Different Spatial Configurations of LED Light Sources Enhance Growth in Tomato Seedlings by Influencing Photosynthesis, CO2 Assimilation, and Endogenous Hormones
Abstract
1. Introduction
2. Results
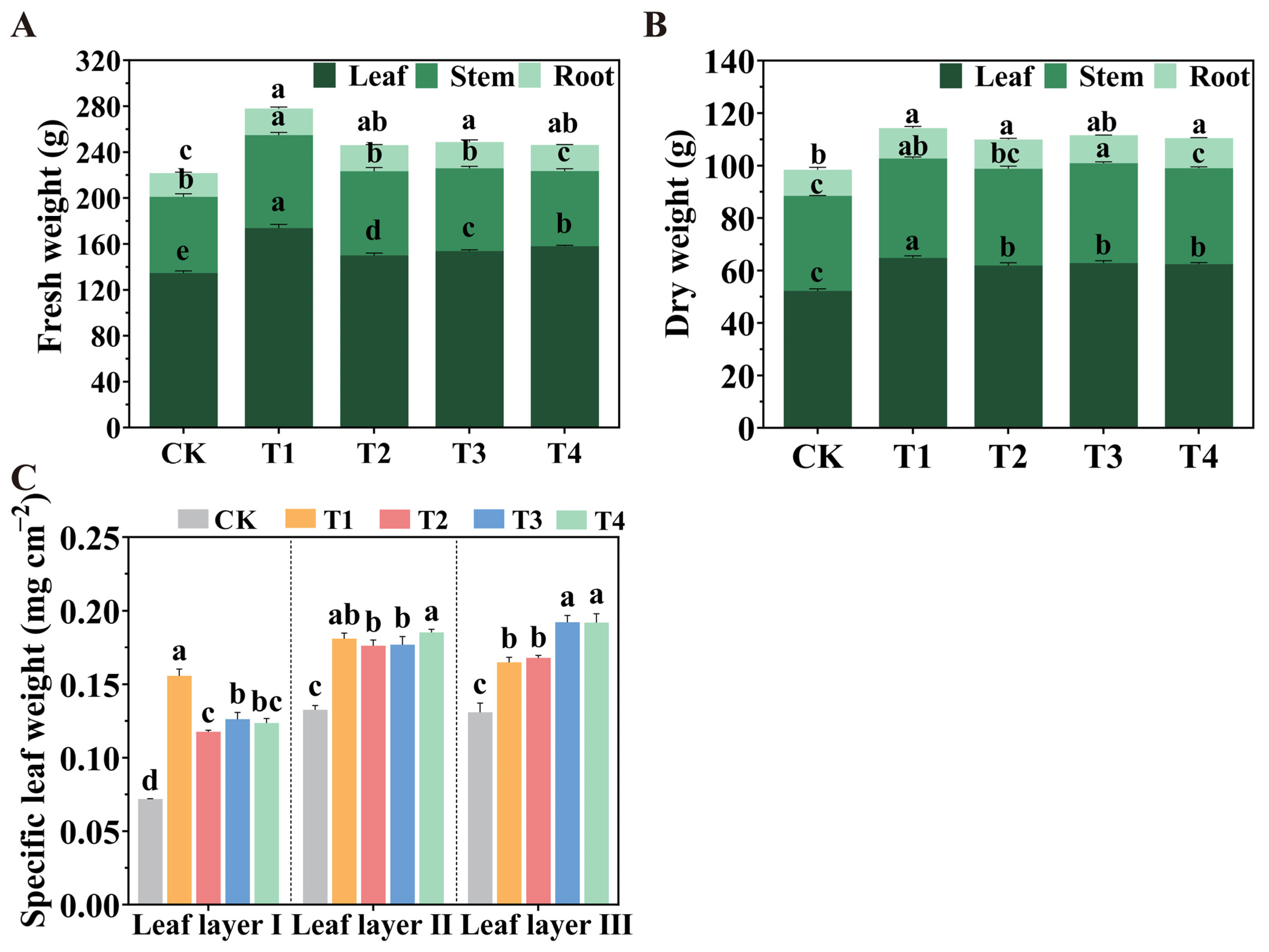
2.1. Accumulation of Biomass Under Various Spatial Arrangements of LED Light Sources
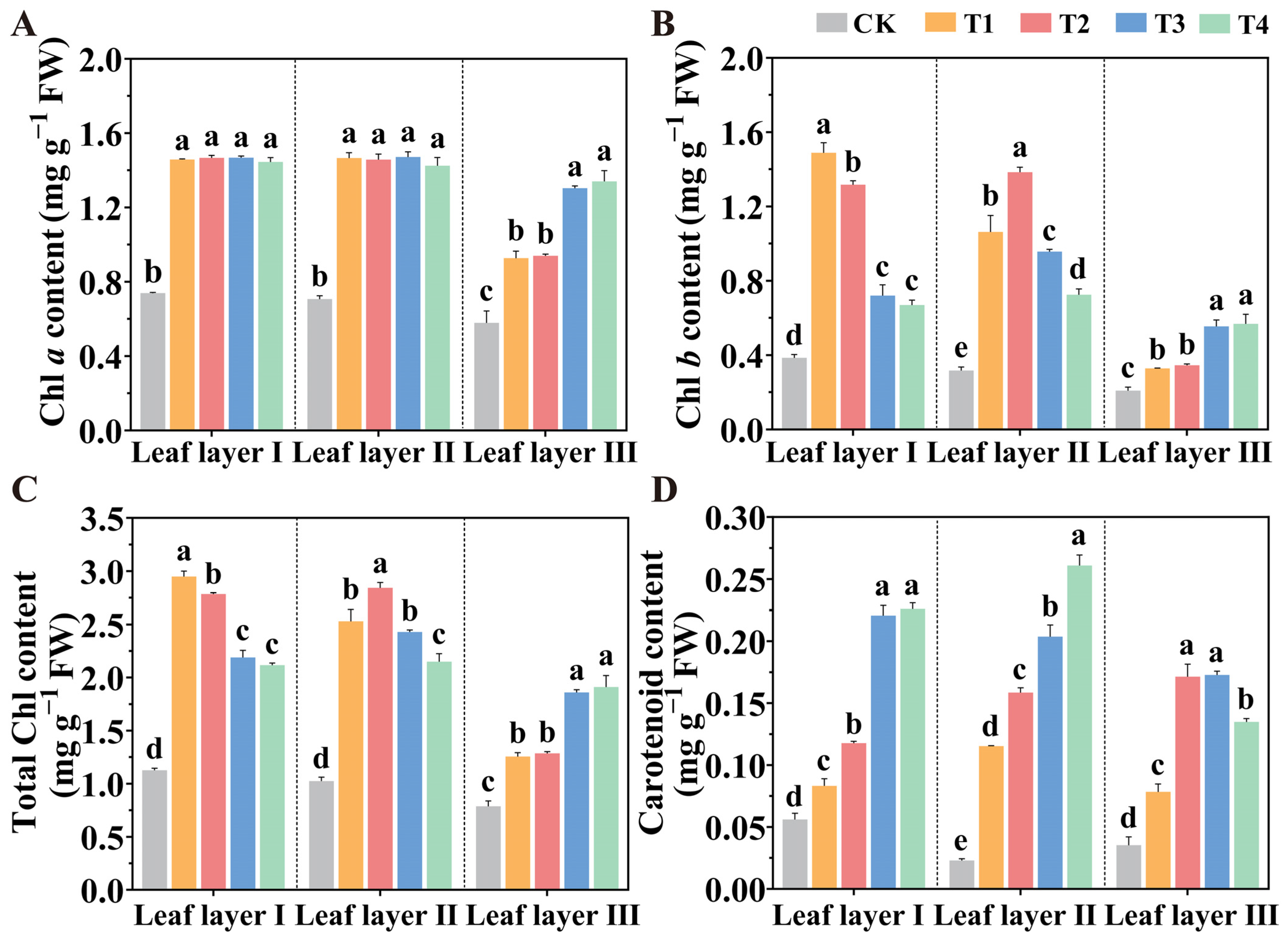
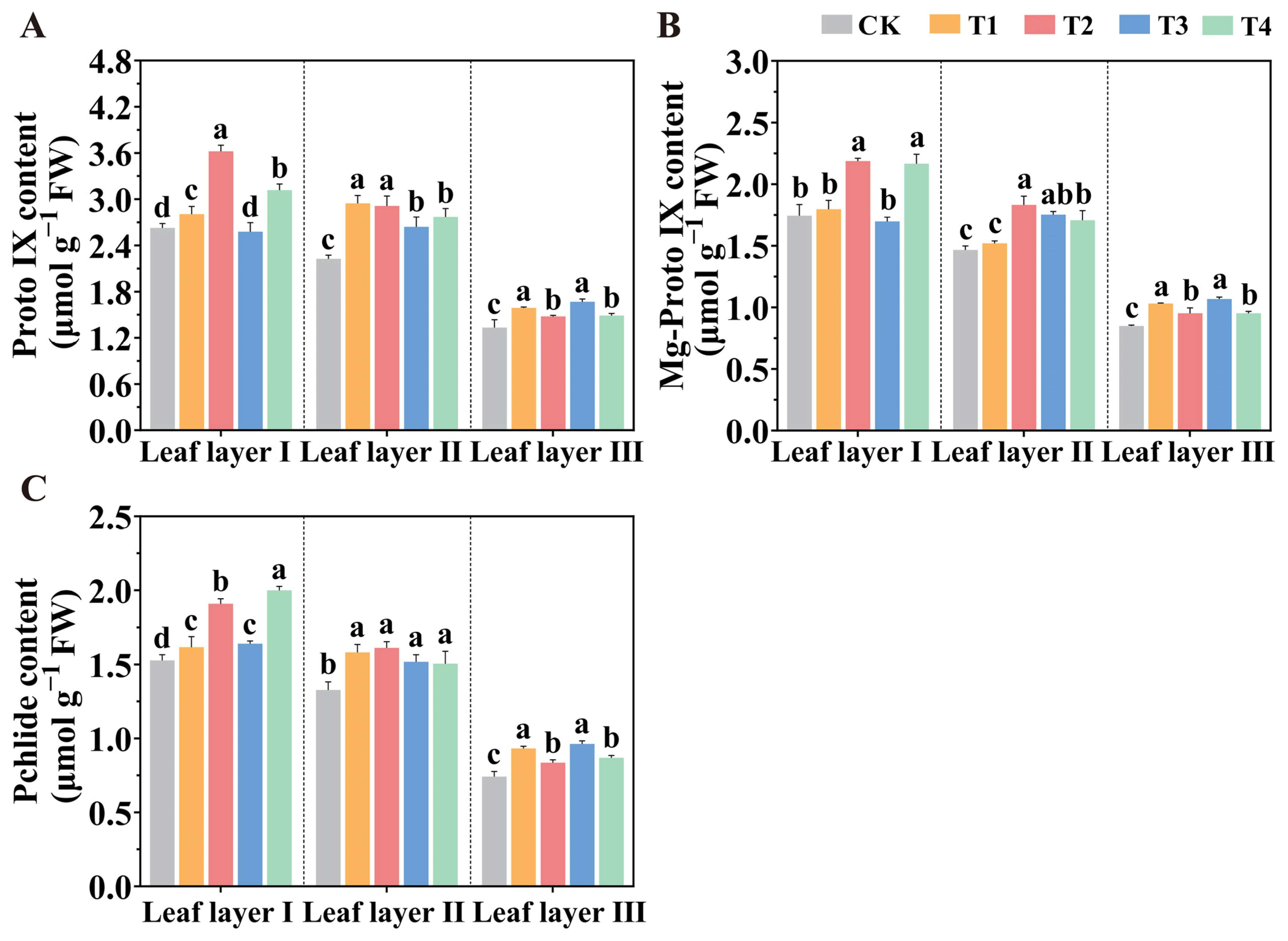
2.2. Spatial Arrangement of LED Light Sources Promotes Photosynthetic Pigment Accumulation
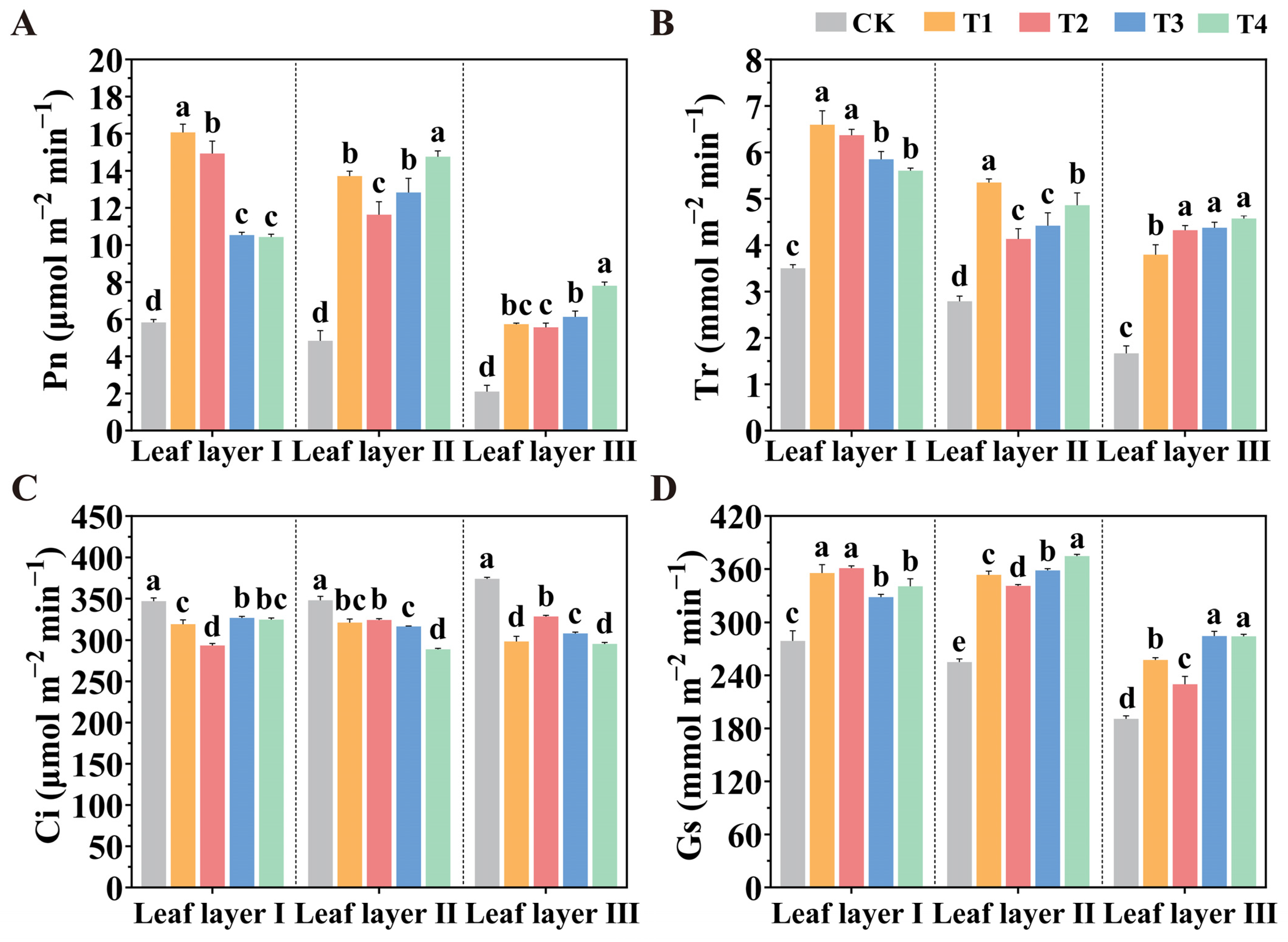
2.3. Manipulation of the Spatial Arrangement of LED Supplemental Lights Improves Photosynthesis
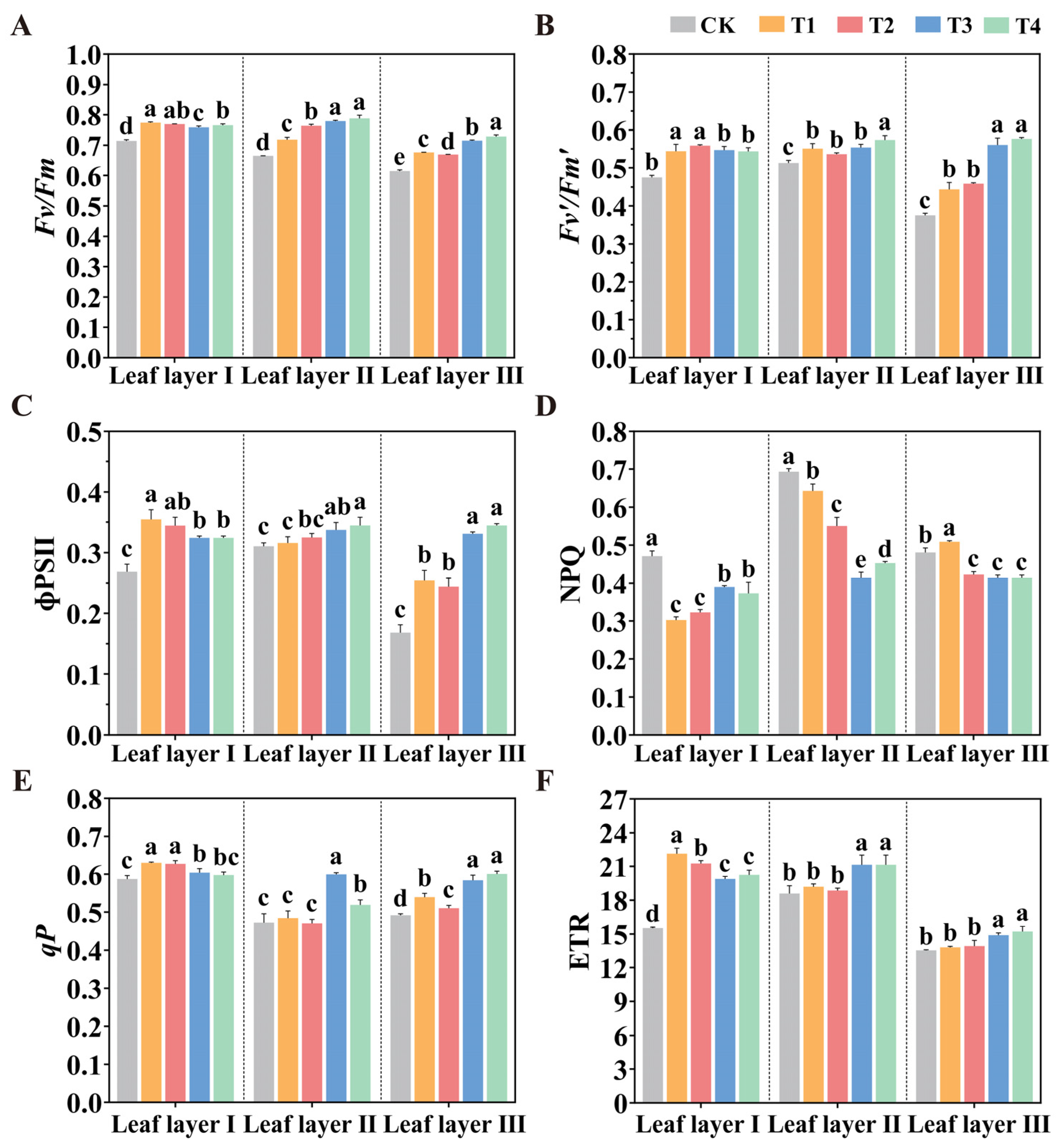
2.4. Manipulation of the Spatial Arrangement of LED Supplemental Light Influences Chlorophyll Fluorescence
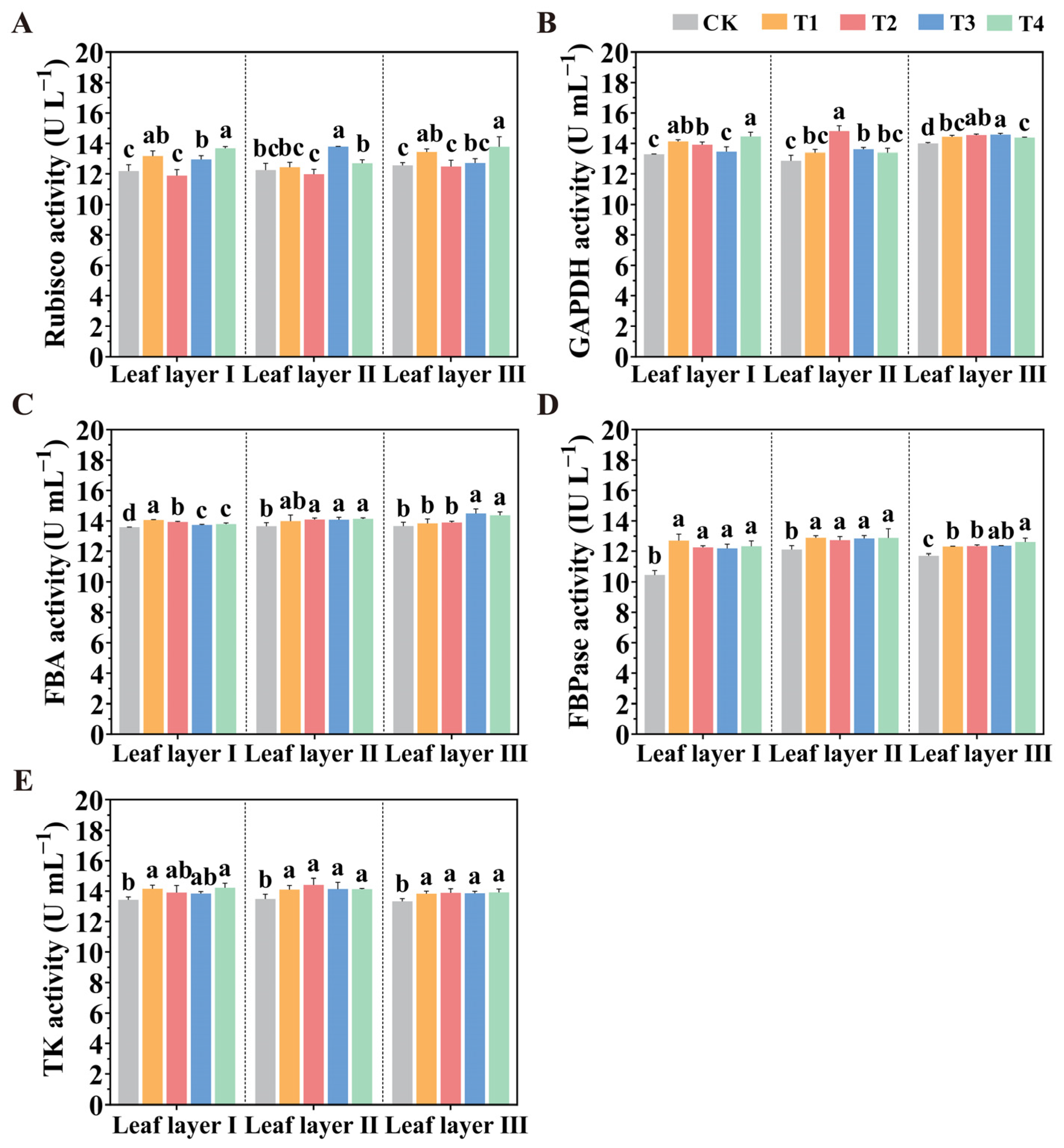
2.5. Manipulation of the Spatial Arrangement of LED Supplemental Light Influences Calvin Cycle Enzyme Activities
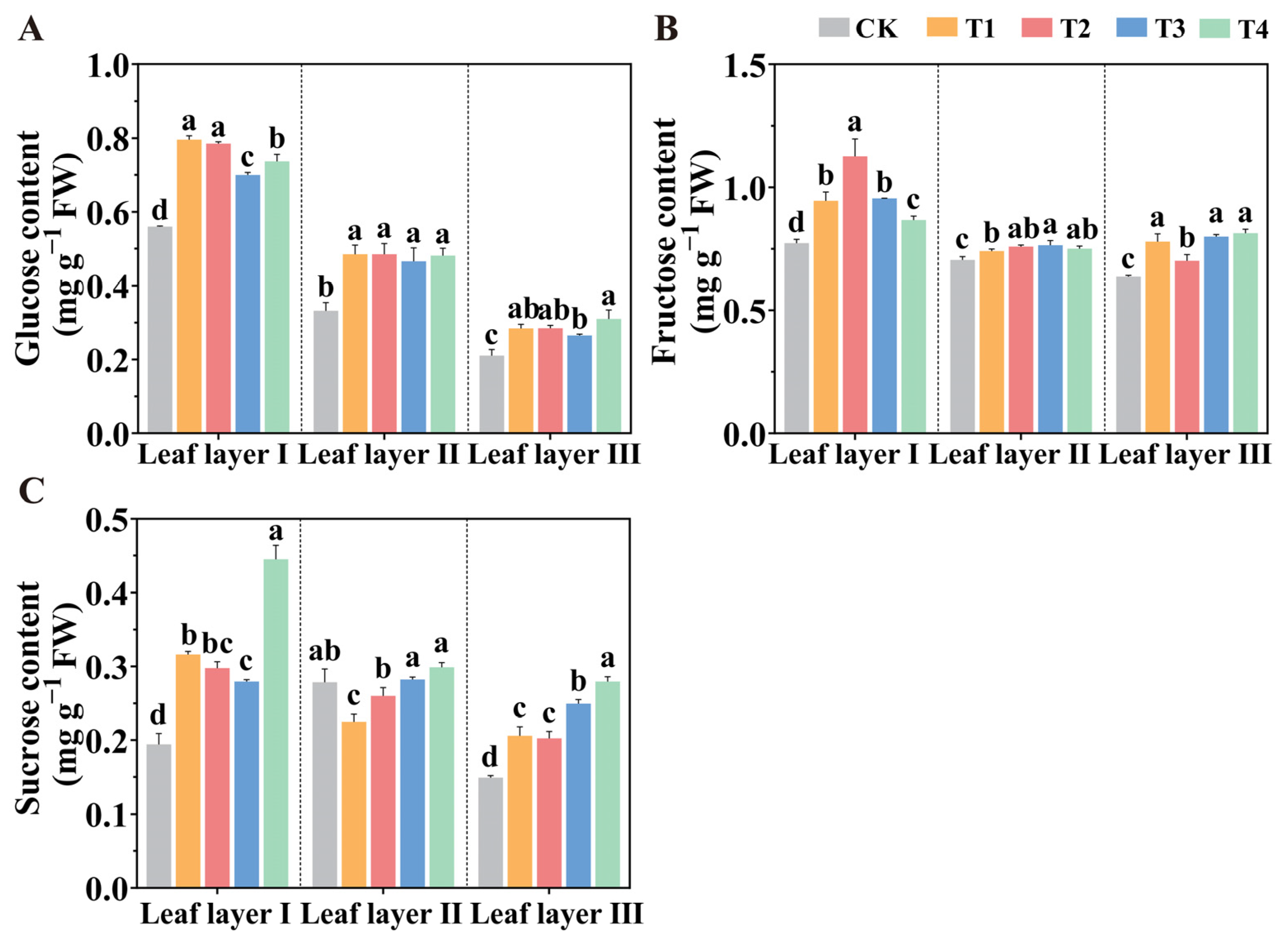
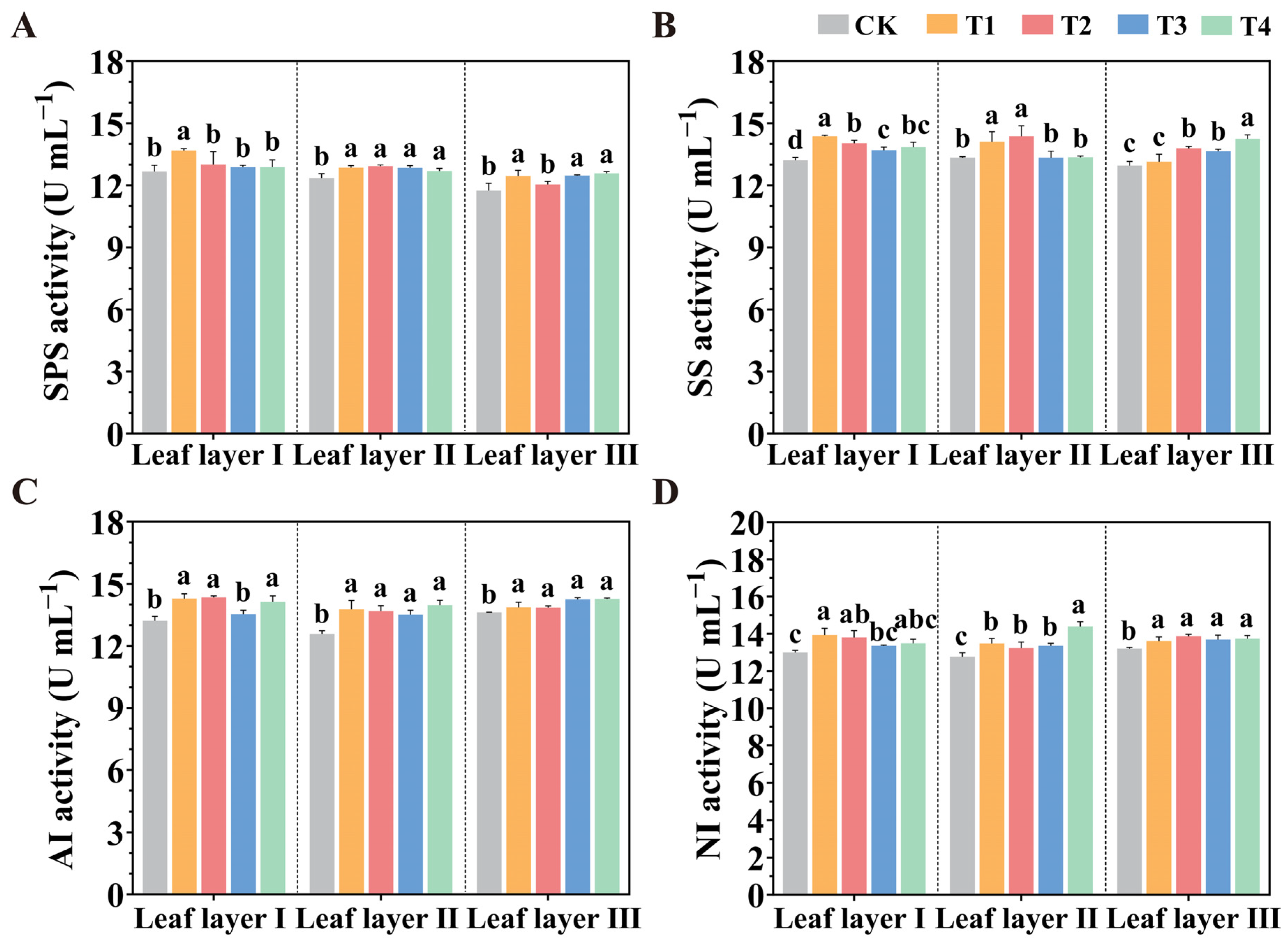
2.6. Carbohydrate Accumulation Under Various Spatial Arrangements of LED Supplemental Lighting
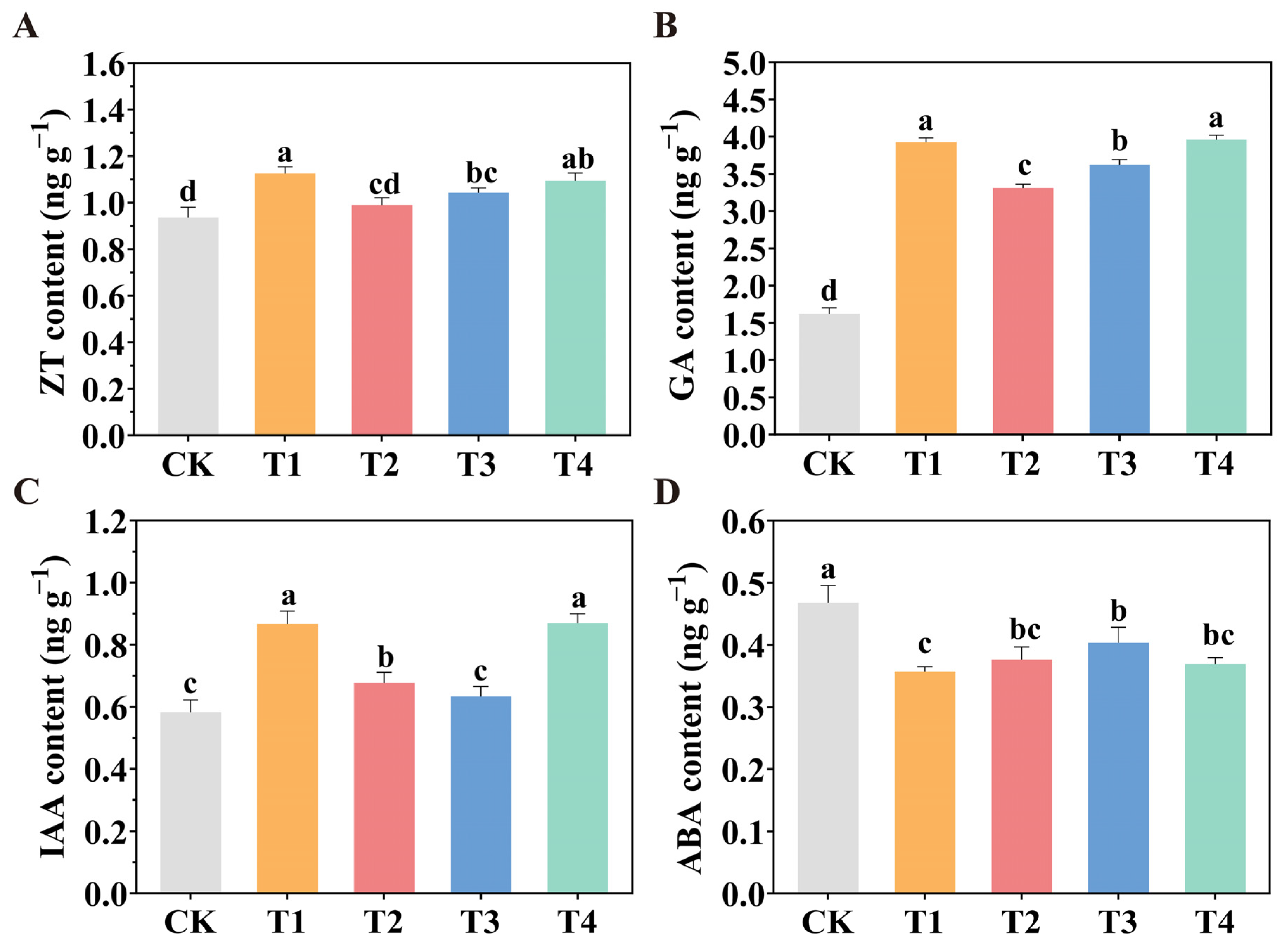
2.7. Endogenous Hormone Contents in Tomato Leaves Under Various LED Light Source Spatial Configurations
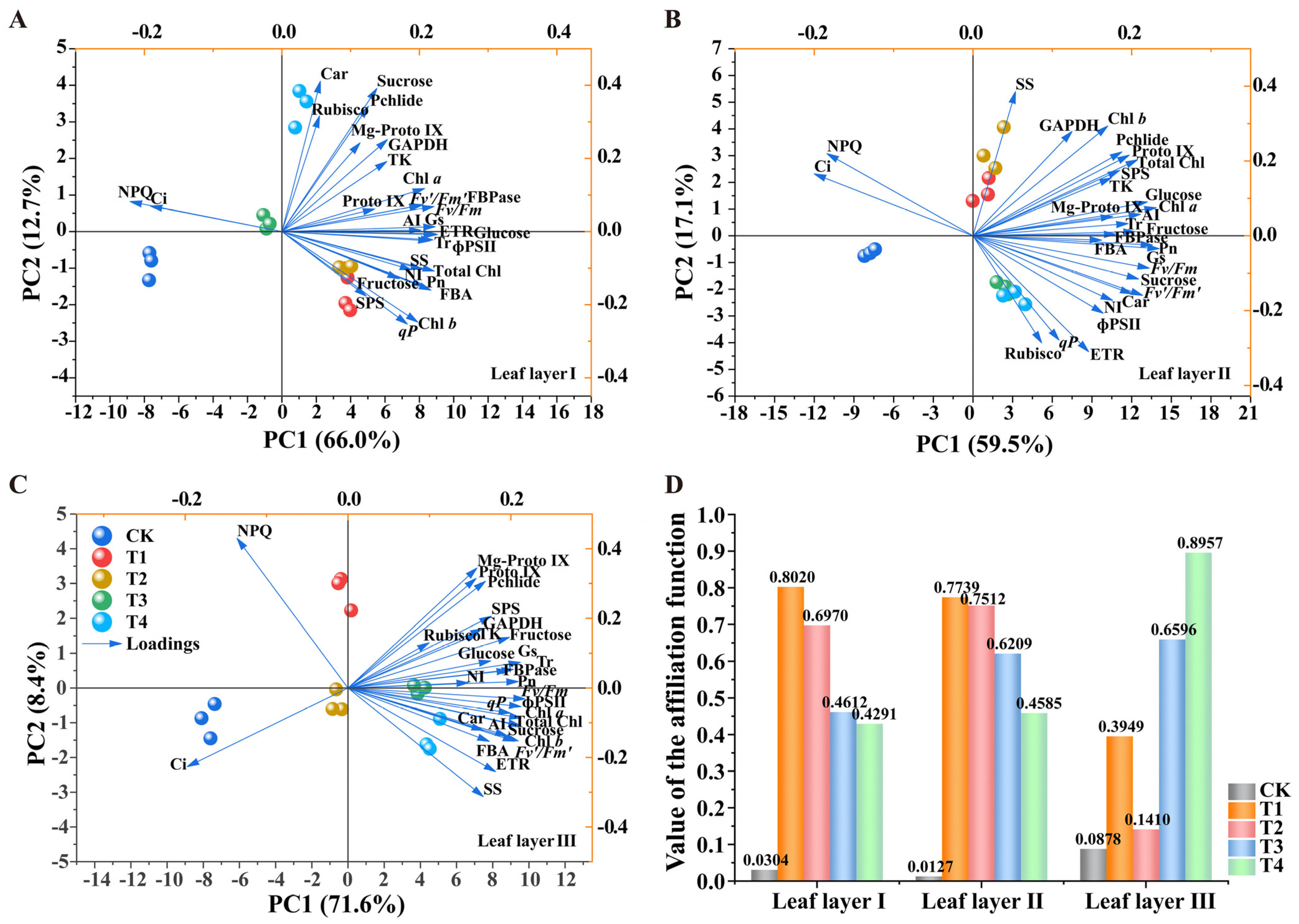
2.8. Principal Component Analyses and Affiliation Function Evaluation Analysis of Photosynthesis-Related Physiological Indices
3. Discussion
4. Materials and Methods
4.1. Tomato Material and Growth Conditions
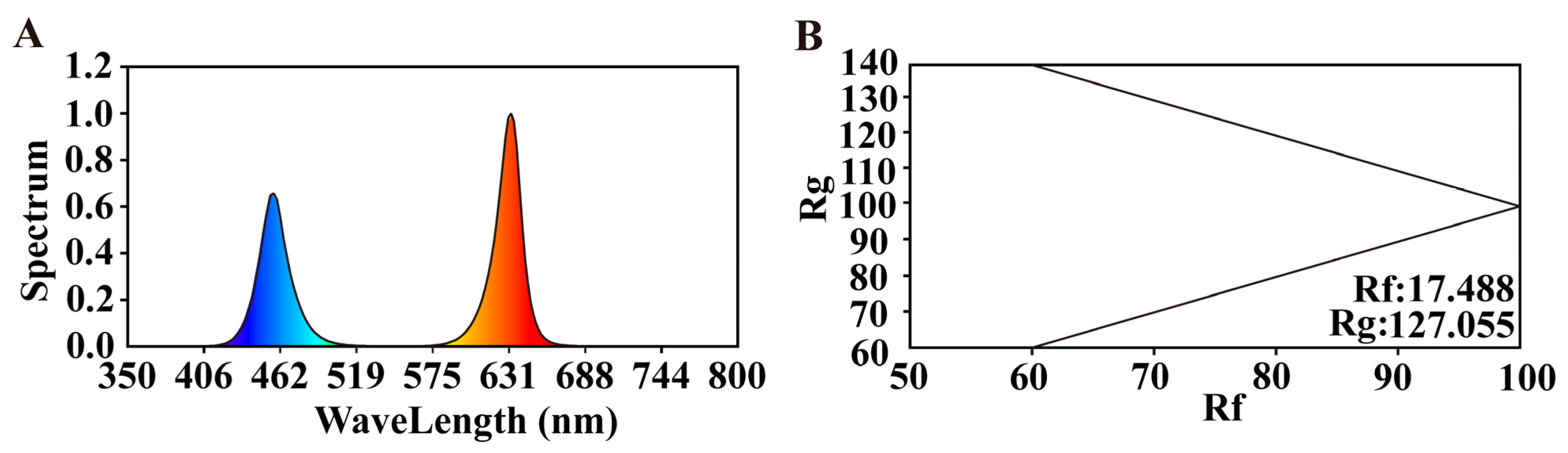
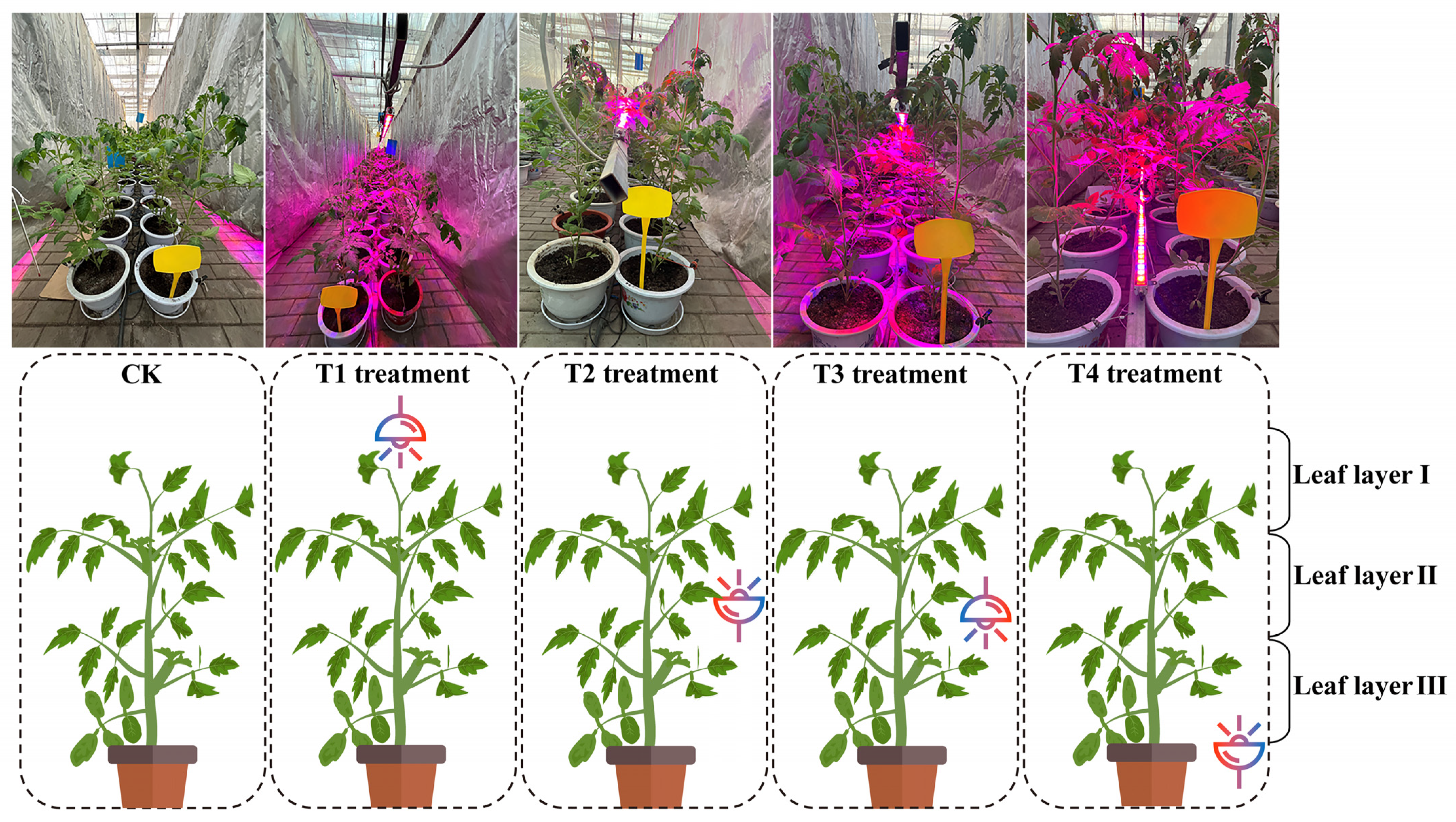
4.2. Light Treatments and Experimental Design
4.3. Measurement of Biomass and Specific Leaf Weight
4.4. Determination of the Contents of Photosynthetic Pigments and Chlorophyll Synthesis Precursors
4.5. Determination of Gas Exchange Parameters
4.6. Determination of Chlorophyll Fluorescence Parameters
4.7. Enzyme Activities Related to Carbon Metabolism in Tomato Leaves
4.8. Carbohydrate Content of Tomato Leaves
4.9. Determination of Hormone Contents
4.10. The Formula for the Affiliation Function Method
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poorter, H.; Fiorani, F.; Pieruschka, R.; Wojciechowski, T.; van der Putten, W.H.; Kleyer, M.; Schurr, U.; Postma, J. Pampered inside, pestered outside? Differences and similarities between plants growing in controlled conditions and in the field. New Phytol. 2016, 212, 838–855. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.D. Plant responses to photoperiod. New Phytol. 2009, 181, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Ilić, Z.S.; Fallik, E. Light quality manipulation improves vegetable quality at harvest and postharvest: A review. Environ. Exp. Bot. 2017, 139, 79–90. [Google Scholar] [CrossRef]
- Gorjian, S.; Calise, F.; Kant, K.; Ahamed, M.S.; Copertaro, B.; Najafi, G.; Zhang, X.; Aghaei, M.; Shamshiri, R.R. A review on opportunities for implementation of solar energy technologies in agricultural greenhouses. J. Clean. Prod. 2021, 285, 124807. [Google Scholar] [CrossRef]
- Kromdijk, J.; Głowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef]
- Trivellini, A.; Toscano, S.; Romano, D.; Ferrante, A. LED lighting to produce high-quality ornamental plants. Plants 2023, 12, 1667. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Wu, B.S.; Hitti, Y.; MacPherson, S.; Orsat, V.; Lefsrud, M.G. Comparison and perspective of conventional and LED lighting for photobiology and industry applications. Environ. Exp. Bot. 2020, 171, 103953. [Google Scholar] [CrossRef]
- Singh, D.; Basu, C. Meinhardt-Wollweber, M.; Roth, B. LEDs for energy efficient greenhouse lighting. Renew. Sustain. Energy Rev. 2015, 49, 139–147. [Google Scholar] [CrossRef]
- Liu, J.; Van Iersel, M.W. Photosynthetic physiology of blue, green, and red light: Light intensity effects and underlying mechanisms. Front. Plant Sci. 2021, 12, 619987. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Zhao, B.; Yuan, X. Improved growth of Artemisia annua L. hairy roots and artemisinin production under red light conditions. Biotechnol. Lett. 2001, 23, 1971–1973. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Paponov, M.; Kechasov, D.; Lacek, J.; Verheul, M.J.; Paponov, I.A. Supplemental light-emitting diode inter-lighting increases tomato fruit growth through enhanced photosynthetic light use efficiency and modulated root activity. Front. Plant Sci. 2020, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xin, G.; Wei, M.; Shi, Q.; Yang, F.; Wang, X. Carbohydrate accumulation and sucrose metabolism responses in tomato seedling leaves when subjected to different light qualities. Sci. Hortic. 2017, 225, 490–497. [Google Scholar] [CrossRef]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, M.O.K. Blue light added with red LEDs enhance growth characteristics, pigments content, and antioxidant capacity in lettuce, spinach, kale, basil, and sweet pepper in a controlled environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef]
- Neo, D.C.J.; Ong, M.M.X.; Lee, Y.Y.; Teo, E.J.; Ong, Q.; Tanoto, H.; Xu, J.; Ong, K.S.; Suresh, V. Shaping and tuning lighting conditions in controlled environment agriculture: A review. ACS Agric. Sci. Technol. 2022, 2, 3–16. [Google Scholar] [CrossRef]
- Slattery, R.A.; Ort, D.R. Perspectives on improving light distribution and light use efficiency in crop canopies. Plant Physiol. 2021, 185, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, Y.; Tian, S.; Liang, Z.; Li, S.; Li, Y.; Wei, M.; Zhang, D. Effect of supplemental lighting on water transport, photosynthetic carbon gain and water use efficiency in greenhouse tomato. Sci. Hortic. 2019, 256, 108630. [Google Scholar] [CrossRef]
- Giliberto, L.; Perrotta, G.; Pallara, P.; Weller, J.L.; Fraser, P.D.; Bramley, P.M.; Fiore, A.; Tavazza, M.; Giuliano, G. Manipulation of the blue light photoreceptor cryptochrome 2 in tomato affects vegetative development, flowering time, and fruit antioxidant content. Plant Physiol. 2005, 137, 199–208. [Google Scholar] [CrossRef]
- Paucek, I.; Appolloni, E.; Pennisi, G.; Quaini, S.; Gianquinto, G.; Orsini, F. LED lighting systems for horticulture: Business growth and global distribution. Sustainability 2020, 12, 7516. [Google Scholar] [CrossRef]
- Eichhorn Bilodeau, S.; Wu, B.S.; Rufyikiri, A.S.; MacPherson, S.; Lefsrud, M. An update on plant photobiology and implications for cannabis production. Front. Plant Sci. 2019, 10, 296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Shen, S.; Takagaki, M.; Kozai, T.; Yamori, W. Supplemental upward lighting from underneath to obtain higher marketable lettuce (Lactuca sativa) leaf fresh weight by retarding senescence of outer leaves. Front. Plant Sci. 2015, 6, 1110. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wei, R.; Xu, L. Optimal greenhouse lighting scheduling using canopy light distribution model: A simulation study on tomatoes. Light. Res. Technol. 2020, 52, 233–246. [Google Scholar] [CrossRef]
- Basha, N.; Nounou, M.; Nounou, H. Multivariate fault detection and classification using interval principal component analysis. J. Comput. Sci. 2018, 27, 1–9. [Google Scholar] [CrossRef]
- Gewers, F.L.; Ferreira, G.R.; Arruda, H.F.D.; Silva, F.N.; Comin, C.H.; Amancio, D.R.; Costa, L.d.F. Principal component analysis: A natural approach to data exploration. ACM Comput. Surv. 2021, 54, 1–34. [Google Scholar] [CrossRef]
- Arena, C.; Tsonev, T.; Doneva, D.; De Micco, V.; Michelozzi, M.; Brunetti, C.; Centritto, M.; Fineschi, S.; Velikova, V.; Loreto, F. The effect of light quality on growth, photosynthesis, leaf anatomy and volatile isoprenoids of a monoterpene-emitting herbaceous species (Solanum lycopersicum L.) and an isoprene-emitting tree (Platanus orientalis L.). Environ. Exp. Bot. 2016, 130, 122–132. [Google Scholar] [CrossRef]
- Lanoue, J.; Leonardos, E.D.; Grodzinski, B. Effects of light quality and intensity on diurnal patterns and rates of photo-assimilate translocation and transpiration in tomato leaves. Front. Plant Sci. 2018, 9, 756. [Google Scholar] [CrossRef]
- Shuang, S.P.; Zhang, J.Y.; Cun, Z.; Wu, H.M.; Hong, J.; Chen, J.W. A comparison of photoprotective mechanism in different light-demanding plants under dynamic light conditions. Front. Plant Sci. 2022, 13, 819843. [Google Scholar] [CrossRef]
- Zhang, X.; He, D.; Niu, G.; Yan, Z.; Song, J. Effects of environment lighting on the growth, photosynthesis, and quality of hydroponic lettuce in a plant factory. Int. J. Agric. Biol. Eng. 2018, 11, 33–40. [Google Scholar] [CrossRef]
- Wang, S.; Meng, X.; Tang, Z.; Wu, Y.; Xiao, X.; Zhang, G.; Hu, L.; Liu, Z.; Lyu, J.; Yu, J. Red and blue LED light supplementation in the morning pre-activates the photosynthetic system of tomato (Solanum lycopersicum L.) leaves and promotes plant growth. Agronomy 2022, 12, 897. [Google Scholar] [CrossRef]
- Liu, X.; Jiao, X.; Chang, T.; Guo, S.; Xu, Z. Photosynthesis and leaf development of cherry tomato seedlings under different LED-based blue and red photon flux ratios. Photosynthetica 2018, 56, 1212–1217. [Google Scholar] [CrossRef]
- Trouwborst, G.; Oosterkamp, J.; Hogewoning, S.W.; Harbinson, J.; Van Ieperen, W. The responses of light interception, photosynthesis and fruit yield of cucumber to LED-lighting within the canopy. Physiol. Plant. 2010, 138, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Paucek, I.; Pennisi, G.; Pistillo, A.; Appolloni, E.; Crepaldi, A.; Calegari, B.; Spinelli, F.; Cellini, A.; Gabarrell, X.; Orsini, F. Supplementary LED interlighting improves yield and precocity of greenhouse tomatoes in the Mediterranean. Agronomy 2020, 10, 1002. [Google Scholar] [CrossRef]
- Davis, P.A.; Caylor, S.; Whippo, C.W.; Hangarter, R.P. Changes in leaf optical properties associated with light-dependent chloroplast movements. Plant Cell Environ. 2011, 34, 2047–2059. [Google Scholar] [CrossRef]
- Mao, L.; Song, Q.; Li, X.; Zheng, H.; Zhu, X.G. Would reducing chlorophyll content result in a higher photosynthesis nitrogen use efficiency in crops? Food Energy Secur. 2024, 13, e576. [Google Scholar] [CrossRef]
- Liang, R.; Wang, L.; Wang, X.; Zhang, J.; Gan, X. Effects of exogenous ALA on leaf photosynthesis, photosynthate transport, and sugar accumulation in Prunus persica L. Forests 2023, 14, 723. [Google Scholar] [CrossRef]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Palmitessa, O.D.; Paciello, P.; Santamaria, P. Supplemental LED increases tomato yield in mediterranean semi-closed greenhouse. Agronomy 2020, 10, 1353. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lou, Y.; Li, H.; Di, X.; Gao, Z. Unveiling the intrinsic mechanism of photoprotection in bamboo under high light. Ind. Crops Prod. 2024, 209, 118049. [Google Scholar] [CrossRef]
- Ferroni, L.; Živčak, M.; Sytar, O.; Kovár, M.; Watanabe, N.; Pancaldi, S.; Baldisserotto, C.; Brestič, M. Chlorophyll-depleted wheat mutants are disturbed in photosynthetic electron flow regulation but can retain an acclimation ability to a fluctuating light regime. Environ. Exp. Bot. 2020, 178, 104156. [Google Scholar] [CrossRef]
- Acevedo-Siaca, L.G.; Coe, R.; Quick, W.P.; Long, S.P. Variation between rice accessions in photosynthetic induction in flag leaves and underlying mechanisms. J. Exp. Bot. 2021, 72, 1282–1294. [Google Scholar] [CrossRef] [PubMed]
- Tausz, M.; Warren, C.R.; Adams, M.A. Dynamic light use and protection from excess light in upper canopy and coppice leaves of Nothofagus cunninghamii in an old growth, cool temperate rainforest in Victoria, Australia. New Phytol. 2005, 165, 143–156. [Google Scholar] [CrossRef]
- Yu, J.Q.; Huang, L.F.; Hu, W.H.; Zhou, Y.H.; Mao, W.H.; Ye, S.F.; Nogués, S. A role for brassinosteroids in the regulation of photosynthesis in Cucumis sativus. J. Exp. Bot. 2004, 55, 1135–1143. [Google Scholar] [CrossRef]
- Kim, S.Y.; Stessman, D.J.; Wright, D.A.; Spalding, M.H.; Huber, S.C.; Ort, D.R. Arabidopsis plants expressing only the redox-regulated Rca-α isoform have constrained photosynthesis and plant growth. Plant J. 2020, 103, 2250–2262. [Google Scholar] [CrossRef]
- Yang, B.; Liu, J.; Ma, X.; Guo, B.; Liu, B.; Wu, T.; Jiang, Y.; Chen, F. Genetic engineering of the Calvin cycle toward enhanced photosynthetic CO2 fixation in microalgae. Biotechnol. Biofuels 2017, 10, 229. [Google Scholar] [CrossRef]
- Silva, M.D.; Vasconcelos, J.M.; da Silva, F.B.; de Oliveira Bailão, A.S.; Guedes, Í.M.R.; da Silva Vilela, M.; Costa, A.C.; Rosa, M.; Silva, F.G. Growing in Red: Impact of Different Light Spectra and Lighting Conditions on Lentil Microgreens Growth in Vertical Farming. Front. Plant Sci. 2024, 15, 1515457. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Nawaz, M.A.; Wei, N.; Cheng, F.; Bie, Z. Suboptimal temperature acclimation enhances chilling tolerance by improving photosynthetic adaptability and osmoregulation ability in watermelon. Hortic. Plant J. 2020, 6, 49–60. [Google Scholar] [CrossRef]
- Huang, W.; Yang, G.; Liu, D.; Li, Q.; Zheng, L.; Ma, J. Metabolomics and transcriptomics analysis of vitro growth in pitaya plantlets with different LED Light spectra treatment. Ind. Crops Prod. 2022, 186, 115237. [Google Scholar] [CrossRef]
- Kläring, H.P.; Klopotek, Y.; Krumbein, A.; Schwarz, D. The effect of reducing the heating set point on the photosynthesis, growth, yield and fruit quality in greenhouse tomato production. Agric. For. Meteorol. 2015, 214, 178–188. [Google Scholar] [CrossRef]
- Guo, Z.; Lv, J.; Zhang, H.; Hu, C.; Qin, Y.; Dong, H.; Zhang, T.; Dong, X.; Du, N.; Piao, F. Red and blue light function antagonistically to regulate cadmium tolerance by modulating the photosynthesis, antioxidant defense system and Cd uptake in cucumber (Cucumis sativus L.). J. Hazard. Mater. 2022, 429, 128412. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Ebihara, M.; Nakaminami, A.; Maruo, T. Photosynthesis, plant growth, and fruit production of single-truss tomato improves with supplemental lighting provided from underneath or within the inner canopy. Sci. Hortic. 2017, 222, 221–229. [Google Scholar] [CrossRef]
- Miret Barrio, J.A.; Munné Bosch, S.; Dijkwel, P.P. ABA signalling manipulation suppresses senescence of a leafy vegetable stored at room temperature. Plant Biotechnol. J. 2018, 16, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, X.; Chen, X.; Han, H.; Shen, B.; Diao, M.; Liu, H.Y. Exogenous selenium promotes the growth of salt-stressed tomato seedlings by regulating ionic homeostasis, activation energy allocation and CO2 assimilation. Front. Plant Sci. 2023, 14, 1206246. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, Q.; Qu, M.; Gao, L.; Hou, L. Blue light alleviates ‘red light syndrome’ by regulating chloroplast ultrastructure, photosynthetic traits and nutrient accumulation in cucumber plants. Sci. Hortic. 2019, 257, 108680. [Google Scholar] [CrossRef]
- Luimstra, V.M.; Schuurmans, J.M.; Verschoor, A.M.; Hellingwerf, K.J.; Huisman, J.; Matthijs, H.C. Blue light reduces photosynthetic efficiency of cyanobacteria through an imbalance between photosystems I and II. Photosynth. Res. 2018, 138, 177–189. [Google Scholar] [CrossRef]
- Kang, L.; Wu, Y.; Zhang, J.; An, Q.; Zhou, C.; Li, D.; Pan, C. Nano-selenium enhances the antioxidant capacity, organic acids and cucurbitacin B in melon (Cucumis melo L.) plants. Ecotoxicol. Environ. Saf. 2022, 241, 113777. [Google Scholar] [CrossRef]
- Miao, C.; Zhang, Y.; Cui, J.; Zhang, H.; Wang, H.; Jin, H.; Lu, P.; He, L.; Zhou, Q.; Yu, J. An enhanced interaction of graft and exogenous SA on photosynthesis, phytohormone, and transcriptome analysis in tomato under salinity stress. Int. J. Mol. Sci. 2024, 25, 10799. [Google Scholar] [CrossRef]
- Casal, J.J. Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 2013, 64, 403–427. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Bashir, T.; Ghosh, R.; Lee, S.K.; Bae, H. An overview of LEDs’ effects on the production of bioactive compounds and crop quality. Molecules 2017, 22, 1420. [Google Scholar] [CrossRef] [PubMed]
- Loi, M.; Villani, A.; Paciolla, F.; Mulè, G.; Paciolla, C. Challenges and opportunities of light-emitting diode (LED) as key to modulate antioxidant compounds in plants. A review. Antioxidants 2020, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.L.; Xu, Q.; Li, F.; Feng, Y.; Qin, F.; Fang, W. Applications of xerophytophysiology in plant production-LED blue light as a stimulus improved the tomato crop. Sci. Hortic. 2012, 148, 190–196. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, C.; Gao, L. Polychromatic supplemental lighting from underneath canopy is more effective to enhance tomato plant development by improving leaf photosynthesis and stomatal regulation. Front. Plant Sci. 2016, 7, 1832. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Hodgins, R.; Van Huystee, R. Rapid simultaneous estimation of protoporphyrin and Mg-porphyrins in higher plants. J. Plant Physiol. 1986, 125, 311–323. [Google Scholar] [CrossRef]
- Zhang, C.; Akhlaq, M.; Yan, H.; Ni, Y.; Liang, S.; Zhou, J.; Xue, R.; Li, M.; Adnan, R.M.; Li, J. Chlorophyll fluorescence parameter as a predictor of tomato growth and yield under CO2 enrichment in protective cultivation. Agric. Water Manag. 2023, 284, 108333. [Google Scholar] [CrossRef]
- Georgelis, N.; Fencil, K.; Richael, C.M. Validation of a rapid and sensitive HPLC/MS method for measuring sucrose, fructose and glucose in plant tissues. Food Chem. 2018, 262, 191–198. [Google Scholar] [CrossRef]
- Dobrev, P.I.; Vankova, R. Quantification of abscisic acid, cytokinin, and auxin content in salt-stressed plant tissues. Plant Salt Toler. Methods Protoc. 2012, 913, 251–261. [Google Scholar] [CrossRef]
- Li, J.; Liu, F.; Wu, Y.; Tang, Z.; Zhang, D.; Lyu, J.; Khan, K.S.; Xiao, X.; Yu, J. Evaluation of nutritional composition, biochemical, and quality attributes of different varieties of tomato (Solanum lycopersicum L.). J. Food Compost. Anal. 2024, 132, 106384. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Wang, S.; Liu, W.; Huang, S.; Xie, Y.; Meng, X.; Li, Z.; Jin, N.; Jin, L.; Lyu, J.; et al. Different Spatial Configurations of LED Light Sources Enhance Growth in Tomato Seedlings by Influencing Photosynthesis, CO2 Assimilation, and Endogenous Hormones. Plants 2025, 14, 1369. https://doi.org/10.3390/plants14091369
Yang X, Wang S, Liu W, Huang S, Xie Y, Meng X, Li Z, Jin N, Jin L, Lyu J, et al. Different Spatial Configurations of LED Light Sources Enhance Growth in Tomato Seedlings by Influencing Photosynthesis, CO2 Assimilation, and Endogenous Hormones. Plants. 2025; 14(9):1369. https://doi.org/10.3390/plants14091369
Chicago/Turabian StyleYang, Xiting, Shuya Wang, Wenkai Liu, Shuchao Huang, Yandong Xie, Xin Meng, Zhaozhuang Li, Ning Jin, Li Jin, Jian Lyu, and et al. 2025. "Different Spatial Configurations of LED Light Sources Enhance Growth in Tomato Seedlings by Influencing Photosynthesis, CO2 Assimilation, and Endogenous Hormones" Plants 14, no. 9: 1369. https://doi.org/10.3390/plants14091369
APA StyleYang, X., Wang, S., Liu, W., Huang, S., Xie, Y., Meng, X., Li, Z., Jin, N., Jin, L., Lyu, J., & Yu, J. (2025). Different Spatial Configurations of LED Light Sources Enhance Growth in Tomato Seedlings by Influencing Photosynthesis, CO2 Assimilation, and Endogenous Hormones. Plants, 14(9), 1369. https://doi.org/10.3390/plants14091369






