Optimization of Sweet Potato (Ipomoea batatas L.) Chlorogenic Acid Extraction Process and Hypoglycemic Effect Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Animals
2.2. Extraction Process of Chlorogenic Acid from Old Leaves of Sweet Potato
2.3. Single-Factor Experimental Design
2.4. Response Surface Experimental Design
2.5. Chlorogenic Acid Standard Curve Plotting
2.6. Calculation of Chlorogenic Acid Extraction Rate
2.7. Determination of CGA Crude Extracts of Sweet Potato Leaf by HPLC
2.8. Collection of Active Components and Targets of Sweet Potato Leaves
2.9. Screening of Disease Targets for Diabetes
2.10. Construction of Protein-Protein Interaction (PPI) Network and Screening of Core Targets
2.11. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment Analysis
2.12. Component–Target Molecular Docking
2.13. Isolation of Primary Mouse Hepatocytes
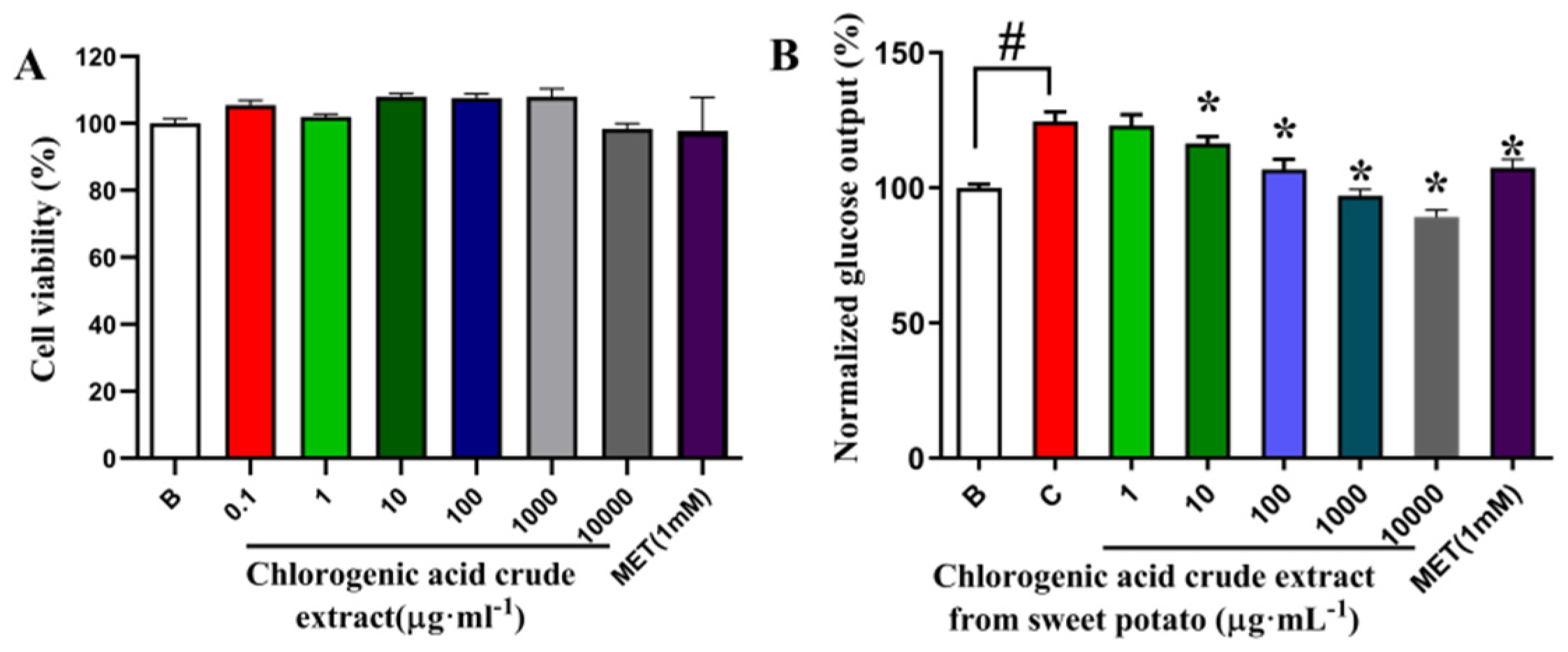
2.14. Cytotoxicity Assay
2.15. Glucose Output
2.16. Data Statistics and Analysis
3. Results and Discussion
3.1. Single-Factor Test Results
3.2. Response Surface Test Results
3.2.1. Significance Test and Variance Analysis of Regression Model
3.2.2. Responsive Surface Experimental Design
3.2.3. Determination of CGA Crude Extract in Sweet Potato Leaves
3.3. Comparison of CGA Crude Extracts in Different Parts and Varieties
3.4. Analysis of Network Pharmacology Results
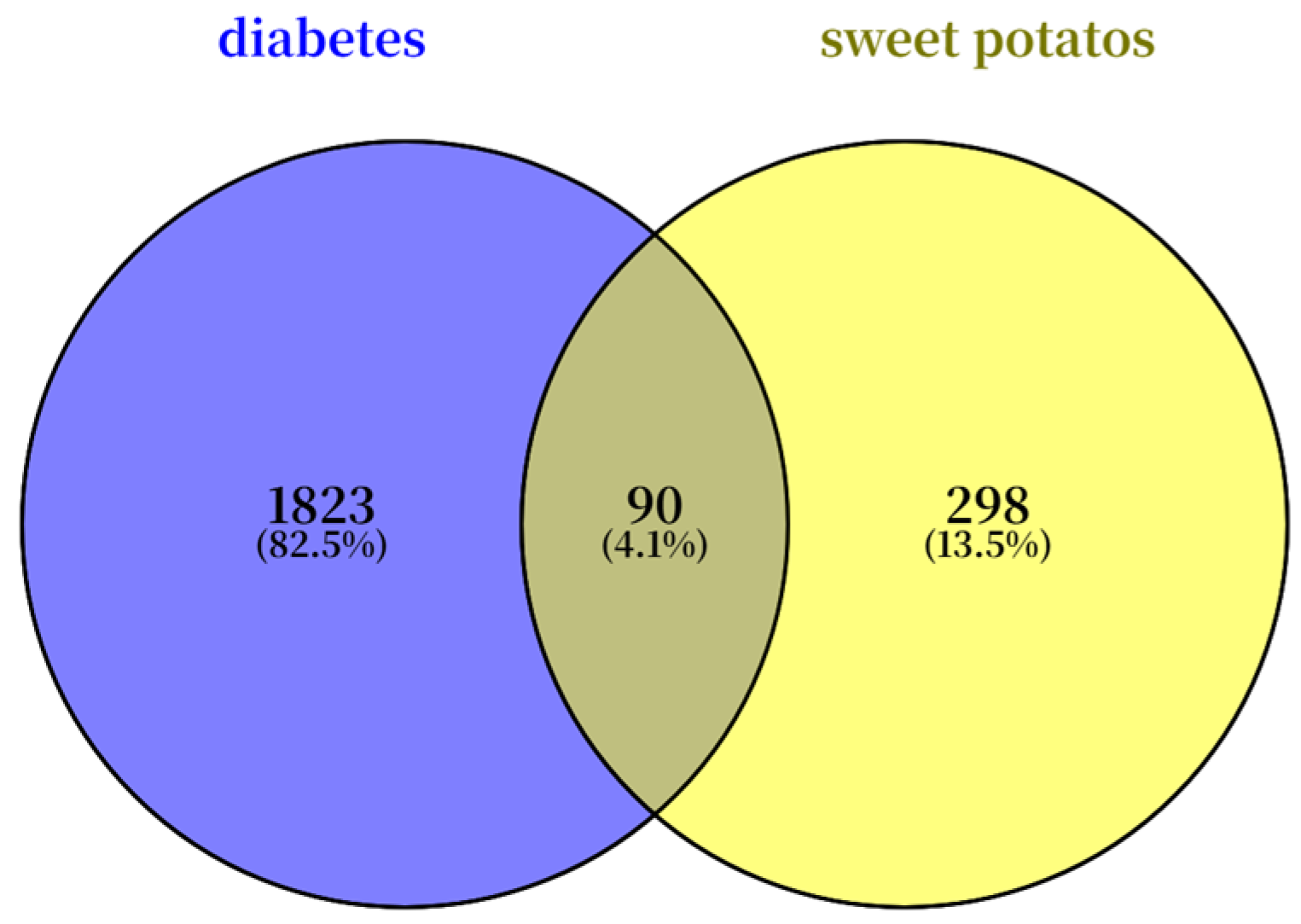
3.4.1. Screening of Active Components, Targets of Sweet Potato Leaves, and Disease Targets
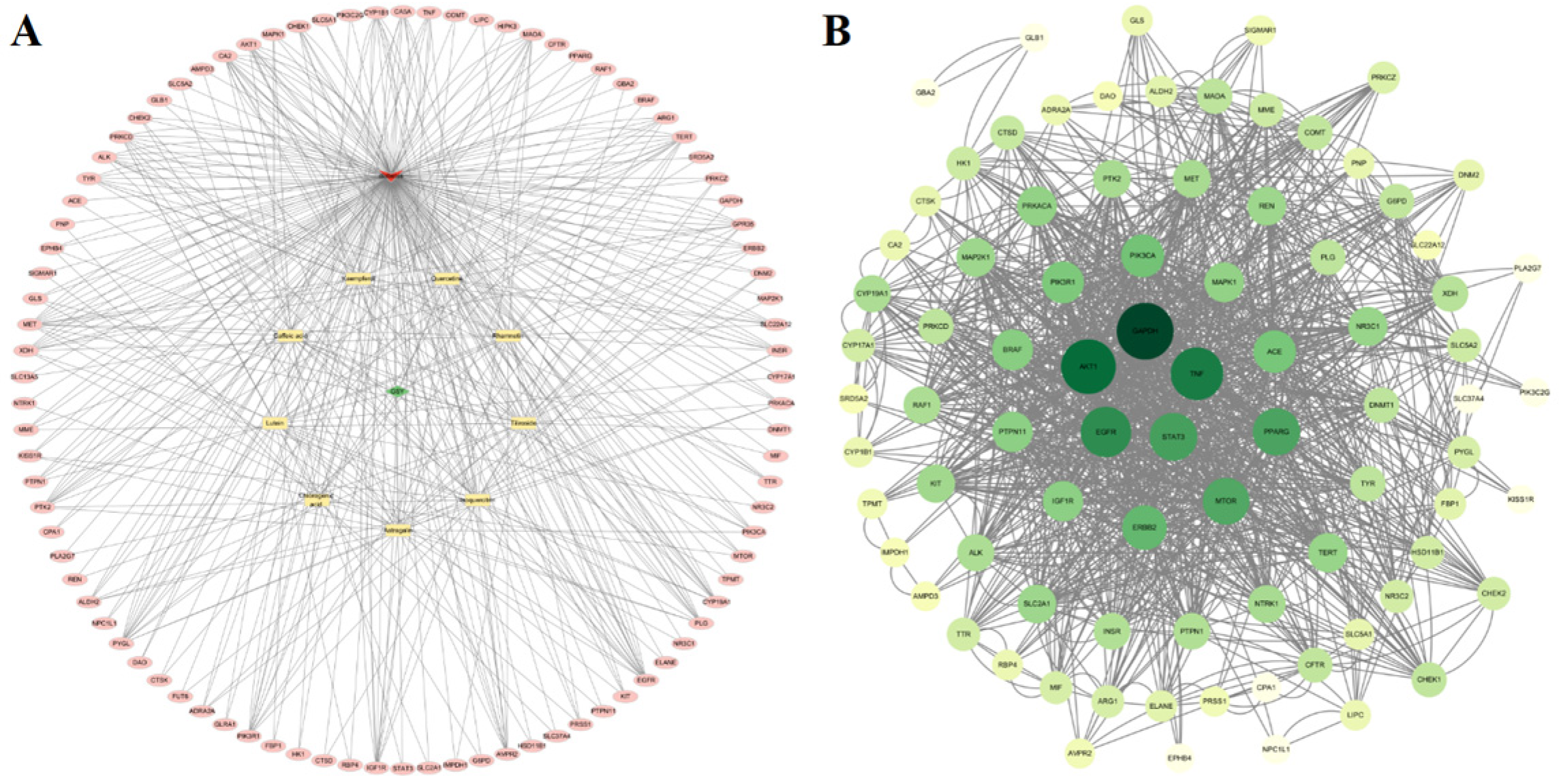
3.4.2. Results of the “Drug-Active Component-Target-Disease” Network Analysis
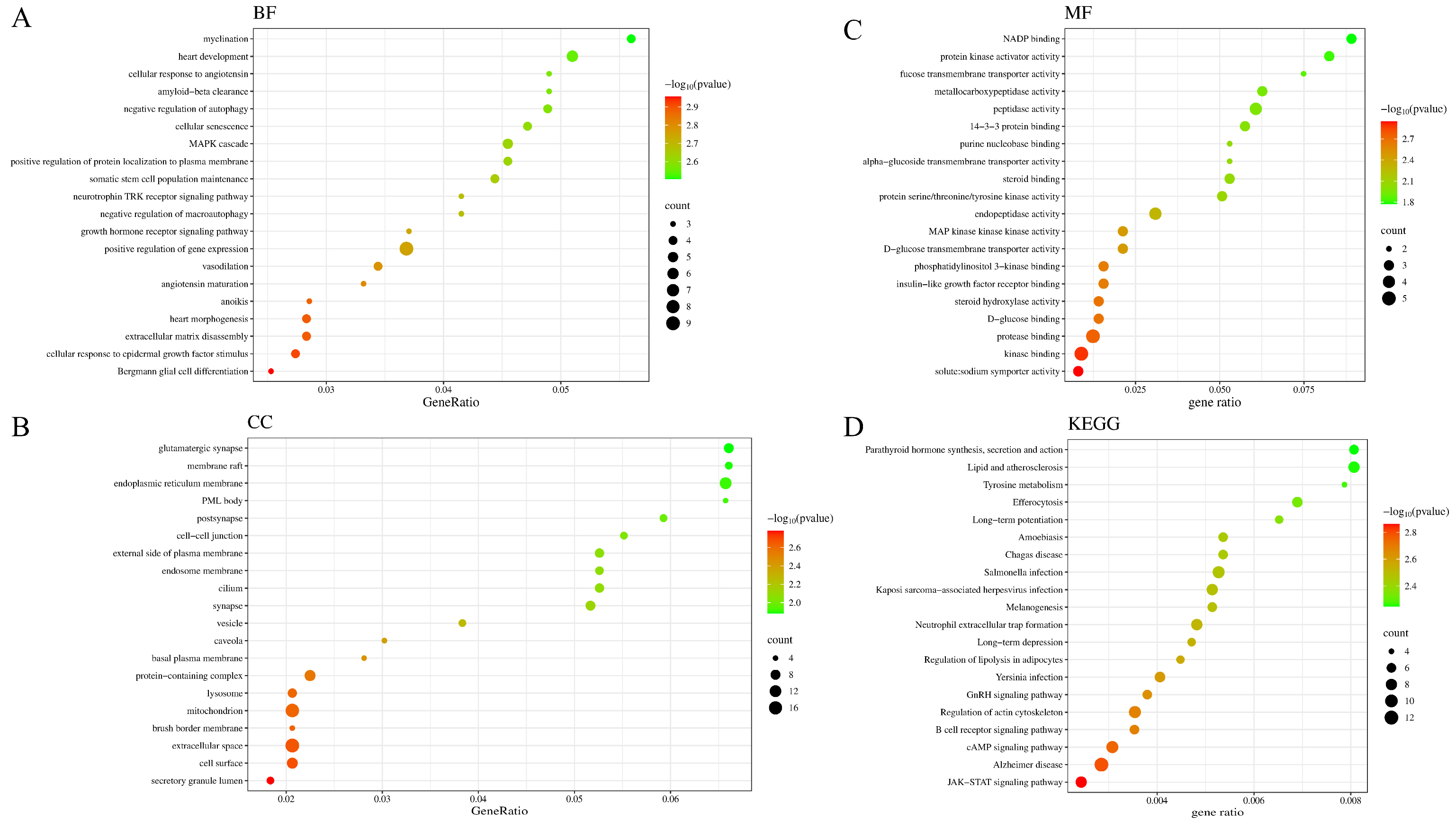
3.4.3. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Functional Enrichment Analysis
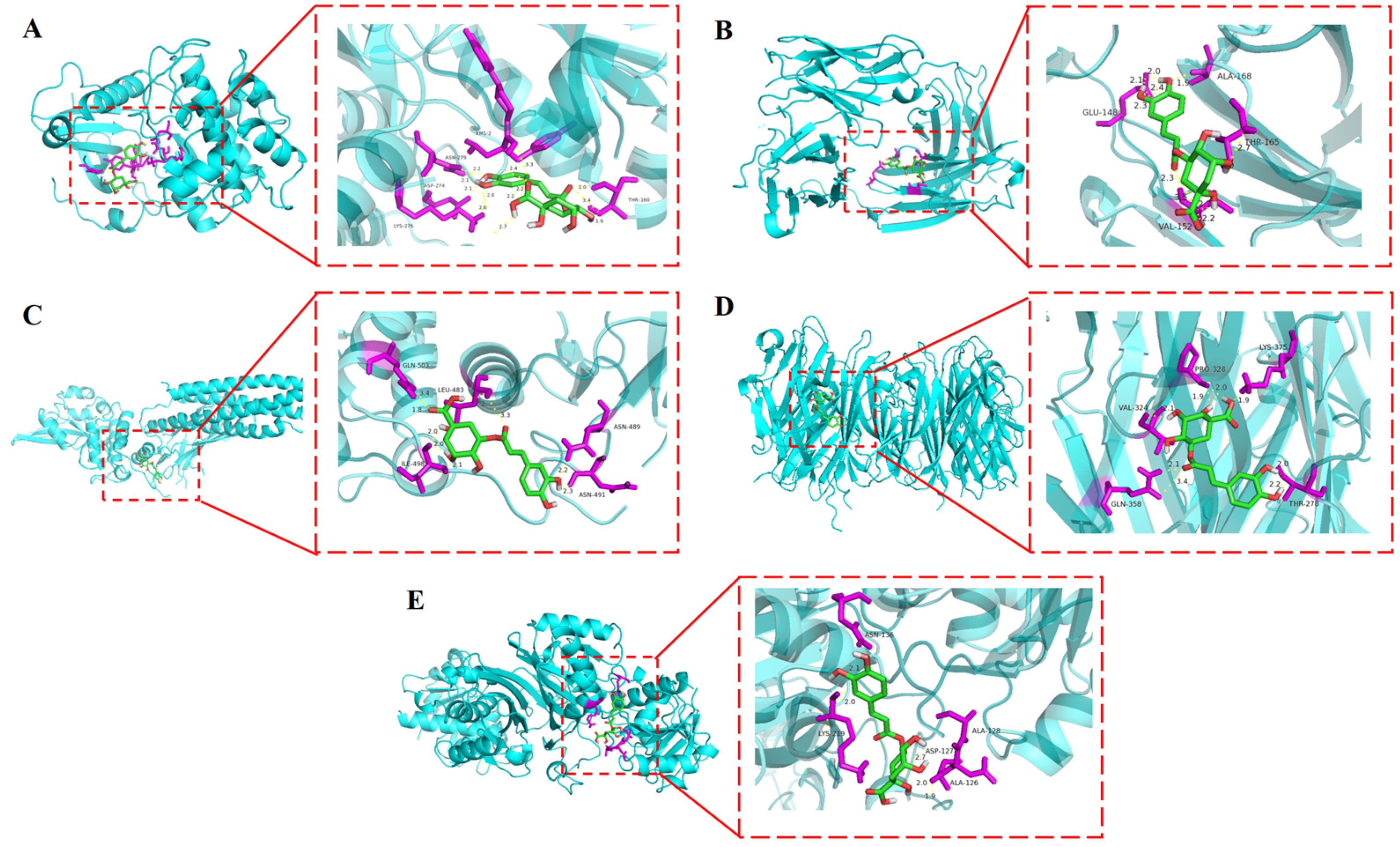
3.5. Molecular Docking Results
3.6. Effect of Crude Extract of Chlorogenic Acid from Sweet Potato on Survival Rate and Glucose Production of Primary Mouse Hepatocytes
3.6.1. Effect of CGA Crude Extract from Sweet Potato Leaves on Survival and Gluconeogenesis of Primary Mouse Hepatocytes
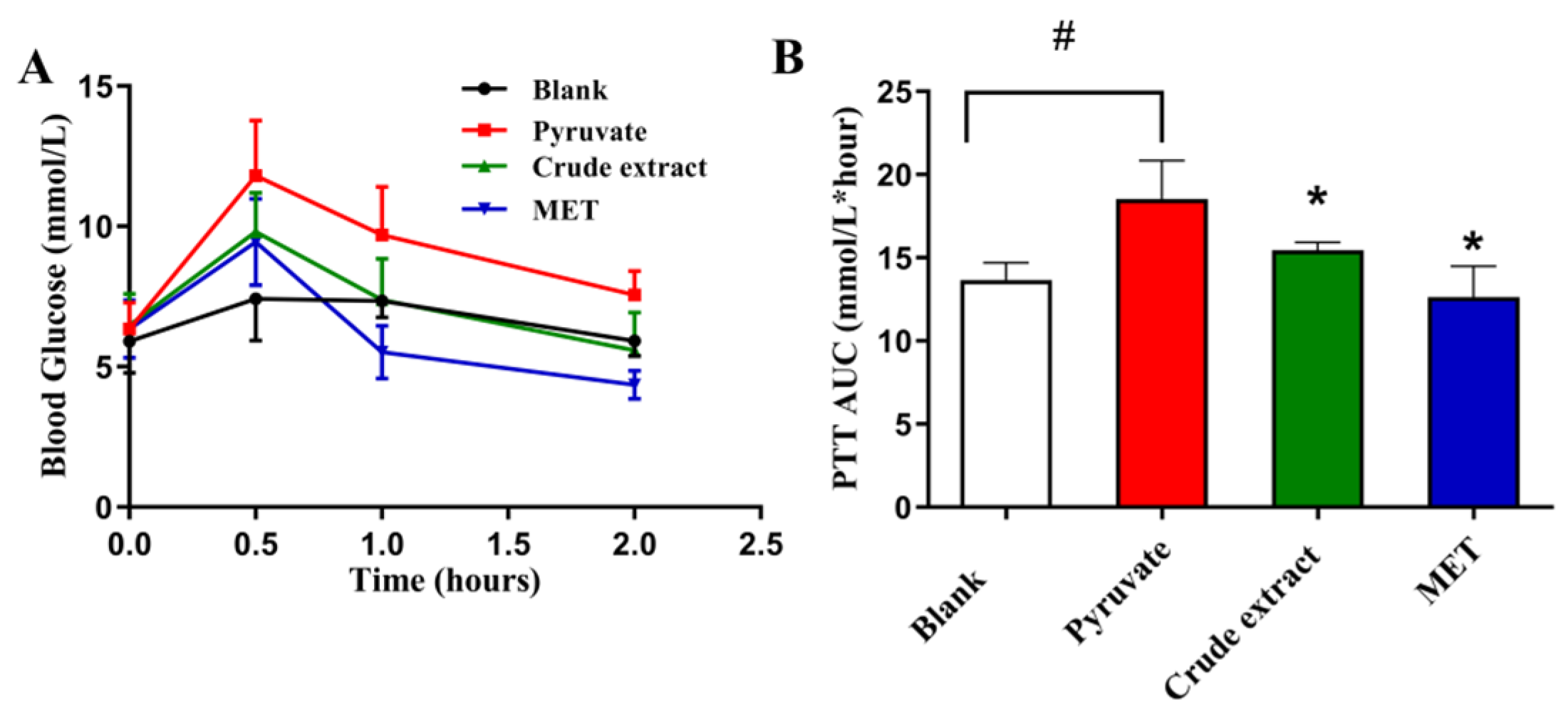
3.6.2. Effects of Sweet Potato CGA Crude Extract on Pyruvate Tolerance in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Zhang, Y.; Deng, J. Exploring the integration and development path of sweet potato industry in yuzhou, Henan Province. Rural. Agric. Farmers (B Ed.) 2022, 8, 7–12. [Google Scholar]
- Hou, F.; Chen, G.; Dong, S.; Xie, B.; Qin, Z.; Li, A.; Zhang, L.; Wang, Q. Comparative study on starch composition, physicochemical properties and noodle quality of different sweet potato varieties. Acta Agric. Nucl. Sin. 2022, 36, 392–401. [Google Scholar]
- Wang, Z.; Li, Z. Biological activity of chlorogenic acid and progress of sweet potato chlorogenic acid research. J. Jiangsu Norm. Univ. (Nat. Sci. Ed.) 2017, 35, 30–34+48. [Google Scholar]
- Shi, Y.; Hu, J.; Li, L. Research progress on nutritional functions and processing utilization of sweet potato. Food Res. Dev. 2022, 43, 205–211. [Google Scholar]
- Miranda, L.; Deußer, H.; Evers, D. The impact of in vitro digestion on bioaccessibility of polyphenols from potatoes and sweet potatoes and their influence on iron absorption by human intestinal cells. Food Funct. 2013, 4, 1595–1601. [Google Scholar] [CrossRef]
- Dai, J.; Li, H.; Gou, L.; Tian, Y.; Cheng, C.; Yuan, F.; Xie, J.; Zhang, L.; Ji, J.; Zhang, L.; et al. Mechanistic study of purple sweet potato anthocyanins: Multifaceted anti-fibrotic effects and targeting of PDGFRβ in liver fibrosis. J. Agric. Food Chem. 2024, 72, 27861–27875. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD—RisC). Worldwide trends in diabetes since 1980: A pooled analysis of 751 population—Based studies with 4.41million participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef] [PubMed]
- Soderling, S.; Beavo, J. Regulation of cAMP and cGMP signaling: New phospho diesterases and new functions. Curr. Opin. Cell Biol. 2000, 12, 174–179. [Google Scholar] [CrossRef]
- Su, W.; Li, A.; Zhang, Y.; Zhang, Y.; Jiao, X. Regulation of gluconeogenesis in liver, kidney and intestine by glucagon. Chin. Pharmac. Bulletin. 2023, 39, 1332–1338. [Google Scholar]
- Capozzi, M.; D’Alessio, D.; Campbell, J. The past, present, and future physiology and pharmacology of glucagon. Cell Metab. 2022, 34, 1654–1674. [Google Scholar] [CrossRef]
- Han, B.; Jiang, P.; Jiang, L.; Li, X.; Ye, X. Three phytosterols from sweet potato inhibit MCF7-xenograft-tumor growth through modulating gut microbiota homeostasis and SCFAs secretion. Food Res. Int. 2021, 141, 110147. [Google Scholar] [CrossRef]
- Liu, C.; Miao, Y.; Zhou, W.; Ma, Y.; Guo, W.; Li, A. Impact of thermal processing on the structure, antioxidant properties and hypoglycemic activities of sweet potato polysaccharides. Foods 2024, 13, 3082. [Google Scholar] [CrossRef]
- Lee, C.; Yoon, Y.; Yoon, Y.; Chung, K.; Kim, M.; Park, G.; Choi, M.; Jang, Y.; Lee, K. Protective effects of a standardized water extract from the stem of Ipomoea batatas L. against high-fat diet-induced obesity. Nutrient 2025, 17, 1643. [Google Scholar] [CrossRef]
- Ammara, A.; Hira, I.; Ayesha, S.; Zulfiqar, T.; Tareen, M.; Amna, D.; Shakir, M.; Hazafa, A.; Naeem, M.; Lorenzo, J.; et al. Comparative study of potato (Solanum tuberosum L.) and sweet potato (Ipomoea batatas L.): Evaluation of proximate composition, polyphenol content, mineral and sntioxidant sctivities. Appl. Sci. 2021, 11, 11844. [Google Scholar]
- Moraga-Babiano, L.; Lucas-González, R.; Domínguez-Valencia, R.; Gaona-Ruiz, M.; Carrillo, C.; Echegaray, N.; Pateiro, M.; Lorenzo, J. Encapsulated purple sweet potato peel extract as antioxidant and sustainable colourant to preserve the quality of beef burgers during the shelf life. Food Chem. 2025, 487, 144657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bian, S.; Hu, J.; Liu, G.; Peng, S.; Chen, H.; Jiang, Z.; Wang, T.; Ye, Q.; Zhu, H. Natural deep eutectic solvent-based microwave-assisted extraction of total flavonoid compounds from spent sweet potato (Ipomoea batatas L.) leaves: Optimization and antioxidant and bacteriostatic Activity. Molecules 2022, 27, 5985. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.; Chen, C.; Varga, V.; Shih, L.; Chen, P.; Lo, S.; Shyur, L.; Li, S. White sweet potato ameliorates hyperglycemia and regenerates pancreatic islets in diabetic mice. Food Nutr. Res. 2020, 64, 10. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Yang, X.; Kou, M.; Lu, C.; Wang, Y.; Peng, J.; Chen, P.; Jiang, J. Selenylation of polysaccharide from the sweet potato and evaluation of antioxidant, antitumor, and antidiabetic activities. J. Agric. Food Chem. 2017, 65, 605–617. [Google Scholar] [CrossRef]
- Luo, D. Hypoglycemic Effect and Mechanism of Polyphenols from Stems and Leaves of Sweet Potato ‘Ximeng 1’. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2020. [Google Scholar]
- Hu, Z.; Lv, G.; Dong, W.; Zheng, F.; Yan, P.; Yu, S.; He, D.; Liu, B.; Gao, Y.; Su, D.; et al. Network pharmacology and molecular docking elucidate the mechanism of phillyrin in colorectal cancer. Food Sci. Nutr. 2025, 13, e71069. [Google Scholar] [CrossRef]
- Bae, J.; Park, W.; Kim, H.; Kim, H.; Kang, K.; Kwak, S.; Ahn, M. Protective effect of carotenoid extract from orange-fleshed sweet potato on gastric ulcer in mice by inhibition of NO, IL-6 and PGE2 Production. Pharmaceuticals 2021, 14, 1320. [Google Scholar] [CrossRef]
- Huang, Q.; Shan, Q.; Ma, F.; Li, S.; Sun, P. Chlorogenic acid mitigates heat stress-induced oxidative damage in bovine mammary epithelial cells by inhibiting NF-κB-mediated NLRP3 inflammasome activation via upregulating the Nrf2 signaling pathway. Int. J. Biol. Macromol. 2025, 301, 140133. [Google Scholar] [CrossRef]
- Tang, S.; Zhong, W.; Li, T.; Li, Y.; Song, G. Isochlorogenic acid A alleviates dextran sulfate sodium-induced ulcerative colitis in mice through STAT3/NF-κB pathway. Int. Immunopharmacol. 2023, 118, 109989. [Google Scholar] [CrossRef]
- Jackson, K.; Rathinasabapathy, T.; Esposito, D.; Komarnytsky, S. Structural constraints and importance of caffeic acid moiety for anti-hyperglycemic effects of caffeoylquinic acids from chicory. Mol. Nutr. Food Res. 2017, 61, 10. [Google Scholar] [CrossRef]
- Henry-Vitrac, C.; Ibarra, A.; Roller, M.; Mérillon, J.; Vitrac, X. Contribution of chlorogenic acids to the inhibition of human hepatic glucose-6-phosphatase activity in vitro by Svetol, a standardized decaffeinated green coffee extract. J. Agric. Food Chem. 2010, 58, 4141–4144. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Zhang, T.; Han, M.; Tian, D.; Liu, J.; Li, S.; Yang, L.; Pan, G. Chlorogenic acid inhibits ceramide accumulation to restrain hepatic glucagon response. Nutrients 2023, 15, 3173. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Cetto, A.; Vázquez, R. Gluconeogenesis inhibition and phytochemical composition of two Cecropia species. J. Ethnopharmacol. 2010, 130, 93–97. [Google Scholar] [CrossRef]
- Abdollahi, M.; Marandi, S.; Ghaedi, K.; Safaeinejad, Z.; Kazeminasab, F.; Shirkhani, S.; Sanei, M.H.; Rezvanian, P.; Nasr-Esfahani, M.H. Insulin-related liver pathways and the therapeutic effects of aerobic training, green coffee, and chlorogenic acid supplementation in prediabetic mice. Oxid. Med. Cell. Longev. 2022, 2022, 5318245. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Miao, Q.; He, M. Optimization of extraction process and stability study of chlorogenic acid from dandelion. China Feed 2023, 23, 54–60. [Google Scholar]
- Mangiapelo, L.; Blasi, F.; Ianni, F.; Barola, C.; Galarini, R.; Abualzulof, G.; Sardella, R.; Volpi, C.; Cossignani, L. Optimization of ultrasound-assisted extraction of chlorogenic acid from potato sprout waste and enhancement of the in vitro total antioxidant capacity. Antioxidants 2023, 12, 348. [Google Scholar] [CrossRef]
- Chen, M.; Liu, B.; Luan, Y.; Zhou, L.; Huang, Y. Optimization of chlorogenic acid extraction from the leaves of Sambucus chinensis Lindl. by response surface methodology. Hunan Agric. Sci. 2022, 7, 84–87+91. [Google Scholar]
- Liu, J.; Duan, H.; Wang, H.; Gao, Q.; Liu, L.; Liang, Y.; He, M.; Xu, L.; Guo, X. Deep eutectic solvent extraction of chlorogenic acid from dandelion with ultrasonic-assisted: Process optimization, purification, and bioactivity. Ultrason. Sonochem. 2025, 122, 107579. [Google Scholar] [CrossRef]
- Gan, J.; Ji, C.; Mao, X.; Wang, J.; Lv, C.; Shi, Y.; Liao, Y.; He, Y.; Shu, L.; Li, L.; et al. Synchronization isolation method for multiple types of cells from mouse liver. Zhonghua Gan Zang Bing Za Zhi 2023, 31, 532–537. [Google Scholar]
- Wang, Y. Extraction and Genotypic Varietion of Antioxidant Functional Components from Sweet Potato Vines. Master’s Thesis, Zhejiang A&F University, Hangzhou, China, 2019. [Google Scholar]
- Chiu, C.; Liu, K.; Lin, H.; Chu, W.; Lai, Y.; Chao, P. Analysis of Chlorogenic Acid in Sweet Potato Leaf Extracts. Plants 2022, 11, 2063. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, J.; Li, X. Determination and analysis of polysaccharide content in stem and leaves of different sweet potato varieties. J. Shanxi Agric. Sci. 2018, 46, 182–186+206. [Google Scholar]
- Wang, W. Study on the Preparation Process Andquality Standards of Senecio cannabifolius Extract; CCUCM: Changchun, China, 2013. [Google Scholar]
- Zhang, X.; Xie, J.; Chen, Y.; Zhang, X. Extraction of chlorogenic acid from folium cortex eucommiae by enzymatic hydrolysis method and determination of its content. China Brew. 2016, 35, 149–152. [Google Scholar]
- Li, J.; Yu, L.; Yang, X.; Guo, J. Extraction process of chlorogenic acid from wild chrysanthemum flower via supercritical fluid extraction. Food Res. Dev. 2012, 33, 30–32. [Google Scholar]
- Shi, J.; Fang, D.; Sui, Y.; Xiong, T.; Chen, X.; Fan, C.; Zhou, D.; Cai, F.; Mei, X. Polyphenol content, antioxidant capacity, and composition in different varieties of sweet potato (Ipomoea batatas L.) leaves during growth stages. Sci. Hortic. 2025, 342, 113925. [Google Scholar] [CrossRef]
- Jahurul, M.; Islam, S. Emerging techniques for the recovery of bioactive compounds from sweet potato leaves [Ipomoea batatas (L.) Lam] and their functional health benefits. Food Bioeng. 2025, 4, 318–339. [Google Scholar] [CrossRef]
- Dewi, N.K.S.M.; Ramona, Y.; Saraswati, M.; Wihandani, D.; Wirasuta, I. The potential of the flavonoid content of Ipomoea batatas L. as an alternative analog GLP-1 for diabetes type 2 treatment-systematic review. Metabolites 2023, 14, 29. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.; Khan, G.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; Xia, F.; Faezeh, M.; et al. Chlorogenic acid(CGA): Apharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Gary, W. Protection against developing type 2 diabetes by coffee consumption: Assessment of the role of chlorogenic acid and metabolites on glycaemic responses. Food Funct. 2020, 11, 4826–4833. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Q.; Shen, L.; Guo, K.; Zhou, X. Chlorogenic acid improves glucose tolerance, lipid metabolism, inflammation and microbiota composition in diabetic db/db mice. Front. Endocrinol. 2022, 13, 1042044. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; Tan, B.; Chen, H.; Lu, Y. Inhibitory effects of chlorogenic acid and isochlorogenic acid from purple sweet potato leaves on α-glucosidase. Mod. Food Sci. Tech. 2014, 30, 103–107. [Google Scholar]
- Siddiqui, S.; Ahmad, R.; Aziz, T.; Khan, A.; Ashraf, H.; Moin, S. (-)-Epigallocatechin-3-gallate and chlorogenic acid in combination with vitamin D as a therapeutic approach for letrozole-induced polycystic ovary syndrome (PCOS) rats: Biochemical and hormonal modulation. J. Steroid Biochem. Mol. Biol. 2025, 252, 106772. [Google Scholar] [CrossRef]
- Han, M.; Tian, D.; Che, X.; Yu, J.; Xiao, A.; Pan, G.; Xiao, N. Mechanism of Lonicerae japonicae Flos in the treatment of diabetes based on netwwork pharmacology. J. Shandong First Med. Uni. (Shandong Acad. Med. Sci.) 2022, 3, 172–177. [Google Scholar]
- Mubarak, A.; Hodgson, J.; Considine, M.; Croft, K.; Matthews, V. Supplementation of a high-fat diet with chlorogenic acid is associated with insulin resistance and hepatic lipid accumulation in mice. J. Agric. Food Chem. 2013, 61, 4371–4378. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Shao, J.; Lai, C.; Huang, E.; Cai, J.; Behrmann, A.; Cheng, S.; Towler, D. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr-/- mice. Arter. Thromb. Vasc. Biol. 2007, 27, 2589–2596. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, S.; Li, X.; Chen, J.; Jiang, Y.; Ye, L. Effect of glucose-control and N-Acetylcysteine on PARP and GAPDH activities in diabetic patients with chronic kidney disease in diabetes. Chin. J. Diabetes 2013, 5, 48–50. [Google Scholar]
- Karthikesan, K.; Pari, L.; Menon, V.P. Antihyperlipidemic effect of chlorogenic acid and tetrahydrocurcumin in rats subjected to diabetogenic agents. Chem.-Biol. Interact. 2010, 188, 643–650. [Google Scholar] [CrossRef]
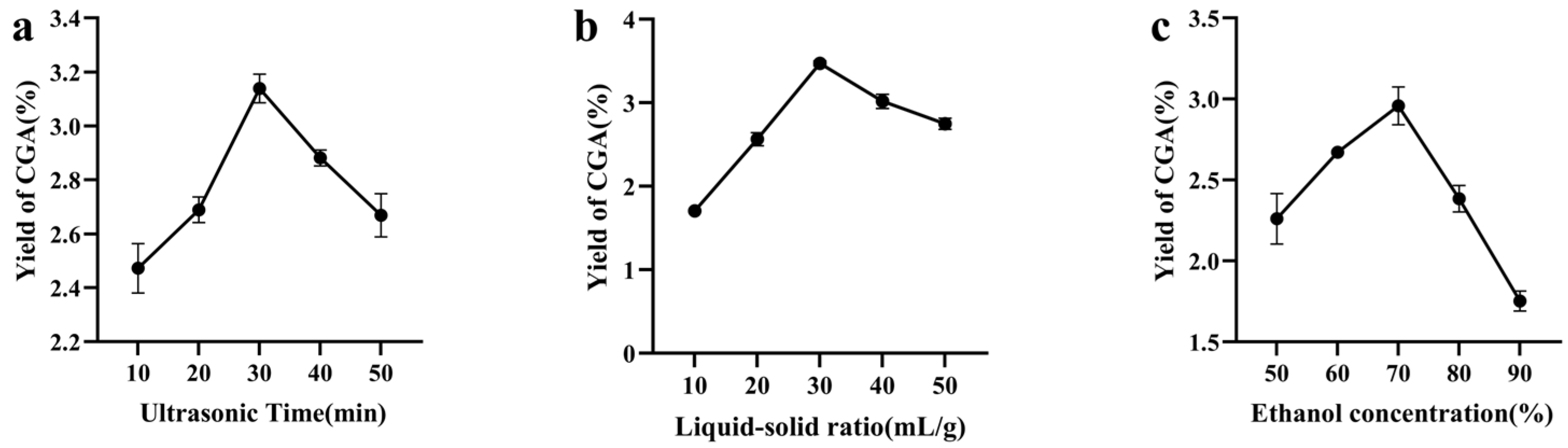
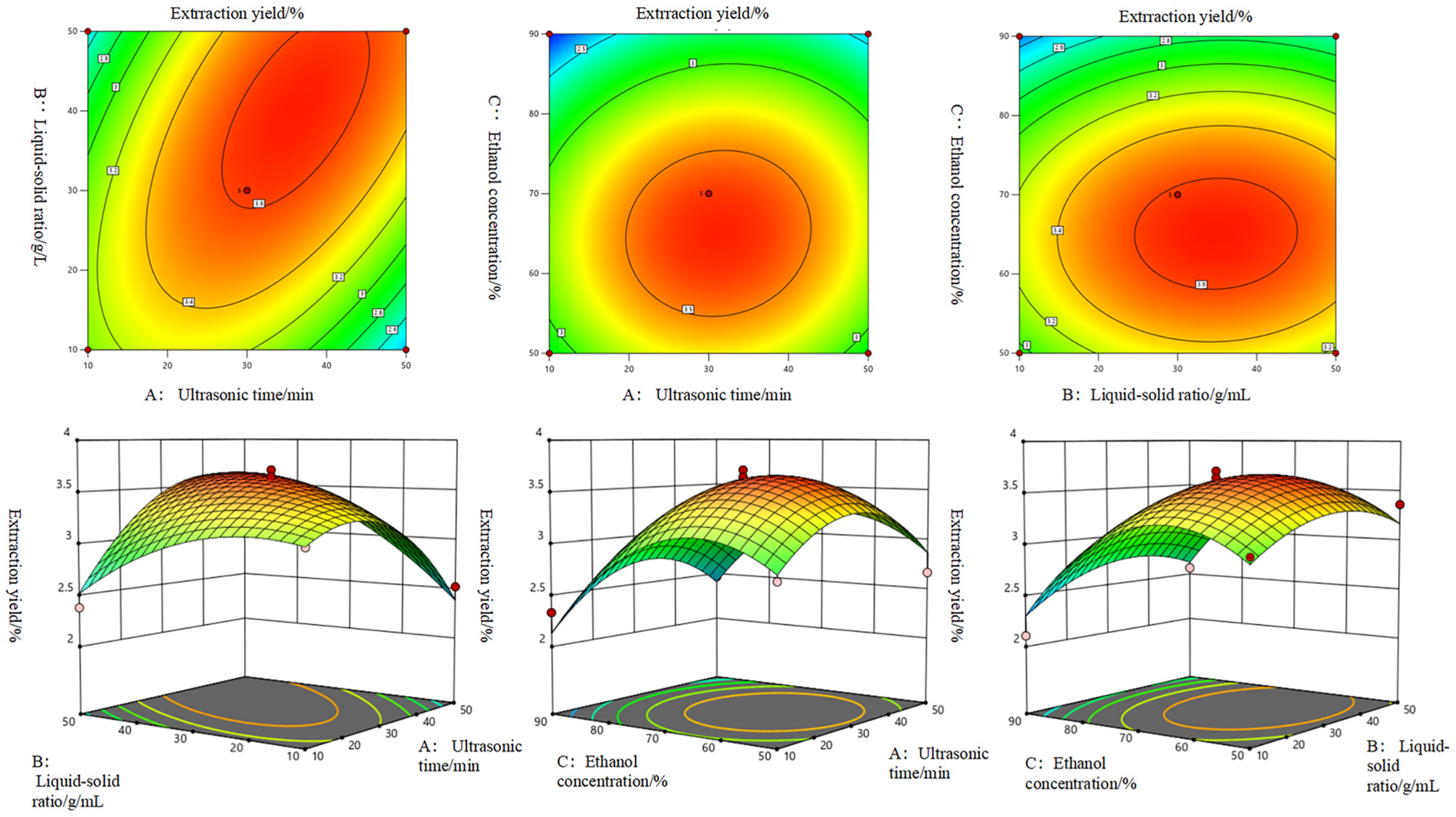
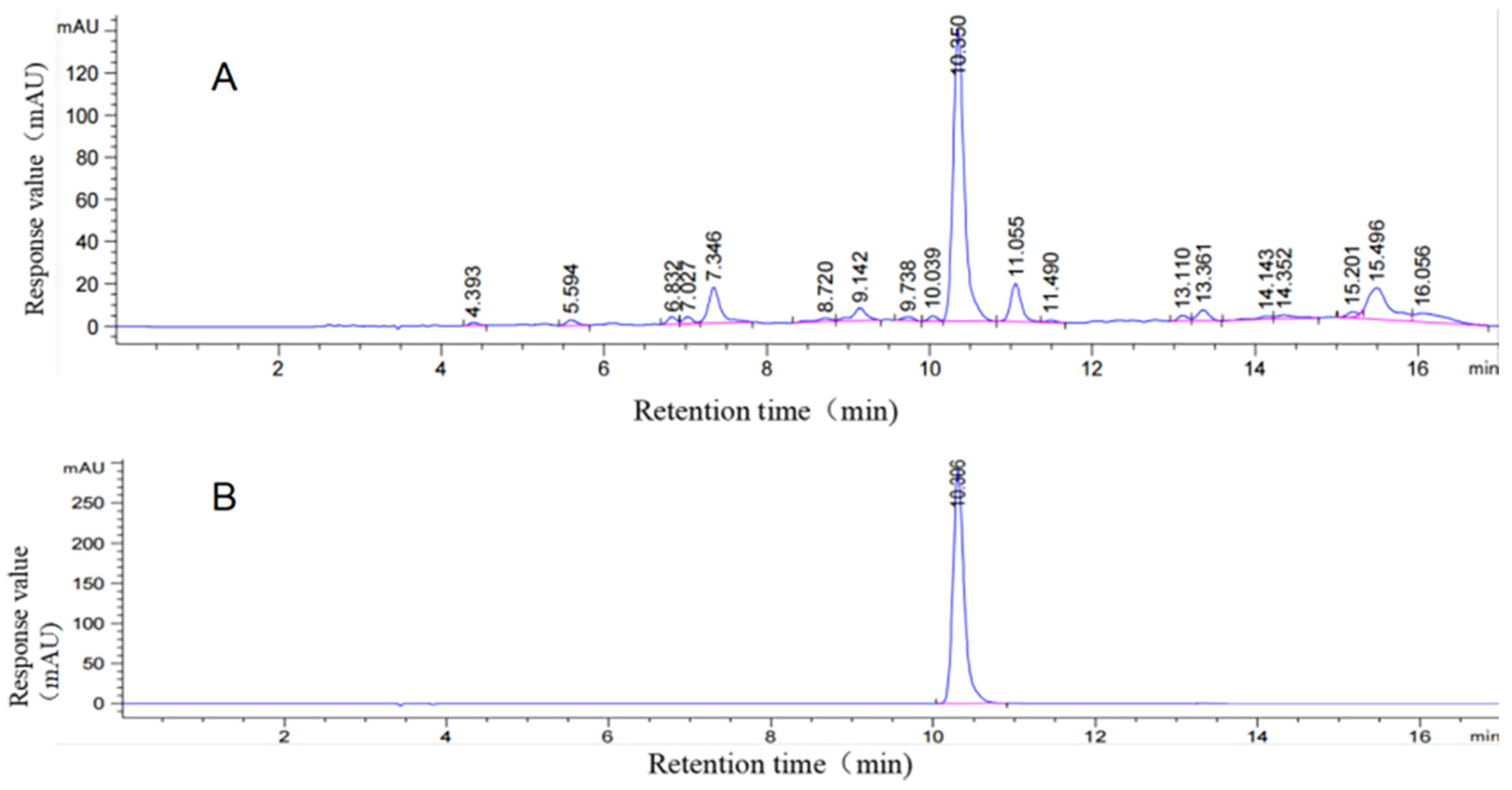
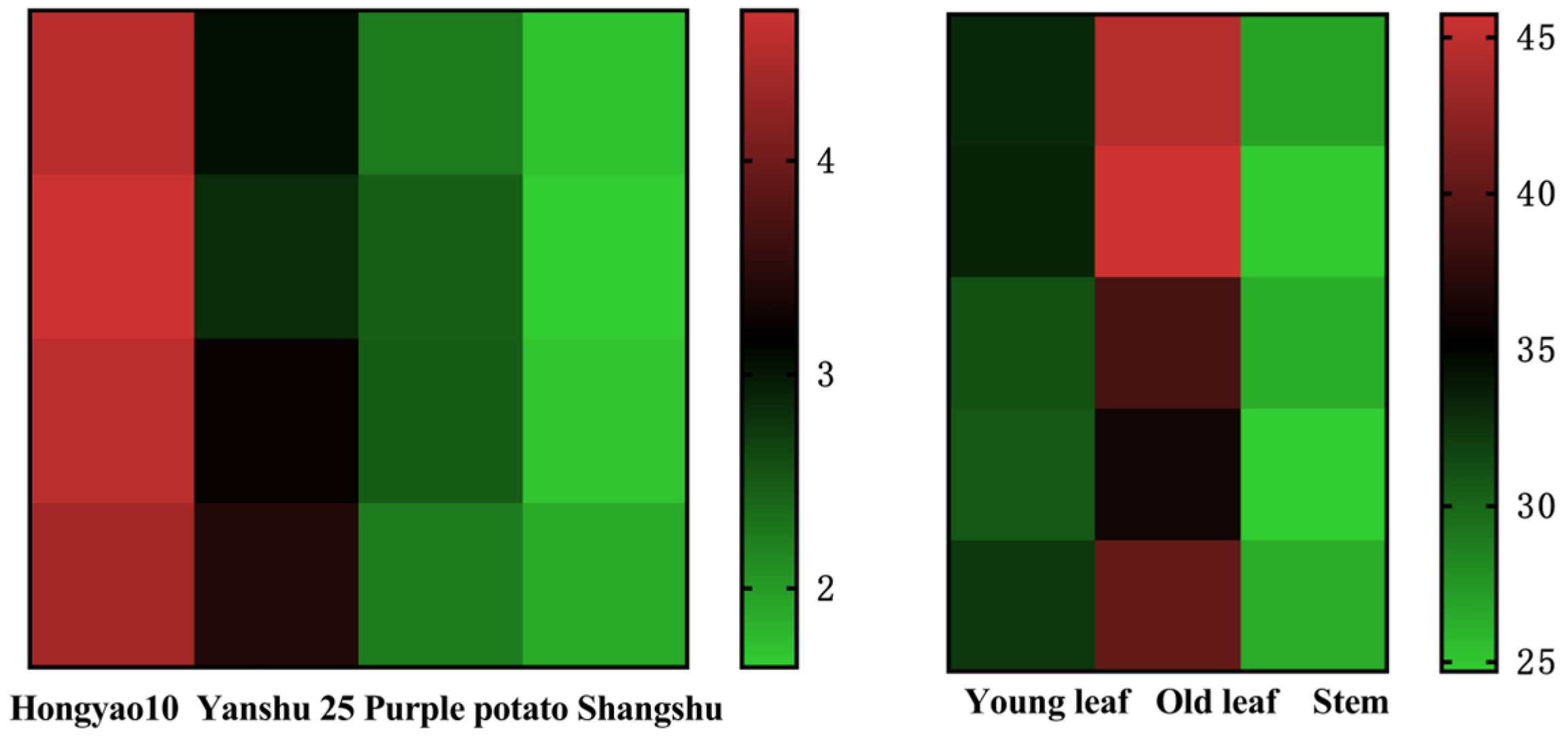
| Design | Ultrasonic Time (A)/min | Solid–Liquid Ratio (B)/(g/mL) | Ethanol Concentration (C)/% |
|---|---|---|---|
| 1 | 10, 20, 30, 40, 50 | 1:30 | 70 |
| 2 | 30 | 1:10, 1:20, 1:30, 1:40, 1:50 | 70 |
| 3 | 30 | 1:30 | 50, 60, 70, 80, 90 |
| Level | A/(min) | B/(g/mL) | C/(%) |
|---|---|---|---|
| −1 | 10 | 1:10 | 50 |
| 0 | 30 | 1:30 | 70 |
| 1 | 50 | 1:50 | 90 |
| Number | A | B | C | Yield of CGA (Y)/% |
|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 3.0903 ± 0.0932 |
| 2 | 1 | −1 | 0 | 2.5221 ± 0.2194 |
| 3 | −1 | 1 | 0 | 2.3798 ± 0.0755 |
| 4 | 1 | 1 | 0 | 3.5263 ± 0.0518 |
| 5 | −1 | 0 | −1 | 2.7959 ± 0.1027 |
| 6 | 1 | 0 | −1 | 2.6667 ± 0.1093 |
| 7 | −1 | 0 | 1 | 2.3326 ± 0.0551 |
| 8 | 1 | 0 | 1 | 2.4675 ± 0.0787 |
| 9 | 0 | −1 | −1 | 3.0138 ± 0.7027 |
| 10 | 0 | 1 | −1 | 3.3620 ± 0.0574 |
| 11 | 0 | −1 | 1 | 2.1054 ± 0.1347 |
| 12 | 0 | 1 | 1 | 2.5717 ± 0.0316 |
| 13 | 0 | 0 | 0 | 3.5726 ± 0.0259 |
| 14 | 0 | 0 | 0 | 3.7070 ± 0.0698 |
| 15 | 0 | 0 | 0 | 3.7894 ± 0.0385 |
| 16 | 0 | 0 | 0 | 3.8841 ± 0.0356 |
| 17 | 0 | 0 | 0 | 3.6392 ± 0.0189 |
| Source | Sum of Squares | Mean Square | df | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Model | 5.2560 | 9 | 0.5840 | 15.1831 | 0.0008 | ** |
| A | 0.0426 | 1 | 0.04263 | 1.1083 | 0.3274 | |
| B | 0.1536 | 1 | 0.1536 | 3.9921 | 0.0859 | |
| C | 0.6969 | 1 | 0.6969 | 18.1177 | 0.0038 | ** |
| AB | 0.7350 | 1 | 0.7350 | 19.1090 | 0.0033 | ** |
| AC | 0.0174 | 1 | 0.0174 | 0.4535 | 0.5223 | |
| BC | 0.0035 | 1 | 0.0035 | 0.0907 | 0.7720 | |
| A2 | 1.1305 | 1 | 1.1305 | 29.3923 | 0.0010 | ** |
| B2 | 0.4329 | 1 | 0.4329 | 11.2550 | 0.0122 | * |
| C2 | 1.6957 | 1 | 1.6957 | 44.0849 | 0.0003 | ** |
| Residual | 0.2692 | 7 | 0.0385 | |||
| Lack of fit | 0.2091 | 3 | 0.0697 | 4.6358 | 0.0863 | |
| Pure error | 0.0601 | 4 | 0.0150 | |||
| Cor total | 5.5252 | 16 |
| Number | Ultrasonic Time (min) | Solid–Liquid Ratio (g/mL) | Ethanol Concentration (%) | Yield of CGA (%) |
|---|---|---|---|---|
| 1 | 50 | 1:40 | 65 | 3.53 |
| 2 | 50 | 1:40 | 65 | 3.56 |
| 3 | 50 | 1:40 | 65 | 3.51 |
| No | Component | MV | OB (%) | DL |
|---|---|---|---|---|
| MOL000414 | Caffeic acid | 180.16 | 54.97 | 0.05 |
| MOL000098 | Quercetine | 302.24 | 46.43 | 0.28 |
| MOL000422 | Kaempferol | 286.24 | 41.88 | 0.24 |
| MOL005889 | Rhamnetin | 316.26 | 36.36 | 0.32 |
| MOL013377 | Lutein | 568.87 | 33.92 | 0.58 |
| MOL000561 | Astragalin | 448.38 | 14.03 | 0.74 |
| MOL001955 | Chlorogenic acid | 354.31 | 11.93 | 0.33 |
| MOL012788 | Tiliroside | 594.52 | 1.94 | 0.66 |
| MOL000437 | Isoquercitrin | 464.38 | 1.87 | 0.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Wang, X.; Zhang, J.; Yang, C.; Yu, X.; Tian, D.; Han, M.; Xiao, N. Optimization of Sweet Potato (Ipomoea batatas L.) Chlorogenic Acid Extraction Process and Hypoglycemic Effect Study. Plants 2026, 15, 120. https://doi.org/10.3390/plants15010120
Wang X, Zhang J, Yang C, Yu X, Tian D, Han M, Xiao N. Optimization of Sweet Potato (Ipomoea batatas L.) Chlorogenic Acid Extraction Process and Hypoglycemic Effect Study. Plants. 2026; 15(1):120. https://doi.org/10.3390/plants15010120
Chicago/Turabian StyleWang, Xiaofei, Jiayu Zhang, Chen Yang, Xiaohan Yu, Dan Tian, Mingli Han, and Na Xiao. 2026. "Optimization of Sweet Potato (Ipomoea batatas L.) Chlorogenic Acid Extraction Process and Hypoglycemic Effect Study" Plants 15, no. 1: 120. https://doi.org/10.3390/plants15010120
APA StyleWang, X., Zhang, J., Yang, C., Yu, X., Tian, D., Han, M., & Xiao, N. (2026). Optimization of Sweet Potato (Ipomoea batatas L.) Chlorogenic Acid Extraction Process and Hypoglycemic Effect Study. Plants, 15(1), 120. https://doi.org/10.3390/plants15010120





