the Triphasic Water Potential Curve: Accounting for Air Vapor Pressure Deficit under Soil Water Stress. Plant Physiol. 2025, 199, 337. [Google Scholar] [CrossRef] [PubMed]
- Knipfer, T.; Bambach, N.; Isabel Hernandez, M.; Bartlett, M.K.; Sinclair, G.; Duong, F.; Kluepfel, D.A.; McElrone, A.J. Predicting Stomatal Closure and Turgor Loss in Woody Plants Using Predawn and Midday Water Potential. Plant Physiol. 2020, 184, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Leuschner, C.; Wedde, P.; Lübbe, T. The Relation between Pressure–Volume Curve Traits and Stomatal Regulation of Water Potential in Five Temperate Broadleaf Tree Species. Ann. For. Sci. 2019, 76, 60. [Google Scholar] [CrossRef]
- Castillo-Argaez, R.; Sapes, G.; Mallen, N.; Lippert, A.; John, G.P.; Zare, A.; Hammond, W.M. Spectral Ecophysiology: Hyperspectral Pressure–Volume Curves to Estimate Leaf Turgor Loss. New Phytol. 2024, 242, 935–946. [Google Scholar] [CrossRef]
- Powell, T.L.; Wheeler, J.K.; de Oliveira, A.A.R.; da Costa, A.C.L.; Saleska, S.R.; Meir, P.; Moorcroft, P.R. Differences in Xylem and Leaf Hydraulic Traits Explain Differences in Drought Tolerance among Mature Amazon Rainforest Trees. Glob. Change Biol. 2017, 23, 4280–4293. [Google Scholar] [CrossRef]
- Martin, A.R.; Li, G.; Cui, B.; Mariani, R.O.; Vicario, K.; Cathline, K.A.; Findlay, A.; Robertson, G. A High-Throughput Approach for Quantifying Turgor Loss Point in Grapevine. Plant Methods 2024, 20, 180. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-Use Efficiency: Advances and Challenges in a Changing Climate. Front. Plant Sci. 2019, 10, 429990. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Vialet-Chabrand, S. Speedy Stomata, Photosynthesis and Plant Water Use Efficiency. New Phytol. 2019, 221, 93–98. [Google Scholar] [CrossRef]
- Leakey, A.D.B.; Ferguson, J.N.; Pignon, C.P.; Wu, A.; Jin, Z.; Hammer, G.L.; Lobell, D.B. Water Use Efficiency as a Constraint and Target for Improving the Resilience and Productivity of C3 and C4 Crops. Annu. Rev. Plant Biol. 2019, 70, 781–808. [Google Scholar] [CrossRef]
- Petrík, P.; Petek-Petrik, A.; Mukarram, M.; Schuldt, B.; Lamarque, L.J. Leaf Physiological and Morphological Constraints of Water-Use Efficiency in C3 Plants. AoB Plants 2023, 15, plad047. [Google Scholar] [CrossRef]
- Medrano, H.; Tomás, M.; Martorell, S.; Flexas, J.; Hernández, E.; Rosselló, J.; Pou, A.; Escalona, J.M.; Bota, J. From Leaf to Whole-Plant Water Use Efficiency (WUE) in Complex Canopies: Limitations of Leaf WUE as a Selection Target. Crop. J. 2015, 3, 220–228. [Google Scholar] [CrossRef]
- Brendel, O.; Epron, D. Are Differences among Forest Tree Populations in Carbon Isotope Composition an Indication of Adaptation to Drought? Tree Physiol. 2022, 42, 26–31. [Google Scholar] [CrossRef]
- Yang, Y.; Guan, H.; Batelaan, O.; McVicar, T.R.; Long, D.; Piao, S.; Liang, W.; Liu, B.; Jin, Z.; Simmons, C.T. Contrasting Responses of Water Use Efficiency to Drought across Global Terrestrial Ecosystems. Sci. Rep. 2016, 6, 23284. [Google Scholar] [CrossRef] [PubMed]
- Buezo, J.; Sanz-Saez, Á.; Moran, J.F.; Soba, D.; Aranjuelo, I.; Esteban, R. Drought Tolerance Response of High-Yielding Soybean Varieties to Mild Drought: Physiological and Photochemical Adjustments. Physiol. Plant 2019, 166, 88–104. [Google Scholar] [CrossRef]
- Kørup, K.; Lærke, P.E.; Baadsgaard, H.; Andersen, M.N.; Kristensen, K.; Münnich, C.; Didion, T.; Jensen, E.S.; Mårtensson, L.M.; Jørgensen, U. Biomass Production and Water Use Efficiency in Perennial Grasses during and after Drought Stress. GCB Bioenergy 2018, 10, 12–27. [Google Scholar] [CrossRef]
- Roby, M.C.; Salas Fernandez, M.G.; Heaton, E.A.; Miguez, F.E.; VanLoocke, A. Biomass Sorghum and Maize Have Similar Water-Use-Efficiency under Non-Drought Conditions in the Rain-Fed Midwest U.S. Agric. For. Meteorol. 2017, 247, 434–444. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, L.; Wang, Y.P.; Canadell, J.G.; Chiew, F.H.S.; Beringer, J.; Li, L.; Miralles, D.G.; Piao, S.; Zhang, Y. Recent Increases in Terrestrial Carbon Uptake at Little Cost to the Water Cycle. Nat. Commun. 2017, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Jezek, M.; Blatt, M.R. The Membrane Transport System of the Guard Cell and Its Integration for Stomatal Dynamics. Plant Physiol. 2017, 174, 487–519. [Google Scholar] [CrossRef]
- Sirichandra, C.; Gu, D.; Hu, H.C.; Davanture, M.; Lee, S.; Djaoui, M.; Valot, B.; Zivy, M.; Leung, J.; Merlot, S.; et al. Phosphorylation of the Arabidopsis AtrbohF NADPH Oxidase by OST1 Protein Kinase. FEBS Lett. 2009, 583, 2982–2986. [Google Scholar] [CrossRef]
- Postiglione, A.E.; Muday, G.K. The Role of ROS Homeostasis in ABA-Induced Guard Cell Signaling. Front. Plant Sci. 2020, 11, 968. [Google Scholar] [CrossRef]
- Spartz, A.K.; Ren, H.; Park, M.Y.; Grandt, K.N.; Lee, S.H.; Murphy, A.S.; Sussman, M.R.; Overvoorde, P.J.; Gray, W.M. SAUR Inhibition of PP2C-D Phosphatases Activates Plasma Membrane H+-ATPases to Promote Cell Expansion in Arabidopsis. Plant Cell 2014, 26, 2129–2142. [Google Scholar] [CrossRef]
- Akiyama, M.; Sugimoto, H.; Inoue, S.I.; Takahashi, Y.; Hayashi, M.; Hayashi, Y.; Mizutani, M.; Ogawa, T.; Kinoshita, D.; Ando, E.; et al. Type 2C Protein Phosphatase Clade D Family Members Dephosphorylate Guard Cell Plasma Membrane H+-ATPase. Plant Physiol. 2022, 188, 2228–2240. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Jin, X.; Albert, R.; Assmann, S.M. Multi-Level Modeling of Light-Induced Stomatal Opening Offers New Insights into Its Regulation by Drought. PLoS Comput. Biol. 2014, 10, e1003930. [Google Scholar] [CrossRef]
- Hiyama, A.; Takemiya, A.; Munemasa, S.; Okuma, E.; Sugiyama, N.; Tada, Y.; Murata, Y.; Shimazaki, K.I. Blue Light and CO2 Signals Converge to Regulate Light-Induced Stomatal Opening. Nat. Commun. 2017, 8, 1284. [Google Scholar] [CrossRef]
- Koolmeister, K.; Merilo, E.; Hõrak, H.; Kollist, H. Stomatal CO2 Responses at Sub- and above-Ambient CO2 Levels Employ Different Pathways in Arabidopsis. Plant Physiol. 2024, 196, 608–620. [Google Scholar] [CrossRef]
- Takemiya, A.; Shimazaki, K.I. Arabidopsis Phot1 and Phot2 Phosphorylate BLUS1 Kinase with Different Efficiencies in Stomatal Opening. J. Plant Res. 2016, 129, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.I.; Kinoshita, T. Blue Light Regulation of Stomatal Opening and the Plasma Membrane H+-ATPase. Plant Physiol. 2017, 174, 531–538. [Google Scholar] [CrossRef]
- Shimazaki, K.I.; Doi, M.; Assmann, S.M.; Kinoshita, T. Light Regulation of Stomatal Movement. Annu. Rev. Plant Biol. 2007, 58, 219–247. [Google Scholar] [CrossRef]
- Haworth, M.; Marino, G.; Cosentino, S.L.; Brunetti, C.; De Carlo, A.; Avola, G.; Riggi, E.; Loreto, F.; Centritto, M. Increased Free Abscisic Acid during Drought Enhances Stomatal Sensitivity and Modifies Stomatal Behaviour in Fast Growing Giant Reed (Arundo donax L.). Environ. Exp. Bot. 2018, 147, 116–124. [Google Scholar] [CrossRef]
- Yan, M.; Yao, Y.; Mou, K.; Dan, Y.; Li, W.; Wang, C.; Liao, W. The Involvement of Abscisic Acid in Hydrogen Gas-Enhanced Drought Resistance in Tomato Seedlings. Sci. Hortic. 2022, 292, 110631. [Google Scholar] [CrossRef]
- Zulfiqar, B.; Raza, M.A.S.; Saleem, M.F.; Ali, B.; Aslam, M.U.; Al-Ghamdi, A.A.; Elshikh, M.S.; Hassan, M.U.; Toleikienė, M.; Ahmed, J.; et al. Abscisic Acid Improves Drought Resilience, Growth, Physio-Biochemical and Quality Attributes in Wheat (Triticum aestivum L.) at Critical Growth Stages. Sci. Rep. 2024, 14, 20411. [Google Scholar] [CrossRef]
- Shabankareh, H.G.; Khorasaninejad, S.; Soltanloo, H.; Asgharipour, M.R. Abscisic Acid Modulation of Drought Tolerance and Essential Oil Biosynthesis in Lavandula Coronopifolia Poir. Sci. Rep. 2025, 15, 33262. [Google Scholar] [CrossRef]
- Tong, X.; Mu, Y.; Zhang, J.; Meng, P.; Li, J. Water Stress Controls on Carbon Flux and Water Use Efficiency in a Warm-Temperate Mixed Plantation. J. Hydrol. 2019, 571, 669–678. [Google Scholar] [CrossRef]
- Qiao, M.; Hong, C.; Jiao, Y.; Hou, S.; Gao, H. Impacts of Drought on Photosynthesis in Major Food Crops and the Related Mechanisms of Plant Responses to Drought. Plants 2024, 13, 1808. [Google Scholar] [CrossRef]
- Walker, B.J.; Vanloocke, A.; Bernacchi, C.J.; Ort, D.R. The Costs of Photorespiration to Food Production Now and in the Future. Annu. Rev. Plant Biol. 2016, 67, 107–129. [Google Scholar] [CrossRef]
- Zadražnik, T.; Moen, A.; Šuštar-Vozlič, J. Chloroplast Proteins Involved in Drought Stress Response in Selected Cultivars of Common Bean (Phaseolus vulgaris L.). 3 Biotech 2019, 9, 331. [Google Scholar] [CrossRef]
- Karami, S.; Shiran, B.; Ravash, R. Molecular Investigation of How Drought Stress Affects Chlorophyll Metabolism and Photosynthesis in Leaves of C3 and C4 Plant Species: A Transcriptome Meta-Analysis. Heliyon 2025, 11, e42368. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Tezara, W. Causes of Decreased Photosynthetic Rate and Metabolic Capacity in Water-Deficient Leaf Cells: A Critical Evaluation of Mechanisms and Integration of Processes. Ann. Bot. 2009, 103, 561. [Google Scholar] [CrossRef]
- Wang, Z.; Li, G.; Sun, H.; Ma, L.; Guo, Y.; Zhao, Z.; Gao, H.; Mei, L. Effects of Drought Stress on Photosynthesis and Photosynthetic Electron Transport Chain in Young Apple Tree Leaves. Biol. Open 2018, 7, bio035279. [Google Scholar] [CrossRef]
- Takahashi, S.; Murata, N. How Do Environmental Stresses Accelerate Photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef]
- Nosalewicz, A.; Okoń, K.; Skorupka, M. Non-Photochemical Quenching under Drought and Fluctuating Light. Int. J. Mol. Sci. 2022, 23, 5182. [Google Scholar] [CrossRef]
- Chen, Y.E.; Liu, W.J.; Su, Y.Q.; Cui, J.M.; Zhang, Z.W.; Yuan, M.; Zhang, H.Y.; Yuan, S. Different Response of Photosystem II to Short and Long-Term Drought Stress in Arabidopsis Thaliana. Physiol. Plant 2016, 158, 225–235. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, L.; Shen, Q.; Yang, J.; Han, X.; Tian, F.; Wu, J. Effects of Water Stress on Photosynthesis, Yield, and Water Use Efficiency in Winter Wheat. Water 2020, 12, 2127. [Google Scholar] [CrossRef]
- Ilioaia, C.; Johnson, M.P.; Duffy, C.D.P.; Pascal, A.A.; Van Grondelle, R.; Robert, B.; Ruban, A.V. Origin of Absorption Changes Associated with Photoprotective Energy Dissipation in the Absence of Zeaxanthin. J. Biol. Chem. 2011, 286, 91–98. [Google Scholar] [CrossRef]
- Rashkov, G.D.; Stefanov, M.A.; Borisova, P.B.; Dobrikova, A.G.; Apostolova, E.L. The Role of the Organization of Light-Harvesting Complex II in the Drought Sensitivity of Pisum sativum L. Int. J. Mol. Sci. 2025, 26, 11078. [Google Scholar] [CrossRef]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and Biochemical Changes during Drought and Recovery Periods at Tillering and Jointing Stages in Wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant Survival under Drought Stress: Implications, Adaptive Responses, and Integrated Rhizosphere Management Strategy for Stress Mitigation. Microbiol. Res. 2021, 242, 126626. [Google Scholar] [CrossRef]
- Yi, X.P.; Zhang, Y.L.; Yao, H.S.; Luo, H.H.; Gou, L.; Chow, W.S.; Zhang, W.F. Rapid Recovery of Photosynthetic Rate Following Soil Water Deficit and Re-Watering in Cotton Plants (Gossypium herbaceum L.) Is Related to the Stability of the Photosystems. J. Plant Physiol. 2016, 194, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Živanović, B.; Komić, S.M.; Tosti, T.; Vidović, M.; Prokić, L.; Jovanović, S.V. Leaf Soluble Sugars and Free Amino Acids as Important Components of Abscisic Acid—Mediated Drought Response in Tomato. Plants 2020, 9, 1147. [Google Scholar] [CrossRef]
- Jiang, Z.; van Zanten, M.; Sasidharan, R. Mechanisms of Plant Acclimation to Multiple Abiotic Stresses. Commun. Biol. 2025, 8, 655. [Google Scholar] [CrossRef]
- Peršić, V.; Ament, A.; Antunović Dunić, J.; Drezner, G.; Cesar, V. PEG-Induced Physiological Drought for Screening Winter Wheat Genotypes Sensitivity—Integrated Biochemical and Chlorophyll a Fluorescence Analysis. Front. Plant Sci. 2022, 13, 987702. [Google Scholar] [CrossRef]
- Urban, L.; Aarrouf, J.; Bidel, L.P.R. Assessing the Effects of Water Deficit on Photosynthesis Using Parameters Derived from Measurements of Leaf Gas Exchange and of Chlorophyll a Fluorescence. Front. Plant Sci. 2017, 8, 304622. [Google Scholar] [CrossRef]
- Zuo, G.; Aiken, R.M.; Feng, N.; Zheng, D.; Zhao, H.; Avenson, T.J.; Lin, X. Fresh Perspectives on an Established Technique: Pulsed Amplitude Modulation Chlorophyll a Fluorescence. Plant-Environ. Interact. 2022, 3, 41. [Google Scholar] [CrossRef]
- Moustaka, J.; Moustakas, M. Early-Stage Detection of Biotic and Abiotic Stress on Plants by Chlorophyll Fluorescence Imaging Analysis. Biosensors 2023, 13, 796. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Moustaka, J. Early Drought Stress Warning in Plants: Color Pictures of Photosystem II Photochemistry. Climate 2022, 10, 179. [Google Scholar] [CrossRef]
- Lotfi, R.; Eslami-Senoukesh, F.; Mohammadzadeh, A.; Zadhasan, E.; Abbasi, A.; Kalaji, H.M. Identification of Key Chlorophyll Fluorescence Parameters as Biomarkers for Dryland Wheat under Future Climate Conditions. Sci. Rep. 2024, 14, 28699. [Google Scholar] [CrossRef]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll Fluorescence, Photoinhibition and Abiotic Stress: Does It Make Any Difference the Fact to Be a C3 or C4 Species? Front. Plant Sci. 2019, 10, 438548. [Google Scholar] [CrossRef]
- Grozeva, S.; Topalova, E.; Ganeva, D.; Tringovska, I. Evaluation of Tomato Landraces for Tolerance to Drought Stress Using Morphological and Physiological Traits. Int. J. Plant Biol. 2024, 15, 1391–1404. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Cao, B.; Cao, D.; Leng, G.; Li, H.; Yin, L.; Shan, L.; Deng, X. Genotypic Variation in Growth and Physiological Response to Drought Stress and Re-Watering Reveals the Critical Role of Recovery in Drought Adaptation in Maize Seedlings. Front. Plant Sci. 2016, 6, 1241. [Google Scholar] [CrossRef]
- Xu, W.; Wuyun, T.; Chen, J.; Yu, S.; Zhang, X.; Zhang, L. Responses of Trollius Chinensis to Drought Stress and Rehydration: From Photosynthetic Physiology to Gene Expression. Plant Physiol. Biochem. 2023, 201, 107841. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Zhuang, Y.; Wang, X.G.; Wang, X.D. Evaluation of Growth, Physiological Response, and Drought Resistance of Different Flue-Cured Tobacco Varieties under Drought Stress. Front. Plant Sci. 2024, 15, 1442618. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll Fluorescence Analysis: A Guide to Good Practice and Understanding Some New Applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Ottosen, C.O.; Rosenqvist, E.; Zhao, L.; Wang, Y.; Yu, W.; Zhao, T.; Wu, Z. Drought Stress Had a Predominant Effect over Heat Stress on Three Tomato Cultivars Subjected to Combined Stress. BMC Plant Biol. 2017, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Li, J.; Harmens, H.; Zheng, X.; Zhang, C. Melatonin Enhances Drought Resistance by Regulating Leaf Stomatal Behaviour, Root Growth and Catalase Activity in Two Contrasting Rapeseed (Brassica napus L.) Genotypes. Plant Physiol. Biochem. 2020, 149, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Lee, J.G. Effect of Drought Stress on Chlorophyll Fluorescence Parameters, Phytochemical Contents, and Antioxidant Activities in Lettuce Seedlings. Horticulturae 2021, 7, 238. [Google Scholar] [CrossRef]
- Hu, C.; Elias, E.; Nawrocki, W.J.; Croce, R. Drought Affects Both Photosystems in Arabidopsis Thaliana. New Phytol. 2023, 240, 663–675. [Google Scholar] [CrossRef]
- Oguz, M.C.; Aycan, M.; Oguz, E.; Poyraz, I.; Yildiz, M. Drought Stress Tolerance in Plants: Interplay of Molecular, Biochemical and Physiological Responses in Important Development Stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Jubany-Marí, T.; Alegre, L. Drought-Induced Senescence Is Characterized by a Loss of Antioxidant Defences in Chloroplasts. Plant Cell Environ. 2001, 24, 1319–1327. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. Die and Let Live: Leaf Senescence Contributes to Plant Survival under Drought Stress. Funct. Plant Biol. 2004, 31, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.R.; Wang, Y.H.; Li, T.; Tan, G.F.; Tao, J.P.; Su, X.J.; Xu, Z.S.; Tian, Y.S.; Xiong, A.S. Effects of Simulated Drought Stress on Carotenoid Contents and Expression of Related Genes in Carrot Taproots. Protoplasma 2021, 258, 379–390. [Google Scholar] [CrossRef]
- Altuntaş, C.; Demiralay, M.; Sezgin Muslu, A.; Terzi, R. Proline-Stimulated Signaling Primarily Targets the Chlorophyll Degradation Pathway and Photosynthesis Associated Processes to Cope with Short-Term Water Deficit in Maize. Photosynth. Res. 2020, 144, 35–48. [Google Scholar] [CrossRef]
- Feller, U. Drought Stress and Carbon Assimilation in a Warming Climate: Reversible and Irreversible Impacts. J. Plant Physiol. 2016, 203, 84–94. [Google Scholar] [CrossRef]
- Borisova-Mubarakshina, M.M.; Vetoshkina, D.V.; Naydov, I.A.; Rudenko, N.N.; Zhurikova, E.M.; Balashov, N.V.; Ignatova, L.K.; Fedorchuk, T.P.; Ivanov, B.N. Regulation of the Size of Photosystem II Light Harvesting Antenna Represents a Universal Mechanism of Higher Plant Acclimation to Stress Conditions. Funct. Plant Biol. 2020, 47, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. [34] Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Tena, N.; Asuero, A.G. Up-To-Date Analysis of the Extraction Methods for Anthocyanins: Principles of the Techniques, Optimization, Technical Progress, and Industrial Application. Antioxidants 2022, 11, 286. [Google Scholar] [CrossRef]
- Taghavi, T.; Patel, H.; Rafie, R. Extraction Solvents Affect Anthocyanin Yield, Color, and Profile of Strawberries. Plants 2023, 12, 1833. [Google Scholar] [CrossRef]
- Yang, Y.; Kilmartin, P.A. Advancing Anthocyanin Extraction: Optimising Solvent, Preservation, and Microwave Techniques for Enhanced Recovery from Merlot Grape Marc. Food Chem. 2025, 472, 142648. [Google Scholar] [CrossRef]
- Taghavi, T.; Patel, H.; Akande, O.E.; Galam, D.C.A. Total Anthocyanin Content of Strawberry and the Profile Changes by Extraction Methods and Sample Processing. Foods 2022, 11, 1072. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, L.; Li, M.; Su, L.; Lian, S.; Zhang, B.; Li, X.; Ge, K.; Li, L. AhGLK1 Affects Chlorophyll Biosynthesis and Photosynthesis in Peanut Leaves during Recovery from Drought. Sci. Rep. 2018, 8, 2250. [Google Scholar] [CrossRef] [PubMed]
- Efeoǧlu, B.; Ekmekçi, Y.; Çiçek, N. Physiological Responses of Three Maize Cultivars to Drought Stress and Recovery. S. Afr. J. Bot. 2009, 75, 34–42. [Google Scholar] [CrossRef]
- Batra, N.G.; Sharma, V.; Kumari, N. Drought-Induced Changes in Chlorophyll Fluorescence, Photosynthetic Pigments, and Thylakoid Membrane Proteins of Vigna radiata. J. Plant Interact. 2014, 9, 712–721. [Google Scholar] [CrossRef]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Harish; Marwal, A. Vital Roles of Carotenoids in Plants and Humans to Deteriorate Stress with Its Structure, Biosynthesis, Metabolic Engineering and Functional Aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Ben Abdallah, M.; Methenni, K.; Nouairi, I.; Zarrouk, M.; Youssef, N. Ben Drought Priming Improves Subsequent More Severe Drought in a Drought-Sensitive Cultivar of Olive Cv. Chétoui. Sci. Hortic. 2017, 221, 43–52. [Google Scholar] [CrossRef]
- Yao, Y.; Xia, L.; Yang, L.; Liu, R.; Zhang, S. Drought Responses and Carbon Allocation Strategies of Poplar with Different Leaf Maturity. Physiol. Plant 2024, 176, e14224. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Lakshmanan, G.M.A.; Gomathinayagam, M.; Panneerselvam, R. Alterations in Morphological Parameters and Photosynthetic Pigment Responses of Catharanthus Roseus under Soil Water Deficits. Colloids Surf. B Biointerfaces 2008, 61, 298–303. [Google Scholar] [CrossRef]
- Mibei, E.K.; Ambuko, J.; Giovannoni, J.J.; Onyango, A.N.; Owino, W.O. Carotenoid Profiling of the Leaves of Selected African Eggplant Accessions Subjected to Drought Stress. Food Sci. Nutr. 2016, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Kang, L.; Kim, H.S.; Xie, T.; Liu, C.; Ji, C.Y.; Kim, S.H.; Park, W.S.; Ahn, M.J.; Wang, S.; et al. Down-Regulation of Lycopene ε-Cyclase Expression in Transgenic Sweetpotato Plants Increases the Carotenoid Content and Tolerance to Abiotic Stress. Plant Sci. 2019, 281, 52–60. [Google Scholar] [CrossRef]
- Gori, A.; Brunetti, C.; Dos Santos Nascimento, L.B.; Marino, G.; Guidi, L.; Ferrini, F.; Centritto, M.; Fini, A.; Tattini, M. Photoprotective Role of Photosynthetic and Non-Photosynthetic Pigments in Phillyrea latifolia: Is Their “Antioxidant” Function Prominent in Leaves Exposed to Severe Summer Drought? Int. J. Mol. Sci. 2021, 22, 8303. [Google Scholar] [CrossRef]
- Jia, H.; Wang, L.; Li, J.; Sun, P.; Lu, M.; Hu, J. Comparative Metabolomics Analysis Reveals Different Metabolic Responses to Drought in Tolerant and Susceptible Poplar Species. Physiol. Plant 2020, 168, 531–546. [Google Scholar] [CrossRef]
- Dauala, G.A.; da Fonseca, B.S.F.; de Sousa, A.B.; Paula-Marinho, S.d.O.; Morais, P.G.C.; Leite, M.R.L.; da Silva, R.F.; de Aviz, R.O.; Duarte, M.H.F.; Nascimento, R.R.; et al. Plant Growth-Promoting Bacteria Modulate Metabolism and Nitrogen Accumulation to Counteract Drought Damage in Cactus Pear Plants. Sci. Rep. 2025, 15, 36432. [Google Scholar] [CrossRef]
- D’Odorico, P.; Schönbeck, L.; Vitali, V.; Meusburger, K.; Schaub, M.; Ginzler, C.; Zweifel, R.; Velasco, V.M.E.; Gisler, J.; Gessler, A.; et al. Drone-Based Physiological Index Reveals Long-Term Acclimation and Drought Stress Responses in Trees. Plant Cell Environ. 2021, 44, 3552–3570. [Google Scholar] [CrossRef]
- Baek, S.G.; Shin, J.W.; Nam, J.I.; Seo, J.M.; Kim, J.M.; Woo, S.Y. Drought and Salinity Stresses Response in Three Korean Native Herbaceous Plants and Their Suitability as Garden Plants. Horticulturae 2024, 10, 1225. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Isayenkov, S.V. The Role of Anthocyanins in Plant Tolerance to Drought and Salt Stresses. Plants 2023, 12, 2558. [Google Scholar] [CrossRef]
- Cirillo, V.; D’Amelia, V.; Esposito, M.; Amitrano, C.; Carillo, P.; Carputo, D.; Maggio, A. Anthocyanins Are Key Regulators of Drought Stress Tolerance in Tobacco. Biology 2021, 10, 139. [Google Scholar] [CrossRef]
- Medina-Lozano, I.; Bertolín, J.R.; Díaz, A. Impact of Drought Stress on Vitamin C and Anthocyanin Content in Cultivated Lettuces (Lactuca sativa L.) and Wild Relatives (Lactuca spp.). Front. Plant Sci. 2024, 15, 1369658. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Asif, S.; Asaf, S.; Lubna; Khan, Z.; Kim, K.M. Unveiling the Protective Role of Anthocyanin in Rice: Insights into Drought-Induced Oxidative Stress and Metabolic Regulation. Front. Plant Sci. 2024, 15, 1397817. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Miao, Y.; Ayyaz, A.; Hannan, F.; Huang, Q.; Ulhassan, Z.; Zhou, Y.; Islam, F.; Hong, Z.; Farooq, M.A.; et al. Purple Stem Brassica napus Exhibits Higher Photosynthetic Efficiency, Antioxidant Potential and Anthocyanin Biosynthesis Related Genes Expression against Drought Stress. Front. Plant Sci. 2022, 13, 936696. [Google Scholar] [CrossRef]
- Zargar, S.M.; Mir, R.A.; Ebinezer, L.B.; Masi, A.; Hami, A.; Manzoor, M.; Salgotra, R.K.; Sofi, N.R.; Mushtaq, R.; Rohila, J.S.; et al. Physiological and Multi-Omics Approaches for Explaining Drought Stress Tolerance and Supporting Sustainable Production of Rice. Front. Plant Sci. 2022, 12, 803603. [Google Scholar] [CrossRef]
- Sarfraz, Z.; Zarlashat, Y.; Ambreen, A.; Mujahid, M.; Iqbal, M.S.; Fatima, S.A.; Iqbal, M.S.; Iqbal, R.; Fiaz, S. Plant Biochemistry in the Era of Omics: Integrated Omics Approaches to Unravel the Genetic Basis of Plant Stress Tolerance. Plant Breed. 2025; early view. [Google Scholar] [CrossRef]
- Wu, C.; Ning, F.; Zhang, Q.; Wu, X.; Wang, W. Enhancing Omics Research of Crop Responses to Drought under Field Conditions. Front. Plant Sci. 2017, 8, 174. [Google Scholar] [CrossRef]
- Varadharajan, V.; Rajendran, R.; Muthuramalingam, P.; Runthala, A.; Madhesh, V.; Swaminathan, G.; Murugan, P.; Srinivasan, H.; Park, Y.; Shin, H.; et al. Multi-Omics Approaches Against Abiotic and Biotic Stress—A Review. Plants 2025, 14, 865. [Google Scholar] [CrossRef]
- Jha, U.C.; Bohra, A.; Nayyar, H. Advances in “Omics” Approaches to Tackle Drought Stress in Grain Legumes. Plant Breed. 2020, 139, 1–27. [Google Scholar] [CrossRef]
- Yue, C.; Cao, H.; Zhang, S.; Shen, G.; Wu, Z.; Yuan, L.; Luo, L.; Zeng, L. Multilayer Omics Landscape Analyses Reveal the Regulatory Responses of Tea Plants to Drought Stress. Int. J. Biol. Macromol. 2023, 253, 126582. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, M.S.S.; Xue, S.; Islam, F.; Ikram, A.U.; Abdullah, M.; Liu, S.; Tappiban, P.; Chen, J. A Comprehensive Overview of Omics-Based Approaches to Enhance Biotic and Abiotic Stress Tolerance in Sweet Potato. Hortic. Res. 2024, 11, uhae014. [Google Scholar] [CrossRef]
- Pascual, L.S.; Serna, E.; Ghani, A.; Lyu, Z.; Immadi, M.S.; Joshi, T.; Verma, M.; Rambla, J.L.; Gómez-Cadenas, A.; Mittler, R.; et al. Multi-Omics-Based Insights into Tomato Adaptation to Multifactorial Stress Combination. Plant Physiol. 2025, 199, kiaf519. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, D.; Wang, H.; Qiao, Y.; Zhao, P.; Cao, Y.; Liu, X.; Yang, Y.; Lin, X.; Xu, S.; et al. Integrative Omics of the Genetic Basis for Wheat WUE and Drought Resilience Reveal the Function of TaMYB7-A1. Nat. Commun. 2025, 16, 8622. [Google Scholar] [CrossRef] [PubMed]
- Sheoran, S. Recent Advances for Drought Stress Tolerance in Maize (Zea mays L.): Present Status and Future Prospects. Front. Plant Sci. 2022, 13, 872566. [Google Scholar] [CrossRef]
- Murmu, S.; Sinha, D.; Chaurasia, H.; Sharma, S.; Das, R.; Jha, G.K.; Archak, S. A Review of Artificial Intelligence-Assisted Omics Techniques in Plant Defense: Current Trends and Future Directions. Front. Plant Sci. 2024, 15, 1292054. [Google Scholar] [CrossRef]
- Amin, A.; Zaman, W.; Park, S.J. Harnessing Multi-Omics and Predictive Modeling for Climate-Resilient Crop Breeding: From Genomes to Fields. Genes 2025, 16, 809. [Google Scholar] [CrossRef]
- Jangra, S.; Chaudhary, V.; Yadav, R.C.; Yadav, N.R. High-Throughput Phenotyping: A Platform to Accelerate Crop Improvement. Phenomics 2021, 1, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Mertens, S.; Verbraeken, L.; Sprenger, H.; De Meyer, S.; Demuynck, K.; Cannoot, B.; Merchie, J.; De Block, J.; Vogel, J.T.; Bruce, W.; et al. Monitoring of Drought Stress and Transpiration Rate Using Proximal Thermal and Hyperspectral Imaging in an Indoor Automated Plant Phenotyping Platform. Plant Methods 2023, 19, 132. [Google Scholar] [CrossRef] [PubMed]
- Genangeli, A.; Avola, G.; Bindi, M.; Cantini, C.; Cellini, F.; Grillo, S.; Petrozza, A.; Riggi, E.; Ruggiero, A.; Summerer, S.; et al. Low-Cost Hyperspectral Imaging to Detect Drought Stress in High-Throughput Phenotyping. Plants 2023, 12, 1730. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Y.; Tomasetto, F.; Yan, W.; Tan, Z.; Liu, J.; Jiang, J. Non-Destructive Measurements of Toona Sinensis Chlorophyll and Nitrogen Content Under Drought Stress Using Near Infrared Spectroscopy. Front. Plant Sci. 2022, 12, 809828. [Google Scholar] [CrossRef]
- Tschurr, F.; Roth, L.; Storni, N.; Zumsteg, O.; Walter, A.; Anderegg, J. Temporal Resolution Trumps Spectral Resolution in UAV-Based Monitoring of Cereal Senescence Dynamics. Plant Methods 2024, 20, 188. [Google Scholar] [CrossRef]
- Zolin, Y.; Popova, A.; Yudina, L.; Grebneva, K.; Abasheva, K.; Sukhov, V.; Sukhova, E. RGB Indices Can Be Used to Estimate NDVI, PRI, and Fv/Fm in Wheat and Pea Plants Under Soil Drought and Salinization. Plants 2025, 14, 1284. [Google Scholar] [CrossRef]
- Briglia, N.; Nuzzo, V.; Petrozza, A.; Summerer, S.; Cellini, F.; Montanaro, G. Preliminary High-Throughput Phenotyping Analysis in Grapevines under Drought. BIO Web Conf. 2019, 13, 02003. [Google Scholar] [CrossRef]
- Danzi, D.; De Paola, D.; Petrozza, A.; Summerer, S.; Cellini, F.; Pignone, D.; Janni, M. The Use of Near-Infrared Imaging (NIR) as a Fast Non-Destructive Screening Tool to Identify Drought-Tolerant Wheat Genotypes. Agriculture 2022, 12, 537. [Google Scholar] [CrossRef]
- Shu, M.; Harfouche, A.L.; Trtílek, M.; Panzarová, K.; Alasia, O.F.; Lagergren, J.H.; Labbé, A.; Engle, N.L.; Clark, M.M.; Chen, J.G.; et al. Leveraging Hyperspectral Phenotyping for Accurate, Non-Destructive Prediction of Metabolite Profiles in Poplar under Drought Stress. Environ. Exp. Bot. 2025, 237, 106218. [Google Scholar] [CrossRef]
- Yu, M.H.; Ding, G.D.; Gao, G.L.; Zhao, Y.Y.; Yan, L.; Sai, K. Using Plant Temperature to Evaluate the Response of Stomatal Conductance to Soil Moisture Deficit. Forests 2015, 6, 3748–3762. [Google Scholar] [CrossRef]
- Ludovisi, R.; Tauro, F.; Salvati, R.; Khoury, S.; Mugnozza, G.S.; Harfouche, A. Uav-Based Thermal Imaging for High-Throughput Field Phenotyping of Black Poplar Response to Drought. Front. Plant Sci. 2017, 8, 252873. [Google Scholar] [CrossRef]
- Pradawet, C.; Khongdee, N.; Pansak, W.; Spreer, W.; Hilger, T.; Cadisch, G. Thermal Imaging for Assessment of Maize Water Stress and Yield Prediction under Drought Conditions. J. Agron. Crop. Sci. 2023, 209, 56–70. [Google Scholar] [CrossRef]
- Yang, Y.; Nan, R.; Mi, T.; Song, Y.; Shi, F.; Liu, X.; Wang, Y.; Sun, F.; Xi, Y.; Zhang, C. Rapid and Nondestructive Evaluation of Wheat Chlorophyll under Drought Stress Using Hyperspectral Imaging. Int. J. Mol. Sci. 2023, 24, 5825. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wu, F.; Ao, Z.; Jin, S.; Qin, F.; Liu, B.; Pang, S.; Liu, L.; Guo, Q. Evaluating Maize Phenotype Dynamics under Drought Stress Using Terrestrial Lidar. Plant Methods 2019, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Mulugeta Aneley, G.; Haas, M.; Köhl, K. LIDAR-Based Phenotyping for Drought Response and Drought Tolerance in Potato. Potato Res. 2022, 66, 1225–1256. [Google Scholar] [CrossRef]
- Thompson, A.L.; Thorp, K.R.; Conley, M.; Andrade-Sanchez, P.; Heun, J.T.; Dyer, J.M.; White, J.W. Deploying a Proximal Sensing Cart to Identify Drought-Adaptive Traits in Upland Cotton for High-Throughput Phenotyping. Front. Plant Sci. 2018, 9, 315692. [Google Scholar] [CrossRef]
- Maesano, M.; Khoury, S.; Nakhle, F.; Firrincieli, A.; Gay, A.; Tauro, F.; Harfouche, A. UAV-Based LiDAR for High-Throughput Determination of Plant Height and Above-Ground Biomass of the Bioenergy Grass Arundo Donax. Remote Sens. 2020, 12, 3464. [Google Scholar] [CrossRef]
- Mazis, A.; Choudhury, S.D.; Morgan, P.B.; Stoerger, V.; Hiller, J.; Ge, Y.; Awada, T. Application of High-Throughput Plant Phenotyping for Assessing Biophysical Traits and Drought Response in Two Oak Species under Controlled Environment. For. Ecol. Manag. 2020, 465, 118101. [Google Scholar] [CrossRef]
- Rossi, R.; Costafreda-Aumedes, S.; Leolini, L.; Leolini, C.; Bindi, M.; Moriondo, M. Implementation of an Algorithm for Automated Phenotyping through Plant 3D-Modeling: A Practical Application on the Early Detection of Water Stress. Comput. Electron. Agric. 2022, 197, 106937. [Google Scholar] [CrossRef]
- Angidi, S.; Madankar, K.; Tehseen, M.M.; Bhatla, A. Advanced High-Throughput Phenotyping Techniques for Managing Abiotic Stress in Agricultural Crops—A Comprehensive Review. Crops 2025, 5, 8. [Google Scholar] [CrossRef]
- He, Z.; Zhang, P.; Jia, H.; Zhang, S.; Nishawy, E.; Sun, X.; Dai, M. Regulatory Mechanisms and Breeding Strategies for Crop Drought Resistance. New Crops 2024, 1, 100029. [Google Scholar] [CrossRef]
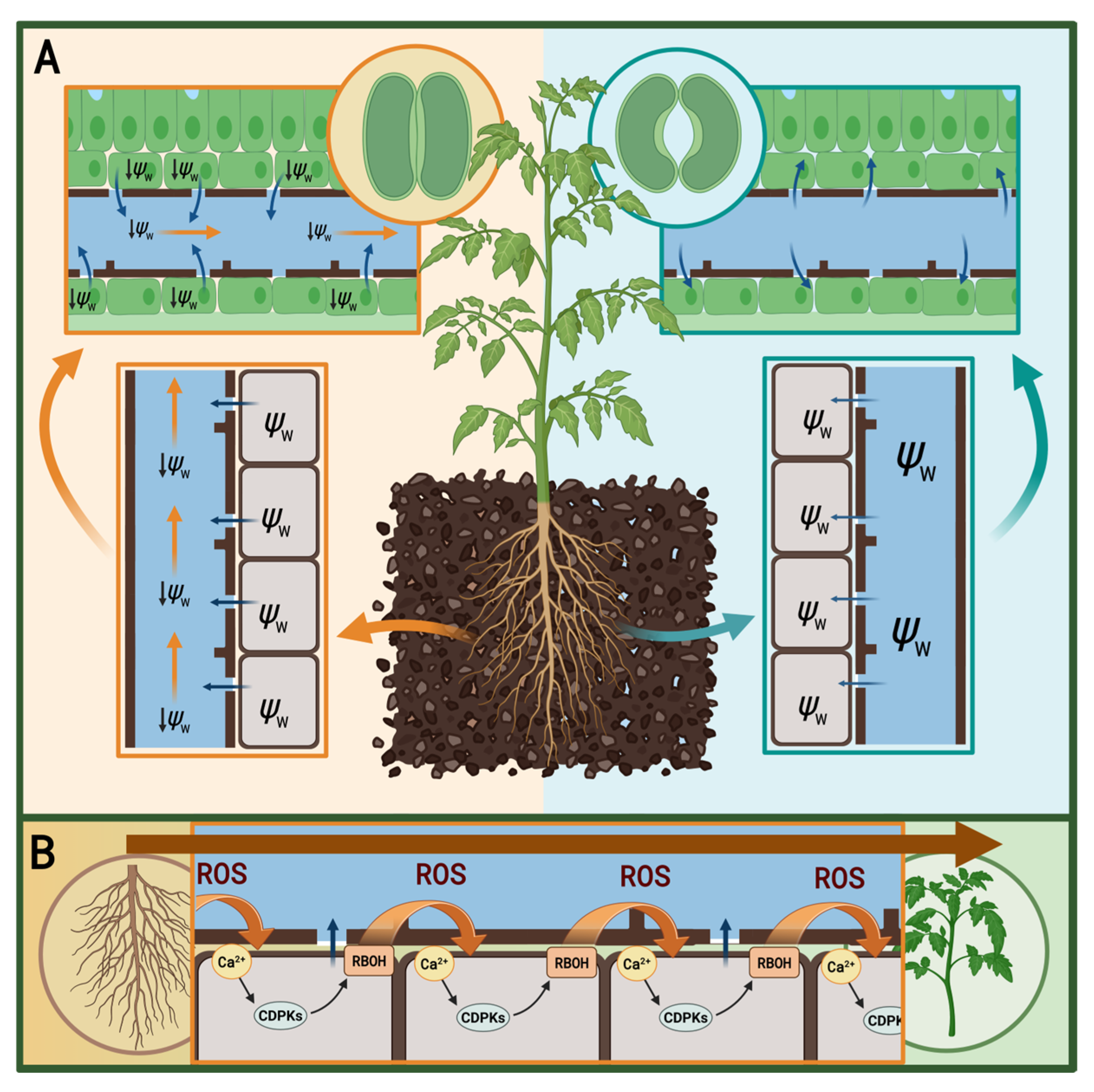

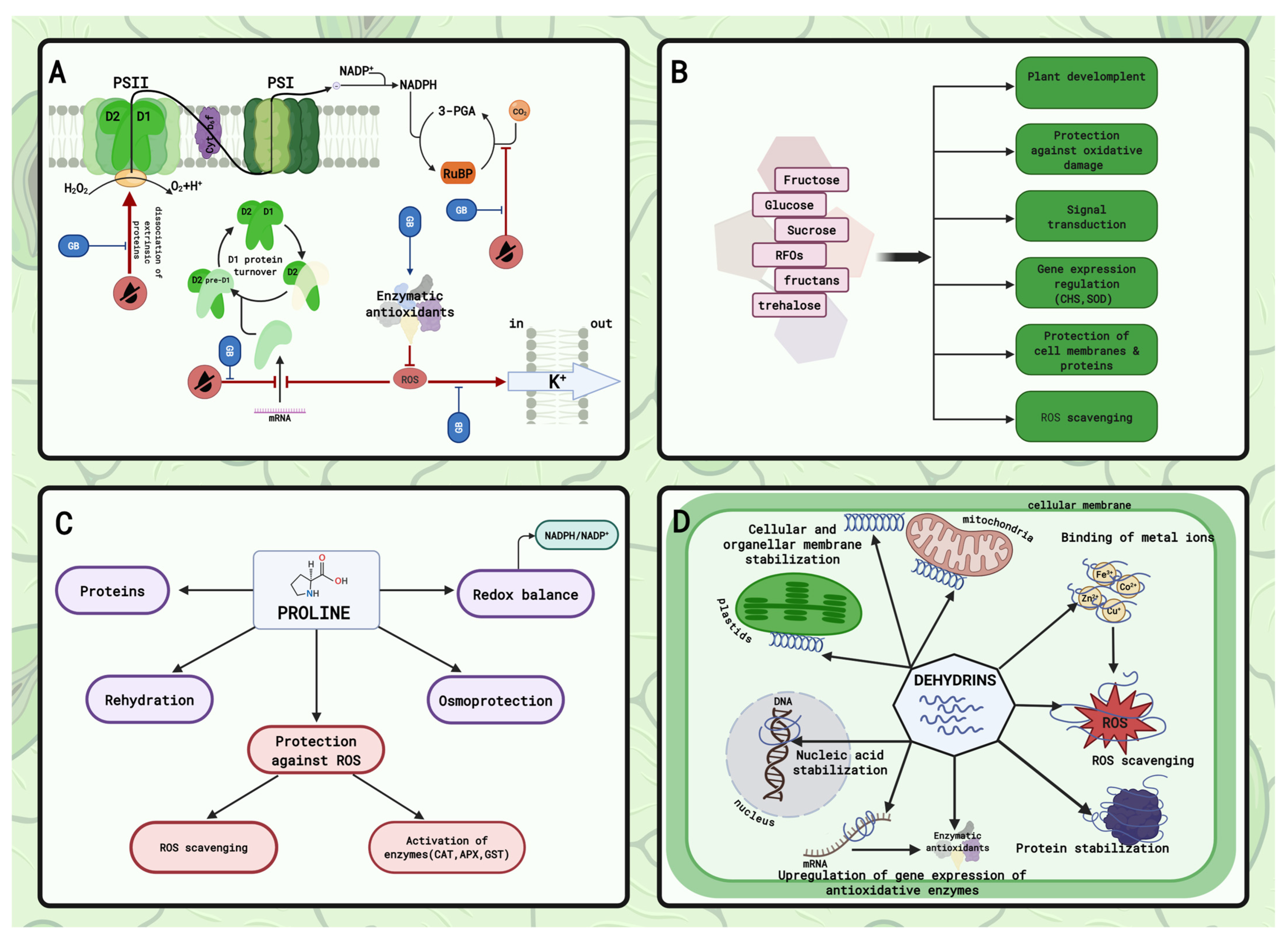


| Phytohormone | Interaction Type | Mechanism of Action | References |
|---|---|---|---|
| Cytokinins (CKs) | Antagonistic | Stomatal aperture: CKs naturally promote stomatal opening. Under drought, CK levels decrease, resulting in downregulation of opening signals and sensitization of guard cells to ABA-induced closure. Growth balance: a low CK/high ABA ratio inhibits shoot growth while maintaining root activity, optimizing the root-to-shoot ratio for survival. | [73] |
| Auxins | Complex | Deep Rooting: ABA promotes local auxin synthesis in root tips to steepen growth angles (gravitropism), enabling reaching deep water. Hydrotropism: To orient towards moist areas, ABA modifies auxin transport, temporarily suppressing gravitropism. Stomata aperture: Auxins generally promote opening. ABA must suppress the sensitivity of guard cells to auxin to ensure timely closure. | [26,74,75] |
| Brassinosteroids (BRs) | Antagonistic (mostly) | BRs promote cell elongation and stomatal opening. High ABA levels are required to override this signal. Note: Exogenous BRs application can improve resistance by stimulating ABA biosynthesis. | [76,77,78,79] |
| Jasmonic Acid (JA) | Synergistic | JA works together with ABA to stimulate stomatal closure. Additionally, JA stimulates de novo ABA biosynthesis. | [80,81,82,83] |
| Strigolactones (SLs) | Synergistic | SLs increase the sensitivity of stomata to ABA. SL-deficient mutants often fail to close stomata efficiently even when ABA is present. | [84,85] |
| Melatonin | Antagonistic/Modulatory | By scavenging ROS and alleviating oxidative stress, it downregulates ABA biosynthesis gene NCED3 and promotes ABA catabolism, preventing excessive senescence and allowing growth recovery. | [86,87] |
| Family of TFs | Plant Species | Protein Name | Regulation | Function/Plant Characteristics | References |
|---|---|---|---|---|---|
| bZIP | Arabidopsis thaliana | AtAREB1/AREB2/ ABF3/ABF1 | Positive | Master transcription factor in ABA-dependent signaling | [96] |
| Oryza sativa | OsABF1 | Positive | Activator in ABA-dependent gene expression | [97] | |
| OsABF2/bZIP46 | Positive | Activator in ABA-dependent gene expression, regulation of WRKY TFs | [97] | ||
| OsbZIP23/62 | Positive | Activator in ABA-dependent gene expression | [97] | ||
| OsbZIP72 | Positive | Activator in ABA-dependent gene expression, including LEA | [98] | ||
| Zea mays | ZmABP9 | Positive | Activator in ABA-dependent gene expression including KIN1, COR15A, PP2C, AZF2, ROS regulation | [99] | |
| ZmbZIP72 | Positive | Activator in ABA-dependent gene expression including RAB18, RD29B, HIS1-3 | [99] | ||
| ZmABI5 | Negative | Regulation of the CAT1, APX and NtERD10A, B, C, D gene expression | [99] | ||
| Triticum aestivum | TaABP1 | Positive | Activator in ABA-dependent gene expression | [97] | |
| TaABL1 | Positive | Activator in ABA-dependent gene expression, regulation of stomatal movement, accumulation of osmolytes | [97] | ||
| TaAREB3 | Positive | Activator in ABA-dependent gene expression | [97] | ||
| Wabi5 | Positive | Activator in ABA-dependent gene expression, e.g., LEA | [97] | ||
| Solanum lycopersicum | SlAREB1 | Positive | Activator in ABA-dependent gene expression including AtRD29A, AtCOR47, and SlCI7-like dehydrin | [100] | |
| SlbZIP1 | Positive | Activator in ABA-dependent gene expression, regulation of chlorophyll content and CAT activity | [101] | ||
| Vitis vinifera | VlbZIP30 | Positive | Activator in ABA-dependent gene expression including stress marker genes and ABA core signaling components | [102] | |
| VlbZIP36 | Positive | Activator in ABA-dependent gene expression, increased activity of antioxidant enzymes | [103] | ||
| VvABF2 | Positive | Activator in ABA-dependent gene expression including RAB18, LEA, RD29B modulation of antioxidative enzymes activity | [104] | ||
| MYB | Arabidopsis thaliana | AtMYB60 | Negative | Stomatal movement | [105] |
| AtMYB96 | Positive | Stomatal movement, modulation of auxin homeostasis during lateral root development, wax synthesis | [105,106] | ||
| AtMYB77 | Positive | Auxin signaling-dependent lateral root growth | [105] | ||
| AtMYB2 | Positive | Activator in ABA-dependent gene expression, including AtRD22 and AtADH10 | [105,106] | ||
| AtMYB15 | Positive | Expression enhancment of genes involved in drought-response (AtADH1, RD22, RD29B, AtEM6), ABA biosynthesis (AtABA1, AtABA2) and signaling (AtABI3) | [107] | ||
| AtMYB44 | Positive | Auxin signaling-dependent lateral root growth, cross-talk between SA and JA (direct regulation of AtWRKY70) | [105,106] | ||
| AtMYB20 | Negative | Stomatal movement | [105] | ||
| Oryza sativa | OsMYB48-1 | Positive | Proline biosynthesis, ABA accumulation, expression enhancement of genes involved in ABA biosynthesis (OsNCED4, OsNCED5), signaling (OsPP2C68, OsRK1) and response (OsRAB21, OsLEA3, OsRAB16C and OsRAB16D) | [105] | |
| OsMYB60 | Positive | Wax synthesis | [106] | ||
| OsMYB1-R1 | Negative | Proline biosynthesis | [108] | ||
| OsMYB2 | Positive | ABA signaling, stomatal movement, regulation of gene expression of OsLEA3, OsRAB16A, and OsDREB2A | [105,108] | ||
| Zea mays | ZmMYB30 | Positive | Upregulation of stress-responsive genes ABF3, ATGolS2, AB15, DREB2A, RD20, RD29B, RD29A, and MYB2 | [99] | |
| ZmMYB56 | Positive | Stomatal movement regulation via transcriptional regulation of ZmTOM7 | [109] | ||
| Triticum aestivum | TaMYB3-R1 | Positive | Stomatal movement | [105] | |
| TaMYB2 | Positive | Upregulation of stress-related genes AtDREB2A, AtRD29B, AtRD22, AtCOR15, AtRab18 and AtABI2 | [105] | ||
| TaMYB19 | Positive | Accumulation of osmolytes, upregulation of stress-related genes including AtRD29A, AtRD22 and AtMYB2 | [105] | ||
| TaMYB30-B | Positive | Accumulation of osmolytes, upregulation of stress-responsive genes including AtRD29A and AtERD1 | [105] | ||
| TaMYB33 | Positive | Maintenance of osmotic balance, increased ROS scavenging | [110] | ||
| TaODORANT1 | Positive | Upregulation of the expression of ROS- and stress-related genes | [111] | ||
| TaMYB44-5A | Negative | Downregulation of the TaRD22-3A, drought- and ABA-responsive gene expression | [112] | ||
| TaPIMP1 | Positive | Increased drought-tolerance and SOD activity, upregulation of defense- and stress-related genes, including RD22 | [105] | ||
| Solanum lycopersicum | SlMYB49 | Positive | Reduced ROS accumulation | [113] | |
| SlMYB1L | Positive | Stomatal movement, proline and H2O2 homeostasis | [114] | ||
| SlMYB78-like | Positive | Regulation of chlorophyll biosynthesis, photosynthesis, ABA biosynthesis and response genes | [115] | ||
| Vitis vinifera | VvMYB60 | Negative | Stomatal movement | [105] | |
| VyMYB24 | Positive | Regulation of root development, antioxidant enzymes, proline accumulation | [116] | ||
| VvMYB14 | Positive | Regulation of POD and CAT expression | [117] | ||
| AP2/ERF (DREB) | Arabidopsis thaliana | AtDREB1A | Positive | Regulation AtRD29A and AtCOR15A expression | [118] |
| AtDREB2A | Positive | Core regulator of ABA-independent drought genes | [119] | ||
| AtTINY | Positive | Stomatal movement, alleviation of BES1 repression of drought-responsive genes. | [120] | ||
| Oryza sativa | OsDREB1A | Positive | Accumulation of proline, maintenance of chlorophyll, regulation of RWC and ion leakage | [121] | |
| OsDREB2A | Positive | Core regulator of ABA-independent drought genes | [119] | ||
| Zea mays | ZmDREB1A | Positive | Expression of drought-responsive genes in both the ABA-independent and ABA-dependent pathways | [99] | |
| ZmDREB2A | Positive | Key regulator of the dehydration-responsive regulon | [99] | ||
| ZmDBF3 | Positive | Improvement of drought tolerance | [99] | ||
| ZmDBP3/4 | Positive | Improvement of drought tolerance | [99] | ||
| Triticum aestivum | TaDREB1 | Positive | Improvement of drought tolerance | [122] | |
| TaAIDFa | Positive | Improvement of drought tolerance | [122] | ||
| TaDREB2 | Positive | Regulation of IAA | [122] | ||
| Solanum lycopersicum | SlDREB1 | Positive | Accumulation of soluble sugars and osmolytes Increased response to drought-stress | [123] | |
| SlDREB2 | Positive | Regulation of stress signaling pathways and proline synthesis | [123] | ||
| SlDREB3 | Negative | Alteration of ABA signaling by negative regulation of the ABA pathway | [123] | ||
| NAC | Arabidopsis thaliana | AtANAC096 | Positive | Interaction with AtABF2 and AtABF4 in the ABA signaling pathway, regulation of AtRD29A gene expression | [124] |
| AtANAC019/055/072 | Positive | Improvement of drought tolerance | [125] | ||
| AtRD26 | Positive | Activator in ABA-dependent gene expression, inhibitor of BES1 expression | [126] | ||
| AtATAF1 | Positive | Regulation of ABA biosynthesis through AtNCED3 and SnRK1 | [127] | ||
| Oryza sativa | SNAC1 | Positive | Stomatal movement, improvement of drought tolerance by regulation of OsPP18 (PP2C) and ROS scavenging enzymes expression in ABA independent pathway | [124,128] | |
| OsNAC5 | Positive | Transcriptional activation of stress-responsive genes, lignin biosynthesis, improvement of lateral root formation | [124] | ||
| OsNAC6 | Positive | Improvement of lateral root formation, upregulation of the expression of genes involved in membrane modification, NA biosynthesis, and GSH relocation. | [124,129] | ||
| OsNAC78 | Positive | Maintenance of ROS homeostasis through activation of OsGSTU37 | [124] | ||
| Zea mays | ZmSNAC1 | Positive | Enhancement of tolerance to drought stress at the germination phase | [99] | |
| ZmNAC55 | Positive | Enhanced tolerance to drought stress through upregulation of genes including RD29B, LEA14, RD17 | [99] | ||
| ZmNAC111 | Positive | Upregulation of drought stress–responsive gene expression | [130] | ||
| ZmNAC20 | Positive | Stomatal movement, activation of drought-responsive genes | [131] | ||
| ZmNAC45/72/18/51 | Positive | Improvement of drought tolerance | [99] | ||
| Triticum aestivum | TaNAC6-3B | Positive | Regulation of NCED, ABA and drought-responsive genes, including LEA | [132] | |
| TaNAC69-5A | Positive | Improvement of root architecture and drought tolerance | [122] | ||
| TaNAC2 | Positive | Stomatal movement, improvement of root architecture and drought tolerance | [122] | ||
| TaNAC29 | Positive | Improvement of drought tolerance by reduction in ROS accumulation | [122] | ||
| TaNAC8-6A | Positive | Stomatal movement | [122] | ||
| Solanum lycopersicum | SlJUB1 | Positive | Regulation of SlDREB1 and SlDREB2 expression and proline synthesis | [133] | |
| SlNAC4 | Positive | Improvement of drought tolerance | [133] | ||
| SlNAC6 | Positive | Accumulation of proline and antioxidant enzymes | [134] | ||
| SlNAP1 | Positive | Regulation of GA and ABA synthesis | [135] | ||
| SlSRN1 | Negative | Gene silencing increases drought tolerance | [136] | ||
| Vitis vinifera | VvNAC17 | Positive | Accumulation of antioxidative enzymes and anthocyanin, regulation of drought-related gene expression including VvDREB1A, VvDREB2A, VvRD29A | [137] | |
| VvNAC33 | Positive | Regulation of the antioxidant enzymes expression including VvCAT1, VvCu/ZnSOD, and VvPOD4 | [138] | ||
| WRKY | Arabidopsis thaliana | AtWRKY57 | Positive | Upregulation of AtRD29A, AtNCED3, and AtABA3 expression | [139] |
| AtWRKY53 | Negative | Stomatal movement: inhibition of stomatal closure via reduced H2O2 content, facilitation of stomatal opening by starch degradation | [140] | ||
| AtWRKY63 (ABO3) | Positive | Regulation of RD29A and COR47A in ABA signaling | [141] | ||
| AtWRKY40 | Negative | Expression inhibition of multiple ABA-induced genes including AtABF4, AtABI4, AtABI5, AtDREB1A, AtMYB2, and AtRAB18 | [142] | ||
| AtWRKY18 | Negative | Suppression enhancement of AtABI4 and AtABI5 transcription induced by WRKY40 | [142] | ||
| Oryza sativa | OsWRKY11 | Positive | Activator of the drought-responsive gene transcription, e.g., OsRAB21 | [143] | |
| OsWRKY5 | Negative | Suppresion of OsMYB2 expression with downregulation of its downstream genes (OsLEA3, OsRAB16A, and OsDREB2A) | [108] | ||
| OsWRKY47 | Positive | Activation of genes involved in inhibition of stress-induced senescence | [144] | ||
| OsWRKY55 | Negative | Negative modulation of drought response via joined transcriptional cascade with OsAP2-39 | [145] | ||
| Zea mays | ZmWRKY58 | Positive | Protection of cell membrane integrity, participation in the ABA and Ca2+ signaling pathway | [146] | |
| ZmWRKY40/106 | Positive | Activation of DREB2B, and RD29A expression in transgenic plants | [146] | ||
| Triticum aestivum | TaWRKY33 | Positive | Improvement of drought tolerance in transgenic plants via ABA synthesis and transduction pathways. | [147] | |
| TaWRKY75-A | Positive | Upregulation of JA biosynthetic genes AtLOX3 and AtAOC1 | [148] | ||
| TaWRKY44 | Positive | Indirect activation of genes associated with cellular antioxidant systems and stress response | [149] | ||
| TaWRKY2-1D | Positive | Regulation of the expression of TaPOD, TaCAT, TaSOD(Fe), and stress-related TaP5CS | [150] | ||
| TaWRKY133 | Negative | Downregulation of transcription of drought-responsive (DREB2A, RD29A, RD29B, ABF1, ABA2, ABI1) and antioxidant enzymes: SOD(Cu/Zn), POD1, and CAT1 genes | [151] | ||
| Solanum lycopersicum | SlWRKY75 | Positive | Enhancement of drought tolerance via JA signaling and regulation of SlARF5 and SlTRY expression responsible for lateral roots and trichome formation | [152,153] | |
| SlWRKY8 | Positive | Accumulation of osmotic substances, upregulation of stress-responsive genes SlAREB, SlDREB2A, and SlRD29. | [154] | ||
| SlWRKY17 | Positive | Upregulation of ROS detoxification-related and drought-responsive genes | [155] | ||
| SlWRKY81 | Negative | Regulation of H2O2–mediated stomatal movement | [156] | ||
| Vitis vinifera | VvWRKY48 | Positive | Increased activity of the antioxidant enzymes, upregulation of the expression of stress-related genes | [157] | |
| VvWRKY18 | Negative | Increased ROS accumulation, lowered activity of antioxidant enzymes, increase in stomatal density | [158] | ||
| VvWRKY13 | Negative | Reduced content of osmolytes, increased ROS accumulation, reduced drought-related gene expression | [159] |
| Type of Detection | Name of the Method | Measured Parameters | Characteristics | Localization | References |
|---|---|---|---|---|---|
| |||||
| Spectrophotometry | Biochemical estimation | chlorophylls, anthocyanins, MDA, proline | Simple, fast, cost-effective, low sensitivity | Not applicable | [236,239] |
| Enzymatic assays | SOD, CAT, APX, GR, MDHAR, DHAR | Not applicable | |||
| Non-enzymatic antioxidant assays | AsA, GSH, vitamins, flavonoids | Not applicable | |||
| Chromatography | HPLC | Proline, glycine betaine, sugars | highly specific, sensitive, time-consuming sample preparation, requirement of complex instrumentation | Not applicable | [236,239] |
| |||||
| Non-fluorescent/colorimetric probes | DAB | H2O2 | Simple, low specificity, irreversible | Nontargeted | [219,236,238] |
| NBT | O2•− | Nontargeted | |||
| Fluorescent probes | CM H2DCF-DA | ROS in general | Irreversible, low specificity, possibility of photooxidation | Intracellular | |
| OxyBurst Green | ROS in general | Irreversible, low specificity, possibility of photooxidation | Extracellular | ||
| DHR | ROS in general | Irreversible, low specificity | Intracellular | ||
| BES H2O2-Ac | H2O2 | Irreversible, slow reactivity | Intracellular | ||
| Amplex Red | H2O2 | Irreversible, pH sensitive, possibility of photooxidation | Extracellular | ||
| boronate-based | H2O2 | Irreversible, high sensitivity, high stability, may react with other ROS | Intracellular, Extracellular | ||
| NBCD | H2O2 | Irreversible, high specificity, high stability | Intracellular | ||
| DHE | O2•− | Irreversible, may react with other ROS | Intracellular | ||
| MitoSOX | O2•− | Irreversible, may react with other ROS | Mitochondria | ||
| DanePy | 1O2 | Irreversible, may react with other ROS, possibility of photobleaching, high photosensitivity | Intracellular, Chloroplasts | ||
| SOSG | 1O2 | Poor penetration, possibility of photobleaching, high photosensitivity, may react with other ROS, can produce 1O2 | Intracellular, Chloroplasts | ||
| Fluorescent protein biosensors | roGFP1, roGFP2 | ROS in general | Reversible, non-invasive, not selective towards specific ROS, enables long-term imaging of ROS, requires plant transformation | Cytosol, Mitochondria, Chloroplasts, ER, Peroxisomes | [219,238] |
| rxYFP | ROS in general | ||||
| roGFP-Orp1 | H2O2 | Reversible, non-invasive, enables long-term imaging of ROS, requires plant transformation, insensitive to changes in pH | Cytosol, Mitochondria, Chloroplasts | ||
| cpYFP | O2•− | Reversible, non-invasive, enables long-term imaging of ROS, requires plant transformation, sensitive to variations in pH | Can be targeted to specific intracellular compartments | ||
| HyPer | H2O2 | Reversible, non-invasive, enables long-term imaging of ROS, requires plant transformation, sensitive to variations in pH | Cytosol, Chloroplasts, Peroxisomes, Nucleus | ||
| Shoot Response (Conservation Strategy) | Root Response (Gain Strategy) | |
|---|---|---|
| Goal | Arrest growth to limit transpiration surface area and preserve energy | Maintain apex elongation to access deep moist soil (hydrotropism) |
| ABA action | High accumulation Closing the stomata, inhibition of PM H+-ATPase | Spatio-temporal regulation of accumulation Activation of PM H+-ATPase in the apex by low concentrations |
| Cell wall elasticity | Stiffening Increase in CW yield threshold (Y) | Loosening Increase in CW extensibility (ϕ) |
| Apoplast pH | Alkalization pH ~6.0 | Acidification in the apex zone pH ~4.5–5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Michalak, A.; Małas, K.; Dąbrowska, K.; Półrolniczak, K.; Bronowska, L.; Misiewicz, A.; Maj, A.; Stabrowska, M.; Wnuk, I.; Kabała, K. Molecular Mechanisms and Experimental Strategies for Understanding Plant Drought Response. Plants 2026, 15, 149. https://doi.org/10.3390/plants15010149
Michalak A, Małas K, Dąbrowska K, Półrolniczak K, Bronowska L, Misiewicz A, Maj A, Stabrowska M, Wnuk I, Kabała K. Molecular Mechanisms and Experimental Strategies for Understanding Plant Drought Response. Plants. 2026; 15(1):149. https://doi.org/10.3390/plants15010149
Chicago/Turabian StyleMichalak, Adrianna, Karolina Małas, Kinga Dąbrowska, Kinga Półrolniczak, Lidia Bronowska, Anna Misiewicz, Angelika Maj, Maja Stabrowska, Iga Wnuk, and Katarzyna Kabała. 2026. "Molecular Mechanisms and Experimental Strategies for Understanding Plant Drought Response" Plants 15, no. 1: 149. https://doi.org/10.3390/plants15010149
APA StyleMichalak, A., Małas, K., Dąbrowska, K., Półrolniczak, K., Bronowska, L., Misiewicz, A., Maj, A., Stabrowska, M., Wnuk, I., & Kabała, K. (2026). Molecular Mechanisms and Experimental Strategies for Understanding Plant Drought Response. Plants, 15(1), 149. https://doi.org/10.3390/plants15010149







