Ursane Triterpenes and Norisoprenoids from Anchusa italica Retz. and Their Chemotaxonomic Significance
Abstract
:1. Introduction
2. Results
2.1. Structure Elucidation
2.2. The Protective Effects of Compounds 1–20 on Hypoxia/Reoxygenation-Injured Neonatal Rat Cardiomyocytes
2.3. Chemotaxonomic Significance
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Acid Hydrolysis of New Compounds
3.5. Quantum Chemical Calculations (Computational NMR and ECD)
3.6. Cell Culture and Hypoxia/Reoxygenation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Teng, L.; Li, M.; Ma, G. Protection of total flavonoids from Anchusa italica Retz. and fourcompounds on hypoxia-reoxygenation inducedinjury in myocardial cells and its mechanism. Chin. Pharmacol. Bull. 2021, 37, 409–416. [Google Scholar]
- Liu, Y.; Hu, B.; Wang, Y.; Bao, F.; Li, H.; Chen, L. Chemical constituents of Anchusa italica Retz. and their protective effects on cardiomyocytes injured by hypoxia/reoxygenation. Phytochem. Lett. 2020, 38, 155–160. [Google Scholar] [CrossRef]
- Hu, B.; Liu, Y.; Zheng, M.; Zhang, R.; Li, M.; Bao, F.; Li, H.; Chen, L. Triterpenoids from Anchusa italica and their protective effects on hypoxia/reoxygenation induced cardiomyocytes injury. Bioorg. Chem. 2020, 97, 103714. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Chen, K.; Deng, X.; Fu, Z.; Chen, D.; Wang, Q. Anti-complementary constituents of Anchusa italica. Nat. Prod. Res. 2017, 31, 2572–2574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Wang, S.; Dong, Y.; Wang, T.; Qu, L.; Li, N.; Wang, T. Bioactive constituents from the aerial parts of Lippia triphylla. Molecules 2015, 20, 21946–21959. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xia, F.; Wang, S.; Wang, K.; Chen, J.; Tu, P. Structural elucidation of two new megastigmane glycosides from the leaves of Aquilaria sinensis. Chin. J. Nat. Med. 2015, 13, 290–294. [Google Scholar] [PubMed]
- Samy, M.N.; Hamed, A.N.; Sugimoto, S.; Otsuka, H.; Kamel, M.S.; Matsunami, K. Officinalioside, a new lignan glucoside from Borago officinalis L. Nat. Prod. Res. 2016, 30, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Tripetch, K.; Ryoji, K.; Phannipha, C.; Yoshikazu, H.; Kazuo, Y. Megastigmane and iridoid glucosides from Clerodendrum inerme. Phytochemistry 2001, 58, 333–336. [Google Scholar]
- Xu, W.; Wang, J.; Ju, B.; Lan, X.; Ying, X.; Stien, D. Seven compounds from Portulaca oleracea L. and their anticholinesterase activities. Nat. Prod. Res. 2022, 36, 2547–2553. [Google Scholar] [CrossRef] [PubMed]
- Stochmal, A.; Simonet, A.M.; Macias, F.A.; Oleszek, W. Alfalfa (Medicago sativa L.) flavonoids. 2. Tricin and chrysoeriol glycosides from aerial parts. J. Agric. Food Chem. 2001, 49, 5310–5314. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Wang, L.; Lin, H.; Shang, E.; Tang, Y.; Yue, S.; Jin, Y.; Tao, W.; Li, S.; Hua, Y.; et al. Hierarchical identification of bioactive components in a medicinal herb by preparative high-performance liquid chromatography and selective knock-out strategy. J. Pharm. Biomed. Anal. 2017, 20, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinsky, L.; Budovsky, A.; Khalfin, B.; Yarmolinsky, L.; Ben-Shabat, S. Medicinal properties of Anchusa strigose and its active compounds. Molecules 2022, 25, 8239. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, G.L.; de Araújo, D.I.; Raimundo e Silva, J.P.; do Nascimento, Y.M.; de Souza, T.A.; Opretzka, L.C.; Villarreal, C.F.; Abreu, L.S.; dos Santos Junior, F.M.; de Melo, J.I.; et al. Sucrose Diester of Aryldihydronaphthalene-Type Lignan with Anti-inflammatory Activity from Heliotropium angiospermum. Rev. Bras. Farmacogn. 2022, 32, 734–740. [Google Scholar] [CrossRef]
- Harput, U.S.; Nagatsu, A.; Saracoglu, I. Antioxidant and cytotoxic effects of Moltkia aurea Boiss. Rec. Nat. Prod. 2012, 6, 62–66. [Google Scholar]
- Hu, X.; Qin, N.; Xue, J.; Li, S.; Huang, X.; Sun, J.; Xu, F.; Li, Z.; Li, D.; Hua, H. Dehydrodiconiferyl alcohol from Silybum marianum (L.) Gaertn accelerates wound healing via inactivating NF-κB pathways in macrophages. J. Pharm. Pharmacol. 2020, 72, 305–317. [Google Scholar] [CrossRef] [PubMed]
- In, S.J.; Seo, K.H.; Song, N.Y.; Lee, D.S.; Kim, Y.C.; Baek, N.I. Lignans and neolignans from the stems of Vibrunum erosum and their neuroprotective and anti-inflammatory activity. Arch. Pharm. Res. 2015, 38, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Giang, L.T.; Park, S.; Lee, S.; Seo, Y.; Van, K.; Tai, B.H.; Hang, N.T.M.; Thao, V.M.; Van, C.; Ban, N.K.; et al. Hepatoprotective Lignan glycosides from the leaves and stems of Symplocos cochinchinensis (Lour.) S. Moore. Chem. Biodivers. 2024, 21, 202400896. [Google Scholar] [CrossRef] [PubMed]
- Brigida, D.A.; Marina, D.G.; Antonio, F.; Pietro, M.; Palma, O.; Fabio, T. Structure elucidation and phytotoxicity of C13 nor-isoprenoids from Cestrum parqui. Phytochemistry 2004, 65, 497–505. [Google Scholar]
- Xu, W.; Yang, J.; Zhu, X.; Hu, Y.; Xu, S.; Li, Y.; Zhao, Y. Ionol derivatives from Euphorbia tirucalli. Rec. Nat. Prod. 2017, 11, 285–289. [Google Scholar]
- Vingre, S.M.G.; Costa, F.L.P. A theoretical study to the loliolide molecule and its isomers: A study by circular dichroism, QTAIM, and NMR theoretical methods. J. Mol. Model. 2021, 27, 116. [Google Scholar] [CrossRef] [PubMed]

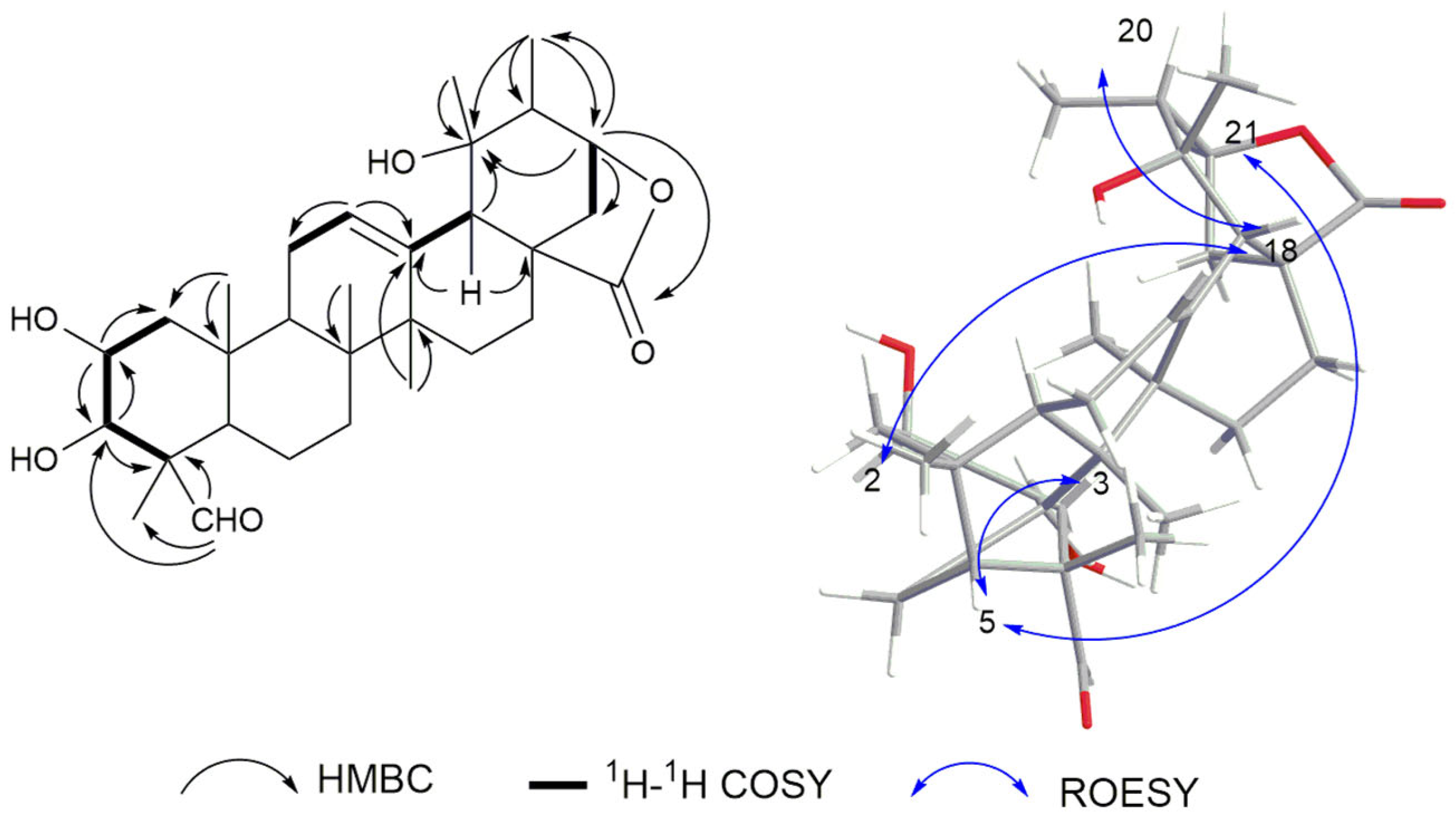

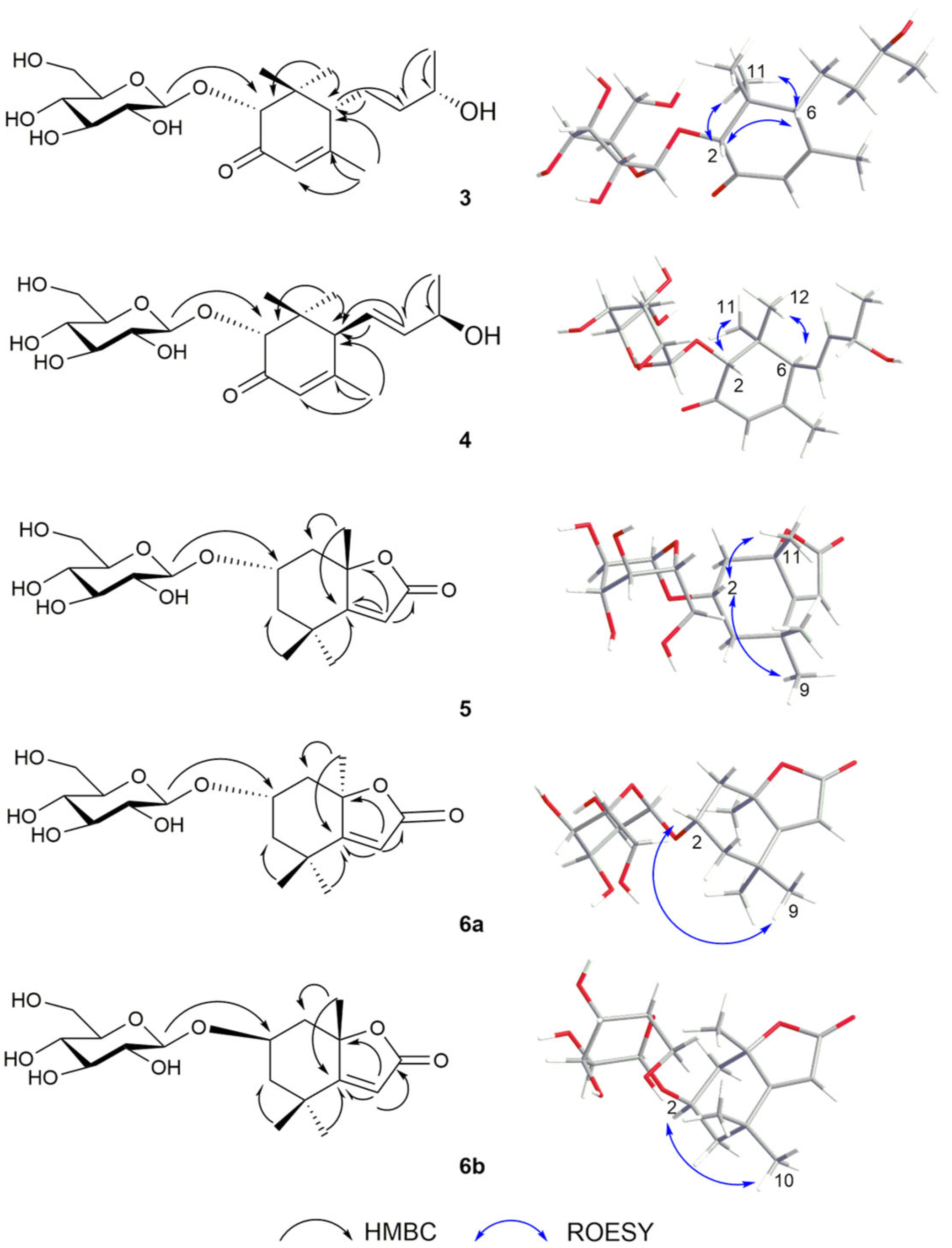

| Position | δC | δH | Position | δC | δH |
|---|---|---|---|---|---|
| 1 | 47.5 | 0.96, 1H, m 2.06, 1H, m | 16 | 29.9 | 1.23, 1H, m 1.81, 1H, m |
| 2 | 69.2 | 4.06, 1H, ddd, 5, 7, 10 | 17 | 45.8 | |
| 3 | 82.6 | 3.11, 1H, d, 9.5 | 18 | 57.0 | 2.40, 1H, s |
| 4 | 55.3 | 19 | 74.3 | ||
| 5 | 58.3 | 1.15, 1H, m | 20 | 45.3 | 1.79, 1H, m |
| 6 | 20.0 | 1.46, 1H, m 1.81, 1H, m | 21 | 83.9 | 4.34, 1H, d, 5.5 |
| 7 | 34.0 | 1.61, 2H, m | 22 | 34.0 | 1.61, 1H, m 3.34, 1H, d, 9.0 |
| 8 | 41.9 | 23 | 208.5 | 9.90, 1H, s | |
| 9 | 47.1 | 1.70, 1H, m | 24 | 21.4 | 1.26, 3H, s |
| 10 | 39.3 | 25 | 18.0 | 0.90, 3H, s | |
| 11 | 25.4 | 1.99, 1H, m 2.08, 1H, m | 26 | 17.4 | 0.95, 3H, s |
| 12 | 131.3 | 5.53, 1H, t, 4.0 | 27 | 26.2 | 1.39, 3H, s |
| 13 | 137.7 | 28 | 185.4 | ||
| 14 | 42.7 | 29 | 13.8 | 1.05, 3H, d, 7.0 | |
| 15 | 29.3 | 1.08, 1H, m 1.89, 1H, m | 30 | 28.4 | 1.21, 3H, s |
| Position | 3 | 4 | 5 | 6a | 6b |
|---|---|---|---|---|---|
| 1 | 1.45, 1H, t, 12.0 2.65, 1H, dd, 2.0, 12.0 | 1.65, 1H, dd, 4.0, 14.0 2.65, 1H, dt, 2.5, 14.5 | 1.77, 1H, dd, 4.0, 14.0 2.67, 1H, dt, 2.5, 14.5 | ||
| 2 | 4.20, 1H, s | 4.17, 1H, s | 4.27, 1H, tt, 4.0, 12.0 | 4.28, 1H, m | 4.30, 1H, m |
| 3 | 1.38, 1H, t, 12.0 2.18, 1H, dd, 2.0, 12.0 | 1.55, 1H, dd, 4.0, 14.0 2.20, 1H, dt, 2.5, 14.5 | 1.44, 1H, dd, 4.0, 14.0 2.21, 1H, dt, 2.5, 14.5 | ||
| 4 | 5.81, 1H, d, 1.0 | 5.91, 1H, d, 1.0 | |||
| 5 | |||||
| 6 | 2.10, 1H, m | 2.77, 1H, d, 7.5 | 5.77, 1H, s | 5.74, 1H, s | 5.74, 1H, s |
| 7 | 1.60, 1H, m 2.11, 1H, m | 5.76, 1H, m | |||
| 8 | 1.62, 2H, m | 5.77, 1H, m | |||
| 9 | 3.87, 1H, m | 4.40, 1H, m | 1.31, 3H, s | 1.27, 3H, s | 1.28, 3H, s |
| 10 | 2.03, 3H, d, 1.0 | 1.92, 3H, d, 10.0 | 1.28, 3H, s | 1.43, 3H, s | 1.45, 3H, s |
| 11 | 0.88, 3H, s | 0.90, 3H, s | 1.59, 3H, s | 1.75, 3H, s | 1.74, 3H, s |
| 12 | 1.19, 3H, s | 1.11, 3H, s | |||
| 13 | 1.19, 3H, d, 6.0 | 1.30, 3H, d, 6.5 | |||
| Glc | |||||
| 1 | 4.33, 1H, d, 8.0 | 4.36, 1H, d, 7.5 | 4.42, 1H, d, 8.0 | 4.37, 1H, d, 8.0 | 4.39, 1H, d, 8.0 |
| 2 | 3.13, 1H, m | 3.17, 1H, m | 3.12, 1H, m | 3.17, 1H, m | 3.17, 1H, m |
| 3 | 3.33, 1H, m | 3.33, 1H, m | 3.29, 1H, m | 3.36, 1H, m | 3.35, 1H, m |
| 4 | 3.24, 1H, m | 3.29, 1H, m | 3.24, 1H, m | 3.27, 1H, m | 3.27, 1H, m |
| 5 | 3.24 1 | 3.22, 1H, m | 3.29 1 | 3.27, 1H, m | 3.27 1 |
| 6 | 3.65, 1H, m 3.83, 1H, m | 3.67, 1H, m 3.83, 1H, m | 3.63, 1H, m 3.89, 1H, m | 3.64, 1H, m 3.85, 1H, m | 3.65, 1H, m 3.86, 1H, m |
| Position | 3 | 4 | 5 | 6a | 6b |
|---|---|---|---|---|---|
| 1 | 43.2 | 42.8 | 45.6 | 42.9 | 45.4 |
| 2 | 77.2 | 77.4 | 72.9 | 74.2 | 74.5 |
| 3 | 201.2 | 200.9 | 48.9 | 46.9 | 44.4 |
| 4 | 124.0 | 125.0 | 35.9 | 37.1 | 37.0 |
| 5 | 168.6 | 164.7 | 183.8 | 185.8 | 185.7 |
| 6 | 54.5 | 58.6 | 113.4 | 113.1 | 113.1 |
| 7 | 25.9 | 128.3 | 173.8 | 174.4 | 174.4 |
| 8 | 38.2 | 138.5 | 88.3 | 88.9 | 88.9 |
| 9 | 75.4 | 77.0 | 30.1 | 30.9 | 31.0 |
| 10 | 24.6 | 23.7 | 25.1 | 26.6 | 26.7 |
| 11 | 21.0 | 25.5 | 25.4 | 27.1 | 27.0 |
| 12 | 24.5 | 21.0 | |||
| 13 | 19.8 | 21.0 | |||
| Glc | |||||
| 1 | 102.0 | 102.5 | 102.7 | 102.9 | 103.2 |
| 2 | 75.1 | 75.3 | 74.8 | 75.3 | 75.3 |
| 3 | 78.1 | 78.1 | 77.8 | 78.5 | 78.4 |
| 4 | 71.7 | 71.5 | 71.5 | 71.7 | 71.7 |
| 5 | 77.8 | 78.0 | 77.8 | 77.9 | 77.9 |
| 6 | 62.9 | 62.6 | 62.6 | 62.8 | 62.8 |
| Number | Compound | Type | A. italica | |
|---|---|---|---|---|
| 1 | 2α,3β,19α-trihydroxy-23-formyl-urs-12-en-28,21β-olide | triterpene | 1, 2 | |
| 2 | 2α,3β,21,24-tetrahydroxyoleanan-12-en-28-oic acid | triterpene | 1 | |
| 3 | (2R,6R,9S)-9-Hydroxy-4-megastigmen-3-one-2-O-β-D-glucopyranoside | norisoprenoids | 1, 2 | |
| 4 | (2R,6S,9S)-9-Hydroxy-megastigman-4,7-dien-3-one-2-O-β-D-glucopyranoside | norisoprenoids | 1, 2 | |
| 5 | (+)-Isololiolide β-D-glucopyranoside | norisoprenoids | 1, 2 | |
| 6a | (2S,8R)-Loliolide β-D-glucopyranoside | norisoprenoids | 1, 2 | |
| 6b | (2R,8S)-Loliolide β-D-glucopyranoside | norisoprenoids | 1, 2 | |
| 7 | Niga-ichigoside F 1 | triterpene | 1 | |
| 8 | Niga-ichigoside F 2 | triterpene | 1 | |
| 9 | Pinfaensin | triterpene | 1, | |
| 10 | Glucosyl tormentate | triterpene | 1 | |
| 11 | 23-Hydroxytormentic acid | triterpene | 1 | |
| 12 | 24-epi-pinfaensic acid | triterpene | 1 | |
| 13 | 19α-Hydroxyasiatic acid | triterpene | 1 | |
| 14 | (+)-Vomifoliol | norisoprenoids | 1 | |
| 15 | Lippianoside E | norisoprenoids | 1, 3 | |
| 16 | 3-oxo-α-ionol-β-D-glucopyranoside | norisoprenoids | 1, 3 | |
| 17 | Asysgangoside | norisoprenoids | 1 | |
| 18 | (6S,9R)-Roseoside | norisoprenoids | 1, 3 | Borago officinalis L. |
| 19 | Sammangaoside B | norisoprenoids | 1, 3 | |
| 20 | (+)-Isololiolide | norisoprenoids | 1, 3 | |
| 21 | Tricin | flavone | 1, 3 | |
| 22 | 6-Hydroxykaempferol 3-β-rutinoside | flavonol | 1, 3 | |
| 23 | Kaempferol 3-O-rutinoside | flavonol | 1, 3 | Anchusa strigosa Banks & Sol. |
| 24 | Narcissin | flavonol | 1, 3 | Heliotropium angiospermum |
| 25 | (+)-Syringaresinol | lignans | 1, 3 | Moltkia aurea Boiss. |
| 26 | (+)-Mediaresinol | lignans | 1 | |
| 27 | Dehydrodiconiferyl alcohol | lignans | 1, 3 | |
| 28 | Vibruresinol | lignans | 1, 3 | |
| 29 | Dehydrodiconiferyl alcohol 4-O-β-D-glucopyranoside | lignans | 1, 3 | |
| 30 | Syringaresinol-4′-O-β-D-glucopyranoside | lignans | 1, 3 | Moltkia aurea Boiss. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, L.; Han, B.; Ma, Z.; Huang, X.; Yang, G.; Zeng, Y.; Liao, M.; Gao, R.; Li, J. Ursane Triterpenes and Norisoprenoids from Anchusa italica Retz. and Their Chemotaxonomic Significance. Plants 2025, 14, 1385. https://doi.org/10.3390/plants14091385
Shen L, Han B, Ma Z, Huang X, Yang G, Zeng Y, Liao M, Gao R, Li J. Ursane Triterpenes and Norisoprenoids from Anchusa italica Retz. and Their Chemotaxonomic Significance. Plants. 2025; 14(9):1385. https://doi.org/10.3390/plants14091385
Chicago/Turabian StyleShen, Linchuang, Bingchen Han, Zhiliang Ma, Xianju Huang, Guangzhong Yang, Yanfeng Zeng, Maochuan Liao, Ruixi Gao, and Jun Li. 2025. "Ursane Triterpenes and Norisoprenoids from Anchusa italica Retz. and Their Chemotaxonomic Significance" Plants 14, no. 9: 1385. https://doi.org/10.3390/plants14091385
APA StyleShen, L., Han, B., Ma, Z., Huang, X., Yang, G., Zeng, Y., Liao, M., Gao, R., & Li, J. (2025). Ursane Triterpenes and Norisoprenoids from Anchusa italica Retz. and Their Chemotaxonomic Significance. Plants, 14(9), 1385. https://doi.org/10.3390/plants14091385






