Increasing the Proportion of Broadleaf Species in Mixed Conifer-Broadleaf Forests Improves Understory Plant Composition and Promotes Soil Carbon Fixation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Understory Plant Surveys and Soil Sample Collection
2.3. Soil Properties Analysis
2.4. Soil DNA Extraction, Bacterial 16S and Fungal ITS Gene Amplification, and Sequencing
2.5. Statistical Analysis
3. Results
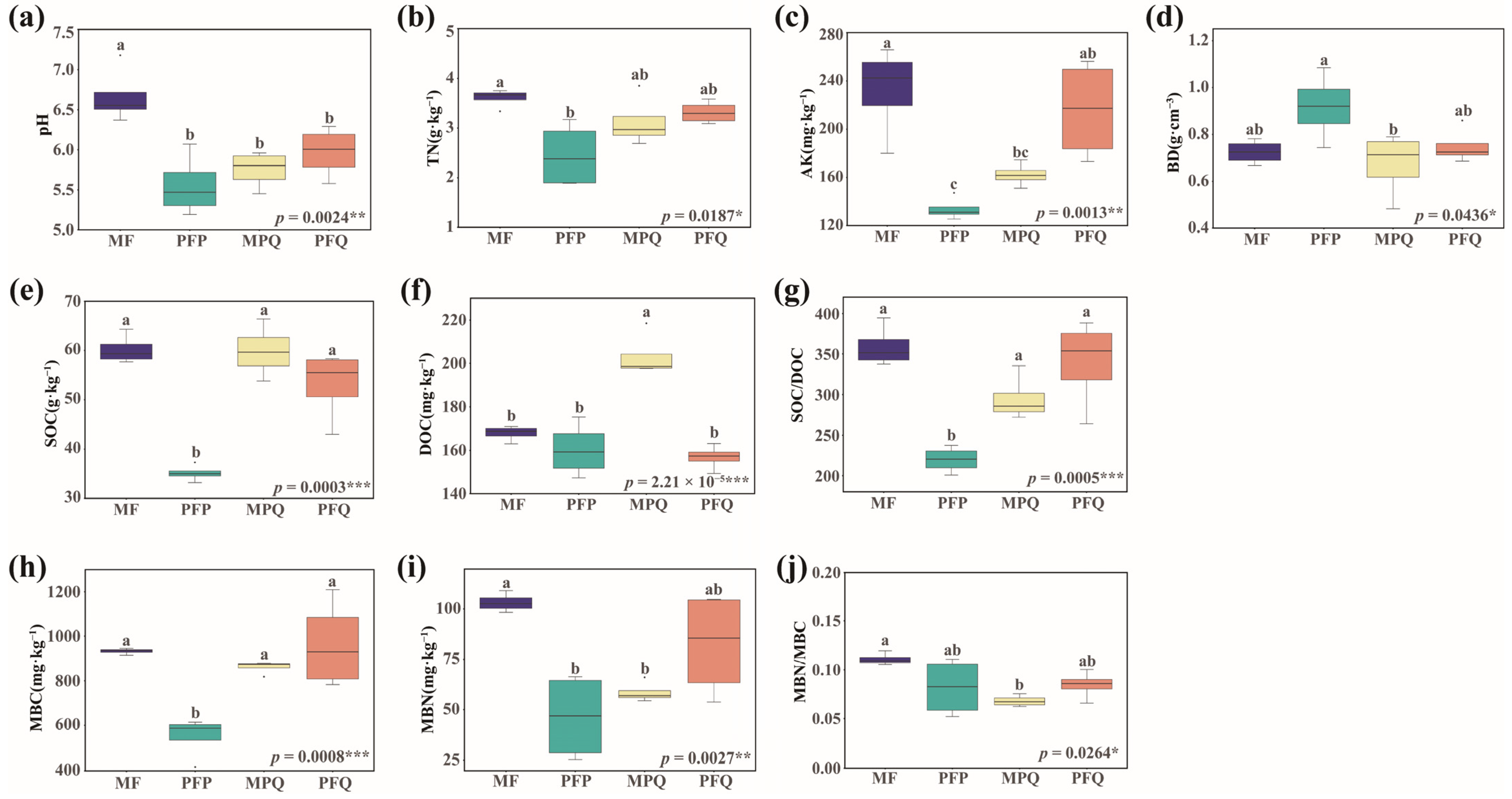
3.1. Soil Characteristics of Different Forest Types
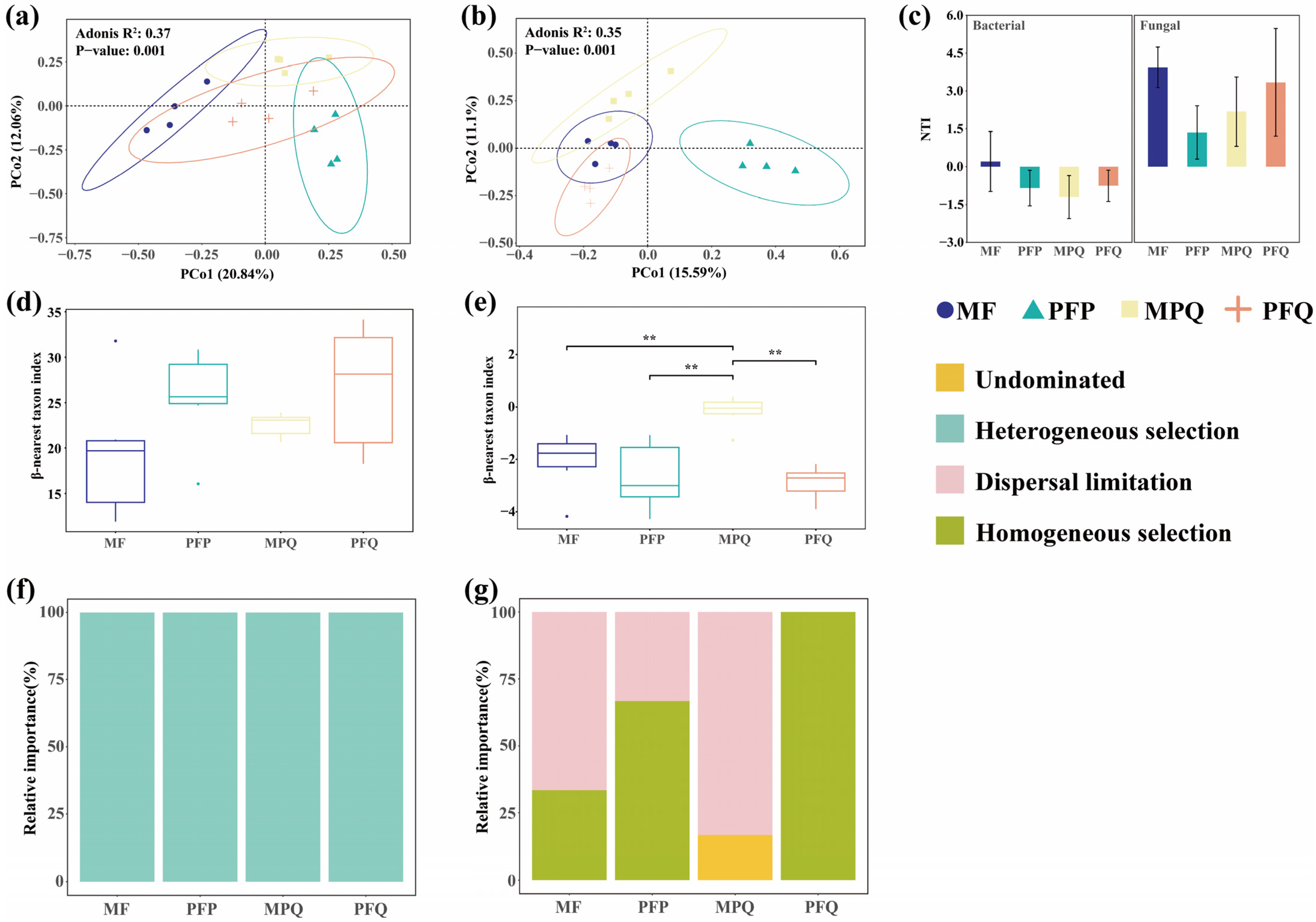
3.2. Soil Microbial β-Diversity in Different Forest Types
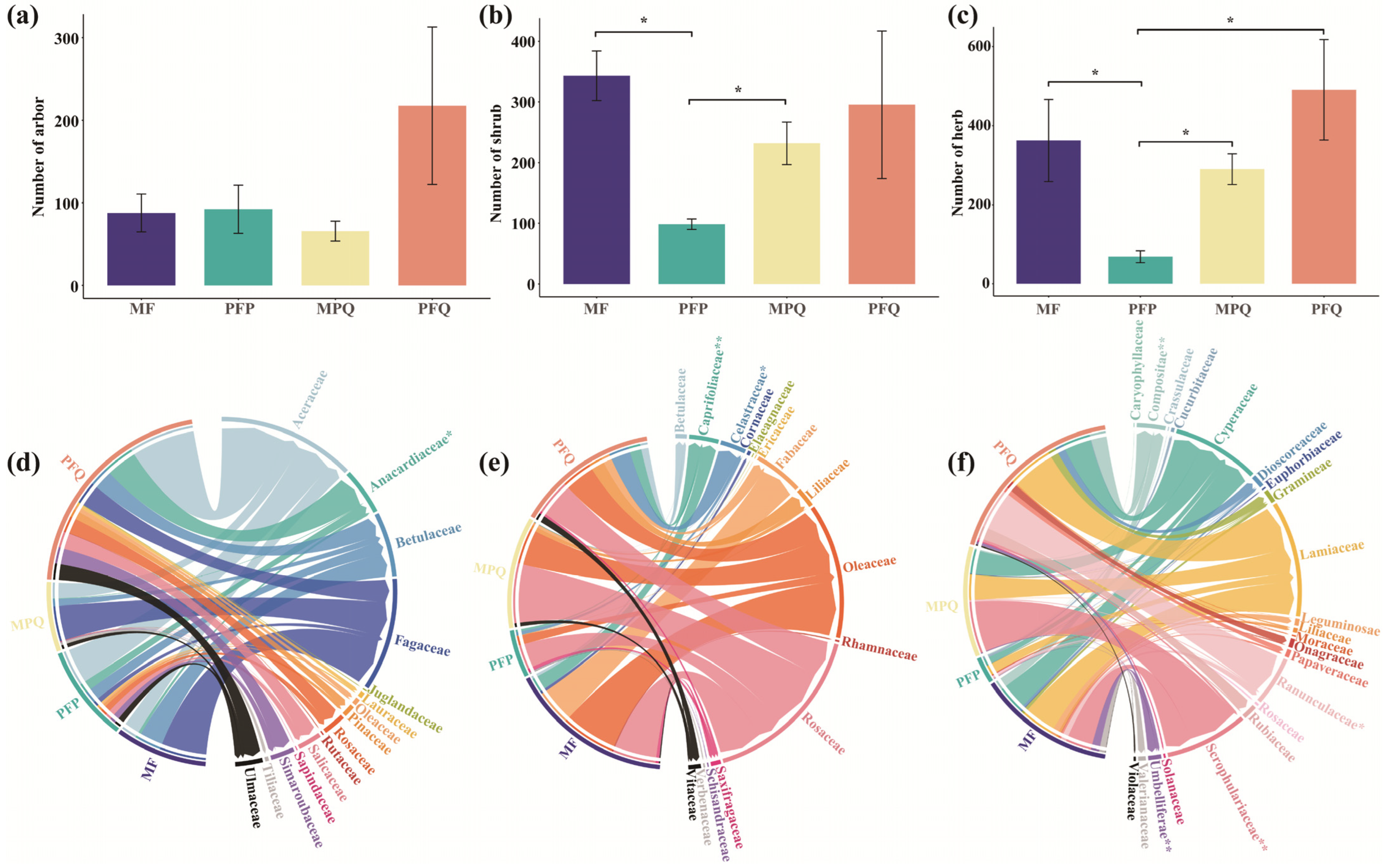
3.3. Understory Plant Composition and Diversity in Different Forest Types
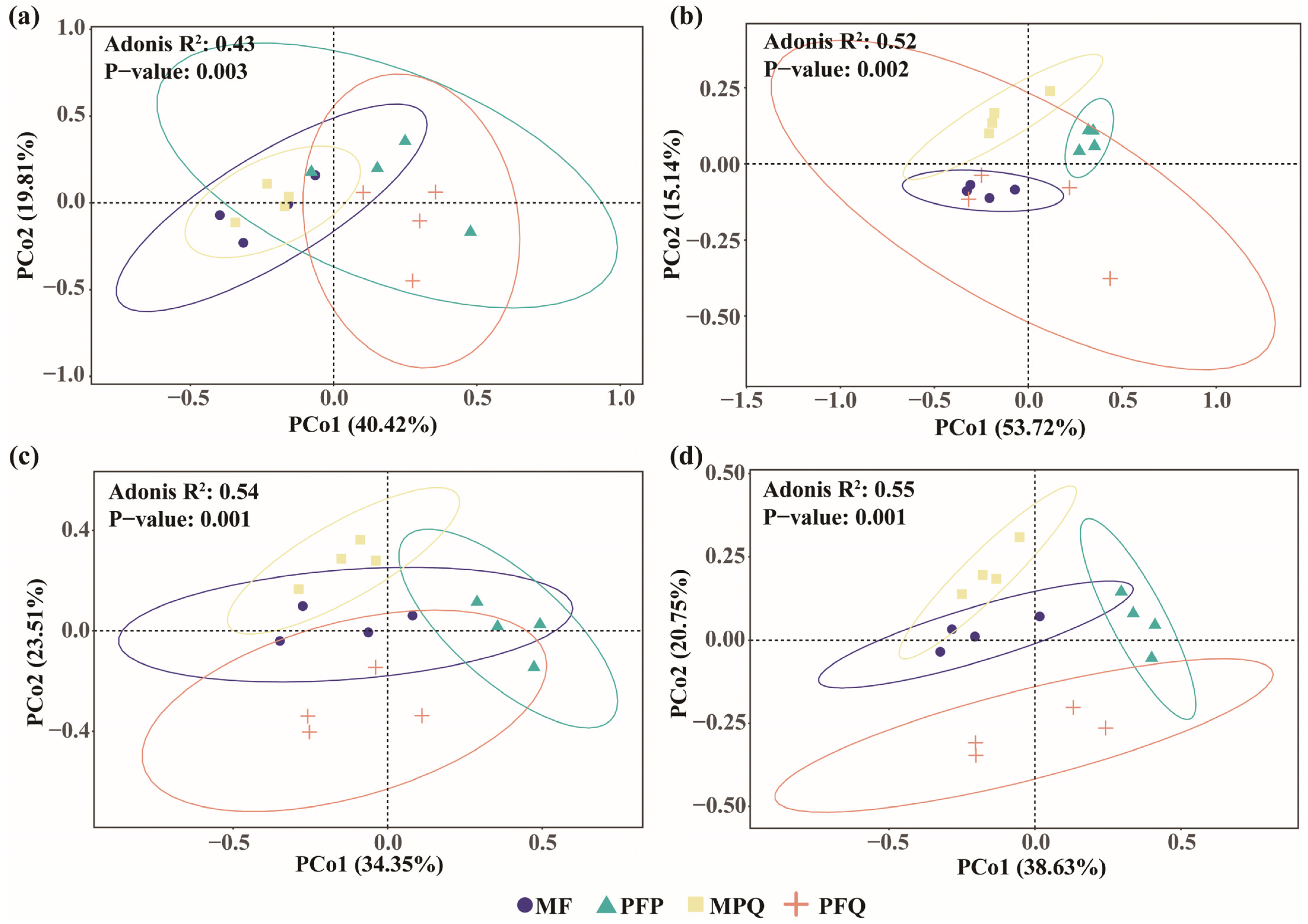
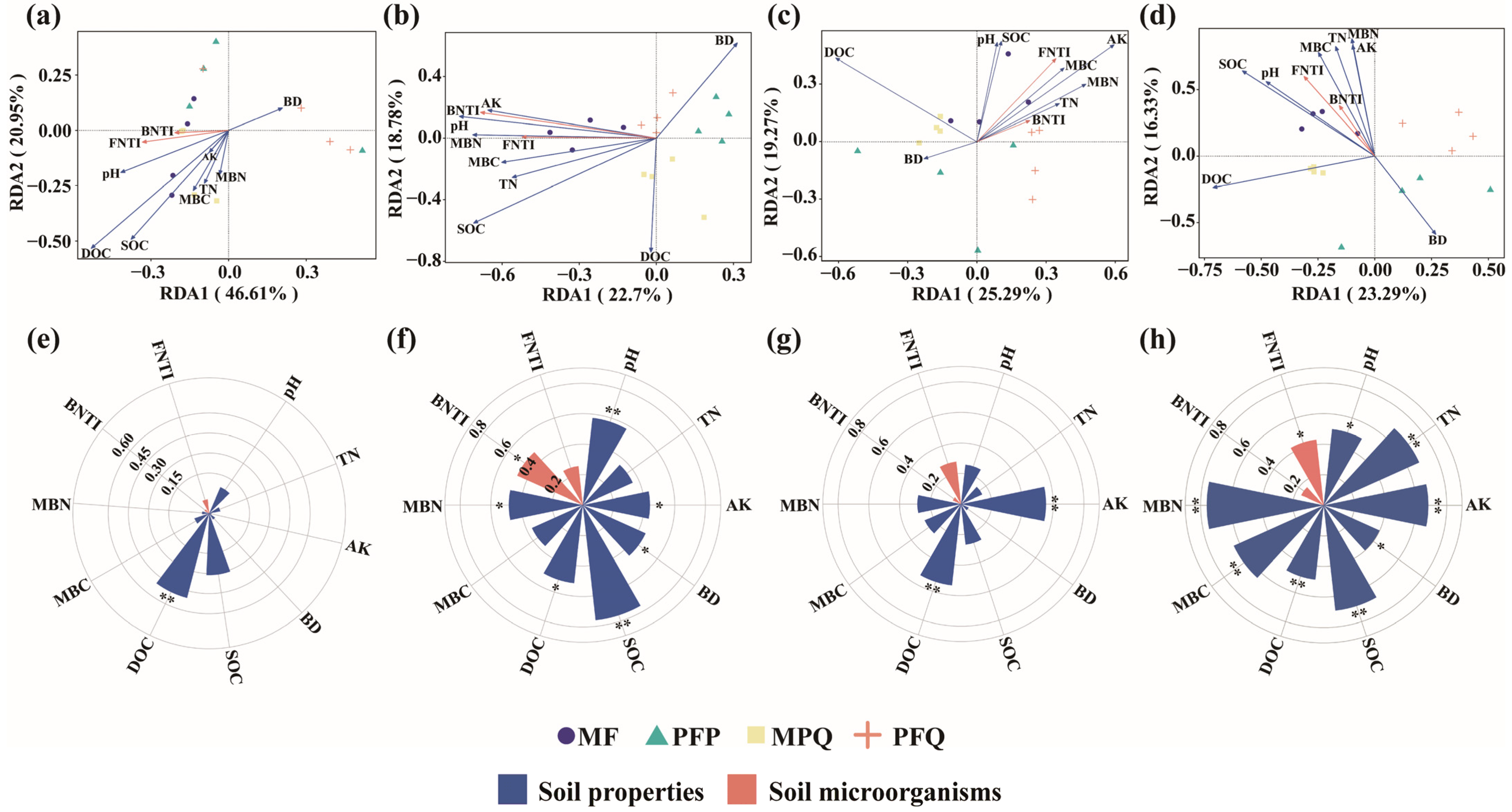
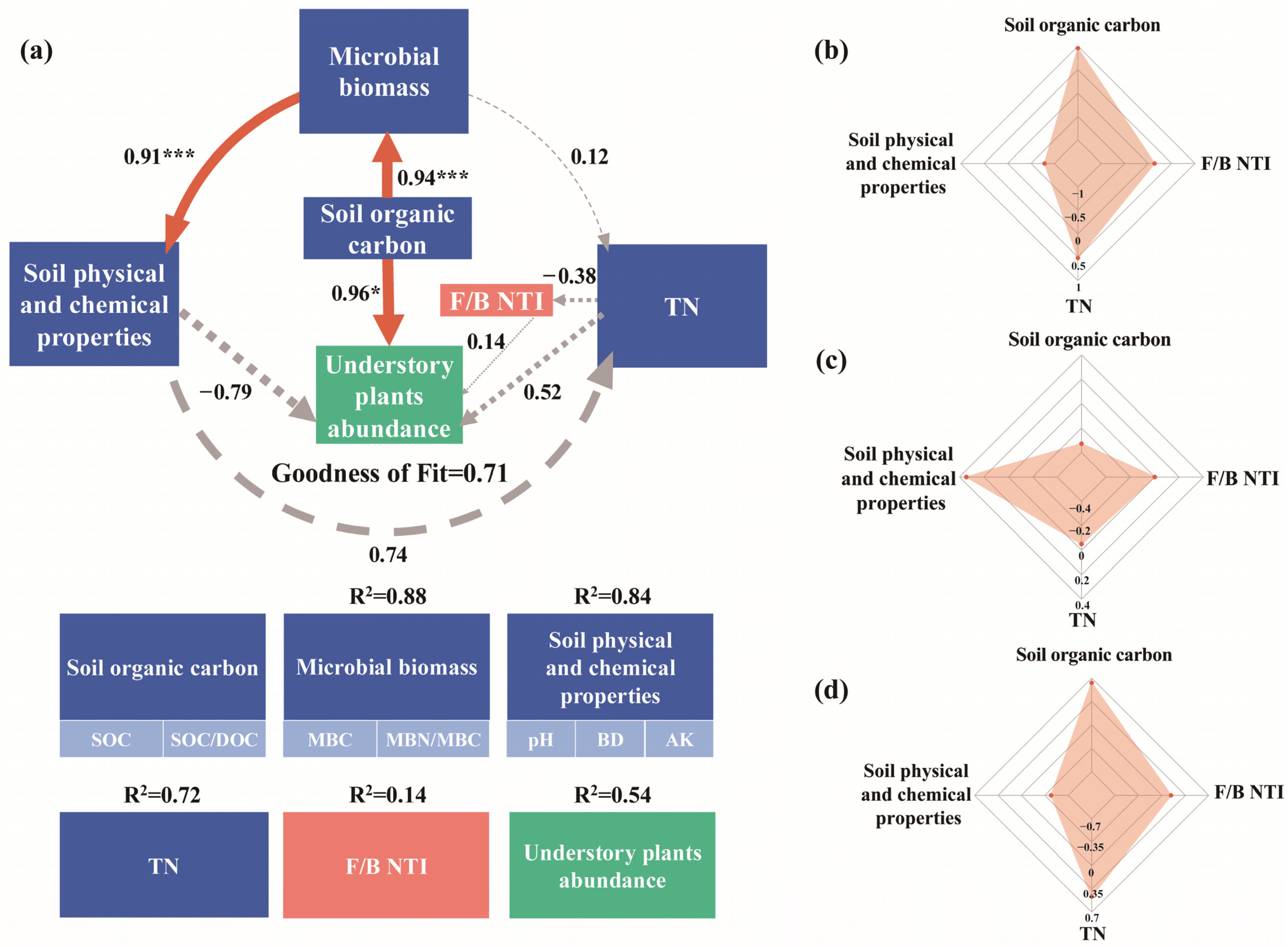
3.4. Influence of Soil Properties and Microbial Diversity on the Composition of Understory Plants
4. Discussion
4.1. Effects of Different Forest Types on the β-Diversity of Soil Microorganisms
4.2. Effects of Different Forest Types on Understory Plant Abundance and β-Diversity
4.3. Relationships Between Soil Properties, Soil Microorganisms, and Understory Plant Community Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Douh, C.; Daïnou, K.; Joël Loumeto, J.; Moutsambote, J.-M.; Fayolle, A.; Tosso, F.; Forni, E.; Gourlet-Fleury, S.; Doucet, J.-L. Soil seed bank characteristics in two central African forest types and implications for forest restoration. For. Ecol. Manag. 2018, 409, 766–776. [Google Scholar] [CrossRef]
- Halofsky, J.E.; Andrews-Key, S.A.; Edwards, J.E.; Johnston, M.H.; Nelson, H.W.; Peterson, D.L.; Schmitt, K.M.; Swanston, C.W.; Williamson, T.B. Adapting forest management to climate change: The state of science and applications in Canada and the United States. For. Ecol. Manag. 2018, 421, 84–97. [Google Scholar] [CrossRef]
- Bonan, G.B.; Shugart, H.H. Environmental factors and ecological processes in boreal forests. Annu. Rev. Ecol. Evol. Syst. 1989, 20, 1–28. [Google Scholar] [CrossRef]
- Guo, X.; Coops, N.C.; Tompalski, P.; Nielsen, S.E.; Bater, C.W.; John Stadt, J. Regional mapping of vegetation structure for biodiversity monitoring using airborne lidar data. Ecol. Inform. 2017, 38, 50–61. [Google Scholar] [CrossRef]
- Depauw, L.; Perring, M.P.; Landuyt, D.; Maes, S.L.; Blondeel, H.; De Lombaerde, E.; Brūmelis, G.; Brunet, J.; Closset-Kopp, D.; Czerepko, J.; et al. Light availability and land-use history drive biodiversity and functional changes in forest herb layer communities. J. Ecol. 2020, 108, 1411–1425. [Google Scholar] [CrossRef]
- Gu, J.; Bol, R.; Wang, Y.; Zhang, H. Controls on soil dissolved organic carbon along the 4000 km North-South forest transect in Eastern China. Catena 2023, 220, 106691. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Z.; Dang, P.; Zhu, H.; Gao, Y.; Ha, V.N.; Zhao, Z. Response of soil microbial community dynamics to Robinia pseudoacacia L. afforestation in the loess plateau: A chronosequence approach. Plant Soil 2017, 423, 327–338. [Google Scholar] [CrossRef]
- Rillig, M.C.; Ryo, M.; Lehmann, A.; Aguilar-Trigueros, C.A.; Buchert, S.; Wulf, A.; Iwasaki, A.; Roy, J.; Yang, G.W. The role of multiple global change factors in driving soil functions and microbial biodiversity. Science 2019, 366, 886–890. [Google Scholar] [CrossRef]
- Mestre, L.; Toro-Manríquez, M.; Soler, R.; Huertas-Herrera, A.; Martínez-Pastur, G.; Lencinas, M.V. The influence of canopy-layer composition on understory plant diversity in southern temperate forests. For. Ecosyst. 2017, 4, 6. [Google Scholar] [CrossRef]
- Jin, Y.-S.; Hu, Y.-K.; Wang, J.; Liu, D.-D.; Lin, Y.-H.; Liu, G.; Zhang, Y.-H.; Zhou, Z.-Q. Diversity of understory communities in Boreal Forests: Influences of forest type, latitude, and spatial scale. Forests 2019, 10, 1003. [Google Scholar] [CrossRef]
- Gmach, M.R.; Cherubin, M.R.; Kaiser, K.; Cerri, C.E.P. Processes that influence dissolved organic matter in the soil: A review. Sci. Agric. 2020, 77, e20180164. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, W.; Chen, H.; Li, D.; Zhao, Y.; Zhao, J.; Xiao, J.; Wu, F.; He, X.; Luo, Y.; et al. Soil carbon accumulation with increasing temperature under both managed and natural vegetation restoration in calcareous soils. Sci Total Environ. 2021, 767, 145298. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Kneeshaw, D.; Peng, C.; Wu, Y.; Feng, L.; Qu, X.; Wang, W.; Pan, C.; Feng, H. Positive effects of species mixing on biodiversity of understory plant communities and soil health in forest plantations. Proc. Natl. Acad. Sci. USA 2025, 122, e2418090122. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, X.; Chen, L.; Kuzyakov, Y.; Wang, R.; Zhang, H.; Han, X.; Jiang, Y.; Sun, O.J. Global pattern of organic carbon pools in forest soils. Glob. Change Biol. 2024, 30, e17386. [Google Scholar] [CrossRef]
- Kalbitz, K.; Kaiser, K. Contribution of dissolved organic matter to carbon storage in forest mineral soils. J. Plant Nutr. Soil Sci. 2008, 171, 52–60. [Google Scholar] [CrossRef]
- Li, X.M.; Chen, Q.L.; He, C.; Shi, Q.; Chen, S.C.; Reid, B.J.; Zhu, Y.G.; Sun, G.X. Organic Carbon Amendments Affect the Chemodiversity of Soil Dissolved Organic Matter and Its Associations with Soil Microbial Communities. Environ. Sci. Technol. 2019, 53, 50–59. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, Z.; Lv, F.; Wang, R.; Wang, Z.; Zhao, Z.; Li, Z.; Zhai, B. Assembly of abundant and rare bacterial and fungal sub-communities in different soil aggregate sizes in an apple orchard treated with cover crop and fertilizer. Soil Biol. Biochem. 2021, 156, 108222. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, R.; Stegen, J.C.; Guo, Z.; Zhang, J.; Li, Z.; Lin, X. Two key features influencing community assembly processes at regional scale: Initial state and degree of change in environmental conditions. Mol. Ecol. 2018, 27, 5238–5251. [Google Scholar] [CrossRef]
- Wu, W.; Logares, R.; Huang, B.; Hsieh, C.H. Abundant and rare picoeukaryotic sub-communities present contrasting patterns in the epipelagic waters of marginal seas in the northwestern Pacific Ocean. Environ. Microbiol. 2017, 19, 287–300. [Google Scholar] [CrossRef]
- Liu, L.; Yang, J.; Yu, Z.; Wilkinson, D.M. The biogeography of abundant and rare bacterioplankton in the lakes and reservoirs of China. ISME J 2015, 9, 2068–2077. [Google Scholar] [CrossRef]
- Jiao, S.; Chen, W.; Wei, G. Biogeography and ecological diversity patterns of rare and abundant bacteria in oil-contaminated soils. Mol. Ecol. 2017, 26, 5305–5317. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zheng, H.; Zhang, K.; Ouyang, Z.; Wu, Y.; Shi, Q.; Li, H. Non-linear impacts of Eucalyptus plantation stand age on soil microbial metabolic diversity. J. Soils Sediments 2013, 13, 887–894. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Meyer, B. Nutrient changes in decomposing beech leaf litter assessed using a solution flux approach. J. Soil Sci. 2006, 41, 279–293. [Google Scholar] [CrossRef]
- Wei, H.; Deng, Y.; Lin, L.; Wang, J.; Huang, J. Improved soil composition promotes nutrient recovery during vegetation restoration in karst peak-cluster depressions. Catena 2023, 222, 106769. [Google Scholar] [CrossRef]
- Mu, C.; Zhang, T.; Zhang, X.; Cao, B.; Peng, X.; Cao, L.; Su, H. Pedogenesis and physicochemical parameters influencing soil carbon and nitrogen of alpine meadows in permafrost regions in the northeastern Qinghai-Tibetan Plateau. Catena 2016, 141, 85–91. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 961–1010. [Google Scholar]
- Jiang, P.K.; Xu, Q.F.; Xu, Z.H.; Cao, Z.H. Seasonal changes in soil labile organic carbon pools within a Phyllostachys praecox stand under high rate fertilization and winter mulch in subtropical China. For. Ecol. Manag. 2006, 236, 30–36. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Mueller, T. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the Ken value. Soil Biol. Biochem. 1996, 28, 33–37. [Google Scholar] [CrossRef]
- Mori, H.; Maruyama, F.; Kato, H.; Toyoda, A.; Dozono, A.; Ohtsubo, Y.; Nagata, Y.; Fujiyama, A.; Tsuda, M.; Kurokawa, K. Design and Experimental Application of a Novel Non-Degenerate Universal Primer Set that Amplifies Prokaryotic 16S rRNA Genes with a Low Possibility to Amplify Eukaryotic rRNA Genes. DNA Res. 2013, 21, 217–227. [Google Scholar] [CrossRef]
- Xiong, W.; Zhao, Q.; Zhao, J.; Xun, W.; Li, R.; Zhang, R.; Wu, H.; Shen, Q. Different Continuous Cropping Spans Significantly Affect Microbial Community Membership and Structure in a Vanilla-Grown Soil as Revealed by Deep Pyrosequencing. Microb. Ecol. 2014, 70, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Wu, C.; Shi, S.; Zhang, H.; Zhu, Z.; Chen, J.; Ge, T. Loss of protistan diversity weakens soil phosphorus availability. Appl. Soil Ecol. 2025, 208, 105976. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.; Fredrickson, J.K.; Chen, X.; Kennedy, D.W.; Murray, C.J.; Rockhold, M.L.; Konopka, A. Quantifying community assembly processes and identifying features that impose them. ISME J. 2013, 7, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ma, Y.; Feng, T.; Kong, X.; Wang, Z.; Zheng, W.; Zhai, B. Assembly processes of abundant and rare microbial communities in orchard soil under a cover crop at different periods. Geoderma 2022, 406, 115543. [Google Scholar] [CrossRef]
- Dong, L.; Li, M.X.; Li, S.; Yue, L.X.; Ali, M.; Han, J.R.; Lian, W.H.; Hu, C.J.; Lin, Z.L.; Shi, G.Y.; et al. Aridity drives the variability of desert soil microbiomes across north-western China. Sci. Total Environ. 2024, 907, 168048. [Google Scholar] [CrossRef]
- Stegen, J.C.; Lin, X.; Fredrickson, J.K.; Konopka, A.E. Estimating and mapping ecological processes influencing microbial community assembly. Front. Microbiol. 2015, 6, 370. [Google Scholar] [CrossRef]
- Feng, Q.; Zhao, W.; Hu, X.; Liu, Y.; Daryanto, S.; Cherubini, F. Trading-off ecosystem services for better ecological restoration: A case study in the Loess Plateau of China. J. Clean. Prod. 2020, 257, 120469. [Google Scholar] [CrossRef]
- Tenenhaus, M.; Vinzi, V.E.; Chatelin, Y.-M.; Lauro, C. PLS path modeling. Comput. Stat. Data Anal. 2005, 48, 159–205. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.; Liu, J.; Liu, J.; Han, L.; Wang, X.; Liu, H.; Xu, M.; Yang, G.; Ren, C.; et al. The contribution of microbial necromass carbon to soil organic carbon in soil aggregates. Appl. Soil Ecol. 2023, 190, 104985. [Google Scholar] [CrossRef]
- Esposito Vinzi, V.; Chin, W.W.; Henseler, J.; Wang, H. PLS Path Modeling: From Foundations to Recent Developments and Open Issues for Model Assessment and Improvement. In Handbook of Partial Least Squares; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Wang, W.; Zhang, F.; Zhao, Q.; Liu, C.; Jim, C.Y.; Johnson, V.C.; Tan, M.L. Determining the main contributing factors to nutrient concentration in rivers in arid northwest China using partial least squares structural equation modeling. J. Environ. Manag. 2023, 343, 118249. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, X.; Wang, T.; Zhang, X.; Feng, Y.; Yang, G.; Zhen, W. Relating land-use/land-cover patterns to water quality in watersheds based on the structural equation modeling. Catena 2021, 206, 105566. [Google Scholar] [CrossRef]
- Tenenhaus, M.; Amato, S.; Vinzi, V.E. A global Goodness–of–Fit index for PLS structural equation modelling. Proc. XLII SIS Sci. Meet. 2004, 1, 739–742. [Google Scholar]
- Cheng, H.; Zhou, X.; Dong, R.; Wang, X.; Liu, G.; Li, Q. Natural vegetation regeneration facilitated soil organic carbon sequestration and microbial community stability in the degraded karst ecosystem. Catena 2023, 222, 106856. [Google Scholar] [CrossRef]
- Hu, Y.H.; Sheng, D.Y.; Xiang, Y.Z.; Yang, Z.J.; Xu, D.P.; Zhang, N.N.; Shi, L.L. The environment, not space, dominantly structures the landscape patterns of the richness and composition of the tropical understory vegetation. PLoS ONE 2013, 8, e81308. [Google Scholar] [CrossRef]
- Emilio, T.; Nelson, B.W.; Schietti, J.; Desmoulière, S.J.M.; Espírito Santo, H.M.V.; Costa, F.R.C. Assessing the relationship between forest types and canopy tree beta diversity in Amazonia. Ecography 2010, 33, 738–747. [Google Scholar] [CrossRef]
- Barbier, S.; Gosselin, F.; Balandier, P. Influence of tree species on understory vegetation diversity and mechanisms involved—A critical review for temperate and boreal forests. For. Ecol. Manag. 2008, 254, 1–15. [Google Scholar] [CrossRef]
- Chávez, V.; Macdonald, S.E. The influence of canopy patch mosaics on understory plant community composition in boreal mixedwood forest. For. Ecol. Manag. 2010, 259, 1067–1075. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, M.; Yue, H.; Li, S.; Lin, G.; Zhang, Y.; Jiang, F.; Huang, Y.; Lin, J. Effects of different artificial vegetation restoration modes on soil microbial community structuree in the soil erosion area of southern China. Catena 2024, 237, 107803. [Google Scholar] [CrossRef]
- Kalbitz, K.; Schmerwitz, J.; Schwesig, D.; Matzner, E. Biodegradation of soil-derived dissolved organic matter as related to its properties. Geoderma 2003, 113, 273–291. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, L.; Zou, B.; Wang, S.; Zheng, Y.; Huang, Z.; He, J.-Z. Soil fungal diversity and functionality changes associated with multispecies restoration of Pinus massoniana plantation in ubtropical China. Forests 2022, 13, 2075. [Google Scholar] [CrossRef]
- Ji, C.; Yang, Y.; Han, W.; He, Y.; Smith, J.; Smith, P. Climatic and edaphic controls on soil pH in Alpine Grasslands on the Tibetan Plateau, China: A Quantitative analysis. Pedosphere 2014, 24, 39–44. [Google Scholar] [CrossRef]
- Mcgroddy, M.E.; Daufresne, T.; Hedin, L.O. Scaling of C:N:P stoichiometry in forests worldwide:implications of terrestrial redfield-type ratios. Ecology 2004, 85, 2390–2401. [Google Scholar] [CrossRef]
- Zhu, D.; Sun, L.; Mao, L.; Li, J.; Yan, B.; Li, B.; Li, X. Combined effects of cropping alfalfa (Medicago sativa L.) on the soil pore structure, microbial communities and organic carbon fractions in saline soils. Appl. Soil Ecol. 2025, 208, 105993. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Dong, L.; Wang, K.; Liu, Y.; Hai, X.; Pan, Y.; Lv, W.; Wang, X.; Shangguan, Z.; et al. Plant productivity and microbial composition drive soil carbon and nitrogen sequestrations following cropland abandonment. Sci. Total Environ. 2020, 744, 140802. [Google Scholar] [CrossRef]

| Sample Plot Type | Dominant Tree Species | Longitude | Latitude | Altitude/m | Slope/° | Aspect | MAT/°C | MAP/mm |
|---|---|---|---|---|---|---|---|---|
| MF | Pinus tabuliformis, Quercus mongolica, Populus davidiana, Betula platyphylla, Carpinus turczaninovii | 112°0′10″ | 35°27′19″ | 1747 | 15 | E | 6.9 | 753 |
| PFP | Pinus tabuliformis | 112°0′57″ | 35°27′50″ | 1613 | 19 | N | 7.4 | 736 |
| MPQ | Pinus tabuliformis, Quercus mongolica | 112°0′29″ | 35°27′32″ | 1675 | 27 | S | 7.1 | 747 |
| PFQ | Quercus mongolica | 112°1′44″ | 35°29′1″ | 1664 | 23 | E | 7.3 | 702 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wang, X.; Huang, Y.; Yang, X.; Wang, R.; Zhang, M. Increasing the Proportion of Broadleaf Species in Mixed Conifer-Broadleaf Forests Improves Understory Plant Composition and Promotes Soil Carbon Fixation. Plants 2025, 14, 1392. https://doi.org/10.3390/plants14091392
Li Z, Wang X, Huang Y, Yang X, Wang R, Zhang M. Increasing the Proportion of Broadleaf Species in Mixed Conifer-Broadleaf Forests Improves Understory Plant Composition and Promotes Soil Carbon Fixation. Plants. 2025; 14(9):1392. https://doi.org/10.3390/plants14091392
Chicago/Turabian StyleLi, Zixing, Xinghao Wang, Yuan Huang, Xinrong Yang, Ran Wang, and Mengtao Zhang. 2025. "Increasing the Proportion of Broadleaf Species in Mixed Conifer-Broadleaf Forests Improves Understory Plant Composition and Promotes Soil Carbon Fixation" Plants 14, no. 9: 1392. https://doi.org/10.3390/plants14091392
APA StyleLi, Z., Wang, X., Huang, Y., Yang, X., Wang, R., & Zhang, M. (2025). Increasing the Proportion of Broadleaf Species in Mixed Conifer-Broadleaf Forests Improves Understory Plant Composition and Promotes Soil Carbon Fixation. Plants, 14(9), 1392. https://doi.org/10.3390/plants14091392







