Effect of Light Intensity and Light Spectrum of LED Light Sources on Photosynthesis and Secondary Metabolite Synthesis in Ocimum basilicum
Abstract
1. Introduction
2. Results
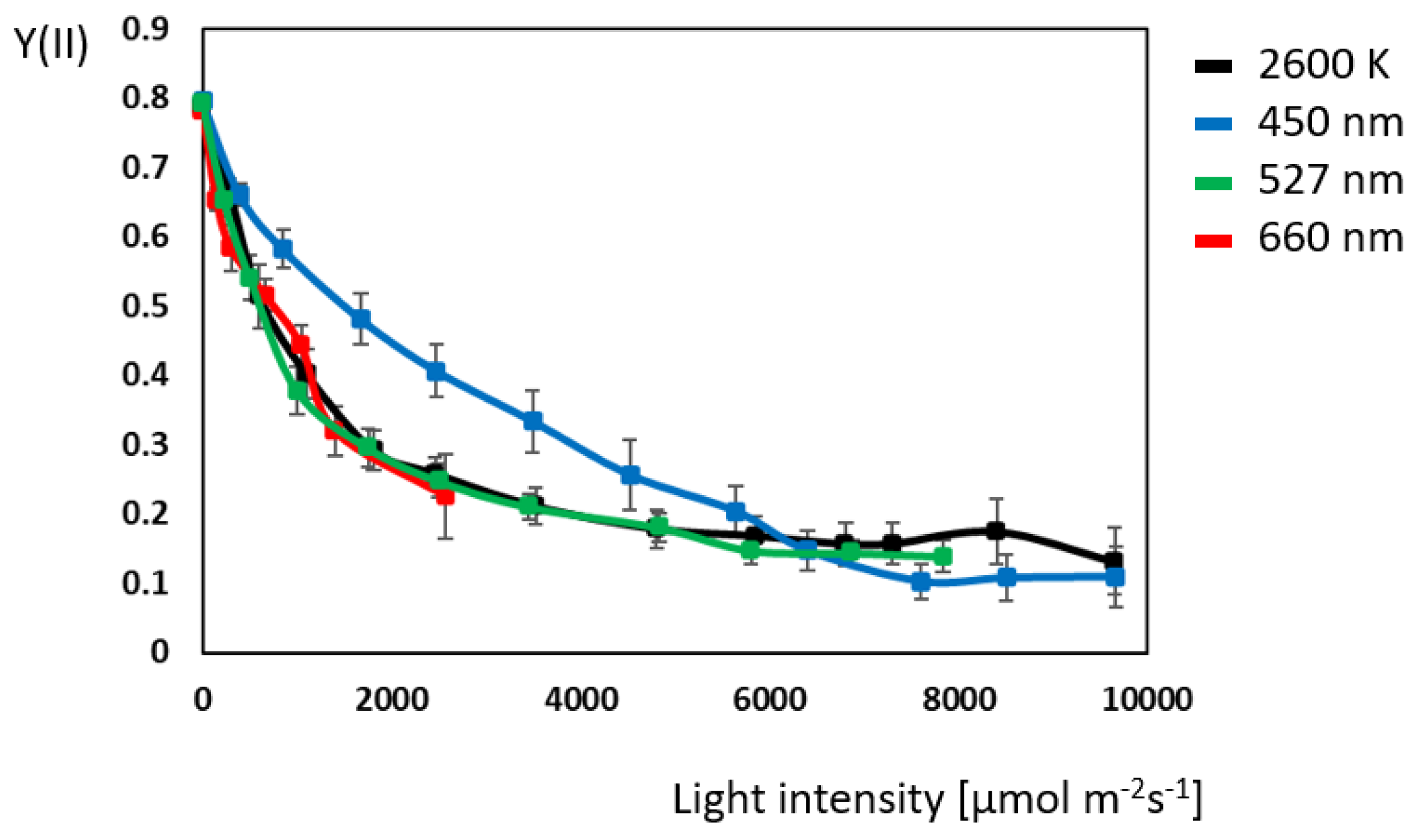
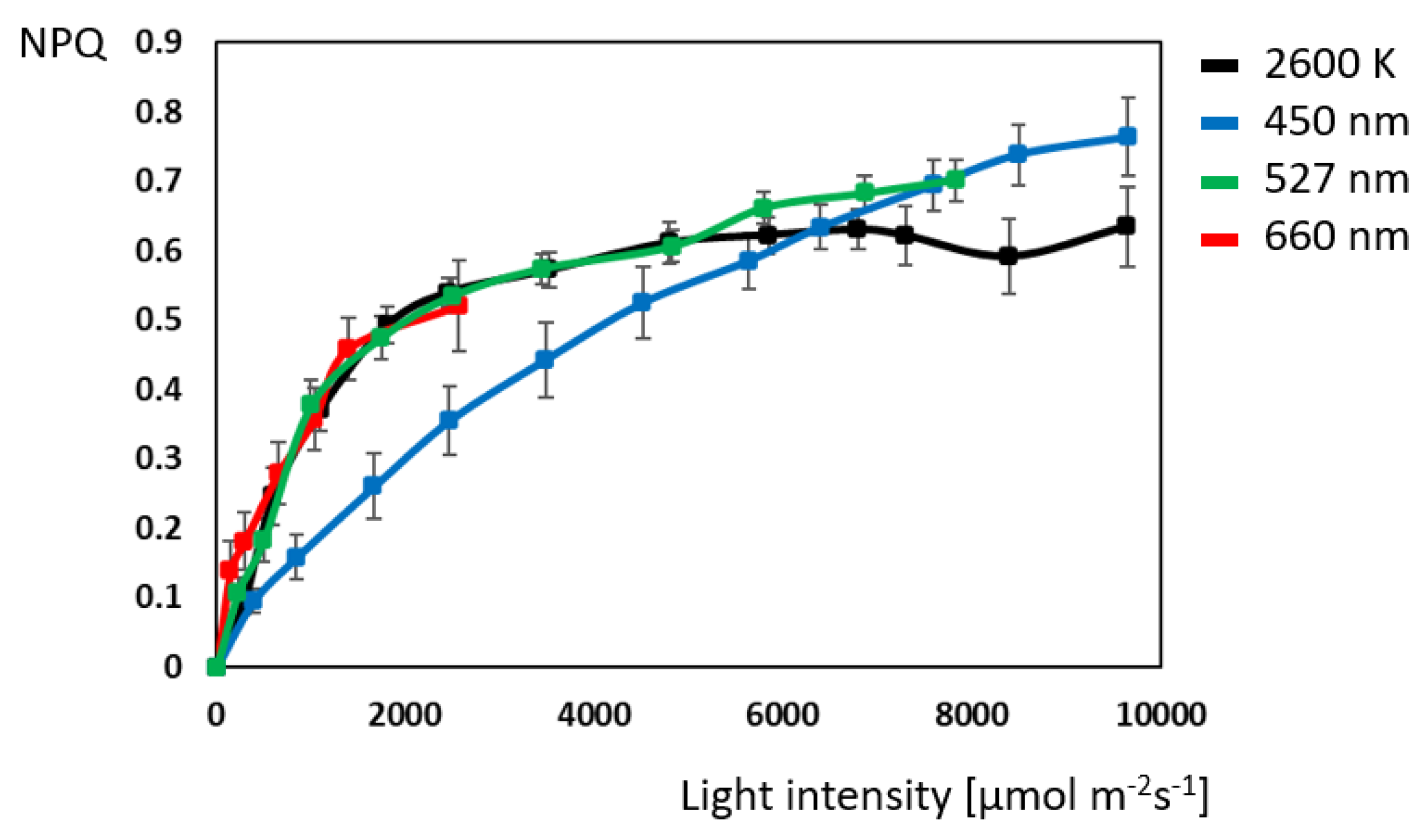
2.1. Chlorophyll Fluorescence: Maximum Electron-Transport-Chain Efficiency with Blue Light
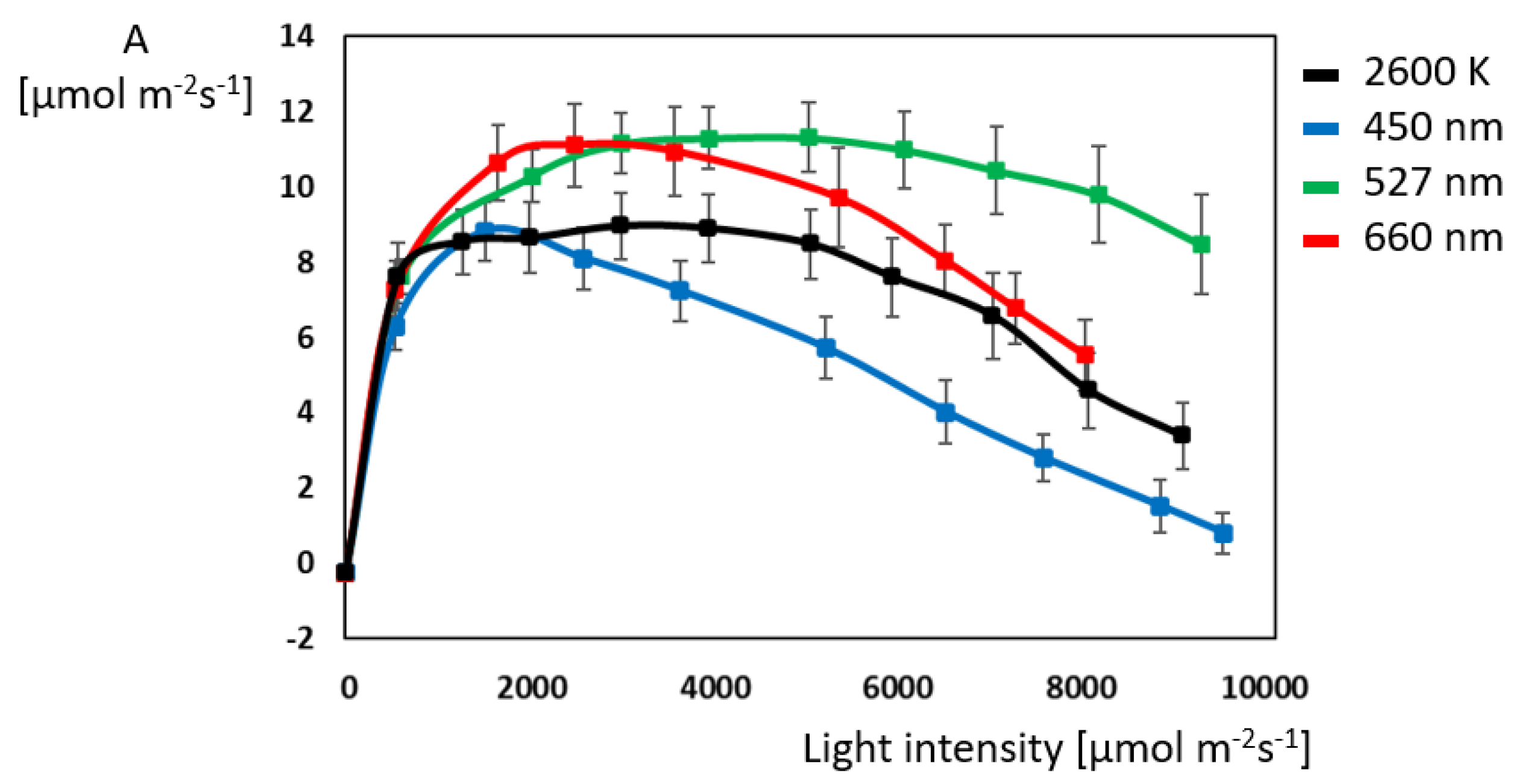
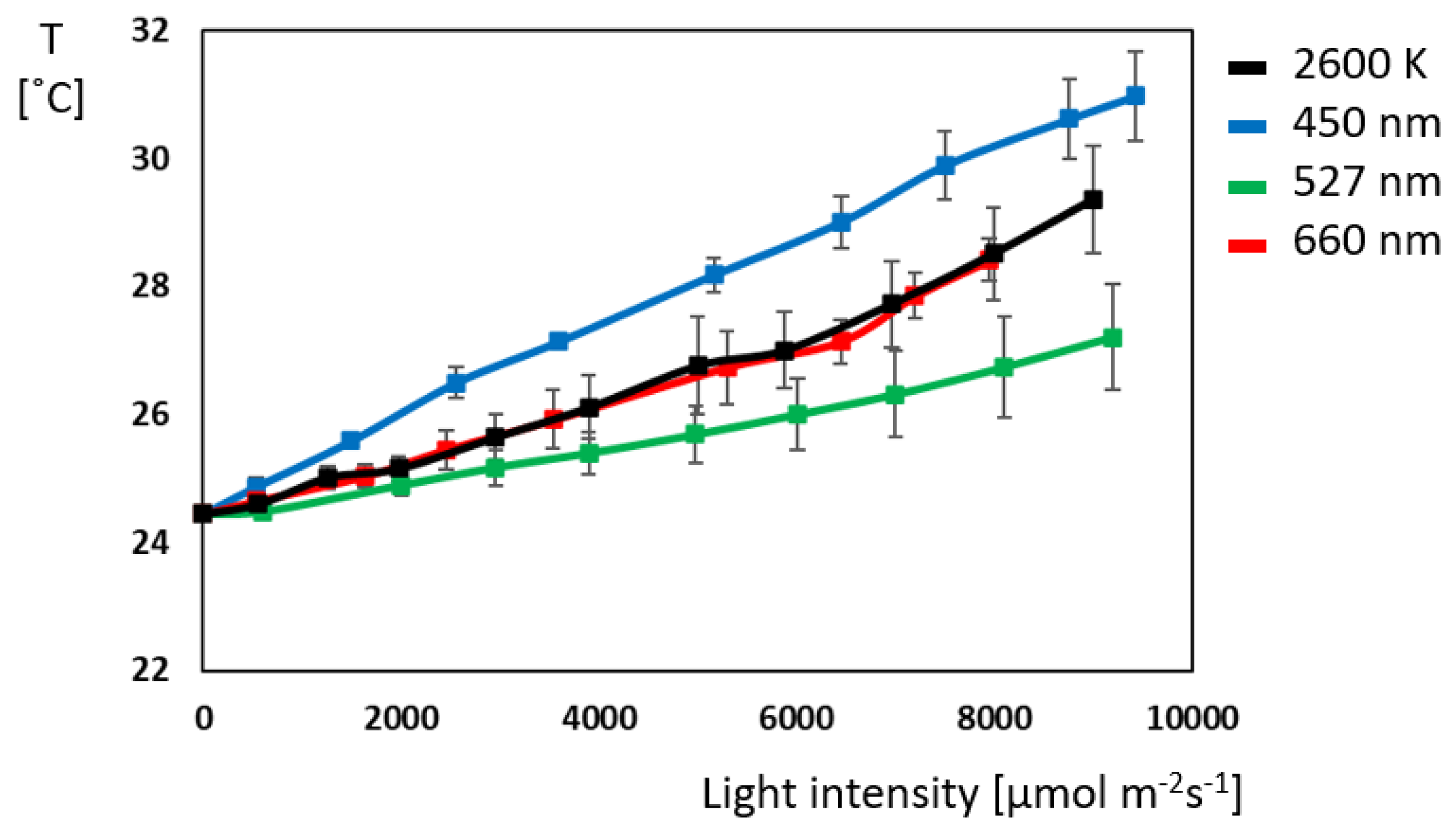
2.2. Gas Exchange: Green and Red Light Outperform Blue Light Despite Its Energy Abundance
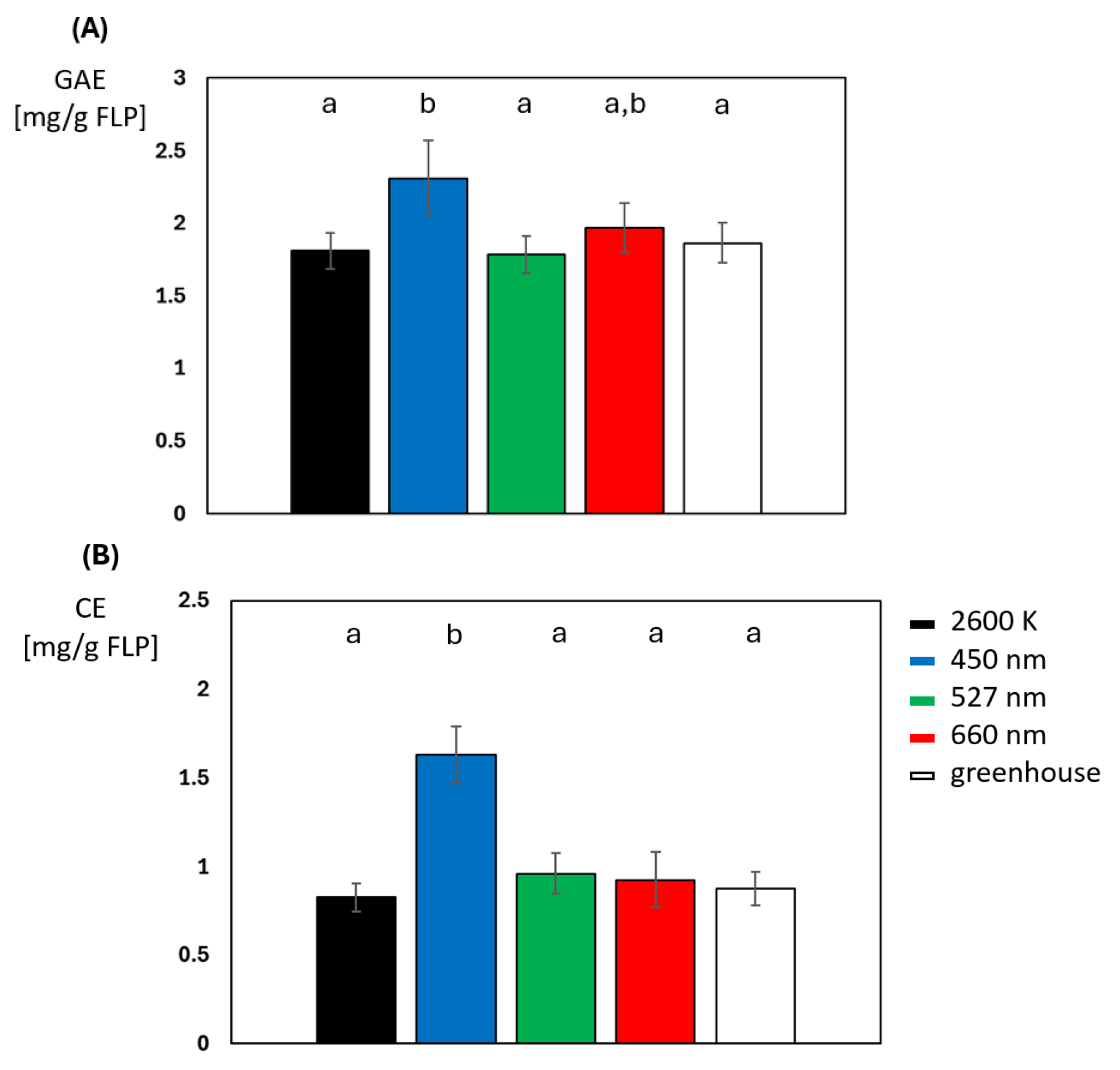
2.3. Secondary Metabolites: How Intensity and Exposure Time of Blue Light Enhances Its Production
2.4. Blue Light Does Not Induce a Uniform Increase in Phenol- or Flavonoid Concentration Throughout the Plant
3. Discussion
3.1. Light Saturation Curves Under Different Light Colors
3.2. Green Light Drives Photosynthesis More Effectively Than Other Light-Colors
3.3. Blue Light Triggers Photo Inhibitory Processes First
3.4. Blue Light Triggers Secondary Metabolism
4. Conclusions
5. Materials and Methods
5.1. Plant Cultivation
5.2. Chlorophyll Fluorescence Analysis
5.3. Gas Exchange Analysis
5.4. Photometric Analysis of Secondary Metabolites
5.4.1. Extraction
5.4.2. Total Phenolic Content (TPC)
5.4.3. Total Flavonoid Content (TFC)
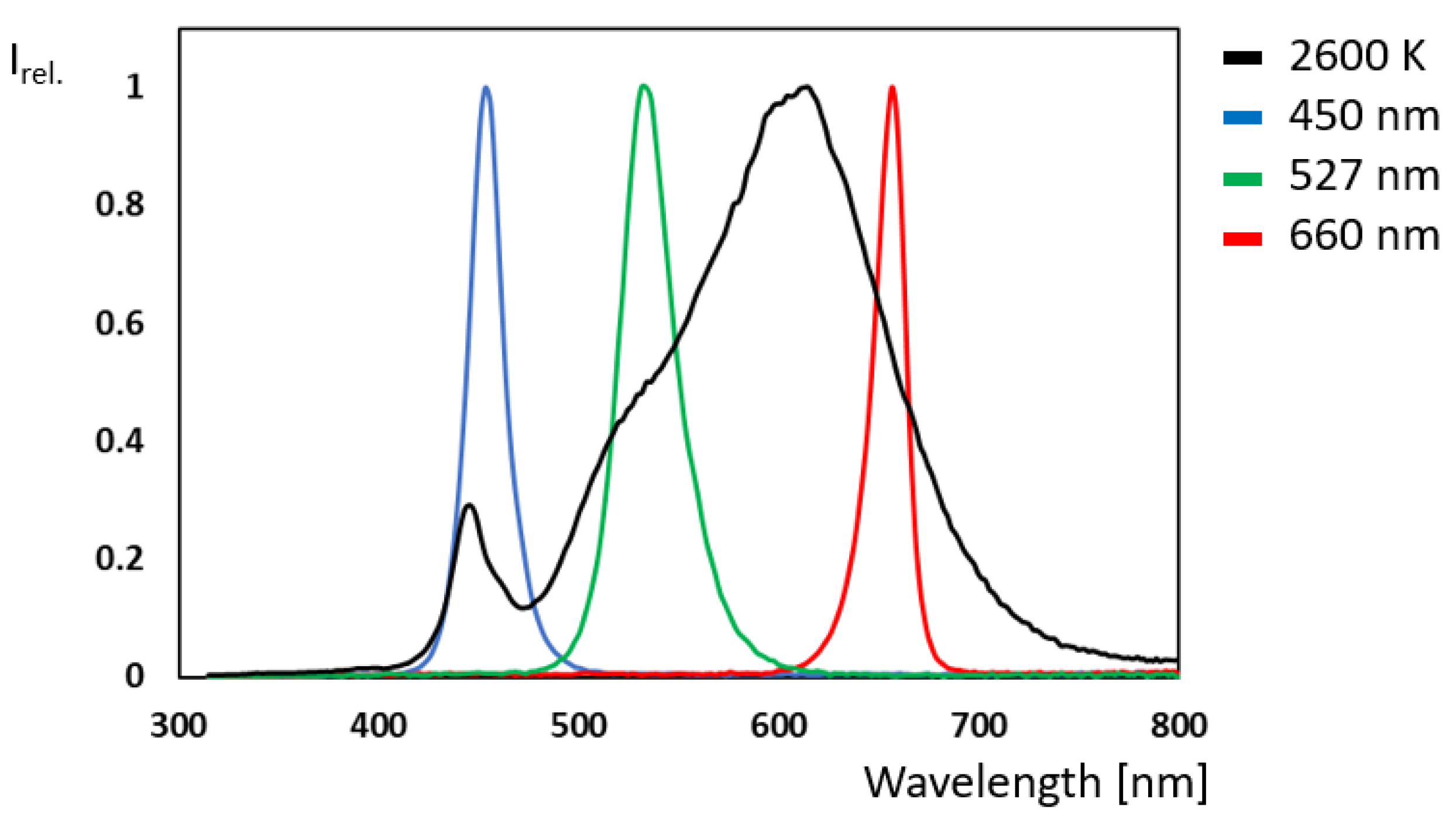
5.5. Light Source
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| LED | Light-emitting diode |
| Y(II) | Quantum yield of photosystem II |
| NPQ | Non-photochemical quenching |
| A | Assimilation rate |
| T | Leave temperature |
| GAE | Gallic acid equivalents |
| CE | Catechin equivalents |
| FLP | Frozen leaf powder |
| TPC | Total phenolic content |
| TFC | Total flavonoid content |
| CCT | Correlated color temperature |
| DLI | Daily light integral |
| Basil | Ocimum basilicum L. |
References
- Klimánková, E.; Holadová, K.; Hajšlová, J.; Čajka, T.; Poustka, J.; Koudela, M. Aroma profiles of five basil (Ocimum basilicum L.) cultivars grown under conventional and organic conditions. Food Chem. 2008, 107, 464–472. [Google Scholar] [CrossRef]
- Makri, O.; Kintzios, S. Ocimum sp. (Basil): Botany, Cultivation, Pharmaceutical Properties, and Biotechnology. J. Herbs Spices Med. Plants 2008, 13, 123–150. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; El-Nakhel, C.; Kyriacou, M.C.; Soteriou, G.A.; Pizzolongo, F.; Romano, R.; de Pascale, S.; Rouphael, Y. Genotype and Successive Harvests Interaction Affects Phenolic Acids and Aroma Profile of Genovese Basil for Pesto Sauce Production. Foods 2020, 10, 278. [Google Scholar] [CrossRef]
- Šimović, M.; Delaš, F.; Gradvol, V.; Kocevski, D.; Pavlović, H. Antifungal effect of eugenol and carvacrol against foodborne pathogens Aspergillus carbonarius and Penicillium roqueforti in improving safety of fresh-cut watermelon. J. Intercult. Ethnopharmacol. 2014, 3, 91–96. [Google Scholar] [CrossRef]
- Lee, S.-J.; Umano, K.; Shibamoto, T.; Lee, K.-G. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 2005, 91, 131–137. [Google Scholar] [CrossRef]
- Baldim, J.L.; Silveira, J.G.F.; Almeida, A.P.; Carvalho, P.L.N.; Rosa, W.; Schripsema, J.; Chagas-Paula, D.A.; Soares, M.G.; Luiz, J.H.H. The synergistic effects of volatile constituents of Ocimum basilicum against foodborne pathogens. Ind. Crop. Prod. 2018, 112, 821–829. [Google Scholar] [CrossRef]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microbiol. 2008, 124, 91–97. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Sherazi, S.T.H.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef]
- Issazadeh, K.; Pahlaviani, K.; Majid, M.R.; Massiha, A.; Bidarigh, S.; Giahi, M.; Muradov, P.Z. Analysis of the phytochemical contents and anti-microbial activity of Ocimum basilicum L. Int. J. Mol. Clin. Microbiol. 2012, 1, 141–147. [Google Scholar]
- Majdi, C.; Pereira, C.; Dias, M.I.; Calhelha, R.C.; Alves, M.J.; Rhourri-Frih, B.; Charrouf, Z.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Phytochemical Characterization and Bioactive Properties of Cinnamon Basil (Ocimum basilicum cv. ‘Cinnamon’) and Lemon Basil (Ocimum×citriodorum). Antioxidants 2020, 9, 369. [Google Scholar] [CrossRef] [PubMed]
- Benedec, D.; Pârvu, A.E.; Oniga, I.; Toiu, A.; Tiperciuc, B. Effects of Ocimum basilicum L. extract on experimental acute inflammation. Rev. Med. Chir. A Soc. Medici Nat. Iasi 2007, 111, 1065–1069. [Google Scholar]
- Eftekhar, N.; Moghimi, A.; Roshan, N.M.; Saadat, S.; Boskabady, M.H. Immunomodulatory and anti-inflammatory effects of hydro-ethanolic extract of Ocimum basilicum leaves and its effect on lung pathological changes in an ovalbumin-induced rat model of asthma. BMC Complement. Altern. Med. 2019, 19, 349. [Google Scholar] [CrossRef]
- Mueller, M.; Hobiger, S.; Jungbauer, A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010, 122, 987–996. [Google Scholar] [CrossRef]
- Szymanowska, U.; Złotek, U.; Karaś, M.; Baraniak, B. Anti-inflammatory and antioxidative activity of anthocyanins from purple basil leaves induced by selected abiotic elicitors. Food Chem. 2015, 172, 71–77. [Google Scholar] [CrossRef]
- Al-Subhi, L.; Waly, M.I. Two Cultivars of Ocimum basilicum Leaves Extracts Attenuate Streptozotocin-mediated Oxidative Stress in Diabetic Rats. Pak. J. Biol. Sci. PJBS 2020, 23, 1010–1017. [Google Scholar] [CrossRef]
- El-Beshbishy, H.; Bahashwan, S. Hypoglycemic effect of basil (Ocimum basilicum) aqueous extract is mediated through inhibition of α-glucosidase and α-amylase activities: An in vitro study. Toxicol. Ind. Health 2011, 28, 42–50. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Nazir, M.; Muhammad, W.; Hashmi, S.S.; Abbasi, R.; Rahman, L.; Hano, C. A Comparative Evaluation of the Antiproliferative Activity against HepG2 Liver Carcinoma Cells of Plant-Derived Silver Nanoparticles from Basil Extracts with Contrasting Anthocyanin Contents. Biomolecules 2019, 9, 320. [Google Scholar] [CrossRef]
- Manikandan, D.B.; Sridhar, A.; Sekar, R.K.; Perumalsamy, B.; Veeran, S.; Arumugam, M.; Karuppaiah, P.; Ramasamy, T. Green fabrication, characterization of silver nanoparticles using aqueous leaf extract of Ocimum americanum (Hoary Basil) and investigation of its in vitro antibacterial, antioxidant, anticancer and photocatalytic reduction. J. Environ. Chem. Eng. 2021, 9, 104845. [Google Scholar] [CrossRef]
- Manosroi, J.; Dhumtanom, P.; Manosroi, A. Anti-proliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines. Cancer Lett. 2006, 235, 114–120. [Google Scholar] [CrossRef]
- Mohammadi, M.; Majd, A.; Nejadsattari, T.; Hashemi, M. Antioxidant and anticancer activities of Ocimum basilicum L. cv. Dark Opal (Lamiaceae). Pharmacogn. Commun. 2014, 4, 48–58. [Google Scholar]
- Nangia-Makker, P.; Raz, T.; Tait, L.; Shekhar, M.P.V.; Li, H.; Balan, V.; Makker, H.; Fridman, R.; Maddipati, K.; Raz, A. Ocimum gratissimum retards breast cancer growth and progression and is a natural inhibitor of matrix metalloproteases. Cancer Biol. Ther. 2013, 14, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Sarahroodi, S.; Esmaeili, S.; Mikaili, P.; Hemmati, Z.; Saberi, Y. The effects of green Ocimum basilicum hydroalcoholic extract on retention and retrieval of memory in mice. Anc. Sci. Life 2012, 31, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Nurzyńska-Wierdak, R. Comparing the growth and flowering of selected basil (Ocimum basilicum L.) varieties. ACTA Agrobot. 2007, 60, 127–131. [Google Scholar] [CrossRef]
- Singh, S.; Singh, M.; Singh, A.K.; Kalra, A.; Yadav, A.; DPatra, D. Enhancing productivity of Indian basil (Ocimum basilicum L.) through harvest management under rainfed conditions of subtropical north Indian plains. Ind. Crop. Prod. 2010, 32, 601–606. [Google Scholar] [CrossRef]
- Formisano, L.; Ciriello, M.; El-Nakhel, C.; Kyriacou, M.C.; Rouphael, Y. Successive Harvests Modulate the Productive and Physiological Behavior of Three Genovese Pesto Basil Cultivars. Agronomy 2021, 11, 560. [Google Scholar] [CrossRef]
- Sipos, L.; Balázs, L.; Székely, G.; Jung, A.; Sárosi, S.; Radácsi, P.; Csambalik, L. Optimization of basil (Ocimum basilicum L.) production in LED light environments—A review. Sci. Hortic. 2021, 289, 110486. [Google Scholar] [CrossRef]
- Absolut Reports. Global Basil Leaves Market Research Report 2020; Absolut Reports: Maharashtra, India, 2020. [Google Scholar]
- Future Market Insights Inc. Basil Leaves Market Outlook—Growth, Demand & Forecast 2023–2033; Future Market Insights Inc.: Pimpri-Chinchwad, India, 2023. [Google Scholar]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Responses of Sweet Basil to Different Daily Light Integrals in Photosynthesis, Morphology, Yield, and Nutritional Quality. HortScience 2018, 53, 496–503. [Google Scholar] [CrossRef]
- Solis-Toapanta, E.; Gómez, C. Growth and Photosynthetic Capacity of Basil Grown for Indoor Gardening under Constant or Increasing Daily Light Integrals. HortTechnology 2019, 29, 880–888. [Google Scholar] [CrossRef]
- Beaman, A.R.; Gladon, R.J.; Schrader, J.A. Sweet Basil Requires an Irradiance of 500 μ mol·m−2·s−1 for Greatest Edible Biomass Production. HortScience 2009, 44, 64–67. [Google Scholar] [CrossRef]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I. Unraveling the Role of Red:Blue LED Lights on Resource Use Efficiency and Nutritional Properties of Indoor Grown Sweet Basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Tabbert, J.M.; Schulz, H.; Krähmer, A.; Riewe, D. Facing energy limitations—Approaches to increase basil (Ocimum basilicum L.) growth and quality by different increasing light intensities emitted by a broadband LED light spectrum (400–780 nm). Front. Plant Sci. 2022, 13, 1055352. [Google Scholar] [CrossRef] [PubMed]
- Aldarkazali, M.; Rihan, H.Z.; Carne, D.; Fuller, M.P. The Growth and Development of Sweet Basil (Ocimum basilicum) and Bush Basil (Ocimum minimum) Grown under Three Light Regimes in a Controlled Environment. Agronomy 2019, 9, 743. [Google Scholar] [CrossRef]
- Hosseini, A.; Mehrjerdi, M.Z.; Aliniaeifard, S.; Seif, M. Photosynthetic and growth responses of green and purple basil plants under different spectral compositions. Physiol. Mol. Biol. Plants 2019, 25, 741–752. [Google Scholar] [CrossRef]
- Kondratieva, V.V.; Voronkova, T.V.; Semenova, M.V.; Olekhnovich, L.S.; Shelepova, O.V. Effect of LEDs on the growth and physiological responses of sweet basil (Ocimum basilicum L.). IOP Conf. Ser. Earth Environ. Sci. 2022, 1045, 12090. [Google Scholar] [CrossRef]
- Lin, K.-H.; Huang, M.-Y.; Hsu, M.-H. Morphological and physiological response in green and purple basil plants (Ocimum basilicum) under different proportions of red, green, and blue LED lightings. Sci. Hortic. 2021, 275, 109677. [Google Scholar] [CrossRef]
- Piovene, C.; Orsini, F.; Bosi, S.; Sanoubar, R.; Bregola, V.; Dinelli, G.; Gianquinto, G. Optimal red:blue ratio in led lighting for nutraceutical indoor horticulture. Sci. Hortic. 2015, 193, 202–208. [Google Scholar] [CrossRef]
- Pennisi, G.; Pistillo, A.; Orsini, F.; Cellini, A.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Crepaldi, A.; Gianquinto, G.; Marcelis, L.F. Optimal light intensity for sustainable water and energy use in indoor cultivation of lettuce and basil under red and blue LEDs. Sci. Hortic. 2020, 272, 109508. [Google Scholar] [CrossRef]
- Chutimanukul, P.; Wanichananan, P.; Janta, S.; Toojinda, T.; Darwell, C.T.; Mosaleeyanon, K. The influence of different light spectra on physiological responses, antioxidant capacity and chemical compositions in two holy basil cultivars. Sci. Rep. 2022, 12, 588. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M. Photosynthesis, Morphology, Yield, and Phytochemical Accumulation in Basil Plants Influenced by Substituting Green Light for Partial Red and/or Blue Light. HortScience 2019, 54, 1769–1776. [Google Scholar] [CrossRef]
- Coutinho, P.W.R.; Echer, M.D.M.; Guimarães, V.F.; Bulegon, L.G.; Abade, M.T.R.; Rocha, M.E.L. Gas exchange and morphometric characteristics of basil according to the times and collection positions in the plant. Rev. Sci. Agrar. Parana. 2022, 21, 90–97. [Google Scholar] [CrossRef]
- Barickman, T.C.; Adhikari, B.; Sehgal, A.; Walne, C.H.; Reddy, K.R.; Gao, W. Drought and Elevated CO2 Impacts Photosynthesis and Biochemicals of Basil (Ocimum basilicum L.). Stresses 2021, 1, 223–237. [Google Scholar] [CrossRef]
- Germano, R.P.; Melito, S.; Cacini, S.; Carmassi, G.; Leoni, F.; Maggini, R.; Montesano, F.F.; Pardossi, A.; Massa, D. Sweet basil can be grown hydroponically at low phosphorus and high sodium chloride concentration: Effect on plant and nutrient solution management. Sci. Hortic. 2022, 304, 111324. [Google Scholar] [CrossRef]
- Kalamartzis, I.; Menexes, G.; Georgiou, P.; Dordas, C. Effect of Water Stress on the Physiological Characteristics of Five Basil (Ocimum basilicum L.) Cultivars. Agronomy 2020, 10, 1029. [Google Scholar] [CrossRef]
- Barickman, T.C.; Olorunwa, O.J.; Sehgal, A.; Walne, C.H.; Reddy, K.R.; Gao, W. Yield, Physiological Performance, and Phytochemistry of Basil (Ocimum basilicum L.) under Temperature Stress and Elevated CO2 Concentrations. Plants 2021, 10, 1072. [Google Scholar] [CrossRef]
- Copolovici, L.; Copolovici, D.M.; Moisa, C.; Lupitu, A. Antagonist Temperature Variation Affects the Photosynthetic Parameters and Secondary Metabolites of Ocimum basilicum L. and Salvia officinalis L. Plants 2022, 11, 1806. [Google Scholar] [CrossRef]
- Park, K.S.; Bekhzod, K.; Kwon, J.K.; Son, J.E. Development of a coupled photosynthetic model of sweet basil hydroponically grown in plant factories. Hortic. Environ. Biotechnol. 2016, 57, 20–26. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV—VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef]
- McCree, K. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Lal, M.A. Plant Physiology, Development and Metabolism, 2nd ed.; Springer: Singapore, 2023; p. 161. [Google Scholar]
- Nishio, J.N. Why are higher plants green? Evolution of the higher plant photosynthetic pigment complement. Plant Cell Environ. 2000, 23, 539–548. [Google Scholar] [CrossRef]
- Moriwaki, T.; Falcioni, R.; Tanaka, F.A.O.; Cardoso, K.A.K.; Souza, L.A.; Benedito, E.; Nanni, M.R.; Bonato, C.M.; Antunes, W.C. Nitrogen-improved photosynthesis quantum yield is driven by increased thylakoid density, enhancing green light absorption. Plant Sci. Int. J. Exp. Plant Biol. 2019, 278, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.G.; Hüner, N.P.A. (Eds.) Introduction to Plant Physiology, 4th ed.; Wiley Textbooks: Hoboken, NJ, USA, 2008; p. 103. [Google Scholar]
- Murchie, E.H.; Hubbart, S.; Chen, Y.; Peng, S.; Horton, P. Acclimation of Rice Photosynthesis to Irradiance under Field Conditions. Plant Physiol. 2002, 130, 1999–2010. [Google Scholar] [CrossRef]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 2017, 69, 2099–2110. [Google Scholar] [CrossRef]
- Vogelmann, T.C.; Han, T. Measurement of gradients of absorbed light in spinach leaves from chlorophyll fluorescence profiles. Plant Cell Environ. 2008, 23, 1287–1405. [Google Scholar] [CrossRef]
- CUI, M.; Vogelmann, T.C.; Smith, W.K. Chlorophyll and light gradients in sun and shade leaves of Spinacia oleracea. Plant Cell Environ. 1991, 14, 493–500. [Google Scholar] [CrossRef]
- Sun, J.; Nishio, J.N.; Vogelmann, T.C. Green Light Drives CO2 Fixation Deep within Leaves. Plant Cell Physiol. 1998, 39, 1020–1026. [Google Scholar] [CrossRef]
- Liu, J.; van Iersel, M.W. Photosynthetic Physiology of Blue, Green, and Red Light: Light Intensity Effects and Underlying Mechanisms. Front. Plant Sci. 2021, 12, 619987. [Google Scholar] [CrossRef]
- Goh, C.-H.; Ko, S.-M.; Koh, S.; Kim, Y.-J.; Bae, H.-J. Photosynthesis and Environments: Photoinhibition and Repair Mechanisms in Plants. J. Plant Biol. 2011, 55, 93–101. [Google Scholar] [CrossRef]
- Kasahara, M.; Kagawa, T.; Oikawa, K.; Suetsugu, N.; Miyao, M.; Wada, M. Chloroplast avoidance movement reduces photodamage in plants. Nature 2002, 420, 829–832. [Google Scholar] [CrossRef]
- Krause, G.H. Photoinhibition of photosynthesis. An evaluation of damaging and protective mechanisms. Physiol. Plant. 1988, 74, 566–574. [Google Scholar] [CrossRef]
- Raven, J.A. The cost of photoinhibition. Physiol. Plant. 2011, 142, 87–104. [Google Scholar] [CrossRef]
- Hakala, M.; Tuominen, I.; Keränen, M.; Tyystjärvi, T.; Tyystjärvi, E. Evidence for the role of the oxygen-evolving manganese complex in photoinhibition of Photosystem II. Biochim. Biophys. Acta 2005, 1706, 68–80. [Google Scholar] [CrossRef]
- Tyystjärvi, T.; Tuominen, I.; Herranen, M.; Aro, E.-M.; Tyystjärvi, E. Action spectrum of psbA gene transcription is similar to that of photoinhibition in Synechocystis sp. PCC 6803. FEBS Lett. 2002, 516, 167–171. [Google Scholar] [CrossRef]
- Berger, S.; Sinha, A.K.; Roitsch, T. Plant physiology meets phytopathology: Plant primary metabolism and plant–pathogen interactions. J. Exp. Bot. 2007, 58, 4019–4026. [Google Scholar] [CrossRef]
- El-Esawi, M.; Arthaut, L.-D.; Jourdan, N.; d’Harlingue, A.; Link, J.; Martino, C.F.; Ahmad, M. Blue-light induced biosynthesis of ROS contributes to the signaling mechanism of Arabidopsis cryptochrome. Sci. Rep. 2017, 7, 13875. [Google Scholar] [CrossRef]
- Min, K.; Ebeler, S.E. Flavonoid effects on DNA oxidation at low concentrations relevant to physiological levels. Food Chem. Toxicol. 2008, 46, 96–104. [Google Scholar] [CrossRef]
- Zvi Rappoport. The Chemistry of Phenols; Wiley: Hoboken, NJ, USA, 2003; pp. 139–141. [Google Scholar]
- Waterhouse, A.L. Determination of Total Phenolics. Curr. Protoc. Food Anal. Chem. 2003, 6, I1.1.1–I1.1.8. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jokic, L.; Pappert, I.; Khanh, T.Q.; Kaldenhoff, R. Effect of Light Intensity and Light Spectrum of LED Light Sources on Photosynthesis and Secondary Metabolite Synthesis in Ocimum basilicum. Plants 2025, 14, 1334. https://doi.org/10.3390/plants14091334
Jokic L, Pappert I, Khanh TQ, Kaldenhoff R. Effect of Light Intensity and Light Spectrum of LED Light Sources on Photosynthesis and Secondary Metabolite Synthesis in Ocimum basilicum. Plants. 2025; 14(9):1334. https://doi.org/10.3390/plants14091334
Chicago/Turabian StyleJokic, Luca, Isabell Pappert, Tran Quoc Khanh, and Ralf Kaldenhoff. 2025. "Effect of Light Intensity and Light Spectrum of LED Light Sources on Photosynthesis and Secondary Metabolite Synthesis in Ocimum basilicum" Plants 14, no. 9: 1334. https://doi.org/10.3390/plants14091334
APA StyleJokic, L., Pappert, I., Khanh, T. Q., & Kaldenhoff, R. (2025). Effect of Light Intensity and Light Spectrum of LED Light Sources on Photosynthesis and Secondary Metabolite Synthesis in Ocimum basilicum. Plants, 14(9), 1334. https://doi.org/10.3390/plants14091334





