Winter Wheat Vernalization Alleles and Freezing Tolerance at the Seedling and Jointing Stages
Abstract
:1. Introduction
2. Results
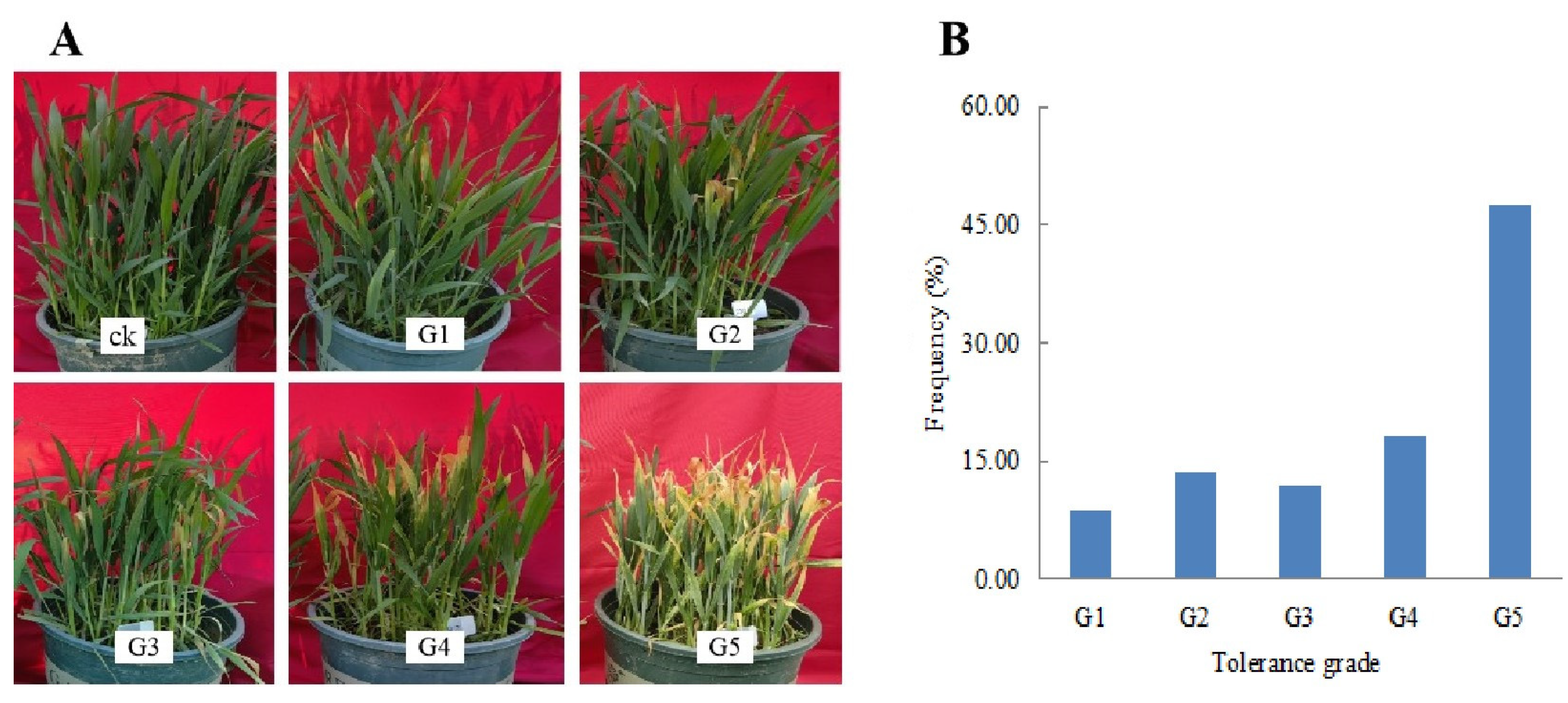
2.1. Analysis of Freezing Tolerance Traits at the Seedling Stage and at the Jointing Stage
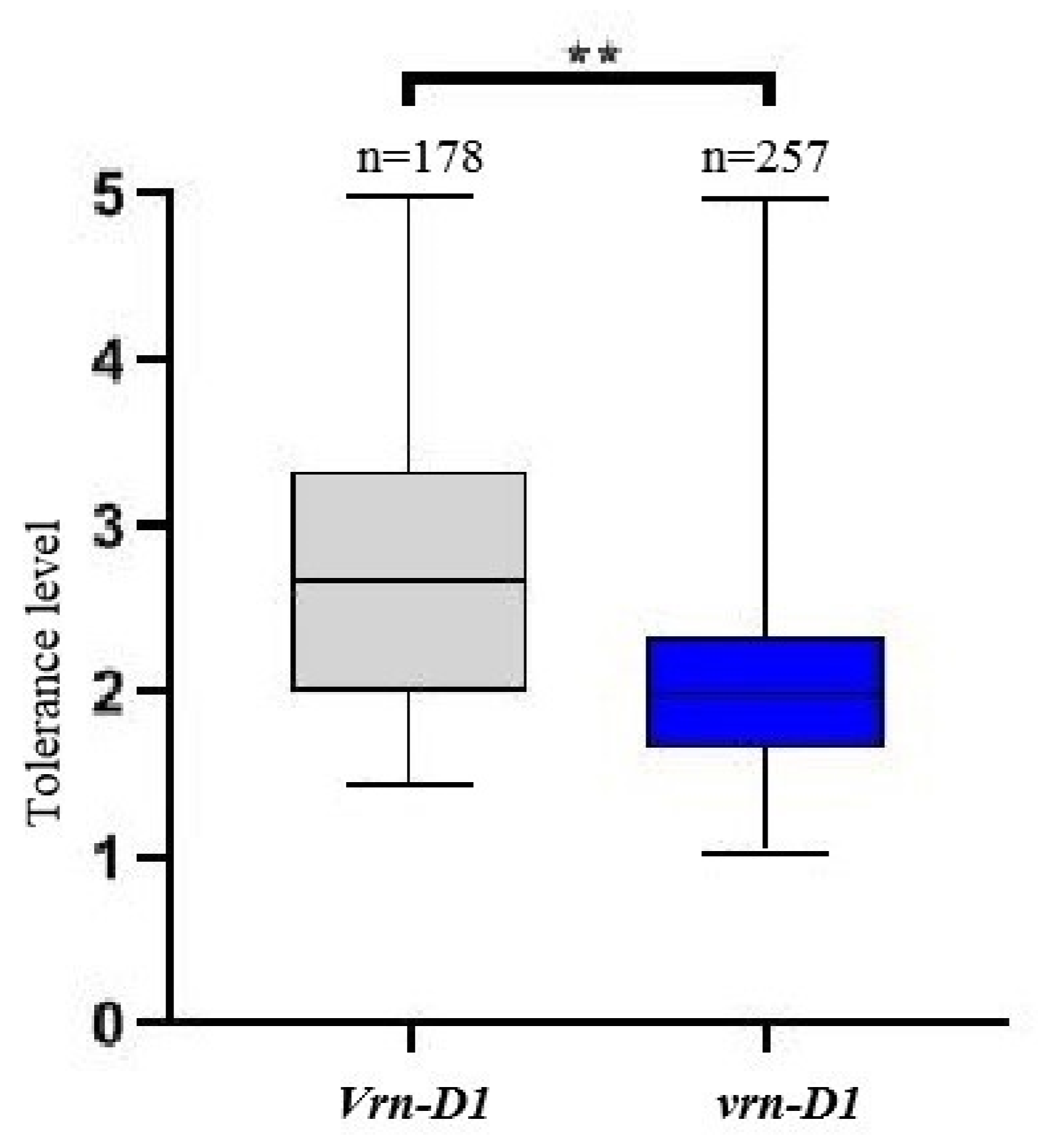
2.2. Association of Seedling Freezing and Vernalization Genotype
2.3. Association of Jointing Freezing and Vernalization Genotype
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Assessing of Freezing Tolerance Traits in Seedlings
4.3. Assessing Freezing Tolerance Traits in Spring
4.4. Molecular Marker Detection
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, Z.H.; Zhuang, Q.S.; Cheng, S.H.; Yu, Z.W.; Zhao, Z.D.; Liu, X. Wheat production and technology improvement in China. J. Agric. 2018, 8, 99–106. [Google Scholar]
- Gang, J.; Hassan, M.A.; Muhammad, N.; Arshad, M.; Chen, X.; Xu, Y.H.; Li, J.C. Comparative Physiology and Transcriptome Analysis of Young Spikes in Response to Late Spring Coldness in Wheat (Triticum aestivum L.). Front. Plant Sci. 2022, 13, 811884. [Google Scholar]
- Wu, Y.F.; Liu, B.H.; Gong, Z.H.; Hu, X.; Ma, J.C.; Ren, D.C.; Liu, H.J.; Ni, Y.J. Predicting yield loss in winter wheat due to frost damage during stem elongation in the central area of the Huang-huai plain in China. Field Crop Res. 2022, 276, 108399. [Google Scholar] [CrossRef]
- Zheng, D.X.; Yang, X.G.; Mínguez, M.I.; Mu, C.Y.; He, Q.; Wu, X. Effect of freezing temperature and duration on winter survival and grain yield of winter wheat. Agric. Forest Meteorol. 2018, 260, 1–8. [Google Scholar] [CrossRef]
- Zheng, B.; Chapman, S.C.; Christopher, J.T.; Frederiks, T.M.; Chenu, K. Frost trends and their estimated impact on yield in the Australian wheat belt. J. Exp. Bot. 2015, 66, 3611–3623. [Google Scholar] [CrossRef]
- Monroy, A.F.; Dryanova, A.; Malette, B.; Oren, D.H.; Ridha, F.M.; Liu, W.C.; Danyluk, J.; Ubayasena, L.W.C.; Kane, K.; Scoles, G.J.; et al. Regulatory gene candidates and gene expression analysis of cold acclimation in winter and spring wheat. Plant Mol. Biol. 2007, 64, 409–423. [Google Scholar] [CrossRef]
- Niu, D.; Gao, Z.; Cui, B.W.; Zhang, Y.X.; He, Y.H. A molecular mechanism for embryonic resetting of winter memory and restoration of winter annual growth habit in wheat. Nat. Plants 2024, 10, 37–52. [Google Scholar] [CrossRef]
- Chepurnov, G.Y.; Ovchinnikova, E.S.; Blinov, A.G.; Chikida, N.N.; Belousova, M.K.; Goncharov, N.P. Analysis of the Structural Organization and Expression of the Vrn-D1 Gene Controlling Growth Habit (Spring vs. Winter) in Aegilops tauschii Coss. Plants 2023, 12, 3596. [Google Scholar] [CrossRef]
- Trevaskis, B. The central role of the VERNALIZA TION1 gene in the vernalization response of cereals. Funct. Plant Biol. 2010, 37, 479–487. [Google Scholar] [CrossRef]
- Yan, L.; Loukoianov, A.; Blechl, A.; Tranquilli, G.; Ramakrishna, W.; Sanmiguel, P.; Bennetzen, J.; Echenique, V.; Dubcovsky, J. The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 2004, 303, 1640–1644. [Google Scholar] [CrossRef]
- Yan, L.; Fu, D.; Li, C.; Blechl, A.; Tranquilli, G.; Bonafede, M.; Sanchez, A.; Valarik, M.; Yasuda, S.; Dubcovsky, J. The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc. Natl. Acad. Sci. USA 2006, 103, 9581–19586. [Google Scholar] [CrossRef] [PubMed]
- Kippes, N.; Debernardi, J.M.; Vasquez-Gross, H.A.; Akpinar, B.A.; Budak, H.; Kato, K.; Chao, S.; Akhunov, E.; Dubcovsky, J. Identiifcation of the VERNALIZATION4 gene reveals the origin of spring growth habit in ancient wheats from South Asia. Proc. Natl. Acad. Sci. USA 2004, 112, 5401–5410. [Google Scholar]
- Yan, L.; Helguera, M.; Kato, K.; Fukuyama, S.; Sherman, J.; Dubcovsky, J. Allelic variation at the VRN-1 promoter region in polyploid wheat. Theor. Appl. Genet. 2004, 109, 1677–1686. [Google Scholar] [CrossRef]
- Sun, Q.M.; Zhou, R.H.; Gao, L.F.; Zhao, G.Y.; Jia, J.Z. The characterization and geographical distribution of the genes responsible for vernalization requirement in Chinese bread wheat. J. Integr. Plant. Biol. 2009, 51, 423–432. [Google Scholar] [CrossRef]
- Dhillon, T.; Pearce, S.P.; Stockinger, E.J. Regulation of freezing tolerance and flowering in temperate cereals: The VRN-1 connection. Plant Physiol. 2010, 153, 1846–1858. [Google Scholar] [CrossRef]
- Koemel, J.E.; Guenzi, A.C.; Anderson, J.A.; Smith, L. Cold hardiness of wheat near-isogenic lines differing in vernalization alleles. Theor. Appl. Genet. 2004, 109, 839–846. [Google Scholar] [CrossRef]
- Reddy, L.; Allan, R.E.; Garland-Campbell, K.A. Evaluation of cold hardiness in two sets of near-isogenic lines of wheat (Triticum aestivum L.) with polymorphic vernalization alleles. Plant Breed. 2006, 125, 448–456. [Google Scholar] [CrossRef]
- Galiba, G.; Vagujfalvi, A.; Li, C.; Soltesz, A.; Dubcovsky, J. Regulatory genes involved in the determination of frost tolerance in temperate cereals. Plant Sci. 2009, 176, 12–19. [Google Scholar] [CrossRef]
- Efremova, T.T.; Chumanova, E.V.; Zhukova, I.M. Winter hardiness analysis of wheat-rye 5R(5A)-substituted lines in Western Siberia. Cereal. Res. Commun. 2021, 50, 25–35. [Google Scholar] [CrossRef]
- Sutka, J.; Galiba, G.; Vagujfalvi, A.; Gill, B.S.; Snape, J.W. Physical mapping of the Vrn-A1 and Fr1 genes on chromosome 5A of wheat using deletion lines. Theor. Appl. Genet. 1999, 99, 199–202. [Google Scholar] [CrossRef]
- Kobayashi, F.; Takumi, S.; Kume, S. Regulation by Vrn-1/Fr-1 chromosomal intervals of CBF-mediated Cor/Lea gene expression and freezing tolerance in common wheat. J. Exp. Bot. 2005, 56, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Winfield, M.O.; Lu, C.; Wilson, I.D. Plant responses to cold: Transcriptome analysis of wheat. Plant Biotechnol. J. 2010, 8, 749–971. [Google Scholar] [CrossRef] [PubMed]
- Laudencia-Chingcuanco, D.; Ganeshan, S.; You, F.; Fowler, B.; Chibbar, R.; Anderson, O. Genome-wide gene expression analysis supports a developmental model of low temperature tolerance gene regulation in wheat (Triticum aestivum L.). BMC Genom. 2011, 12, 299. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Peral, M.M.; Oliver, S.N.; Casao, M.C.; Greenup, A.A.; Trevaskis, B. The promoter of the cereal VERNALIZATION1 gene is sufficient for transcriptional induction by prolonged cold. PLoS ONE 2011, 6, e29456. [Google Scholar] [CrossRef]
- Linmin, A.E.; Fowler, D.B. Low-temperature tolerance and genetic potential in wheat (Triticum aestivum L.): Response to photoperiod vernalization, and plant development. Planta 2006, 224, 360–366. [Google Scholar] [CrossRef]
- Zhu, J.; Pearce, S.; Burke, A.; See, D.R.; Skinner, Z.D.; Dubcovsky, J.; Garland-Campbell, K. Copy number and haplotype variation at the VRN-A1 and central FR-A2 loci are associated with frost tolerance in hexaploid wheat. Theor. Appl. Genet. 2014, 127, 1183–1197. [Google Scholar] [CrossRef]
- Zhang, J.J.; Shahidul, I.M.D.; Zhao, Y.; Anwar, M.; Alhabbar, Z.; She, M.Y.; Ben, B.; Lu, M.Q.; Mayer, J.E.; Ma, W.J. Non-escaping frost tolerant QTL linked genetic loci at reproductive stage in six wheat DH populations. Crop J. 2022, 10, 147–165. [Google Scholar] [CrossRef]
- Fu, D.P.; Szücs, L.; Yan, M.; Helguera, J.S.; Skinner, J.V.; Zitzewitz, P.M.; Hayes, D.J. Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Mol. Genet. Genom. 2005, 273, 54–65. [Google Scholar] [CrossRef]
- Milec, Z.; Tomková, L.; Sumíková, T.; Pánková, K. A new multiplex PCR test for the determination of Vrn-B1 alleles in bread wheat (Triticum aestivum L.). Mol. Breeding 2012, 30, 317–323. [Google Scholar] [CrossRef]
- Yang, F.P.; Guo, Y.; Lv, Y.C.; Dong, Y.C.; Li, Y.; Hua, Q.C.; Hu, M.X.; Liu, J.D. Distribution frequency of vernalization and photoperiod genes in Gansu wheat landraces and winter hardness analysis. J. Plant Genet. Resour. 2023, 24, 1558–1567. [Google Scholar]
- Kirill, O.P.; Alexandra, I.K.; Ekaterina, S.O.; Sergey, A.L.; Nikolay, P.G. Analysis of the Effects of the Vrn-1 and Ppd-1 Alleles on Adaptive and Agronomic Traits in Common Wheat (Triticum aestivum L.). Plants 2024, 13, 1453. [Google Scholar] [CrossRef] [PubMed]
- Palomino, C.; Cabrera, A. Evaluation of the Allelic Variations in Vernalisation (VRN1) and Photoperiod (PPD1) Genes and Genetic Diversity in a Spanish Spelt Wheat Collection. Int. J. Mol. Sci. 2023, 24, 16041. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.F.; Wan, Y.X.; Cao, W.X.; Li, Y.; Zhang, Q.Q.; Li, Y.; Zhang, P.Z. Evaluation method of late spring coldness tolerance in wheat. Acta Agron. Sin. 2023, 49, 438–446. [Google Scholar]
- NY/T 1301-2007; Technical Procedures for Wheat Variety Regional Trials. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2007; pp. 1–17.
- Liu, F.F.; Wan, Y.X.; Cao, W.X.; Zhang, Q.Q.; Li, Y.; Li, Y.; Zhang, P.Z. Advances on identification of wheat freezing tolerance in spring. J. Plant Genet. Resour. 2021, 22, 1193–1199. [Google Scholar]
- Zhong, X.L.; Wang, D.L.; Ji, T.J.; Hu, X.; Zhao, P.; Han, L.S.; Wang, X.G.; Huang, S.H.; Huang, J.Y.; Sun, Z.F. Analysis on the factors affecting frost tolerance for winter wheat. Acta Agron. Sin. 2007, 33, 1810–1814. [Google Scholar]
- Zhang, X.K.; Xiao, Y.G.; Zhang, Y.; Xia, X.C.; Dubcovsky, J.; He, Z.H. Allelic variation at the vernalization genes Vrn-A1, Vrn-B1, Vrn-D1 and Vrn-B3 in Chinese wheat cultivars and their association with growth habit. Crop Sci. 2008, 48, 458–470. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, L.Z.; Hu, Y.G. Distribution of vernalization genes in Chinese wheat landraces and their relationship with winter hardness. Sci. Agric. Sin. 2010, 43, 2619–2632. [Google Scholar]
- Li, Z.; Yang, J.; Li, R.B.; Wang, X.L.; Zhang, X.K.; Zhang, L.L. Composition and distribution of major vernalization genes in wheat cultivars from main production areas in China. J. Northwest Agric. For. Univ. 2018, 46, 35–40. [Google Scholar]
- Zhang, H.J.; Xue, X.H.; Guo, J.; Huang, Y.W.; Dai, X.R.; Li, T.; Hu, J.H.; Qu, Y.F.; Yu, L.Q.; Mai, C.Y.; et al. Association of the recessive allele vrn-D1 with winter frost tolerance in bread wheat. Front. Plant Sci. 2022, 13, 879768–879780. [Google Scholar] [CrossRef]
- You, G.X.; Sun, G.Z.; Zhang, X.Y.; Xiao, S.H. Cold hardiness and its relationship with the VRN1 genotypes in wheat accessions in the Yellow-Huai-Hai river valley region of China. Acta Agron. Sin. 2015, 41, 557–564. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Shou, L.L. A rapid modified CTAB method of extracting genomic DNA from wheat leaf. Chin. Agric. Sci. Bull. 2012, 28, 46–49. [Google Scholar]
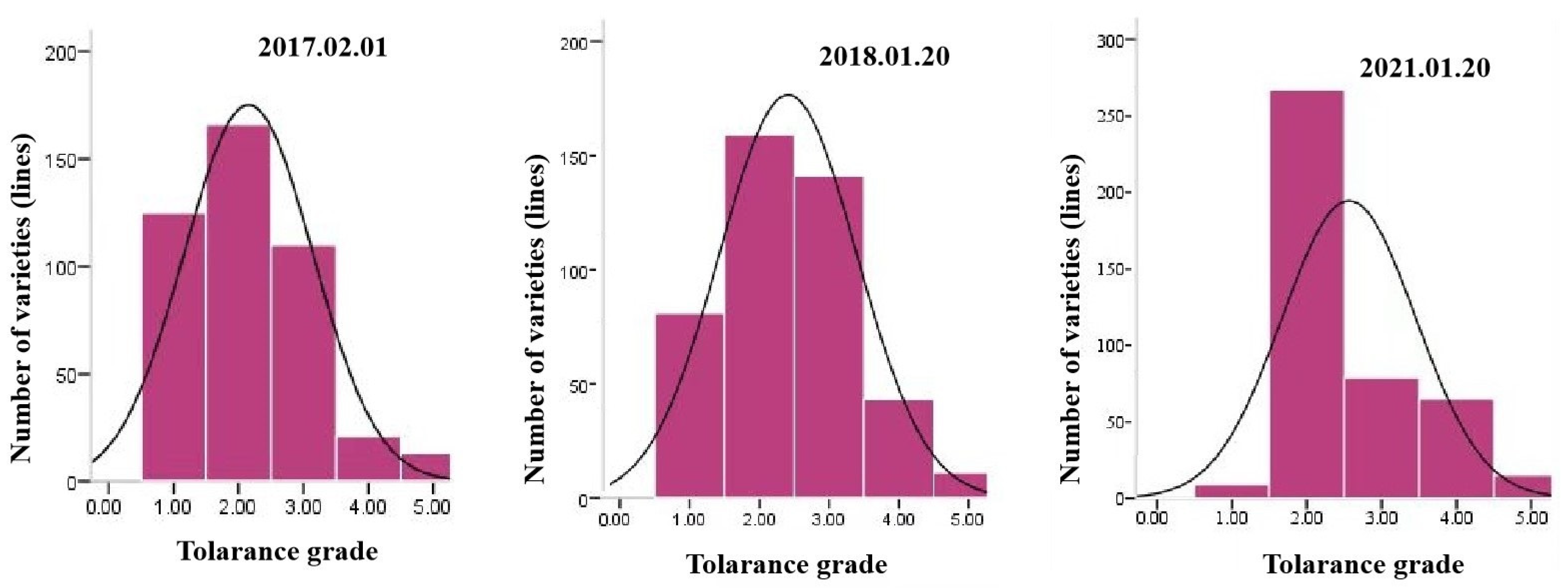


| Years | Minimum Temperature (°C) | Grade Range | Mean | SD | CV (%) |
|---|---|---|---|---|---|
| 2017 | −8 | 1–5 | 2.15 | 0.99 | 46.04 |
| 2018 | −12 | 1–5 | 2.41 | 0.98 | 40.76 |
| 2021 | −12 | 1–5 | 12.56 | 0.89 | 34.78 |
| Freezing Tolerance | Gene Type | ||||
|---|---|---|---|---|---|
| 2017 | 2018 | 2021 | Vrn-D1 | Vrn-B1 | |
| 2017 | 1 | ||||
| 2018 | 0.773 ** | 1 | |||
| 2021 | 0.699 ** | 0.702 ** | 1 | ||
| Vrn-D1 | 0.288 ** | 0.280 ** | 0.503 ** | 1 | — |
| Vrn-B1 | 0.050 | 0.078 | 0.141 ** | — | 1 |
| Accession | Seedling-Freezing Grade (In the Field) | Jointing-Freezing Grade (In the Climate Chamber) | ||
|---|---|---|---|---|
| 2017 | 2018 | 2021 | ||
| Fengdecunmai 5 | 3 | 2 | 2 | 1 |
| Henong 825 | 2 | 2 | 2 | 1 |
| Handan 6172 | 1 | 1 | 1 | 1 |
| Huaimai 22 | 1 | 1 | 2 | 1 |
| Xiaoyan 6 | 2 | 1 | 2 | 1 |
| Annong1124 | 3 | 3 | 4 | 2 |
| Bainon g3217 | 2 | 2 | 2 | 2 |
| Chuanmai 42 | 3 | 4 | 4 | 2 |
| Jimai 22 | 1 | 1 | 2 | 2 |
| Huaimai 30 | 2 | 3 | 3 | 2 |
| Huaimai 29 | 1 | 1 | 2 | 2 |
| Huiyan 22 | 1 | 2 | 3 | 2 |
| Neimai 8 | 5 | 4 | 4 | 2 |
| Yannong 21 | 1 | 1 | 2 | 2 |
| An1243 | 1 | 2 | 2 | 2 |
| Bainong 207 | 3 | 3 | 2 | 2 |
| Huaimai 18 | 1 | 1 | 2 | 3 |
| Huaimai 20 | 1 | 1 | 2 | 3 |
| Luyuan 502 | 2 | 3 | 3 | 3 |
| Yannong 19 | 1 | 1 | 2 | 3 |
| Huaimai 25 | 1 | 1 | 3 | 3 |
| Shijiazhuang 8 | 1 | 1 | 1 | 3 |
| Yangnuomai 1 | 5 | 5 | 5 | 3 |
| Zhengmai 366 | 2 | 2 | 2 | 3 |
| Su 553 | 1 | 2 | 2 | 4 |
| Aikang 58 | 1 | 2 | 2 | 4 |
| Fanmai 5 | 2 | 2 | 3 | 4 |
| Yanzhan 4110 | 2 | 2 | 3 | 4 |
| Jimai 73 | 1 | 1 | 2 | 4 |
| Zhengmai 9023 | 1 | 2 | 2 | 4 |
| Wanmai 38 | 1 | 1 | 2 | 4 |
| Zhoumai 18 | 2 | 3 | 2 | 4 |
| Yannong 999 | 2 | 2 | 2 | 4 |
| Jinan 17 | 1 | 1 | 2 | 4 |
| Zhongmai 895 | 3 | 2 | 2 | 4 |
| Luanxuan 988 | 3 | 2 | 2 | 5 |
| Xinong 889 | 2 | 2 | 2 | 5 |
| Qianmai 18 | 3 | 3 | 2 | 5 |
| Guomai 8 | 1 | 1 | 2 | 5 |
| Huiyan 77 | 1 | 2 | 3 | 5 |
| Xinmai 18 | 2 | 3 | 2 | 5 |
| Jimai 20 | 2 | 2 | 2 | 5 |
| Kaimai 18 | 3 | 3 | 2 | 5 |
| Yangmai 20 | 3 | 4 | 3 | 5 |
| Liangxing 99 | 1 | 1 | 2 | 5 |
| Mianmai 39 | 3 | 2 | 3 | 5 |
| Guoshengmai 1 | 3 | 4 | 4 | 5 |
| Yangmai 158 | 4 | 3 | 4 | 5 |
| Wanmai 52 | 1 | 2 | 2 | 5 |
| Ligao 6 | 1 | 2 | 2 | 5 |
| Guinong 775 | 2 | 2 | 1 | 5 |
| Neimai 836 | 5 | 4 | 4 | 5 |
| Gene Type | Freezing Grade (Mean ± SD) | Number of Accessions | Frequency (%) |
|---|---|---|---|
| vrn-A1 + vrn-B1 + vrn-D1 + vrn-B3 | 2.05 ± 0.69 a 1 | 242 | 55.63 |
| vrn-A1 + vrn-B1 + Vrn-D1 + vrn-B3 | 2.78 ± 0.90 b | 175 | 40.23 |
| vrn-A1 + Vrn-B1 + vrn-D1 + vrn-B3 | 2.87 ± 0.77 b | 15 | 3.45 |
| vrn-A1 + Vrn-B1 + Vrn-D1 + vrn-B3 | 3.00 ± 0.33 b | 3 | 0.69 |
| Tolerance Grade | Dead Stem Rate | Tolerance Type |
|---|---|---|
| 1 | 0.00~0.13 | Extremely strong |
| 2 | 0.14~0.28 | Strong |
| 3 | 0.29~0.42 | Moderate |
| 4 | 0.43~0.65 | Weak |
| 5 | 0.66~1.00 | Extremely weak |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Cao, W.; Zhang, Q.; Li, Y.; Zhou, H.; Wan, Y. Winter Wheat Vernalization Alleles and Freezing Tolerance at the Seedling and Jointing Stages. Plants 2025, 14, 1350. https://doi.org/10.3390/plants14091350
Liu F, Cao W, Zhang Q, Li Y, Zhou H, Wan Y. Winter Wheat Vernalization Alleles and Freezing Tolerance at the Seedling and Jointing Stages. Plants. 2025; 14(9):1350. https://doi.org/10.3390/plants14091350
Chicago/Turabian StyleLiu, Fangfang, Wenxin Cao, Qiqi Zhang, Yao Li, Heng Zhou, and Yingxiu Wan. 2025. "Winter Wheat Vernalization Alleles and Freezing Tolerance at the Seedling and Jointing Stages" Plants 14, no. 9: 1350. https://doi.org/10.3390/plants14091350
APA StyleLiu, F., Cao, W., Zhang, Q., Li, Y., Zhou, H., & Wan, Y. (2025). Winter Wheat Vernalization Alleles and Freezing Tolerance at the Seedling and Jointing Stages. Plants, 14(9), 1350. https://doi.org/10.3390/plants14091350





