Leaf-Fruit Trait Decoupling Along Environmental Gradients in Tropical Cryptocaryeae (Lauraceae)
Abstract
1. Introduction
2. Results
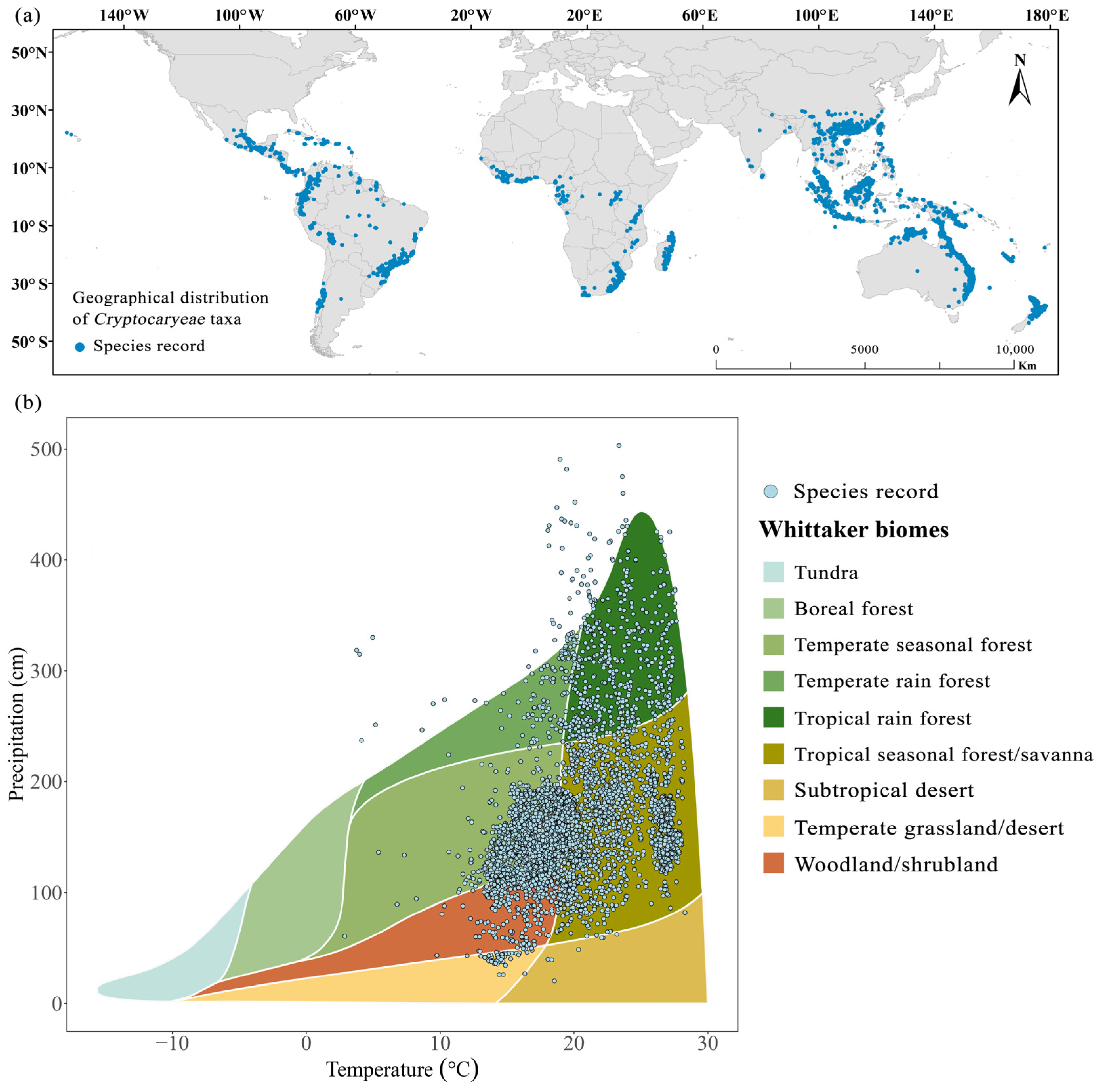
2.1. Global Patterns of Species Distribution of Cryptocaryeae Trees
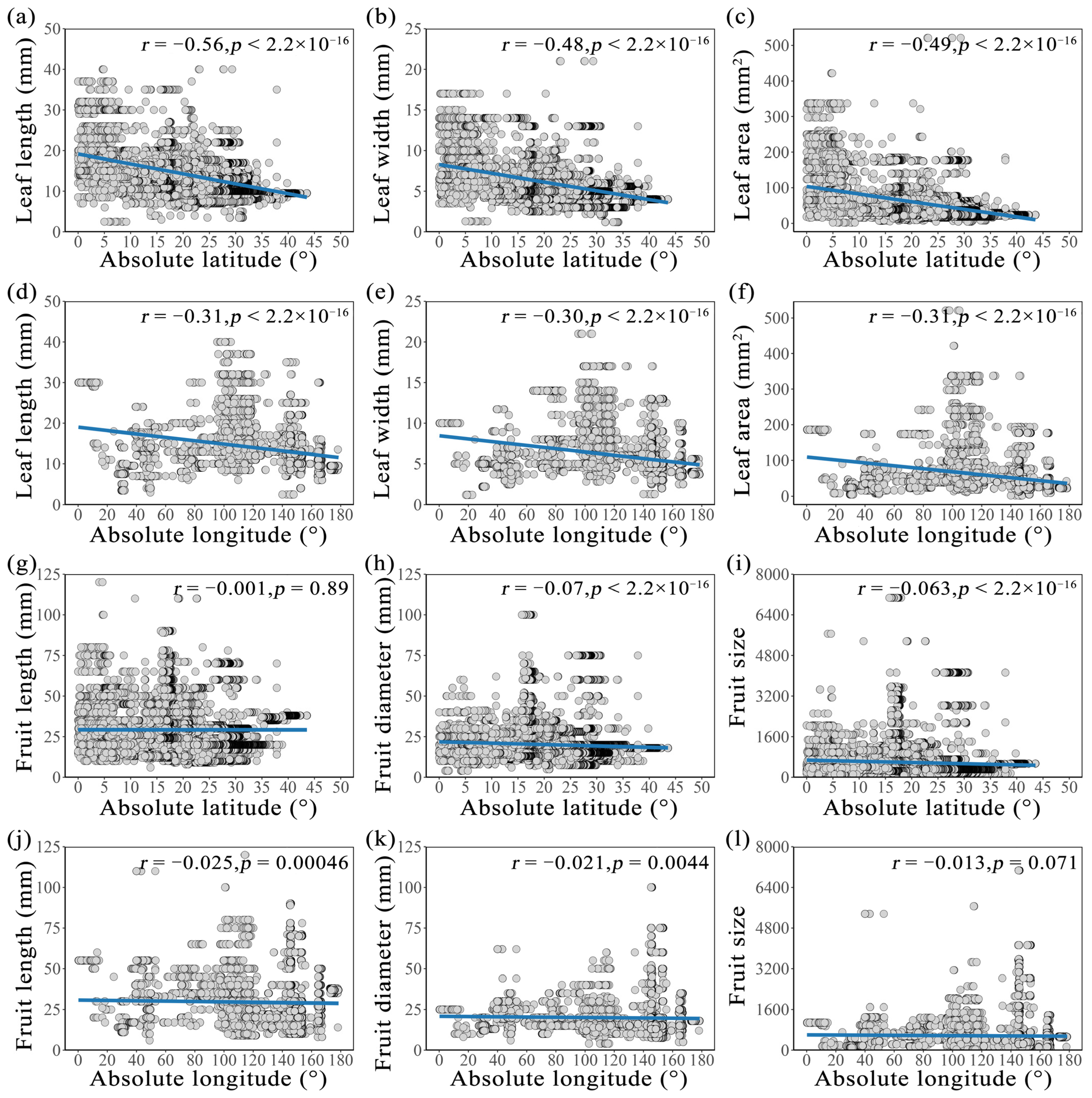
2.2. Longitudinal and Latitudinal Gradients of Leaf and Fruit Morphological Traits of Cryptocaryeae Trees
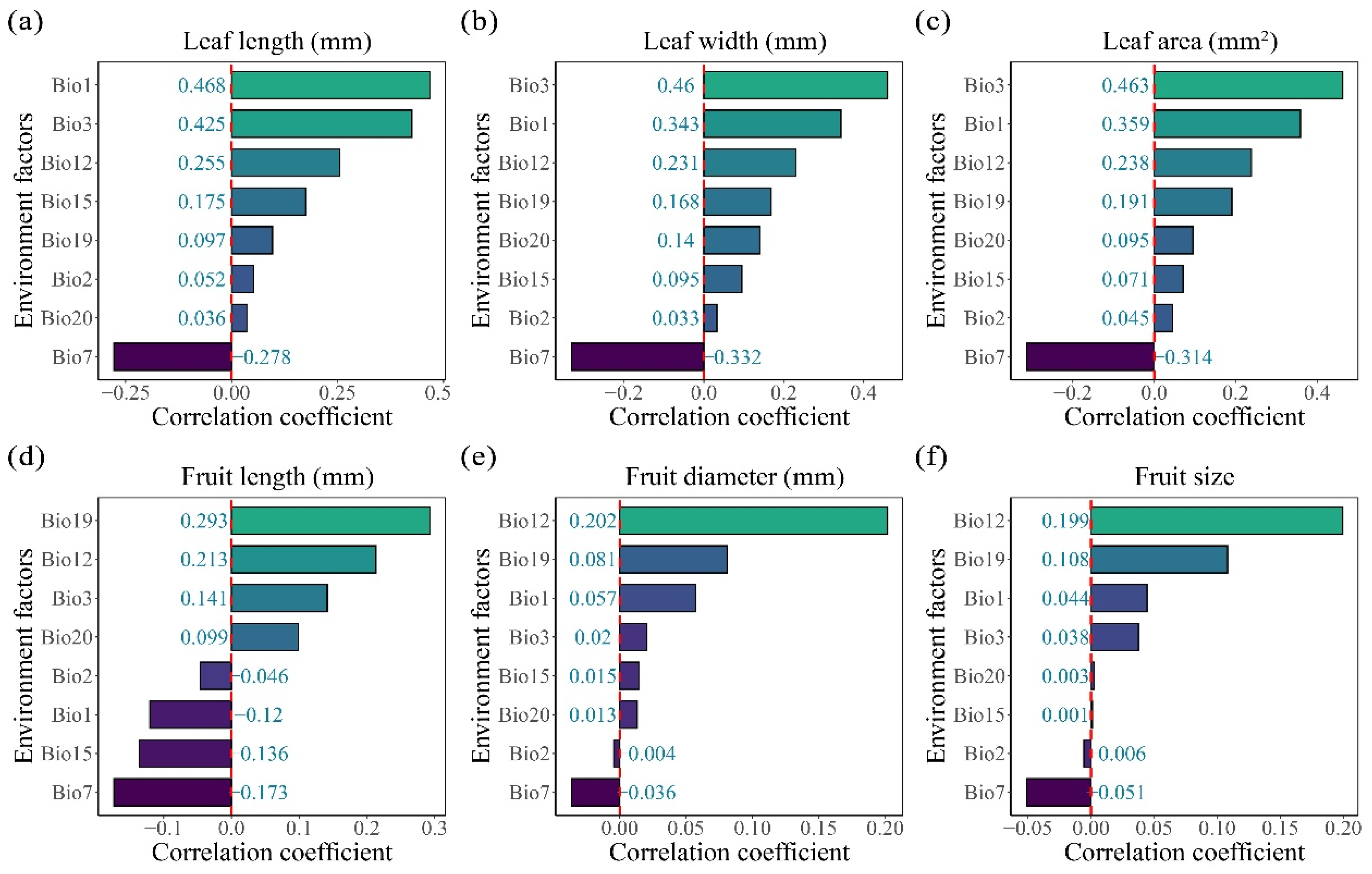
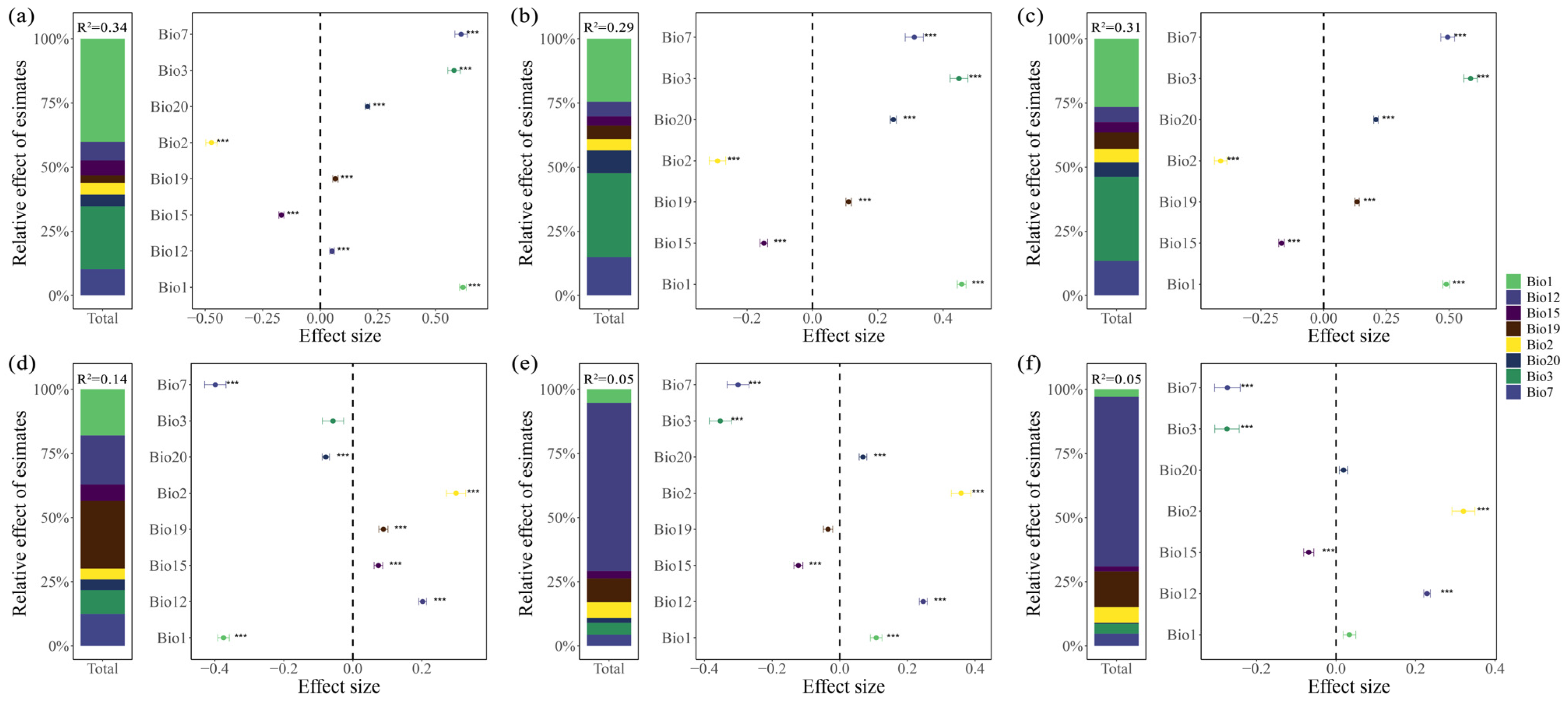
2.3. Climatic Influences on the Leaf and Fruit Morphological Traits of Cryptocaryeae Trees
3. Discussions
4. Materials and Methods
4.1. Morphological Trait Data
4.2. Environmental Factors Data
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boyko, J.D.; Hagen, E.R.; Beaulieu, J.M.; Vasconcelos, T. The evolutionary responses of life-history strategies to climatic variability in flowering plants. New Phytol. 2023, 240, 1587–1600. [Google Scholar] [CrossRef]
- Liu, H.; Yin, D.; He, P.; Cadotte, M.W.; Ye, Q. Linking plant functional traits to biodiversity under environmental change. Biol. Divers. 2024, 1, 22–28. [Google Scholar] [CrossRef]
- Field, R.D.; van der Werf, G.R.; Fanin, T.; Fetzer, E.J.; Fuller, R.; Jethva, H.; Levy, R.; Livesey, N.J.; Luo, M.; Torres, O.; et al. Indonesian fire activity and smoke pollution in 2015 show persistent nonlinear sensitivity to El Niño-induced drought. Proc. Natl. Acad. Sci. USA 2016, 113, 9204–9209. [Google Scholar] [CrossRef] [PubMed]
- van Oldenborgh, G.J.; Krikken, F.; Lewis, S.; Leach, N.J.; Lehner, F.; Saunders, K.R.; van Weele, M.; Haustein, K.; Li, S.; Wallom, D.; et al. Attribution of the Australian bushfire risk to anthropogenic climate change. Nat. Hazards Earth Syst. Sci. 2021, 21, 941–960. [Google Scholar] [CrossRef]
- Boisvert-Marsh, L.; de Blois, S. Unravelling potential northward migration pathways for tree species under climate change. J. Biogeogr. 2021, 48, 1088–1100. [Google Scholar] [CrossRef]
- Alexander, J.M.; Chalmandrier, L.; Lenoir, J.; Burgess, T.I.; Essl, F.; Haider, S.; Kueffer, C.; McDougall, K.; Milbau, A.; Nuñez, M.A.; et al. Lags in the response of mountain plant communities to climate change. Glob. Change Biol. 2018, 24, 563–579. [Google Scholar] [CrossRef]
- Ash, J.D.; Givnish, T.J.; Waller, D.M. Tracking lags in historical plant species’ shifts in relation to regional climate change. Glob. Change Biol. 2017, 23, 1305–1315. [Google Scholar] [CrossRef]
- Fox, R.J.; Donelson, J.M.; Schunter, C.; Ravasi, T.; Gaitán-Espitia, J.D. Beyond buying time: The role of plasticity in phenotypic adaptation to rapid environmental change. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180174. [Google Scholar] [CrossRef] [PubMed]
- Murren, C.J.; Auld, J.R.; Callahan, H.; Ghalambor, C.K.; Handelsman, C.A.; Heskel, M.A.; Kingsolver, J.G.; Maclean, H.J.; Masel, J.; Maughan, H.; et al. Constraints on the evolution of phenotypic plasticity: Limits and costs of phenotype and plasticity. Heredity 2015, 115, 293–301. [Google Scholar] [CrossRef]
- Pigliucci, M.; Murren, C.J.; Schlichting, C.D. Phenotypic plasticity and evolution by genetic assimilation. J. Exp. Biol. 2006, 209, 2362–2367. [Google Scholar] [CrossRef]
- Gratani, L. Plant Phenotypic Plasticity in Response to Environmental Factors. Adv. Bot. 2014, 2014, 208747. [Google Scholar] [CrossRef]
- Fritz, M.A.; Rosa, S.; Sicard, A. Mechanisms Underlying the Environmentally Induced Plasticity of Leaf Morphology. Front. Genet. 2018, 9, 478. [Google Scholar] [CrossRef]
- Schlichting, C.D. The Evolution of Phenotypic Plasticity in Plants. Annu. Rev. Ecol. Syst. 1986, 17, 667–693. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, L.; Qi, D. Variation in leaf traits at different altitudes reflects the adaptive strategy of plants to environmental changes. Ecol. Evol. 2020, 10, 8166–8175. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Guo, X.; Liu, S.; Yu, T.; Guo, W.; Wang, R.; Ye, S.; Lambertini, C.; Brix, H.; Eller, F. Intraspecific variation in Phragmites australis: Clinal adaption of functional traits and phenotypic plasticity vary with latitude of origin. J. Ecol. 2020, 108, 2531–2543. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.-K. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef] [PubMed]
- van der Merwe, S.; Greve, M.; Olivier, B.; le Roux, P.C. Testing the role of functional trait expression in plant–plant facilitation. Funct. Ecol. 2021, 35, 255–265. [Google Scholar] [CrossRef]
- Stotz, G.C.; Salgado-Luarte, C.; Escobedo, V.M.; Valladares, F.; Gianoli, E. Phenotypic plasticity and the leaf economics spectrum: Plasticity is positively associated with specific leaf area. Oikos 2022, 2022, e09342. [Google Scholar] [CrossRef]
- Yang, J.; Chong, P.; Chen, G.; Xian, J.; Liu, Y.; Yue, Y. Shifting plant leaf anatomical strategic spectra of 286 plants in the eastern Qinghai-Tibet Plateau: Changing gears along 1050–3070 m. Ecol. Indic. 2023, 146, 109741. [Google Scholar] [CrossRef]
- Desmond, S.C.; Garner, M.; Flannery, S.; Whittemore, A.T.; Hipp, A.L. Leaf shape and size variation in bur oaks: An empirical study and simulation of sampling strategies. Am. J. Bot. 2021, 108, 1540–1554. [Google Scholar] [CrossRef]
- Rawat, M.; Arunachalam, K.; Arunachalam, A.; Alatalo, J.M.; Pandey, R. Assessment of leaf morphological, physiological, chemical and stoichiometry functional traits for understanding the functioning of Himalayan temperate forest ecosystem. Sci. Rep. 2021, 11, 23807. [Google Scholar] [CrossRef]
- Fricke, E.C.; Tewksbury, J.J.; Rogers, H.S. Linking intra-specific trait variation and plant function: Seed size mediates performance tradeoffs within species. Oikos 2019, 128, 1716–1725. [Google Scholar] [CrossRef]
- Smith, T.M.; Sherman, C.D.H.; Cumming, E.E.; York, P.H.; Jarvis, J.C. Size matters: Variations in seagrass seed size at local scales affects seed performance. Hydrobiologia 2022, 849, 2335–2352. [Google Scholar] [CrossRef]
- Song, Y.; Xia, S.-W.; Tan, Y.-H.; Yu, W.-B.; Yao, X.; Xing, Y.-W.; Corlett, R.T. Phylogeny and biogeography of the Cryptocaryeae (Lauraceae). Taxon 2023, 72, 1244–1261. [Google Scholar] [CrossRef]
- Song, Y.; Yu, W.-B.; Tan, Y.-H.; Jin, J.-J.; Wang, B.; Yang, J.-B.; Liu, B.; Corlett, R.T. Plastid phylogenomics improve phylogenetic resolution in the Lauraceae. J. Syst. Evol. 2020, 58, 423–439. [Google Scholar] [CrossRef]
- Cao, Z.; Yang, L.; Xin, Y.; Xu, W.; Li, Q.; Zhang, H.; Tu, Y.; Song, Y.; Xin, P. Comparative and phylogenetic analysis of complete chloroplast genomes from seven Neocinnamomum taxa (Lauraceae). Front. Plant Sci. 2023, 14, 1205051. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, B.; Yang, Y.; Ferguson, D.K. Phylogeny and taxonomy of Cinnamomum (Lauraceae). Ecol. Evol. 2022, 12, e9378. [Google Scholar] [CrossRef] [PubMed]
- Henn, J.J.; Buzzard, V.; Enquist, B.J.; Halbritter, A.H.; Klanderud, K.; Maitner, B.S.; Michaletz, S.T.; Pötsch, C.; Seltzer, L.; Telford, R.J.; et al. Intraspecific Trait Variation and Phenotypic Plasticity Mediate Alpine Plant Species Response to Climate Change. Front. Plant Sci. 2018, 9, 1548. [Google Scholar] [CrossRef]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global climatic drivers of leaf size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef]
- Cox, A.J.F.; González-Caro, S.; Meir, P.; Hartley, I.P.; Restrepo, Z.; Villegas, J.C.; Sanchez, A.; Mercado, L.M. Variable thermal plasticity of leaf functional traits in Andean tropical montane forests. Plant Cell Environ. 2024, 47, 731–750. [Google Scholar] [CrossRef]
- Vendramini, F.; Díaz, S.; Gurvich, D.E.; Wilson, P.J.; Thompson, K.; Hodgson, J.G. Leaf traits as indicators of resource-use strategy in floras with succulent species. New Phytol. 2002, 154, 147–157. [Google Scholar] [CrossRef]
- McDonald, P.G.; Fonseca, C.R.; Overton, J.M.; Westoby, M. Leaf-size divergence along rainfall and soil-nutrient gradients: Is the method of size reduction common among clades? Funct. Ecol. 2003, 17, 50–57. [Google Scholar] [CrossRef]
- Peppe, D.J.; Royer, D.L.; Cariglino, B.; Oliver, S.Y.; Newman, S.; Leight, E.; Enikolopov, G.; Fernandez-Burgos, M.; Herrera, F.; Adams, J.M.; et al. Sensitivity of leaf size and shape to climate: Global patterns and paleoclimatic applications. New Phytol. 2011, 190, 724–739. [Google Scholar] [CrossRef]
- Pérez, F.; Hinojosa, L.F.; Ossa, C.G.; Campano, F.; Orrego, F. Decoupled evolution of foliar freezing resistance, temperature niche and morphological leaf traits in Chilean Myrceugenia. J. Ecol. 2014, 102, 972–980. [Google Scholar] [CrossRef]
- Eller, C.B.; Meireles, L.D.; Sitch, S.; Burgess, S.S.O.; Oliveira, R.S. How Climate Shapes the Functioning of Tropical Montane Cloud Forests. Curr. For. Rep. 2020, 6, 97–114. [Google Scholar] [CrossRef]
- Feng, X.; Zhong, L.; Wang, C.; Yang, Q.; Zhou, H.; Zhao, W. Acquisitive to conservative resource use strategy and increased site-specific trait variance contribute to Sophora moorcroftiana dominance along an altitudinal gradient in Qinghai–Tibet Plateau. Plant Ecol. 2023, 224, 1075–1087. [Google Scholar] [CrossRef]
- Poorter, L.; Bongers, F. Leaf Traits Are Good Predictors of Plant Performance Across 53 Rain Forest Species. Ecology 2006, 87, 1733–1743. [Google Scholar] [CrossRef]
- Meira-Neto, J.A.A.; Cândido, H.M.N.; Miazaki, Â.; Pontara, V.; Bueno, M.L.; Solar, R.; Gastauer, M. Drivers of the growth–survival trade-off in a tropical forest. J. Veg. Sci. 2019, 30, 1184–1194. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Bloom, A.J.; Field, C.B.; Waring, R.H. Plant Responses to Multiple Environmental Factors: Physiological ecology provides tools for studying how interacting environmental resources control plant growth. BioScience 1987, 37, 49–57. [Google Scholar] [CrossRef]
- Wang, J.; Gao, J.; Wu, Y.; Xu, B.; Shi, F.; Zhou, H.; Bisht, N.; Wu, N. Effects of Heterogeneous Environment After Deforestation on Plant Phenotypic Plasticity of Three Shrubs Based on Leaf Traits and Biomass Allocation. Front. Ecol. Evol. 2021, 9, 608663. [Google Scholar] [CrossRef]
- Chitwood, D.H.; Sinha, N.R. Evolutionary and Environmental Forces Sculpting Leaf Development. Curr. Biol. 2016, 26, R297–R306. [Google Scholar] [CrossRef]
- Bjorkman, A.D.; Elmendorf, S.C.; Beamish, A.L.; Vellend, M.; Henry, G.H.R. Contrasting effects of warming and increased snowfall on Arctic tundra plant phenology over the past two decades. Glob. Change Biol. 2015, 21, 4651–4661. [Google Scholar] [CrossRef] [PubMed]
- JHudson, M.G.; Henry, G.H.R.; Cornwell, W.K. Taller and larger: Shifts in Arctic tundra leaf traits after 16 years of experimental warming. Glob. Change Biol. 2011, 17, 1013–1021. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide ‘fast–slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Lomáscolo, S.B.; Levey, D.J.; Kimball, R.T.; Bolker, B.M.; Alborn, H.T. Dispersers shape fruit diversity in Ficus (Moraceae). Proc. Natl. Acad. Sci. USA 2010, 107, 14668–14672. [Google Scholar] [CrossRef] [PubMed]
- Corlett, R.T. Frugivory and Seed Dispersal. In Plant-Animal Interactions: Source of Biodiversity; Del-Claro, K., Torezan-Silingardi, H.M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 175–204. [Google Scholar]
- Gonçalves, B. Case not closed: The mystery of the origin of the carpel. Evodevo 2021, 12, 14. [Google Scholar] [CrossRef]
- González-Varo, J.P.; Onrubia, A.; Pérez-Méndez, N.; Tarifa, R.; Illera, J.C. Fruit abundance and trait matching determine diet type and body condition across frugivorous bird populations. Oikos 2022. [Google Scholar] [CrossRef]
- Quintero, E.; Pizo, M.A.; Jordano, P. Fruit resource provisioning for avian frugivores: The overlooked side of effectiveness in seed dispersal mutualisms. J. Ecol. 2020, 108, 1358–1372. [Google Scholar] [CrossRef]
- Rehling, F.; Jaroszewicz, B.; Braasch, L.V.; Albrecht, J.; Jordano, P.; Schlautmann, J.; Farwig, N.; Schabo, D.G. Within-Species Trait Variation Can Lead to Size Limitations in Seed Dispersal of Small-Fruited Plants. Front. Ecol. Evol. 2021, 9, 698885. [Google Scholar] [CrossRef]
- Brodie, J.F. Evolutionary cascades induced by large frugivores. Proc. Natl. Acad. Sci. USA 2017, 114, 11998–12002. [Google Scholar] [CrossRef]
- Janson, C.H. Adaptation of Fruit Morphology to Dispersal Agents in a Neotropical Forest. Science 1983, 219, 187–189. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.C.P.; Nunes, A.; Rodrigues, R.G.; Branquinho, C. The response of plant functional traits to aridity in a tropical dry forest. Sci. Total Environ. 2020, 747, 141177. [Google Scholar] [CrossRef]
- Pueyo, Y.; Kefi, S.; Alados, C.L.; Rietkerk, M. Dispersal strategies and spatial organization of vegetation in arid ecosystems. Oikos 2008, 117, 1522–1532. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef]
- Hofhansl, F.; Chacón-Madrigal, E.; Brännström, Å.; Dieckmann, U.; Franklin, O. Mechanisms driving plant functional trait variation in a tropical forest. Ecol. Evol. 2021, 11, 3856–3870. [Google Scholar] [CrossRef] [PubMed]
- Maugarny-Calès, A.; Laufs, P. Getting leaves into shape: A molecular, cellular, environmental and evolutionary view. Development 2018, 145, dev161646. [Google Scholar] [CrossRef]
- Li, Y.; Reich, P.B.; Schmid, B.; Shrestha, N.; Feng, X.; Lyu, T.; Maitner, B.S.; Xu, X.; Li, Y.; Zou, D.; et al. Leaf size of woody dicots predicts ecosystem primary productivity. Ecol. Lett. 2020, 23, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Wright, I.J.; Cavender-Bares, J.; Craine, J.M.; Oleksyn, J.; Westoby, M.; Walters, M.B. The Evolution of Plant Functional Variation: Traits, Spectra, and Strategies. Int. J. Plant Sci. 2003, 164, S143–S164. [Google Scholar] [CrossRef]
- Costa, D.S.; Zotz, G.; Hemp, A.; Kleyer, M. Trait patterns of epiphytes compared to other plant life-forms along a tropical elevation gradient. Funct. Ecol. 2018, 32, 2073–2084. [Google Scholar] [CrossRef]
- Eisenring, M.; Unsicker, S.B.; Lindroth, R.L. Spatial, genetic and biotic factors shape within-crown leaf trait variation and herbivore performance in a foundation tree species. Funct. Ecol. 2021, 35, 54–66. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Moles, A.T.; Leitch, I.J.; Bennett, M.D.; Dickie, J.B.; Knight, C.A. Correlated evolution of genome size and seed mass. New Phytol. 2007, 173, 422–437. [Google Scholar] [CrossRef]
- Moles, A.T.; Ackerly, D.D.; Webb, C.O.; Tweddle, J.C.; Dickie, J.B.; Pitman, A.J.; Westoby, M. Factors that shape seed mass evolution. Proc. Natl. Acad. Sci. USA 2005, 102, 10540–10544. [Google Scholar] [CrossRef] [PubMed]
- Sukhorukov, A.P.; Sousa-Baena, M.S.; Romanov, M.S.; Wang, X. Editorial: Fruit and seed evolution in angiosperms. Front. Plant Sci. 2023, 14, 1196443. [Google Scholar] [CrossRef]
- Zheng, J.; Guo, Z.; Wang, X. Seed mass of angiosperm woody plants better explained by life history traits than climate across China. Sci. Rep. 2017, 7, 2741. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü. Leaf Trait Plasticity and Evolution in Different Plant Functional Types. Annu. Plant Rev. Online 2020, 3, 473–522. [Google Scholar]
- Encinas-Viso, F.; Revilla, T.A.; van Velzen, E.; Etienne, R.S. Frugivores and cheap fruits make fruiting fruitful. J. Evol. Biol. 2014, 27, 313–324. [Google Scholar] [CrossRef]
- Sun, M.; Folk, R.A.; Gitzendanner, M.A.; Soltis, P.S.; Chen, Z.; Soltis, D.E.; Guralnick, R.P. Recent accelerated diversification in rosids occurred outside the tropics. Nat. Commun. 2020, 11, 3333. [Google Scholar] [CrossRef] [PubMed]
- O’Donnel, M.S.; Ignizio, D.A. Bioclimatic Predictors for Supporting Ecological Applications in the Conterminous United States, Data Series; US Geological Survey: Reston, VA, USA, 2012; p. 17.
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Lobo, J.M.; Hortal, J. The effect of prevalence and its interaction with sample size on the reliability of species distribution models. Community Ecol. 2009, 10, 196–205. [Google Scholar] [CrossRef]
- Feng, X.; Park, D.S.; Liang, Y.; Pandey, R.; Papeş, M. Collinearity in ecological niche modeling: Confusions and challenges. Ecol. Evol. 2019, 9, 10365–10376. [Google Scholar] [CrossRef]
- Bartoń, K. _MuMIn: Multi-Model Inference. R package version 1.48.4. 2024. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 6 November 2025).
- Lai, J.; Zou, Y.; Zhang, J.; Peres-Neto, P.R. Generalizing hierarchical and variation partitioning in multiple regression and canonical analyses using the rdacca.hp R package. Methods Ecol. Evol. 2022, 13, 782–788. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 6 November 2025).
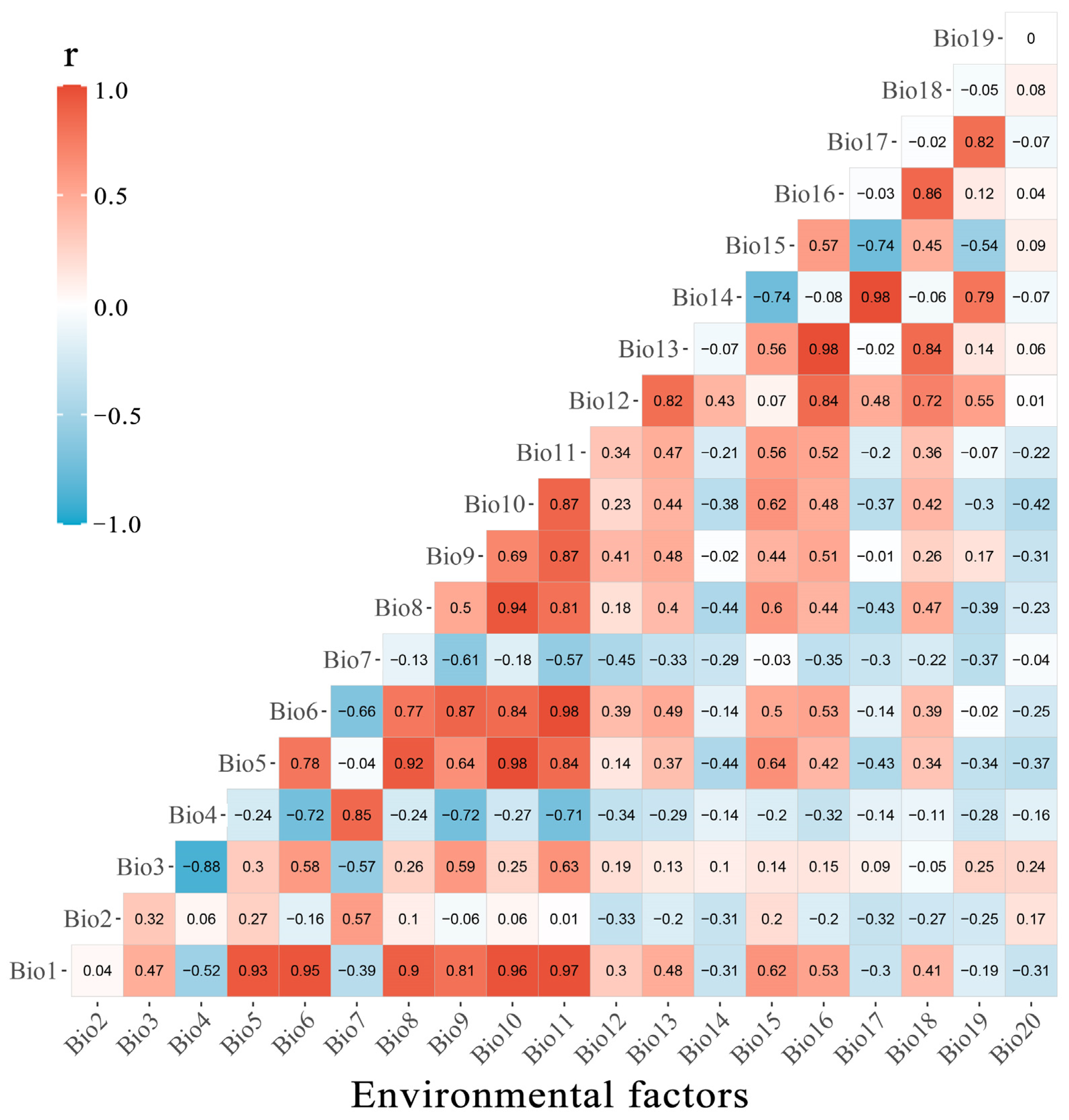
| Code Name | Environmental Factors |
|---|---|
| Bio1 | Annual Mean Temperature |
| Bio2 | Mean Diurnal Range |
| Bio3 | Isothermality |
| Bio7 | Annual Temperature Range |
| Bio12 | Annual Precipitation |
| Bio15 | Precipitation Seasonality |
| Bio19 | Precipitation of Coldest Quarter |
| Bio20 | Digital Elevaton Model |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Zhao, W.; Wang, L.; Song, Y.; Jiang, H.; Guo, X. Leaf-Fruit Trait Decoupling Along Environmental Gradients in Tropical Cryptocaryeae (Lauraceae). Plants 2026, 15, 126. https://doi.org/10.3390/plants15010126
Zhao W, Wang L, Song Y, Jiang H, Guo X. Leaf-Fruit Trait Decoupling Along Environmental Gradients in Tropical Cryptocaryeae (Lauraceae). Plants. 2026; 15(1):126. https://doi.org/10.3390/plants15010126
Chicago/Turabian StyleZhao, Wendi, Lifang Wang, Yu Song, Honglei Jiang, and Xiali Guo. 2026. "Leaf-Fruit Trait Decoupling Along Environmental Gradients in Tropical Cryptocaryeae (Lauraceae)" Plants 15, no. 1: 126. https://doi.org/10.3390/plants15010126
APA StyleZhao, W., Wang, L., Song, Y., Jiang, H., & Guo, X. (2026). Leaf-Fruit Trait Decoupling Along Environmental Gradients in Tropical Cryptocaryeae (Lauraceae). Plants, 15(1), 126. https://doi.org/10.3390/plants15010126






