Morphological, Biochemical, and Cytological Analyses of Deep-Sowing Tolerance in Sorghum Seeds
Abstract
1. Introduction
2. Lines and Methods
2.1. Lines
2.2. Deep-Sowing Emergence Test
2.3. Phytohormone Detection
2.4. Assay of Starch, Phytohormones, Cell Morphogenesis-Related Enzyme Activity
2.5. Soluble Sugar and Glucose Content Analysis
2.6. ATP and Energy Charge Analysis
2.7. Morphology Observation of Sorghum Mesocotyl
2.8. Quantitative RT-PCR of Metabolism-Related Genes
2.9. Statistical Analysis
3. Results
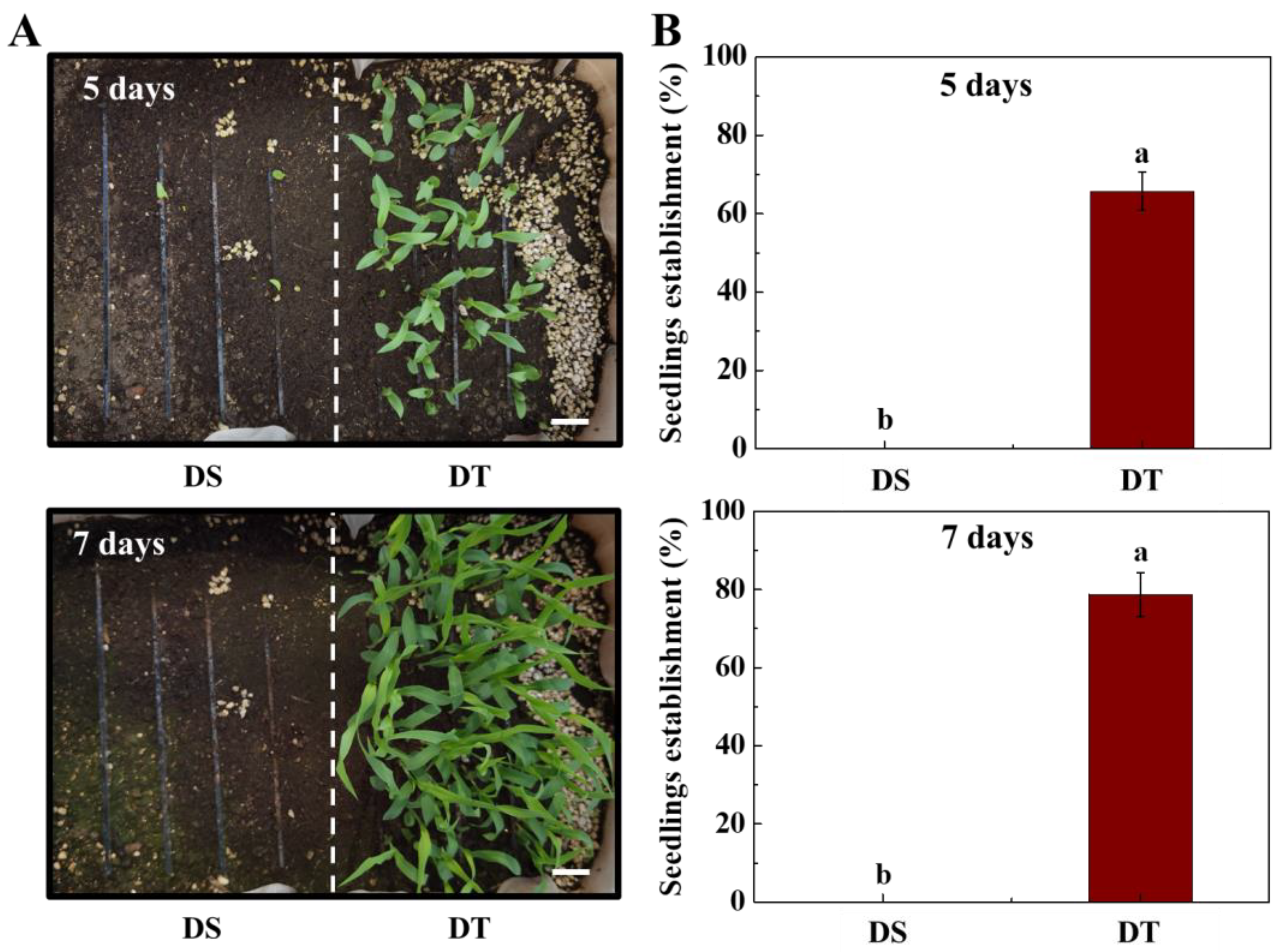
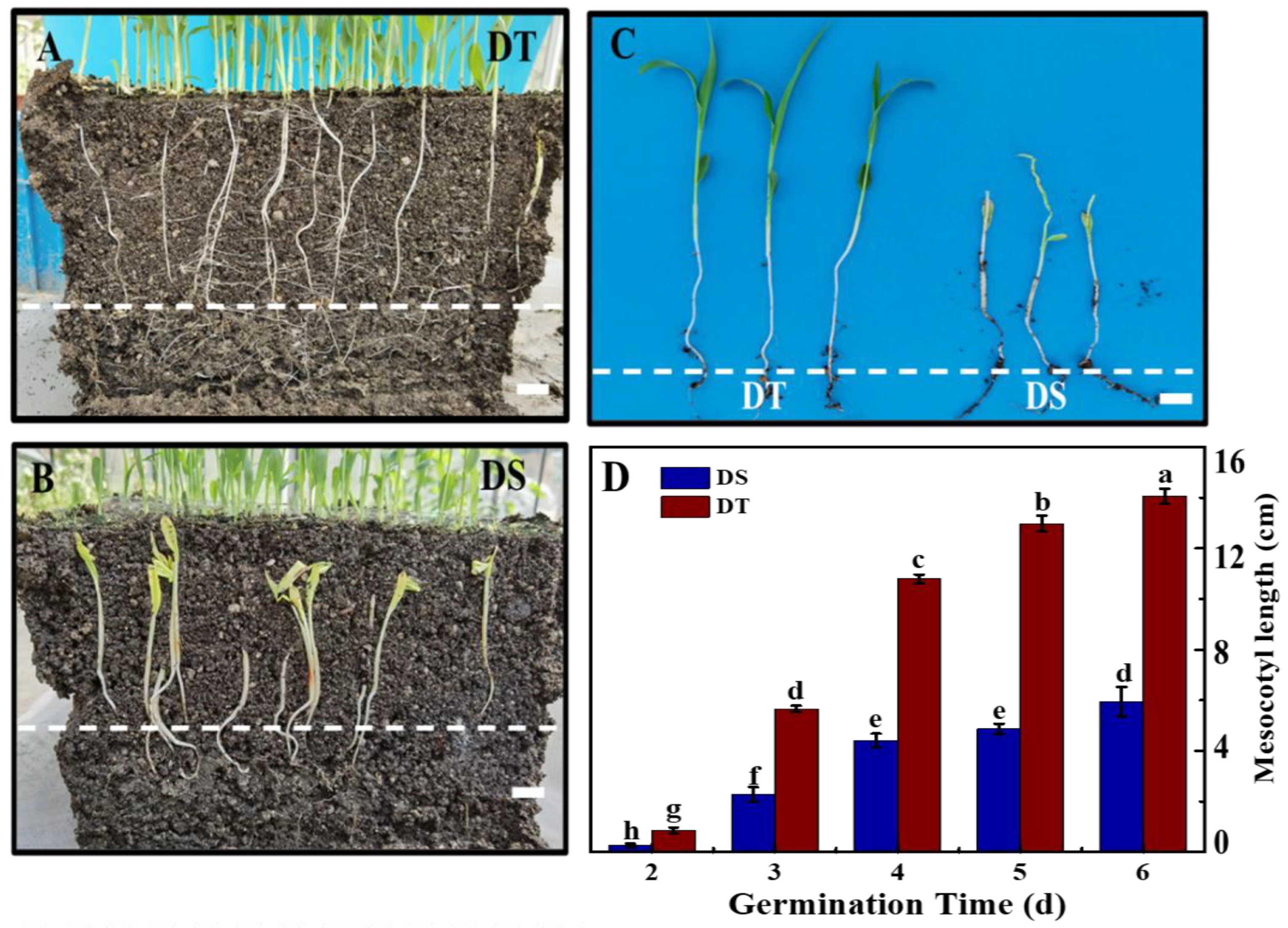
3.1. Dynamic Changes of Seedlings at Deep-Sowing Condition
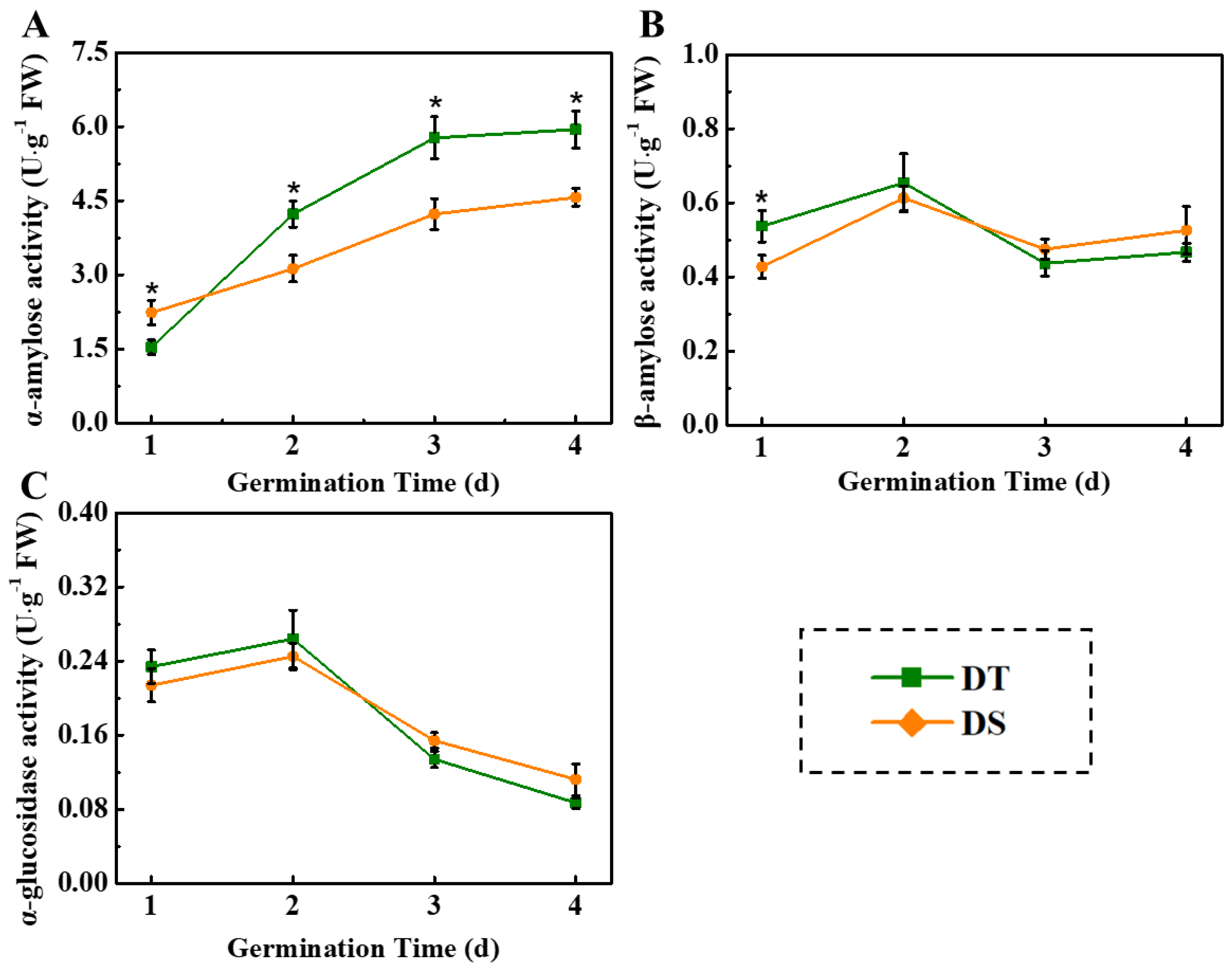
3.2. Dynamic Changes of Starch Degradation in Sorghum Seeds at Deep-Sowing Condition
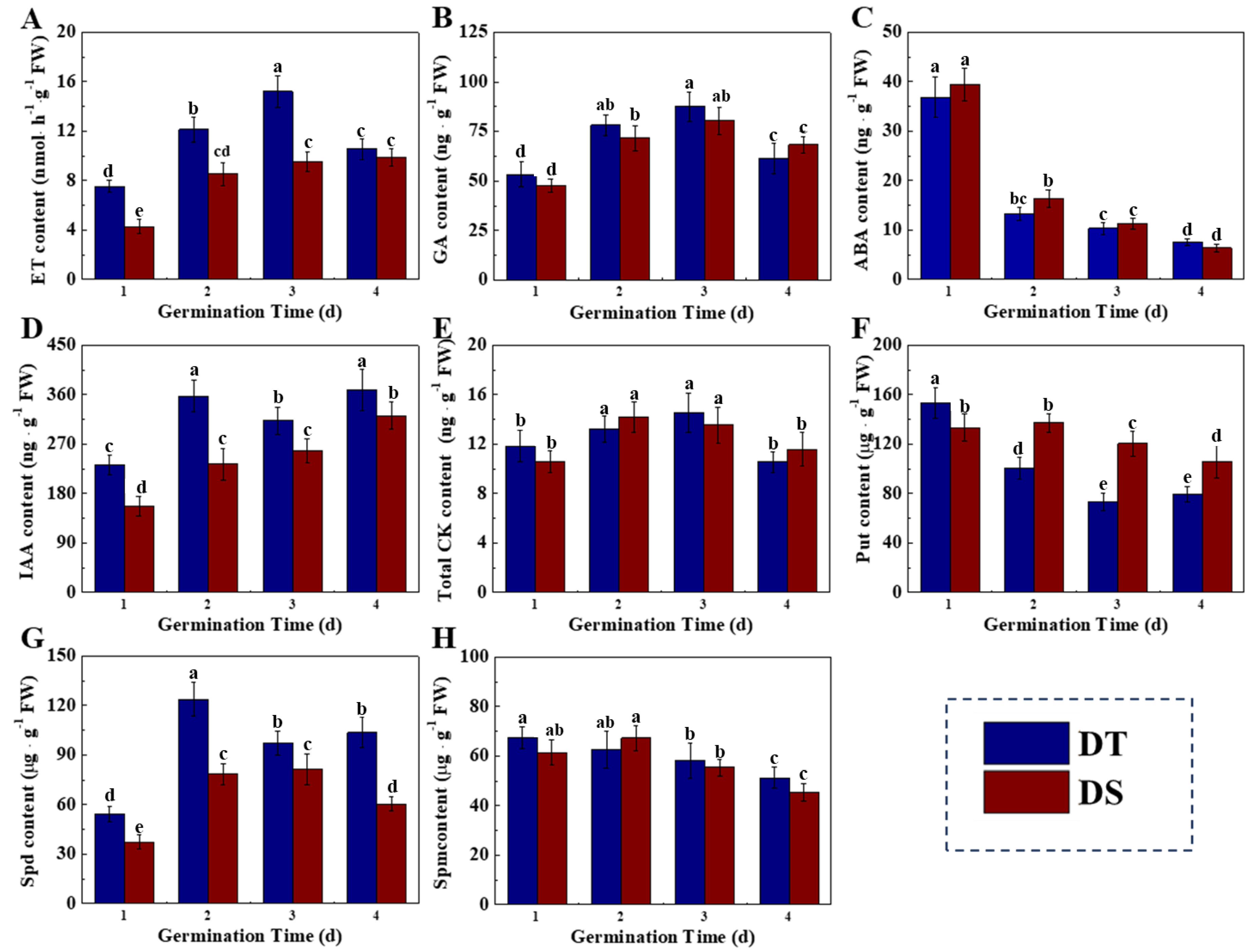
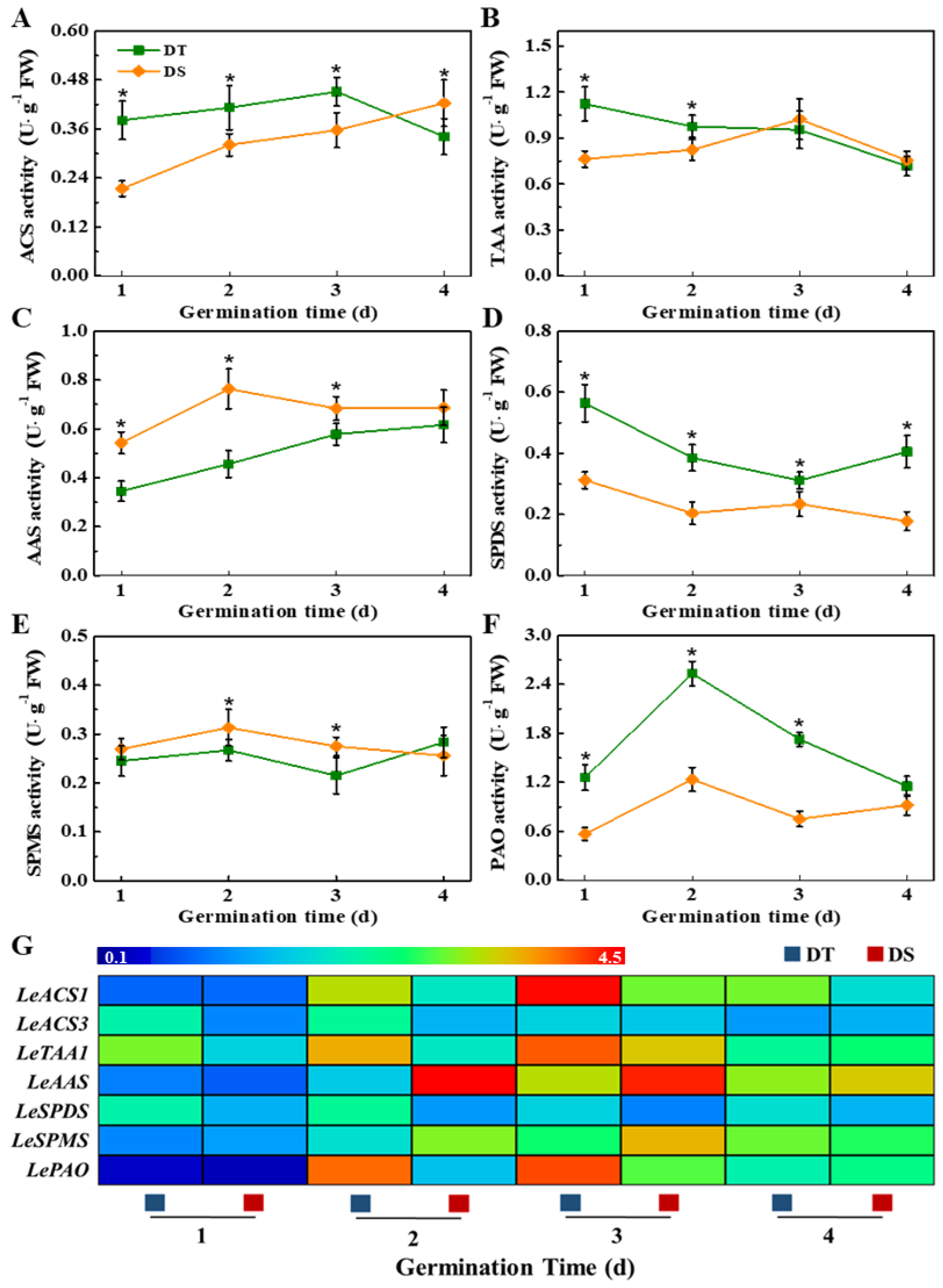
3.3. Dynamic Changes of Phytohormones in Sorghum Mesocotyl Under the Deep Seeding Condition
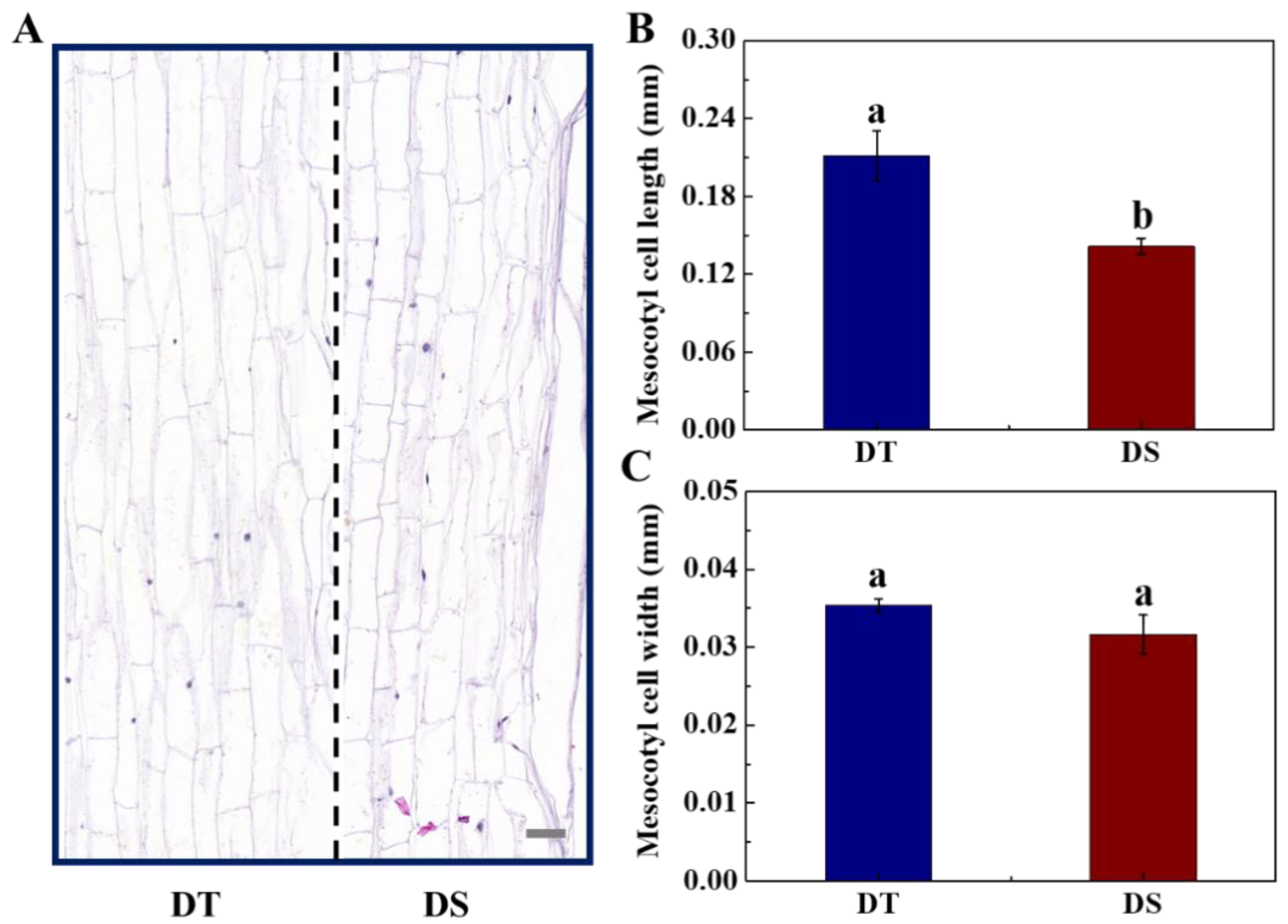
3.4. Cytological Changes of Mesocotyl Under the Deep-Sowing Condition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, R.; Sharma, S.; Bhandari, M.; Bobade, H.; Sohu, R.; Singh, B. Characterization of bioprocessed white and red sorghum flours: Anti-nutritional and bioactive compounds, functional properties, molecular, and morphological features. J. Food Sci. 2023, 88, 3287–3301. [Google Scholar] [CrossRef]
- Rotundo, J.L.; Salinas, A.; Gomara, N.; Borras, L.; Messina, C. Maize outyielding sorghum under drought conditions helps explain land use changes in the US. Field Crops Res. 2024, 308, 109298. [Google Scholar] [CrossRef]
- Chen, B.X.; Li, J.H.; Li, S.J.; Hu, X.L.; Gao, M.; Yao, W.S.; Xu, N.; Gao, S.J. Analysis on mesocotyl elongation of different types of sorghum [Sorghum bicolor(L.) Moench]. Crops 2016, 3, 63–67. [Google Scholar]
- Zou, G.H.; Zhou, L.B.; Zhai, G.W.; Ding, Y.Q.; Lu, P.; Liu, H.Q.; Zhen, X.Q.; Liu, X.H.; Zhang, L.Y.; Xin, Z.G.; et al. A high throughput method for screening deep-seeding tolerance in sorghum. Genet. Resour. Crop Evol. 2019, 66, 1643–1651. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.T.; Yang, R.F.; Wang, F.H.; Fu, J.; Yang, Y.B.; Bai, T.; Wang, S.X.; Yin, H.Q. Effects of gibberellin priming on seedling emergence and transcripts involved in mesocotyl elongation in rice under deep direct-seeding conditions. J. Zhejiang Univ.-Sci. B (Biomed. Biotechnol.) 2021, 22, 1002–1021. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Zhang, L.; Wang, J.C.; Li, C.S.; Zeng, X.; Xie, S.P.; Zhang, Y.Z.; Liu, S.S.; Hu, S.L.; Wang, J.H. Quantitative trait locus analysis for deep-sowing germination ability in the maize IBM Syn10 population. Front. Plant Sci. 2017, 8, 813–825. [Google Scholar] [CrossRef]
- Ceseski, A.R.; Al-Khatib, K. Seeding depth effects on elongation, emergence, and early development of California rice cultivars. Crop Sci. 2021, 61, 2012–2022. [Google Scholar] [CrossRef]
- Kato, F.; Araki, M.; Miyazawa, Y.; Fujii, N.; Takeda, K.; Suge, H.; Takahashi, H. Factors responsible for deep-sowing tolerance in wheat seedlings: Varietal differences in cell proliferation and the co-ordinated synchronization of epidermal cell expansion and cortical cell division for the gibberellin-mediated elongation of first. Ann. Bot. 2011, 108, 439–447. [Google Scholar] [CrossRef]
- Niu, L.J.; Hao, R.Q.; Wu, X.L.; Wang, W. Maize mesocotyl: Role in response to stress and deep-sowing tolerance. Plant Breed. 2019, 19, 466–473. [Google Scholar] [CrossRef]
- Elena, S.; Tatiana, B.; Elena, T.; Galina, S.; Andrej, F.; Sergei, M. Red light-induced inhibition of maize (Zea mays) mesocotyl elongation: Evaluation of apoplastic metabolites. Funct. Plant Biol. 2023, 50, 532–539. [Google Scholar]
- Meng, Y.; Zhan, J.H.; Liu, H.Y.; Liu, J.D.; Wang, Y.M.; Guo, Z.; He, S.; Nie, L.X.; Kohli, A.; Ye, G.Y. Natural variation of OsML1, a mitochondrial transcription termination factor, contributes to mesocotyl length variation in rice. Plant J. 2023, 115, 910–925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Lai, Y.C.; Meng, Y.; Tang, A.; Dong, W.J.; Liu, Y.H.; Liu, K.; Wang, L.Z.; Yang, X.L.; Wang, W.L.; et al. Analyses and identifications of quantitative trait loci and candidate genes controlling mesocotyl elongation in rice. J. Integr. Agric. 2023, 22, 325–340. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, Y.T.; Liu, Y.Y.; Demar, L.; Li, D.D.; Wang, P.X.; Xu, J.L.; Zhen, S.H.; Lu, J.W.; Peng, Y.L.; et al. Dissecting the genetic basis of maize deep-sowing tolerance by combining association mapping and gene expression analysis. J. Integr. Agric. 2022, 21, 1266–1277. [Google Scholar] [CrossRef]
- Xiong, Q.; Ma, B.; Lu, X.; Huang, Y.H.; He, S.J.; Yang, C.; Yin, C.C.; Zhao, H.; Zhou, Y.; Zhang, W.K.; et al. Ethylene-inhibited jasmonic acid biosynthesis promotes mesocotyl/coleoptile elongation of etiolated rice seedlings. Plant Cell 2017, 29, 1053–1072. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.J.; Mei, H.W.; Fan, P.Q.; Li, Y.N.; Xu, X.Y.; Wei, H.B.; Yan, M.; Luo, L.J. Dynamic transcriptome and phytohormone profiling along the time of light exposure in the mesocotyl of rice seedling. Sci. Rep. 2017, 7, 11961. [Google Scholar] [CrossRef]
- Claeys, H.; De Bodt, S.; Inze, D. Gibberellins and DELLAs: Central nodes in growth regulatory networks. Trends Plant Sci. 2014, 19, 231–239. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Zhong, Y. Research progress of physiological response and molecular genetic mechanism under deep-seeding stress in maize seeds. Mol. Plant Breed. 2021, 19, 2381–2390. [Google Scholar]
- Jiang, J.F.; Jin, L.C.; Wang, L.R. Research progress in elongation of mesocotyl in rice. Acta Agric. Jiangxi 2017, 29, 23–26. [Google Scholar]
- Hu, Z.Y.; Yamauchi, T.; Yang, J.H.; Jikumaru, Y.; Tsuchida-Mayama, T.; Ichikawa, H.; Takamure, I.; Nagamura, Y.; Tsutsumi, N.; Yamaguchi, S.; et al. Strigolactone and cytokinin act antagonistically in regulating rice mesocotyl elongation in darkness. Plant Cell Physiol. 2014, 55, 30–41. [Google Scholar] [CrossRef]
- Yue, J.R.; He, Y.J.; Qiu, T.Q.; Guo, N.N.; Han, X.P.; Wang, X.L. Research advances in the molecular mechanisms of plant microtubules in regulating hypocotyl elongation. Chin. Bull. Bot. 2021, 56, 363–371. [Google Scholar]
- Tian, J.; Kong, Z.S. The role of the augmin complex in establishing microtubule arrays. J. Exp. Bot. 2019, 70, 3035–3041. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Wang, C.; Ma, D.R.; Li, L.; Cui, Z.B.; Wang, X.X.; Qian, Q.; Cai, B.D.; Feng, Y.Q.; Chen, W.F. Cortical microtubule disorganized related to an endogenous gibberellin increase plays an important role in rice mesocotyl elongation. Plant Biotechnol. 2016, 33, 59–69. [Google Scholar] [CrossRef]
- Ma, Q.Q.; Wang, X.H.; Sun, J.B.; Mao, T.L. Coordinated regulation of hypocotyl cell elongation by light and ethylene through a microtubule destabilizing protein. Plant Physiol. 2018, 176, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Lampugnani, E.R.; Khan, G.A.; Somssich, M.; Persson, S. Building a plant cell wall at a glance. J. Cell Sci. 2018, 131, jcs207373. [Google Scholar] [CrossRef]
- Zhang, B.C.; Gao, Y.H.; Zhang, L.J.; Zhou, Y.H. The plant cell wall: Biosynthesis, construction, and functions. J. Integr. Plant Biol. 2021, 63, 251–272. [Google Scholar] [CrossRef]
- Li, R.; Wang, Q.; Zhao, G.L.; Peng, H.; Zhang, D.J.; Li, Z.J. Effects of germination time on phenolics, antioxidant capacity, in vitro phenolic bioaccessibility and starch digestibility in sorghum. Int. J. Food Sci. Technol. 2022, 57, 5175–5185. [Google Scholar] [CrossRef]
- Wang, Y.L.; Bai, Y.X.; Ji, H.Y.; Dong, J.J.; Li, X.X.; Liu, J.L.; Jin, Z. Insights into rice starch degradation by maltogenic α-amylase: Effect of starch structure on its rheological properties. Food Hydrocoll. 2022, 124, 107289. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Wang, T. Analysis of the relationship between a-amylase and germinating rate of rice seeds during the process of seed germination. Chin. Bull. Bot 2001, 18, 226–230. [Google Scholar]
- Kim, S.K.; Son, T.K.; Park, S.Y.; Lee, I.J.; Lee, B.H.; Kim, H.Y.; Lee, S.C. Influences of gibberellin and auxin on endogenous plant hormone and starch mobilization during rice seed germination under salt stress. J. Environ. Biol. 2006, 27, 181–186. [Google Scholar]
- Liu, X.H.; Zou, G.H.; Zhai, G.W.; Liu, H.Q.; Zhen, X.Q.; Chen, H.Y. Association analysis between SSR markers and germination-related traits in sorghum germplasm resources. Acta Agric. Zhejiangensis 2021, 33, 965–973. [Google Scholar]
- Huang, Y.T.; Lin, C.; He, F.; Li, Z.; Guan, Y.J.; Hu, Q.J.; Hu, J. Exogenous spermidine improves seed germination of sweet corn via involvement in phytohormone interactions, H2O2 and relevant gene expression. BMC Plant Biol. 2017, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Farrow, S.C.; Emery, R.J.N. Concurrent profiling of indole-3-acetic acid, abscisic acid, and cytokinins and structurally related purines by high-performance-liquid-chromatography tandem electrospray mass spectrometry. Plant Methods 2012, 8, 42–60. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Perry, L.; Kisiala, A.; Olechowski, H.; Emery, R.J.N. Cytokinin activity during early kernel development corresponds positively with yield potential and later stage ABA accumulation in field-grown wheat (Triticum aestivum L.). Planta 2020, 252, 76. [Google Scholar] [CrossRef]
- Zhu, L.W.; Cao, D.D.; Hu, Q.J.; Guan, Y.J.; Hu, W.M.; Nawaz, A.; Hu, J. Physiological changes and sHSPs genes relative transcription in relation to the acquisition of seed germination during maturation of hybrid rice seed. J. Sci. Food Agric. 2015, 96, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, L.H.; Liu, H.B.; Yuan, M. Impaired SWEET-mediated sugar transportation impacts starch metabolism in developing rice seeds. Crop J. 2022, 10, 98–108. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, Y.M.; Luo, Y.B.; Jiang, W.B. A simple and rapid determination of ATP, ADP and AMP concentrations in pericarp tissue of litchi fruit by high performance liquid chromatography. Food Technol. Biotechnol. 2006, 4, 531–534. [Google Scholar]
- Li, B.; Li, D.D.; Zhang, J.; Huang, G.Q.; Li, X.B. Modification of resin semithin section staining method in plant tissues. Plant Physiol. J. 2011, 47, 1207–1212. [Google Scholar] [CrossRef]
- Zhou, W.G.; Chen, F.; Zhao, S.H.; Yang, C.Q.; Meng, Y.J.; Shuai, H.W.; Luo, X.F.; Dai, Y.J.; Yin, H.; Du, J.B.; et al. DA-6 promotes germination and seedling emergence from aged soybean seeds by mediating fatty acid metabolism and glycometabolism. J. Exp. Bot. 2019, 70, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Yuri, S.; Hironobu, S. IRIEK Genotypic variation in coleoptile or mesocotyl lengths of upland rice (Oryza sativa L.) and seedling emergence in deep sowing. Afr. J. Agric. Res. 2012, 7, 6239–6248. [Google Scholar] [CrossRef]
- Lee, H.S.; Sasaki, K.; Kang, J.W.; Sato, T.; Song, W.Y.; Ahn, S.N. Mesocotyl elongation is essential for seedling emergence under deep-seeding condition in rice. Rice 2017, 10, 32. [Google Scholar] [CrossRef]
- Lee, H.S.; Kang, J.W.; Chung, N.J.; Choi, K.S.; Ahn, S.N. Identification of molecular markers for mesocotyl elongation in weedy rice. Korean J. Breed. Sci. 2012, 44, 144–150. [Google Scholar]
- Cao, L.Y.; Yuan, S.J.; Zhou, P.H.; Zhan, X.D.; Wu, W.M.; Gao, J.X.; Cheng, S.H. Effect of different hormones on mesocotyl length in Oryza sativa L. Aata Agron. Sin. 2005, 31, 1098–1100. [Google Scholar]
- Zhong, S.; Shi, H.; Xue, C.; Wei, N.; Guo, H.W.; Deng, X.W. Ethylene-orchestrated circuitry coordinates a seedling’s response to soil cover and etiolated growth. Proc. Natl. Acad. Sci. USA 2014, 111, 3913–3920. [Google Scholar] [CrossRef]
- Liu, J.H.; Wang, W.; Wu, H.; Gong, X.Q.; Moriguchi, T. Polyamines function in stress tolerance: From synthesis to regulation. Front. Plant Sci. 2015, 6, 827. [Google Scholar] [CrossRef]
- Tao, Y.J.; Wang, J.; Miao, J.; Chen, J.; Wu, S.J.; Zhu, J.Y.; Zhang, D.P.; Gu, H.W.; Cui, H.; Shi, S.Y.; et al. The spermine synthase OsSPMS1 regulates seed germination, grain size, and yield. Plant Physiol. 2018, 178, 1522–1536. [Google Scholar] [CrossRef]
- Alcaázar, R.; Fortes, A.M.; Tiburcio, A.F. Editorial: Polyamines in plant biotechnology, food nutrition, and human health. Front. Plant Sci. 2020, 11, 120. [Google Scholar] [CrossRef]
- Huang, Y.T.; Mei, G.F.; Cao, D.D.; Qin, Y.B.; Yang, L.; Ruan, X.L. Spermidine enhances heat tolerance of rice seeds during mid-filling stage and promote subsequent seed germination. Front. Plant Sci. 2023, 14, 1230331. [Google Scholar] [CrossRef]
- Ma, G.J.; Zhang, M.D.; Xu, J.L.; Zhou, W.X.; Cao, L.W. Transcriptomic analysis of short-term heat stress response in Pinellia ternata provided novel insights into the improved thermotolerance by spermidine and melatonin. Ecotoxicol. Environ. Saf. 2020, 202, 110877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.Z.; Li, Y.S.; Li, Y.; Yao, H.M.; Zhao, J.; Wang, C.; Zhao, Y.; Wang, H.N.; Fang, Y.F.; Hu, J. Physiological mechanism regulating light-induced mesocotyl elongation by polyamine oxidase (PAO) in maize. Acta Agron. Sin. 2016, 42, 734–742. [Google Scholar] [CrossRef]
- Gorim, L.; Asch, F. Seed coating reduces respiration losses and affects sugar metabolism during germination and early seedling growth in cereals. Funct. Plant Biol. 2014, 42, 209–218. [Google Scholar] [CrossRef]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef]
- Catusse, J.; Meinhard, J.; Job, C.; Strub, J.M.; Fischer, U.; Pestsova, E.; Westhoff, P.; Dorsselaer, A.V.; Job, D. Proteomics reveals potential biomarkers of seed vigor in sugarbeet. Proteomics 2011, 11, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Mao, B.G.; Peng, Y.; Shao, Y.; Hu, Y.Y.; Wu, T.H.; Zhao, B.R. Research progresses of the mechanism of mesocotyl eongation in rice. Hybrid Rice 2018, 33, 1–6. [Google Scholar]
- Bethke, G.; Thao, A.; Xiong, G.Y.; Li, B.H.; Soltis, N.E.; Hatsugai, N.; Hillmer, R.A.; Katagiri, F.; Kliebenstein, D.J.; Pauly, M.; et al. Pectin biosynthesis is critical for cell wall integrity and immunity in Arabidopsis thaliana. Plant Cell 2016, 28, 537–556. [Google Scholar] [CrossRef]
- Gigli-Bisceglia, N.; Engelsdorf, T.; Hamann, T. Plant cell wall integrity maintenance in model plants and crop species-relevant cell wall components and underlying guiding principles. Cell. Mol. Life Sci. 2020, 77, 2049–2077. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Fernández, R.; Rodríguez-Gacio, M.D.C.; Barrero-Sicilia, C.; Carbonero, P. Three endo-β-mannanase genes expressed in the micropylar endosperm and in the radicle influence germination of Arabidopsis thaliana seeds. Planta 2010, 233, 25–36. [Google Scholar] [CrossRef]
- Chen, X.L.; Jin, C.J.; Wang, P.W.; Chen, K.; Zhang, X.X.; Li, J.F.; Gong, C.; Wang, A.X. Genome-wide analysis and endo-β-mannanase gene family expression profiling of tomato and soybean. Nord. J. Bot. 2018, 36, e01580. [Google Scholar] [CrossRef]
- Song, S.H.; Song, S.Q.; Wu, P.; Meng, S.C.; Xing, B.T. Thermoinhibition of Brassica rapa ssp. chinensis seed germination in relation to degrading enzymes of cell walls. Acta Hortic. Sin. 2014, 41, 1115–1124. [Google Scholar]
- Ren, Y.F.; He, J.Y.; Wang, X.F. Changes in activities of three enzymes degrading galactomannan during and foll owing rice seed germination. Chin. J. Rice Sci. 2007, 21, 275–280. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; You, Z.; Chen, H.; Liu, X.; Mei, G.; Liu, H.; Cao, D.; Zheng, X.; Zou, G. Morphological, Biochemical, and Cytological Analyses of Deep-Sowing Tolerance in Sorghum Seeds. Plants 2025, 14, 1366. https://doi.org/10.3390/plants14091366
Huang Y, You Z, Chen H, Liu X, Mei G, Liu H, Cao D, Zheng X, Zou G. Morphological, Biochemical, and Cytological Analyses of Deep-Sowing Tolerance in Sorghum Seeds. Plants. 2025; 14(9):1366. https://doi.org/10.3390/plants14091366
Chicago/Turabian StyleHuang, Yutao, Zhaotong You, Heyun Chen, Xiuhui Liu, Gaofu Mei, Heqin Liu, Dongdong Cao, Xueqiang Zheng, and Guihua Zou. 2025. "Morphological, Biochemical, and Cytological Analyses of Deep-Sowing Tolerance in Sorghum Seeds" Plants 14, no. 9: 1366. https://doi.org/10.3390/plants14091366
APA StyleHuang, Y., You, Z., Chen, H., Liu, X., Mei, G., Liu, H., Cao, D., Zheng, X., & Zou, G. (2025). Morphological, Biochemical, and Cytological Analyses of Deep-Sowing Tolerance in Sorghum Seeds. Plants, 14(9), 1366. https://doi.org/10.3390/plants14091366



