Self-Inhibition Effects of Litter-Mediated Plant-Phyllosphere Feedback on Seedling Growth in Invasive and Native Congeneric Species
Abstract
1. Introduction
2. Results
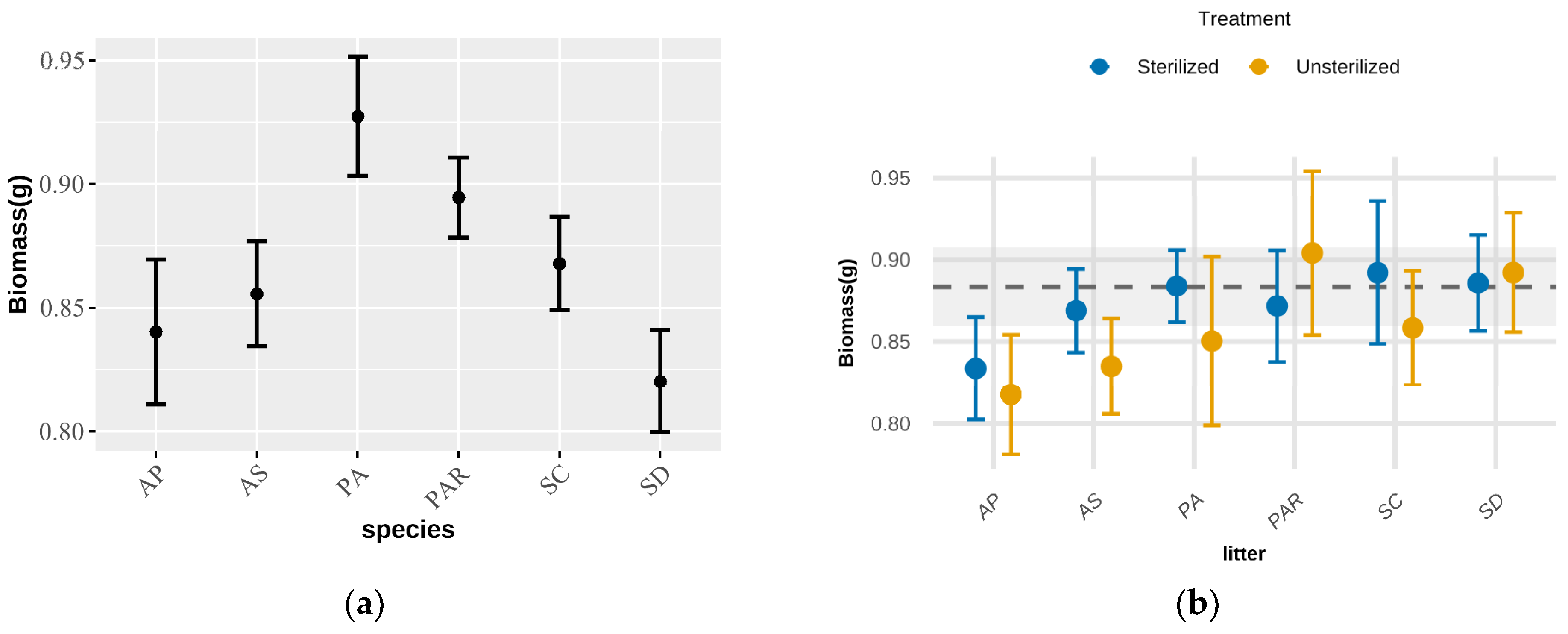
2.1. Phyllosphere Feedback Effects
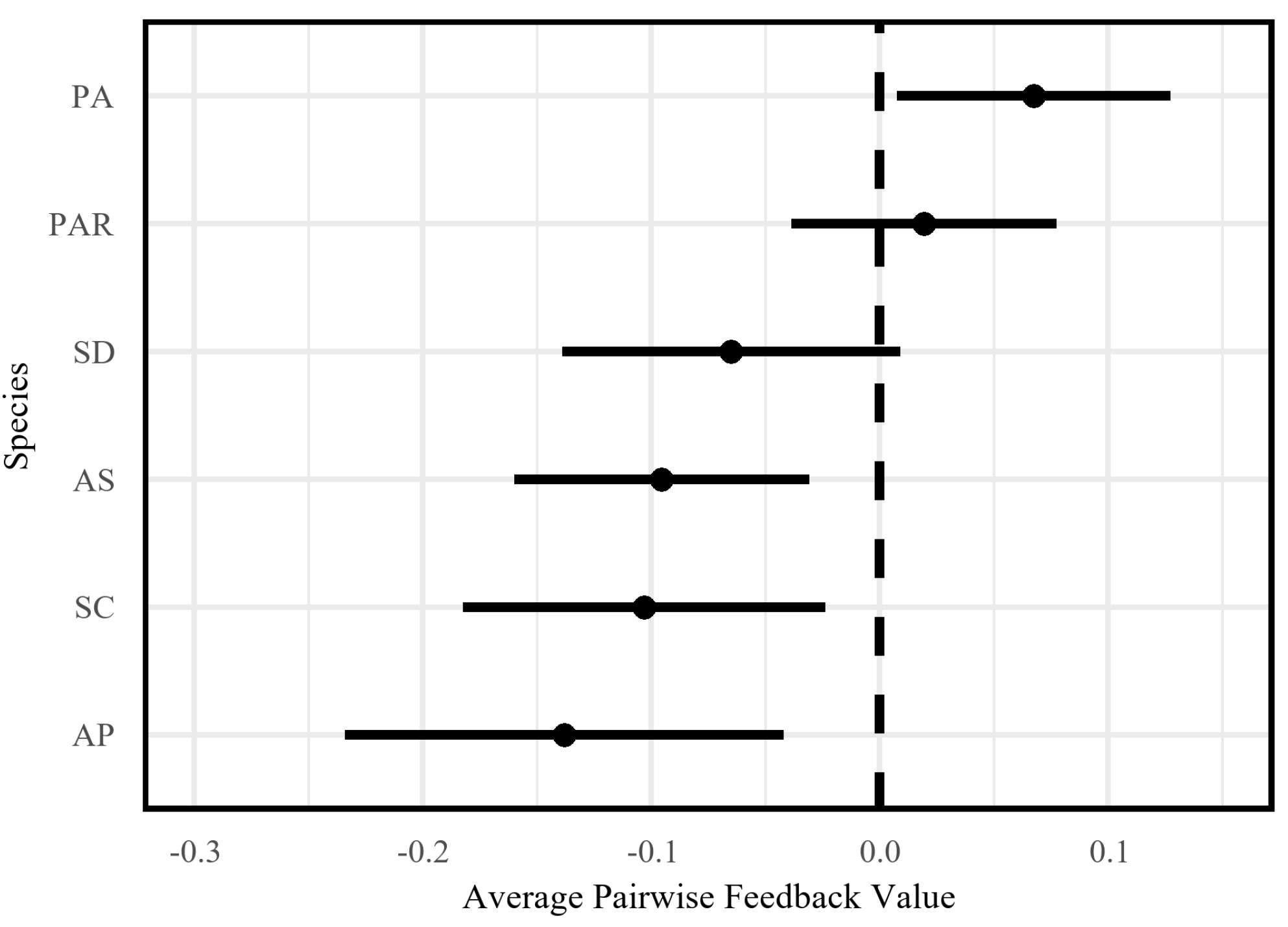
2.2. Species-Specific Feedback Patterns
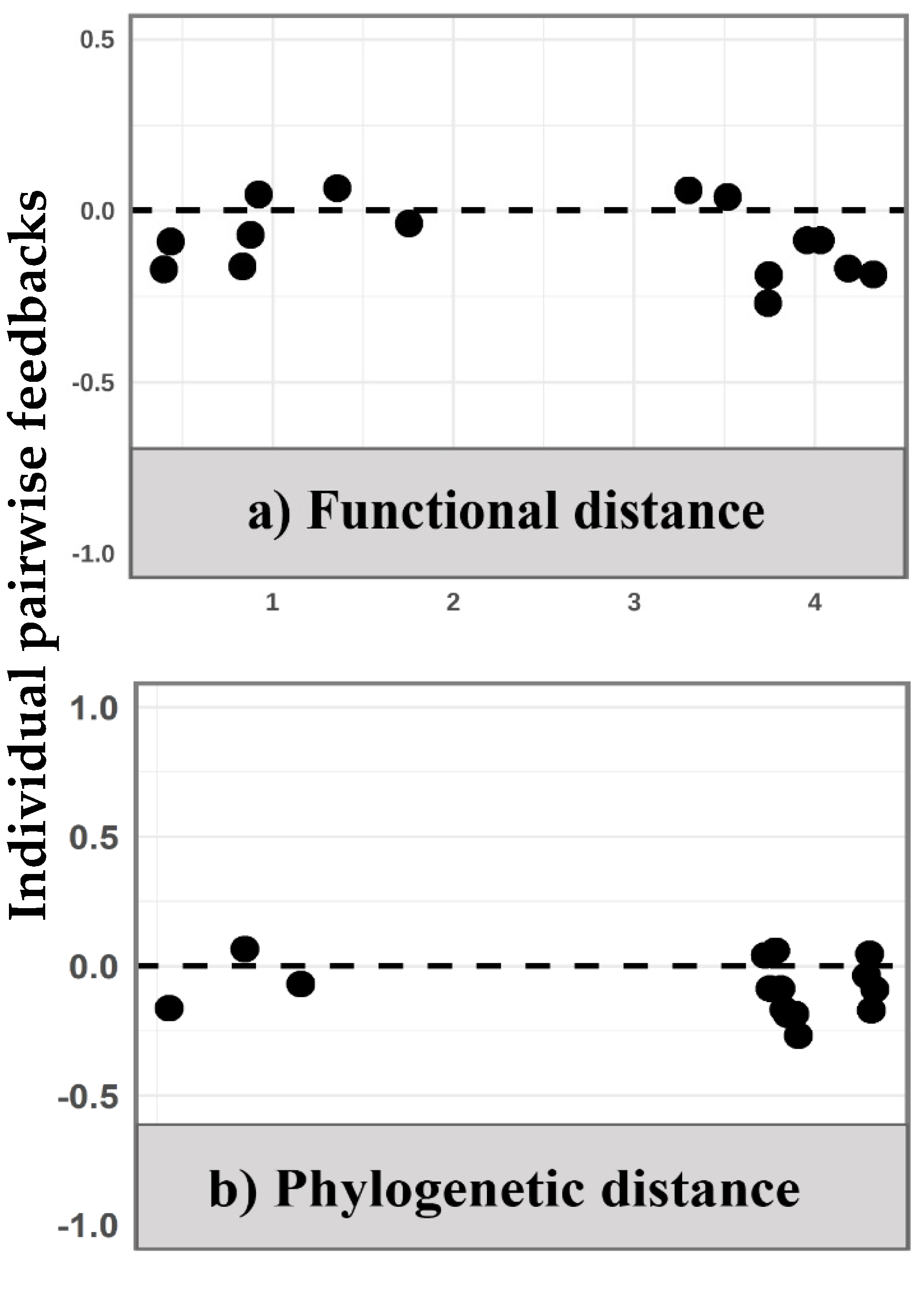
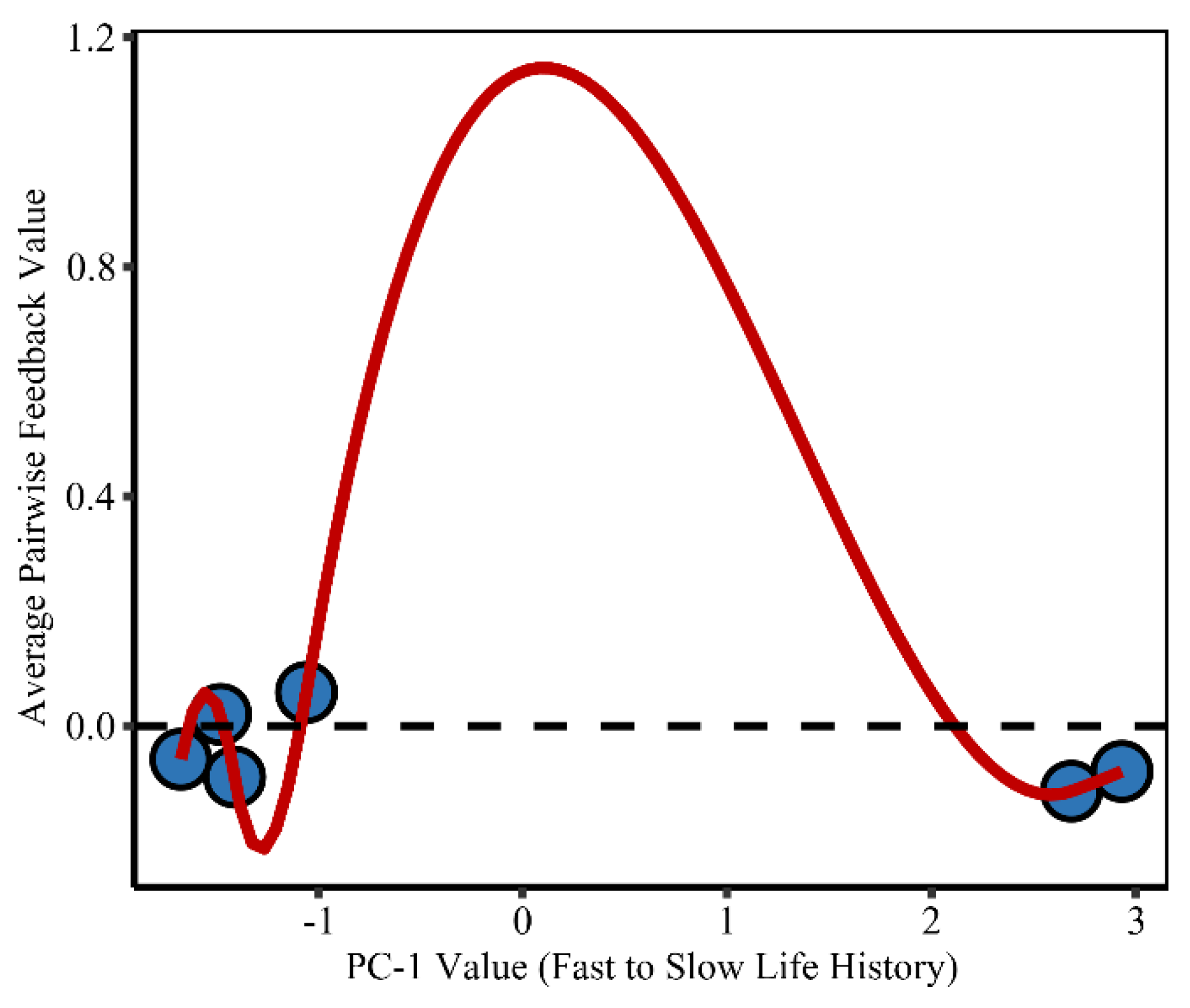
2.3. Functional and Phylogenetic Associations with Feedback Dynamics
3. Discussion
4. Materials and Methods
4.1. Study Species Selection and Phylogenetic Validation
4.2. Soil Preprocessing and Substrate Standardization
4.3. Experimental Design and Litter-Mediated Interaction Manipulation
4.4. Phenotypic Measurement and Functional Trait Quantification
4.5. Statistical Analyses
4.5.1. Multiscale Drivers of Phyllosphere Feedback
4.5.2. Feedback Intensity Metrics
4.5.3. Trait/Phylogeny Relationships
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GLMM | generalized linear mixed model |
| PPF | plant-phyllosphere feedback |
Appendix A

| Latin Name | Abbreviation | Life History | Family | Origin |
|---|---|---|---|---|
| Solidago canadensis | SC | Perennial | Asteraceae | Exotic |
| Alternanthera philoxeroides | AP | Perennial | Amaranthaceae | Exotic |
| Phytolacca americana | PA | Perennial | Phytolaccaceae | Exotic |
| Solidago decurrens | SD | Perennial | Asteraceae | Native |
| Alternanthera sessilis | AS | Perennial | Amaranthaceae | Native |
| Phytolacca acinosa | PAR | Perennial | Phytolaccaceae | Native |

| Species | Accession Number | Author Citation | Base Pair Length |
|---|---|---|---|
| Alternanthera philoxeroides | KY968872.1 | Xu, S.Z., Jin, X.H. and Li, Z.Y. [44] | 715 |
| Alternanthera sessilis | EU497240.1 | Heenan, P.B., et al. [45] | 731 |
| Phytolacca acinosa | MK602343.1 | Grosjean, N. and Blaudez, D | 703 |
| Phytolacca americana | MW843828.1 | Wang, J. | 716 |
| Solidago canadensis | HQ142591.1 | Laureto, P.J. and Barkman, T.J. [46] | 811 |
| Solidago decurrens | MN947289.1 | Lin, Q. | 685 |
References
- Bever, J.D.; Westover, K.M.; Antonovics, J. Incorporating the soil community into plant population dynamics: The utility of the feedback approach. J. Ecol. 1997, 85, 561–573. [Google Scholar] [CrossRef]
- Keane, R.M.; Crawley, M.J. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 2002, 17, 164–170. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Bardgett, R.D.; van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Bever, J.D.; Mangan, S.A.; Alexander, H.M. Main tenance of plant species diversity by pathogens. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 305–325. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Liu, Y.J.; Brunel, C.; van Kleunen, M. Evidence for Elton’s diversity-invasibility hypothesis from belowground. Ecology 2020, 101, e03187. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Brunel, C.; van Kleunen, M. Soil-microorganism-mediated invasional meltdown in plants. Nat. Ecol. Evol. 2020, 4, 1612–1621. [Google Scholar] [CrossRef] [PubMed]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef]
- Grady, K.; Sorensen, J.; Stopnisek, N.; Guittar, J.; Shade, A. Assembly and seasonality of core phyllosphere microbiota on perennial biofuel crops. Nat. Commun. 2019, 10, 4135. [Google Scholar] [CrossRef]
- Xu, N.; Zhao, Q.; Zhang, Z.; Zhang, Q.; Wang, Y.; Qin, G.; Qian, H. Phyllosphere microorganisms: Sources, drivers, and their interactions with plant hosts. J. Agric. Food Chem. 2022, 70, 4860–4870. [Google Scholar] [CrossRef]
- Guerreiro, M.A.; Brachmann, A.; Beferow, D.; Peršoh, D. Transient leaf endophytes are the most active fungi in 1-year-old beech leaf litter. Fungal Divers. 2018, 89, 237–251. [Google Scholar] [CrossRef]
- Voriskova, J.; Baldrian, P. Fungal community on decomposing leaf litter undergoes rapid successional changes. ISME J. 2013, 7, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Szink, I.; Davis, E.L.; Ricks, K.D.; Koide, R.T. New evidence for broad trophic status of leaf endophytic fungi of Quercus gambelii. Fungal Ecol. 2016, 22, 2–9. [Google Scholar] [CrossRef]
- Clay, K.; Schardl, C. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am. Nat. 2002, 160, S99–S127. [Google Scholar] [CrossRef] [PubMed]
- Christian, N.; Herre, E.A.; Mejia, L.C.; Clay, K. Exposure to the leaf litter microbiome of healthy adults protects seedlings from pathogen damage. Proc. R. Soc. B–Biol. Sci. 2017, 284, 20170641. [Google Scholar] [CrossRef] [PubMed]
- Monteil, C.L.; Guilbaud, C.; Glaux, C.; Lafolie, F.; Soubeyrand, S.; Morris, C.E. Emigration of the plant patho gen Pseudomonas syringae from leaf litter contributes to its population dynamics in alpine snowpack. Environ. Microbiol. 2012, 14, 2099–2112. [Google Scholar] [CrossRef]
- Kerdraon, L.; Laval, V.; Suffert, F. Microbiomes and pathogen survival in crop residues, an ecotone between plant and soil. Phytobiomes J. 2019, 3, 246–255. [Google Scholar] [CrossRef]
- Whitaker, B.K.; Bauer, J.T.; Bever, J.D.; Clay, K. Negative plant-phyllosphere feedbacks in native Asteraceae hosts-a novel extension of the plant-soil feedback framework. Ecol. Lett. 2017, 20, 1064–1073. [Google Scholar] [CrossRef]
- Zaret, M.M.; Bauer, J.T.; Clay, K.; Whitaker, B.K. Conspecific leaf litter induces negative feedbacks in Asteraceae seedlings. Ecology 2021, 102, e03557. [Google Scholar] [CrossRef]
- Fang, K.; Chen, L.; Zhou, J.; Yang, Z.-P.; Dong, X.-F.; Zhang, H.-B. Plant-soil-foliage feedbacks on seed germination and seedling growth of the invasive plant Ageratina adenophora. Proc. R. Soc. B 2019, 286, 20191520. [Google Scholar] [CrossRef]
- van der Putten, W.H.; Bardgett, R.D.; Bever, J.D.; Bezemer, T.M.; Casper, B.B.; Fukami, T.; Kardol, P.; Klironomos, J.N.; Kulmatiski, A.; Schweitzer, J.A.; et al. Plant-soil feedbacks: The past, the present and future challenges. J. Ecol. 2013, 101, 265–276. [Google Scholar] [CrossRef]
- Cortois, R.; Schroder–Georgi, T.; Weigelt, A.; van der Putten, W.H.; De Deyn, G.B. Plant–soil feedbacks: Role of plant functional group and plant traits. J. Ecol. 2016, 104, 1608–1617. [Google Scholar] [CrossRef]
- Crawford, K.M.; Bauer, J.T.; Comita, L.S.; Eppinga, M.B.; Johnson, D.J.; Mangan, S.A.; Queenborough, S.A.; Strand, A.E.; Suding, K.N.; Umbanhowar, J.; et al. When and where plant-soil feedback may promote plant coexistence: A meta-analysis. Ecol. Lett. 2019, 22, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Singh, D.; Lai-Hoe, A.; Go, R.; Rahim, R.A.; An, A.; Chun, J.; Adams, J.M. Distinctive phyllosphere bacterial communities in tropical trees. Microb. Ecol. 2012, 63, 674–681. [Google Scholar] [CrossRef]
- Kembel, S.W.; O’Connor, T.K.; Arnold, H.K.; Hubbell, S.P.; Wright, S.J.; Green, J.L. Relationships between phyllosphere bacterial communities and plant functional traits in a neotropical forest. Proc. Natl. Acad. Sci. USA 2014, 111, 13715–13720. [Google Scholar] [CrossRef]
- Parker, I.M.; Saunders, M.; Bontrager, M.; Weitz, A.P.; Hendricks, R.; Magarey, R.; Suiter, K.; Gilbert, G.S. Phylogenetic structure and host abundance drive disease pressure in communities. Nature 2015, 520, 542–544. [Google Scholar] [CrossRef] [PubMed]
- Laforest-Lapointe, I.; Paquette, A.; Messier, C.; Kembel, S.W. Leaf bacterial diversity mediates plant diversity and ecosystem function relationships. Nature 2017, 546, 145–147. [Google Scholar] [CrossRef]
- Janzen, D.H. Herbivores and the number of tree species in tropical forests. Am. Nat. 1970, 104, 501–528. [Google Scholar] [CrossRef]
- Connell, J.H. On the role of natural enemies in prevent ing competitive exclusion in some marine animals and in rain forest trees. Dyn. Popul. 1971, 298, 312. [Google Scholar]
- Liu, Y.Q.; Mao, L.Y.; Yan, R.L.; He, X.Y.; Liao, Y. Study on extraction and insecticidal activity of total saponins from Phytolacca americana leaves. J. Plant Resour. Environ. 2018, 27, 33–38. [Google Scholar]
- Chai, X.; Zhao, H.; Li, X.L. Research progress on chemical constituents, pharmacological effects, toxicity, and heavy metal enrichment characteristics of Phytolacca plants. China J. Chin. Mater. Medica 2024, 49, 4359–4371. [Google Scholar]
- Hickman, D.T.; Rasmussen, A.; Ritz, K.; Birkett, M.A.; Neve, P. Allelochemicals as multi-kingdom plant defence compounds: Towards an integrated approach. Pest Manag. Sci. 2021, 77, 1121–1131. [Google Scholar] [CrossRef]
- Schandry, N.; Becker, C. Allelopathic plants: Models for studying plant-interkingdom interactions. Trends Plant Sci. 2020, 25, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Facelli, J.M.; Williams, R.; Fricker, S.; Ladd, B. Establishment and growth of seedlings of Eucalyptus obliqua: Interactive effects of litter, water, and pathogens. Aust. J. Ecol. 1999, 24, 484–494. [Google Scholar] [CrossRef]
- Purahong, W.; Wubet, T.; Lentendu, G.; Schloter, M.; Pecyna, M.J.; Kapturska, D.; Hofrichter, M.; Krüger, D.; Buscot, F. Life in leaf litter: Novel insights into community dynamics of bacteria and fungi during litter decomposition. Mol. Ecol. 2016, 25, 4059–4074. [Google Scholar] [CrossRef]
- Veen, G.F.; Fry, E.L.; ten Hooven, F.C.; Kardol, P.; Morriën, E.; De Long, J.R. The role of plant litter in driving plant-soil feedbacks. Front. Environ. Sci. 2019, 7, 168. [Google Scholar] [CrossRef]
- Christian, N.; Whitaker, B.K.; Clay, K. Microbiomes: Unifying animal and plant systems through the lens of community ecology theory. Front. Microbiol. 2015, 6, 869. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.H. Study on the Alien Plants in Jiangxi Province, China; Jiangxi Agricultural University: Nanchang, China, 2021. [Google Scholar]
- Xu, G.Y.; Li, H.Y.; Mo, X.Q.; Meng, W.-Q. Composition and spatial-temporal distribution of Chinese naturalized plants. Chin. J. Plant Ecol. 2019, 43, 601–610. [Google Scholar] [CrossRef]
- Yang, B. Alien terrestrial herbs in China: Diversity and ecological insights. Biodivers. Sci. 2010, 18, 660. [Google Scholar]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Poorter, H. Interspecific variation in relative growth rate: On ecological causes and physiological consequences. Causes Conseq. Var. Growth Rate Product. High. Plants 1989, 24, 45–68. [Google Scholar]
- Bauer, J.T.; Mack, K.M.L.; Bever, J.D. Plant-soil feedbacks as drivers of succession: Evidence from remnant and restored tallgrass prairies. Ecosphere 2015, 6, 1–12. [Google Scholar] [CrossRef]
- Mangan, S.A.; Schnitzer, S.A.; Herre, E.A.; Mack, K.M.L.; Valencia, M.C.; Sanchez, E.I.; Bever, J.D. Negative plant–soil feedback predicts tree-species relative abundance in a tropical forest. Nature 2010, 466, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Z.; Li, Z.Y.; Jin, X.H. DNA barcoding of invasive plants in China: A resource for identifying invasive plants. Mol. Ecol. Resour. 2018, 18, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Heenan, P.L.; Peter, J.K. Alternanthera nahui, a new species of Amaranthaceae indigenous to New Zealand. N. Z. J. Bot. 2009, 47, 97–105. [Google Scholar] [CrossRef]
- Laureto, P. Nuclear and Chloroplast DNA Suggest a Complex Single Origin for the Threatened Allopolyploid Solidago houghtonii (Asteraceae) Involving Reticulate Evolution and Introgression. Syst. Bot. 2011, 36, 209–226. [Google Scholar] [CrossRef]
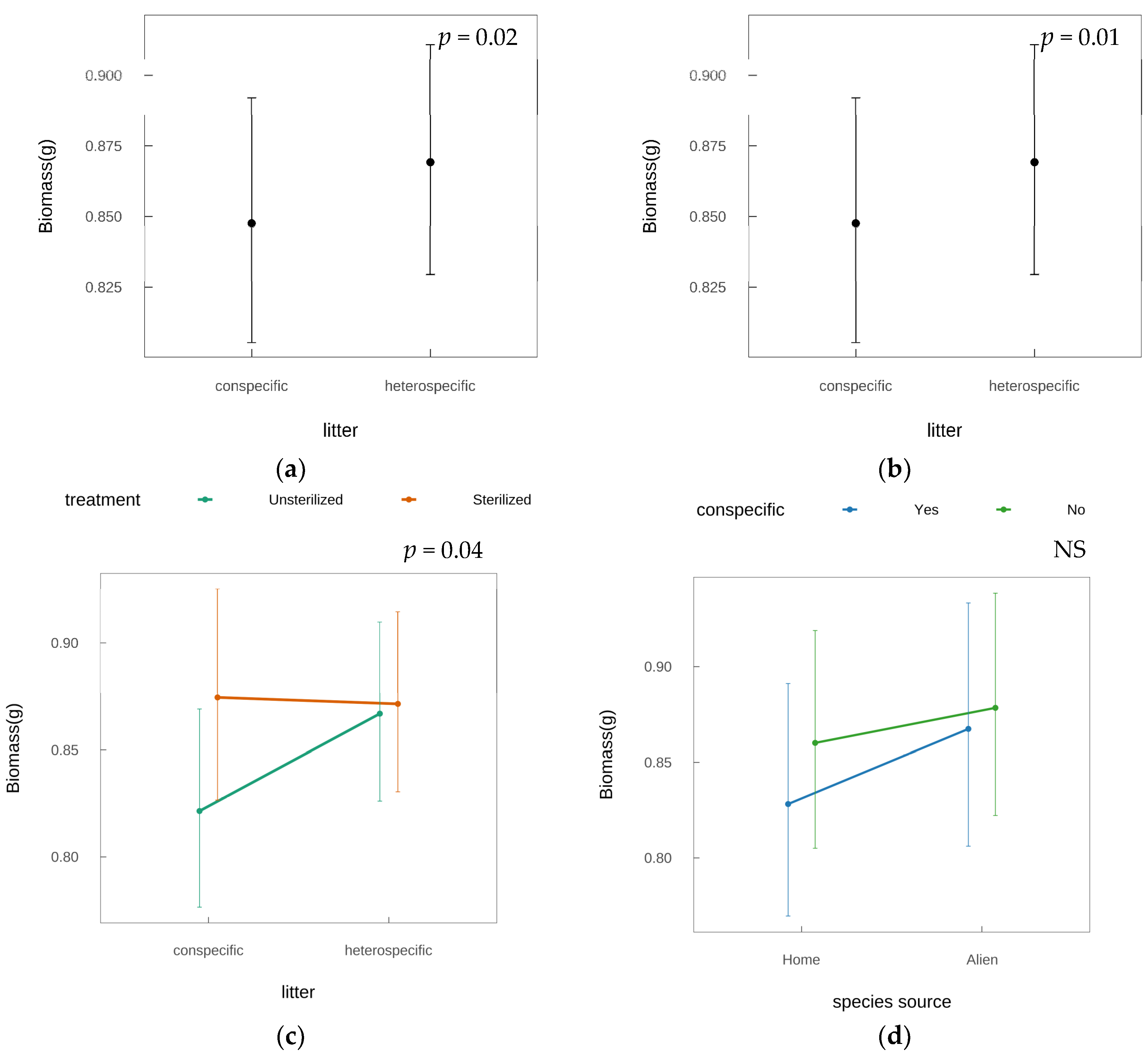
| Chisq | D.F. | F.p-Value | Variance | |
|---|---|---|---|---|
| Fixed effects | ||||
| Intercept | 17.22 | 1 | <0.01 * | - |
| treatment | 6.64 | 1 | 0.01 * | - |
| conspecific | 7.27 | 1 | 0.01 * | - |
| species_source | 0.75 | 1 | 0.39 | - |
| litter_source | 5.60 | 1 | 0.02 * | - |
| treatment×conspecific | 4.45 | 1 | 0.04 * | - |
| conspecific×species source | 0.66 | 1 | 0.42 | |
| Random effects | ||||
| species | - | - | - | <0.01 |
| Residual | - | - | - | 0.01 |


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, K.; Shi, P.; Xu, X.; Wang, J. Self-Inhibition Effects of Litter-Mediated Plant-Phyllosphere Feedback on Seedling Growth in Invasive and Native Congeneric Species. Plants 2025, 14, 1355. https://doi.org/10.3390/plants14091355
Cao K, Shi P, Xu X, Wang J. Self-Inhibition Effects of Litter-Mediated Plant-Phyllosphere Feedback on Seedling Growth in Invasive and Native Congeneric Species. Plants. 2025; 14(9):1355. https://doi.org/10.3390/plants14091355
Chicago/Turabian StyleCao, Kaili, Peili Shi, Xingliang Xu, and Jingsheng Wang. 2025. "Self-Inhibition Effects of Litter-Mediated Plant-Phyllosphere Feedback on Seedling Growth in Invasive and Native Congeneric Species" Plants 14, no. 9: 1355. https://doi.org/10.3390/plants14091355
APA StyleCao, K., Shi, P., Xu, X., & Wang, J. (2025). Self-Inhibition Effects of Litter-Mediated Plant-Phyllosphere Feedback on Seedling Growth in Invasive and Native Congeneric Species. Plants, 14(9), 1355. https://doi.org/10.3390/plants14091355


.png)




