The Prolonged Application of Organic Fertilizers Increases the Quality and Yield of Tea Crops
Abstract
1. Introduction
2. Results
2.1. Effects of Organic-Fertilizer Substitution on Tea Yield and Quality
2.2. Effects of Organic-Fertilizer Substitution on Soil Properties and the SQI
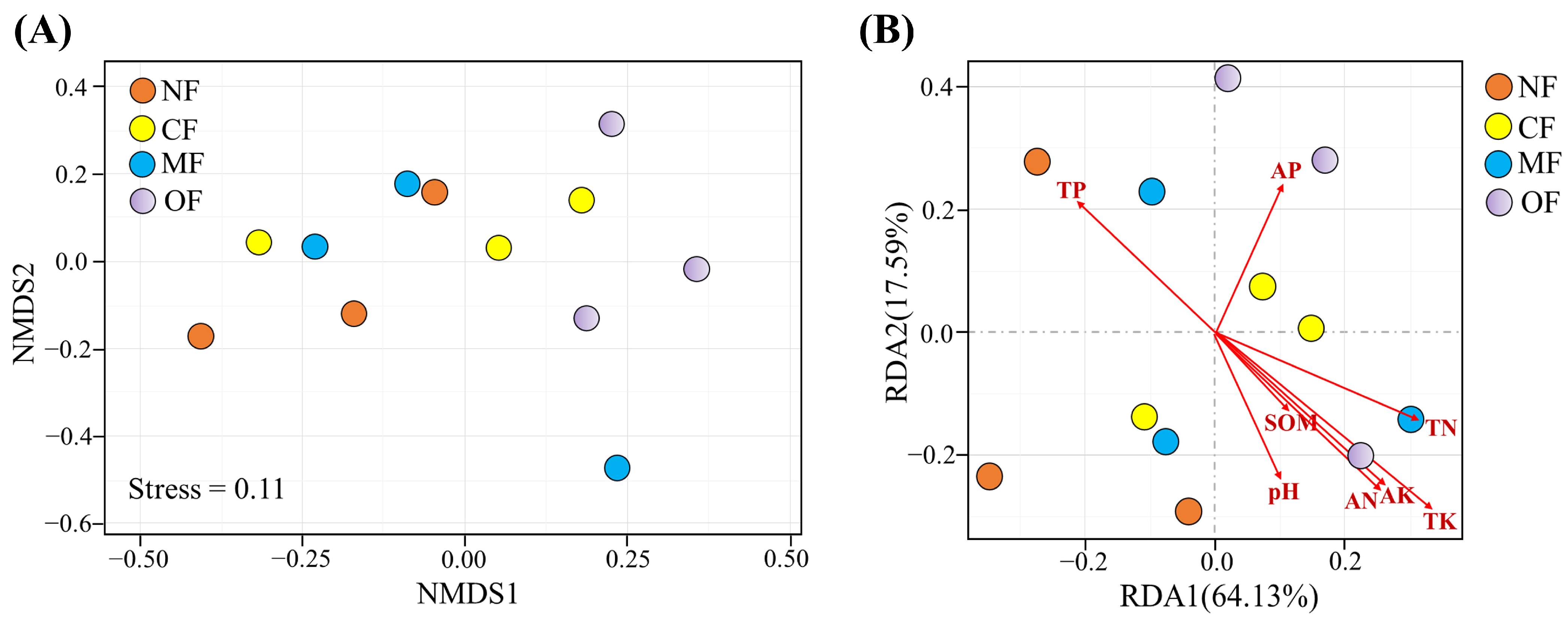
2.3. Effects of Organic-Fertilizer Substitution on Soil Bacterial Community Diversity
2.4. Shifts in Soil Bacterial Community Composition
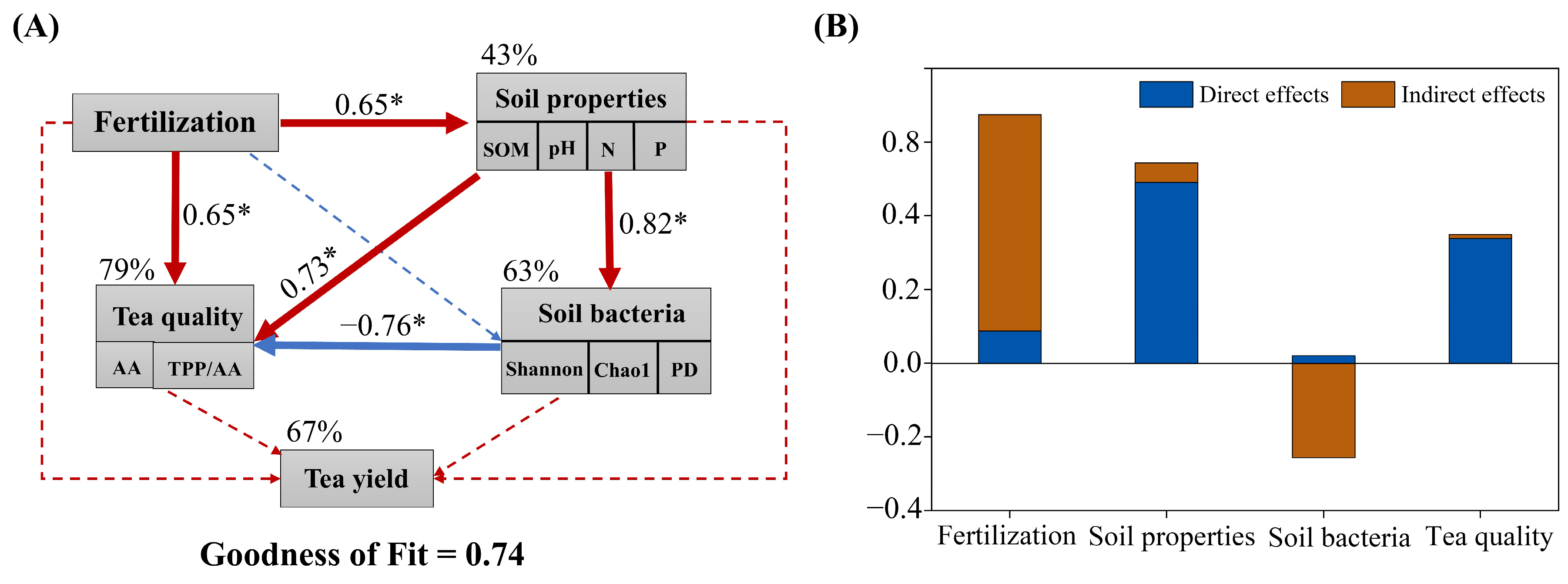
2.5. Relationships Among Soil Properties, Bacteria, and Tea Performance
3. Discussion
3.1. Organic-Fertilizer Substitutions Affect Soil Properties
3.2. Organic-Fertilizer Substitutions Affects Yield and Quality of Tea
3.3. Organic-Fertilizer Substitutions Affect Soil Bacteria
4. Materials and Methods
4.1. Site Description and Experiment Design
4.2. Soil Sampling and Analysis
4.3. Soil Quality Evaluation
4.4. Yield and Quality of Tea
4.5. Soil DNA Extraction and High-Throughput Sequencing
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mei, Y.; Liang, X. Analysis of China’s Tea Production, Sales, Import and Export Situation in 2023. China Tea 2024, 46, 18–26. [Google Scholar]
- Chen, Y.; Wang, F.; Wu, Z.; Jiang, F.; Yu, W.; Yang, J.; Chen, J.; Jian, G.; You, Z.; Zeng, L. Effects of long-term nitrogen fertilization on the formation of metabolites related to tea quality in subtropical China. Metabolites 2021, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Liao, W.; Yi, X.; Niu, S.; Ma, L.; Shi, Y.; Zhang, Q.; Liu, M.; Ruan, J. Fertilization status and reduction potential in tea gardens of China. J. Plant Nutr. Fertil. 2019, 25, 421–432. [Google Scholar]
- Yang, X.D.; Ni, K.; Shi, Y.Z.; Yi, X.Y.; Zhang, Q.F.; Fang, L.; Ma, L.; Ruan, J. Effects of long-term nitrogen application on soil acidification and solution chemistry of a tea plantation in China. Agr. Ecosyst. Environ. 2018, 252, 74–82. [Google Scholar] [CrossRef]
- Raza, S.; Miao, N.; Wang, P.; Ju, X.; Chen, Z.; Zhou, J.; Kuzyakov, Y. Dramatic loss of inorganic carbon by nitrogen-induced soil acidification in Chinese croplands. Global Change Biol. 2020, 26, 3738–3751. [Google Scholar] [CrossRef]
- Morugán-Coronado, A.; Pérez-Rodríguez, P.; Insolia, E.; Soto-Gómez, D.; Fernández-Calvino, D.; Zornoza, R. The impact of crop diversification, tillage and fertilization type on soil total microbial, fungal and bacterial abundance: A worldwide meta-analysis of agricultural sites. Agr. Ecosyst. Environ. 2022, 329, 107867. [Google Scholar] [CrossRef]
- Menšík, L.; Hlisnikovský, L.; Pospíšilová, L.; Kunzová, E. The effect of application of organic manures and mineral fertilizers on the state of soil organic matter and nutrients in the long-term field experiment. J. Soils Sediment. 2018, 18, 2813–2822. [Google Scholar] [CrossRef]
- Mockeviciene, I.; Repsiene, R.; Amaleviciute-Volunge, K.; Karcauskiene, D.; Slepetiene, A.; Lepane, V. Effect of long-term application of organic fertilizers on improving organic matter quality in acid soil. Arch. Agron. Soil Sci. 2022, 68, 1192–1204. [Google Scholar] [CrossRef]
- Sun, R.; Guo, X.; Wang, D.; Chu, H. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Geng, Y.H.; Cao, G.J.; Wang, L.C.; Wang, S.H. Effects of equal chemical fertilizer substitutions with organic manure on yield, dry matter, and nitrogen uptake of spring maize and soil nitrogen distribution. PLoS ONE 2019, 14, e0219512. [Google Scholar] [CrossRef]
- Liu, H.; Du, X.; Li, Y.; Han, X.; Li, B.; Zhang, X.; Li, Q.; Liang, W. Organic substitutions improve soil quality and maize yield through increasing soil microbial diversity. J. Clean. Prod. 2022, 347, 131323. [Google Scholar] [CrossRef]
- Tang, Q.; Cotton, A.; Wei, Z.; Xia, Y.; Daniell, T.; Yan, X. How does partial substitution of chemical fertiliser with organic forms increase sustainability of agricultural production? Sci. Total Environ. 2022, 803, 149933. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Lam, S.K.; Yan, X.; Chen, D. How does recycling of livestock manure in agroecosystems affect crop productivity, reactive nitrogen losses, and soil carbon balance? Environ. Sci. Technol. 2017, 51, 7450–7457. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhu, B.; Wang, S.; Zhu, X.; Vereecken, H.; Brüggemann, N. Stimulation of N2O emission by manure application to agricultural soils may largely offset carbon benefits: A global meta-analysis. Glob. Chang. Biol. 2017, 23, 4068–4083. [Google Scholar] [CrossRef]
- Hartmann, M.; Frey, B.; Mayer, J.; Mäder, P.; Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 2015, 9, 1177–1194. [Google Scholar] [CrossRef]
- Hoffland, E.; Kuyper, T.W.; Comans, R.N.J.; Creamer, R.E. Eco-functionality of organic matter in soils. Plant Soil 2020, 455, 1–22. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, S.; Wang, X.; Xu, X.; Ai, C.; Liang, G.; Zhu, P.; Zhou, W. Enzymatic stoichiometry reveals phosphorus limitation-induced changes in the soil bacterial communities and element cycling: Evidence from a long-term field experiment. Geoderma 2022, 426, 116124. [Google Scholar] [CrossRef]
- Bünemann, E.; Bongiorno, G.; Bai, Z.; Creamer, R.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Maurya, S.; Abraham, J.S.; Somasundaram, S.; Toteja, R.; Gupta, R.; Makhija, S. Indicators for assessment of soil quality: A mini-review. Environ. Monit. Assess. 2020, 192, 604. [Google Scholar] [CrossRef]
- Ji, L.; Ni, K.; Wu, Z.; Zhang, J.; Yi, X.; Yang, X.; Ling, N.; You, Z.; Guo, S.; Ruan, J. Effect of organic substitution rates on soil quality and fungal community composition in a tea plantation with long-term fertilization. Biol. Fertil. Soils 2020, 56, 633–646. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Mei, H.; Li, J.; Chen, X.; Huang, Q.; Li, X.; Tang, J. Effects of long-term application of earthworm bio-organic fertilization technology on soil quality and organo-mineral complex in tea garden. Forests 2023, 14, 225. [Google Scholar] [CrossRef]
- Yi, X.; Ji, L.; Hu, Z.; Yang, X.; Li, H.; Jiang, Y.; He, T.; Yang, Y.; Ni, K.; Ruan, J. Organic amendments improved soil quality and reduced ecological risks of heavy metals in a long-term tea plantation field trial on an Alfisol. Sci. Total Environ. 2022, 838, 156017. [Google Scholar] [CrossRef] [PubMed]
- Adomako, M.O.; Roiloa, S.; Yu, F.H. Potential roles of soil microorganisms in regulating the effect of soil nutrient heterogeneity on plant performance. Microorganisms 2022, 10, 2399. [Google Scholar] [CrossRef] [PubMed]
- Blagodatskaya, E.; Kuzyakov, Y. Active microorganisms in soil: Critical review of estimation criteria and approaches. Soil Biol. Biochem. 2013, 67, 192–211. [Google Scholar] [CrossRef]
- Wani, F.S.; Ahmad, L.; Ali, T.; Mushtaq, A. Role of microorganisms in nutrient mobilization and soil health—A review. J. Pure. Appl. Microbiol. 2015, 9, 1401–1410. [Google Scholar]
- Yang, Y.; Li, X.; Liu, J.; Zhou, Z.; Zhang, T.; Wang, X. Bacterial diversity as affected by application of manure in red soils of subtropical China. Biol. Fertil. Soils 2017, 53, 639–649. [Google Scholar] [CrossRef]
- Sivojiene, D.; Kacergius, A.; Baksiene, E.; Maseviciene, A.; Zickiene, L. The influence of organic fertilizers on the abundance of soil microorganism communities, agrochemical indicators, and yield in East Lithuanian light soils. Plants 2021, 10, 2648. [Google Scholar] [CrossRef]
- Huang, X.; Zheng, Y.; Li, P.; Cui, J.; Sui, P.; Chen, Y.; Gao, Y. Organic management increases beneficial microorganisms and promotes the stability of microecological networks in tea plantation soil. Front. Microbiol. 2023, 14, 1237842. [Google Scholar] [CrossRef]
- Siddiqui, Z.A. Effects of plant growth promoting bacteria and composed organic fertilizers on the reproduction of Meloidogyne incognita and tomato growth. Bioresour. Technol. 2004, 95, 223–227. [Google Scholar] [CrossRef]
- Holík, L.; Hlisnikovský, L.; Honzík, R.; Trögl, J.; Burdová, H.; Popelka, J. Soil microbial communities and enzyme activities after long-term application of inorganic and organic fertilizers at different depths of the soil profile. Sustainability 2019, 11, 3251. [Google Scholar] [CrossRef]
- Zhong, W.; Gu, T.; Wang, W.; Zhang, B.; Lin, X.; Huang, Q.; Shen, W. The effects of mineral fertilizer and organic manure on soil microbial community and diversity. Plant Soil 2010, 326, 511–522. [Google Scholar] [CrossRef]
- Ji, L.; Si, H.; He, J.; Fan, L.; Li, L. The shifts of maize soil microbial community and networks are related to soil properties under different organic fertilizers. Rhizosphere 2021, 19, 100388. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. A review. Sustain. Agric. Vol. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- Köninger, J.; Lugato, E.; Panagos, P.; Kochupillai, M.; Orgiazzi, A.; Briones, M.J. Manure management and soil biodiversity: Towards more sustainable food systems in the EU. Agr. Syst. 2021, 194, 103251. [Google Scholar] [CrossRef]
- Hu, X.; Gu, H.; Liu, J.; Wei, D.; Zhu, P.; Zhou, B.; Chen, X.; Jin, J.; Liu, X. Metagenomics reveals divergent functional profiles of soil carbon and nitrogen cycling under long-term addition of chemical and organic fertilizers in the black soil region. Geoderma 2022, 418, 115846. [Google Scholar] [CrossRef]
- Xia, M.; Ma, X.; Liu, J.; Wu, M.; Li, Z.; Liu, M. Potential effect of key soil bacterial taxa on the increase of rice yield under milk vetch rotation. Front. Microbiol. 2023, 14, 1150505. [Google Scholar] [CrossRef]
- Dai, X.; Song, D.; Zhou, W.; Liu, G.; Liang, G.; He, P.; Sun, G.; Yuan, Y.; Liu, Z.; Yao, Y.; et al. Partial substitution of chemical nitrogen with organic nitrogen improves rice yield, soil biochemical indictors and microbial composition in a double rice cropping system in south China. Soil Till. Res. 2021, 205, 104753. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Zhao, J.; Nie, J.; Zang, H.; Zeng, Z.; Olesen, J.E. Yield benefits from replacing chemical fertilizers with manure under water deficient conditions of the winter wheat–summer maize system in the North China plain. Eur. J. Agron. 2020, 119, 126118. [Google Scholar] [CrossRef]
- Ye, J.; Wang, Y.; Wang, Y.; Hong, L.; Jia, X.; Kang, J.; Lin, S.; Wu, Z.; Wang, H. Improvement of soil acidification in tea plantations by long-term use of organic fertilizers and its effect on tea yield and quality. Front. Plant Sci. 2022, 13, 1055900. [Google Scholar] [CrossRef]
- Luo, G.W.; Li, L.; Friman, V.P.; Guo, J.; Guo, S.; Shen, Q.; Ling, N. Organic amendments increase crop yields by improving microbe-mediated soil functioning of agroecosystems: A meta-analysis. Soil Biol. Biochem. 2018, 124, 105–115. [Google Scholar] [CrossRef]
- Sedlář, O.; Balík, J.; Černý, J.; Smatanová, M. Long-term application of organic fertilizers in relation to soil organic matter quality. Agronomy 2023, 13, 175. [Google Scholar] [CrossRef]
- He, H.; Peng, M.; Lu, W.; Hou, Z.; Li, J. Commercial organic fertilizer substitution increases wheat yield by improving soil quality. Sci. Total Environ. 2022, 851, 158132. [Google Scholar] [CrossRef] [PubMed]
- Sarma, B.; Farooq, M.; Gogoi, N.; Borkotoki, B.; Kataki, R.; Garg, A. Soil organic carbon dynamics in wheat-green gram crop rotation amended with vermicompost and biochar in combination with inorganic fertilizers: A comparative study. J. Clean. Prod. 2018, 201, 471–480. [Google Scholar] [CrossRef]
- Guo, Z.; Han, J.; Li, J.; Xu, Y.; Wang, X. Effects of long-term fertilization on soil organic carbon mineralization and microbial community structure. PLoS ONE 2019, 14, e0211163. [Google Scholar]
- Wu, H.; Cai, A.; Xing, T.; Huai, S.; Zhu, P.; Xu, M.; Lu, C. Fertilization enhances mineralization of soil carbon and nitrogen pools by regulating the bacterial community and biomass. J. Soil Sediments 2021, 21, 1633–1643. [Google Scholar] [CrossRef]
- Liu, J.; Shu, A.; Song, W.; Shi, W.; Li, M.; Zhang, W.; Li, Z.; Liu, G.; Yuan, F.; Zhang, S.; et al. Long-term organic fertilizer substitution increases rice yield by improving soil properties and regulating soil bacteria. Geoderma 2021, 404, 115287. [Google Scholar] [CrossRef]
- Salehi, A.; Fallah, S.; Sourki, A.A. Organic and inorganic fertilizer effect on soil CO2 flux, microbial biomass, and growth of Nigella sativa L. Int. Agrophys. 2017, 31, 103–116. [Google Scholar] [CrossRef]
- Wang, B.; Wang, S.; Li, G.; Fu, L.; Chen, H.; Yin, M.; Chen, J. Reducing nitrogen fertilizer usage coupled with organic substitution improves soil quality and boosts tea yield and quality in tea plantations. J. Sci. Food Agric. 2025, 105, 1228–1238. [Google Scholar] [CrossRef]
- Wang, Y.J.; Hu, X.; Jin, G.; Hou, Z.; Ning, J.; Zhang, Z. Rapid prediction of chlorophylls and carotenoids content in tea leaves under different levels of nitrogen application based on hyperspectral imaging. J. Sci. Food Agric. 2019, 99, 1997–2004. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, B.; Zhao, X.; Hu, J.; Dong, Z.; Xiao, H.; Yuan, Y.; Guo, F.; Wang, F.; Ni, D.; et al. Novel insights into the role of leaf in the cutting process of Camellia sinensis using physiological, biochemical and transcriptome analyses. Tree Physiol. 2023, 43, 2031–2045. [Google Scholar] [CrossRef]
- Yao, L.; Liu, X.; Jiang, Y.; Caffin, N.; D’Arcy, B.; Singanusong, R.; Datta, N.; Xu, Y. Compositional analysis of teas from Australian supermarkets. Food Chem. 2006, 94, 115–122. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci. 2007, 81, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Kuhr, S.; Engelhardt, U.H. Influence of catechins and theaflavins on the astringent taste of black tea brews. Z. Lebensm. Unters. Forsch. 1992, 195, 108–111. [Google Scholar] [CrossRef]
- Hara, Y.; Luo, S.J.; Wickremasinghe, R.L. Special issue on tea. Food Rev. Int. 1995, 11, 371–545. [Google Scholar]
- Yao, X.; Qi, Y.; Chen, H.; Zhang, B.; Chen, Z.; Lu, L. Study of Camellia sinensis diploid and triploid leaf development mechanism based on transcriptome and leaf characteristics. PLoS ONE 2023, 18, e0275652. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, X.; Guo, X.; Wang, D.; Chu, H. Effects of long-term application of chemical and organic fertilizers on the abundance of microbial communities involved in the nitrogen cycle. Appl. Soil Ecol. 2015, 95, 171–178. [Google Scholar] [CrossRef]
- Gu, S.; Hua, Q.; Cheng, Y.; Bai, L.; Liu, Z.; Xiao, W.; Gong, Z.; Wu, Y.; Feng, K.; Deng, Y.; et al. Application of organic fertilizer improves microbial community diversity and alters microbial network structure in tea (Camellia sinensis) plantation soils. Soil Till. Res. 2019, 195, 104356. [Google Scholar] [CrossRef]
- Feng, Y.; Delgado-Baquerizo, M.; Zhu, Y.; Han, X.; Han, X.; Xin, X.; Li, W.; Guo, Z.; Dang, T.; Li, C.; et al. Responses of Soil Bacterial Diversity to Fertilization are Driven by Local Environmental Context Across China. Engineering 2022, 12, 164–170. [Google Scholar] [CrossRef]
- Ma, L.; Zhuo, R. Response of tea yield, quality and soil bacterial characteristics to long-term nitrogen fertilization in an eleven-year field experiment. Appl. Soil Ecol. 2021, 166, 103976. [Google Scholar] [CrossRef]
- Dang, P.; Li, C.; Lu, C.; Zhang, M.; Huang, T.; Wan, C.; Wang, H.; Chen, Y.; Qin, X.; Liao, Y.; et al. Effect of fertilizer management on the soil bacterial community in agroecosystems across the globe. Agr. Ecosyst. Environ. 2022, 326, 107795. [Google Scholar] [CrossRef]
- Qi, N.; Lin, C.A.; Jia, Z.; Zhang, C.; Ma, D.; Li, F.; Zhang, J.; Li, D.; Han, X.; Cai, Z.; et al. Multiple long-term observations reveal a strategy for soil pH-dependent fertilization and fungal communities in support of agricultural production. Agr. Ecosyst. Environ. 2020, 293, 106837. [Google Scholar]
- Zhang, S.; Sun, L.; Wang, Y.; Fan, K.; Xu, Q.; Li, Y.; Ma, Q.; Wang, J.; Ren, W.; Ding, Z. Cow manure application effectively regulates the soil bacterial community in tea plantation. BMC Microbiol. 2020, 20, 190. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Zhang, G.; Jiang, L.; Han, X. Mechanisms of soil acidification reducing bacterial diversity. Soil Biol. Biochem. 2015, 81, 275–281. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, Z.; Xiao, X.; Zhang, N.; Wang, X.; Yang, Z.; Xu, K.; Liang, Y. Structural changes of soil organic matter and the linkage to rhizosphere bacterial communities with biochar amendment in manure fertilized soils. Sci. Total Environ. 2019, 692, 333–343. [Google Scholar] [CrossRef]
- Ding, J.; Jiang, X.; Ma, M.; Zhou, B.; Guan, D.; Zhao, B.; Zhou, J.; Cao, F.; Li, L.; Li, J. Effect of 35 years inorganic fertilizer and manure amendment on structure of bacterial and archaeal communities in black soil of northeast China. Appl. Soil Ecol. 2016, 105, 187–195. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Yi, X.; Zheng, H.; Ni, K.; Ma, Q.; Cai, Y.; Ma, L.; Shi, Y.; Yang, X.; et al. Partially replacing chemical fertilizer with manure improves soil quality and ecosystem multifunctionality in a tea plantation. Agr. Ecosyst. Environ. 2025, 378, 109284. [Google Scholar] [CrossRef]
- Wang, Z.; Geng, Y.; Liang, T.; Hu, X. Effects of Chemical fertilizer reduction and chemical fertilizer applied with organic fertilizer on tea garden soil nutrients and tea yield and quality. Ecol. Environ. Sci. 2018, 27, 2243–2251. [Google Scholar]
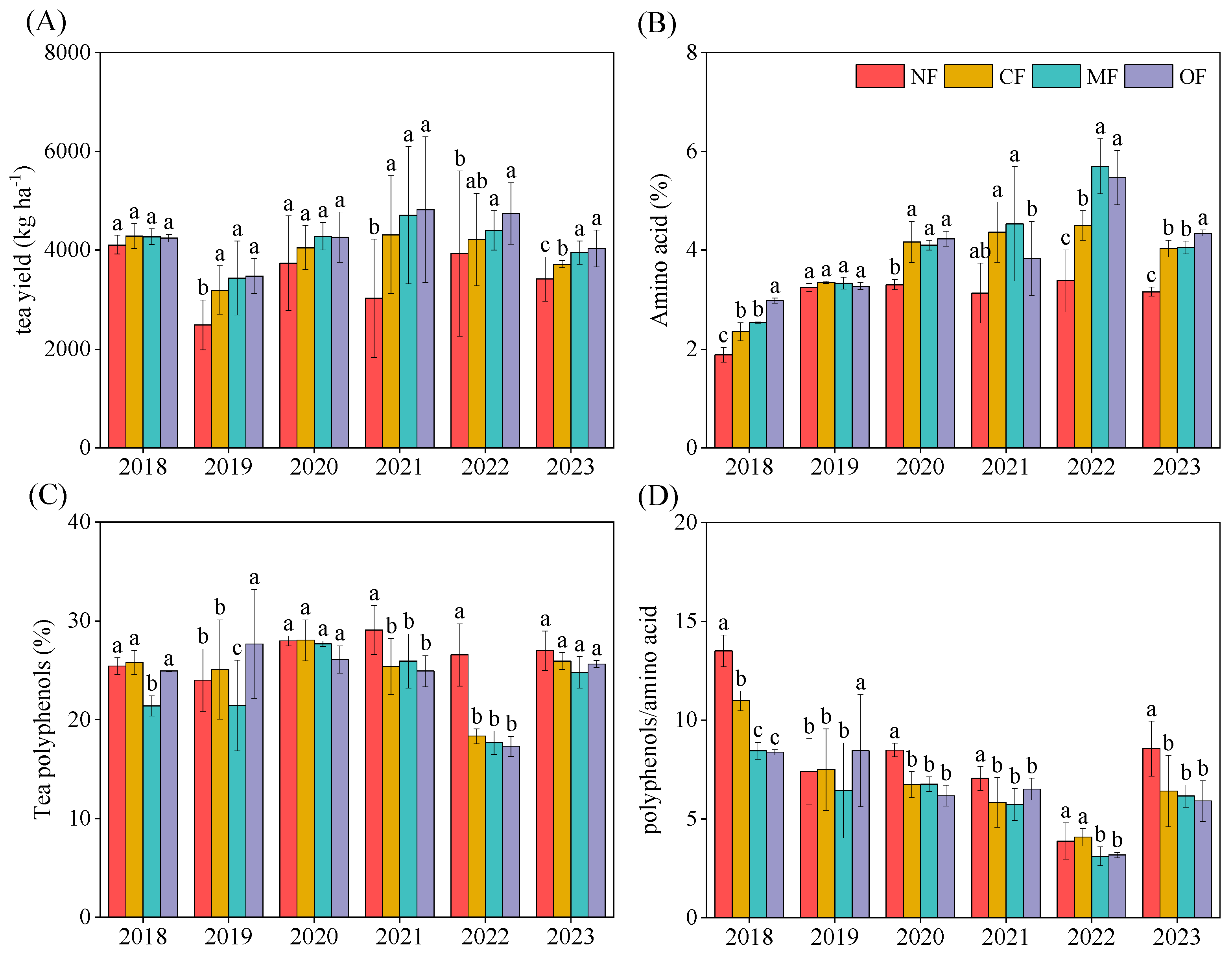

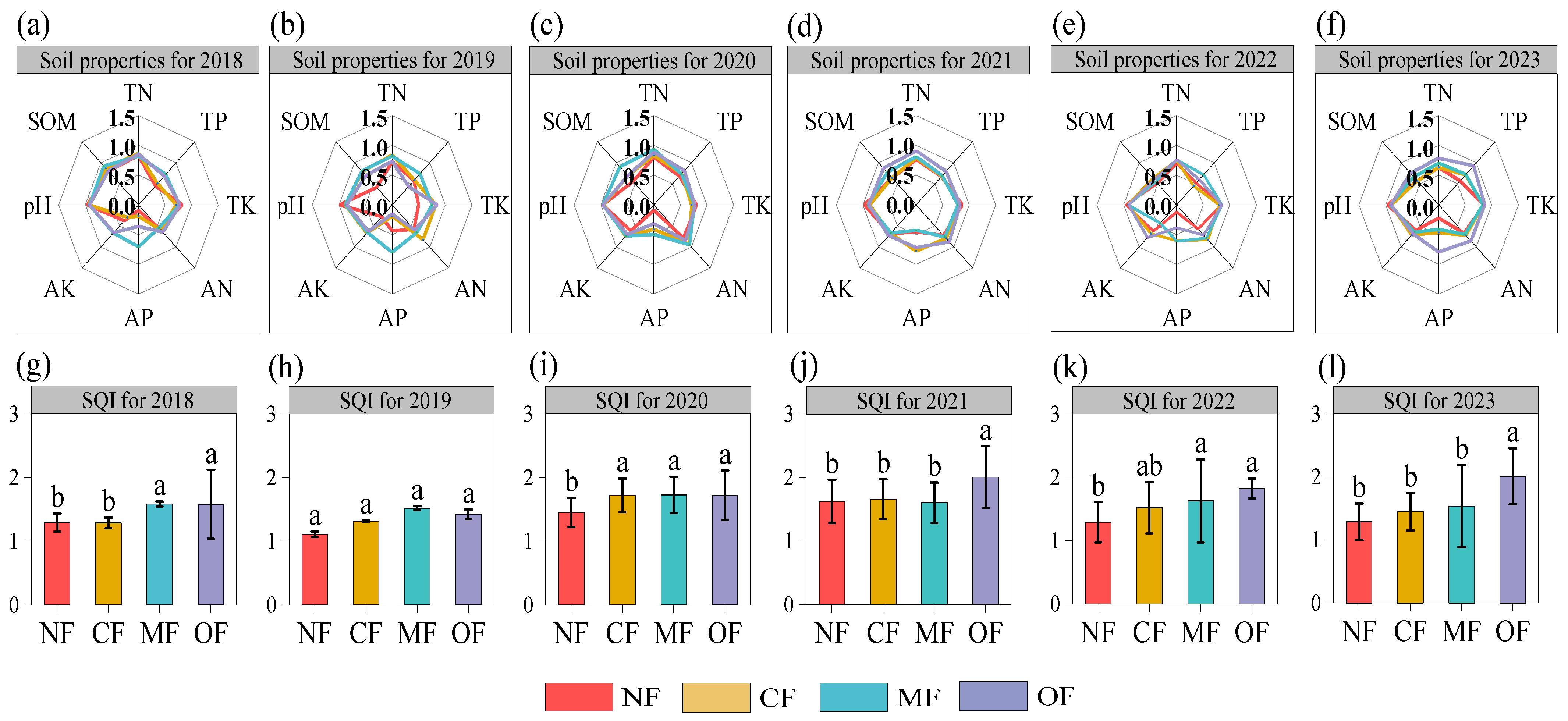
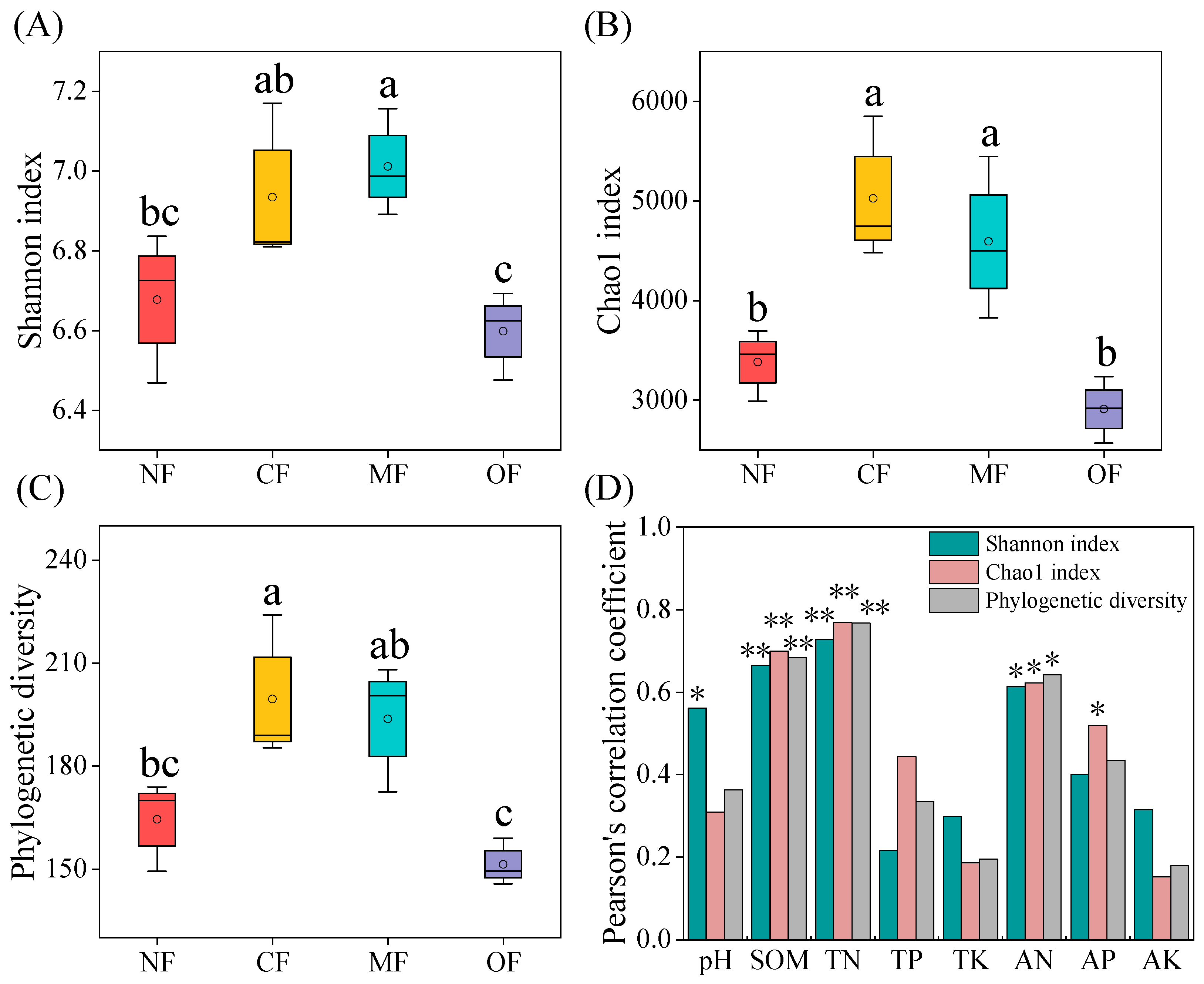
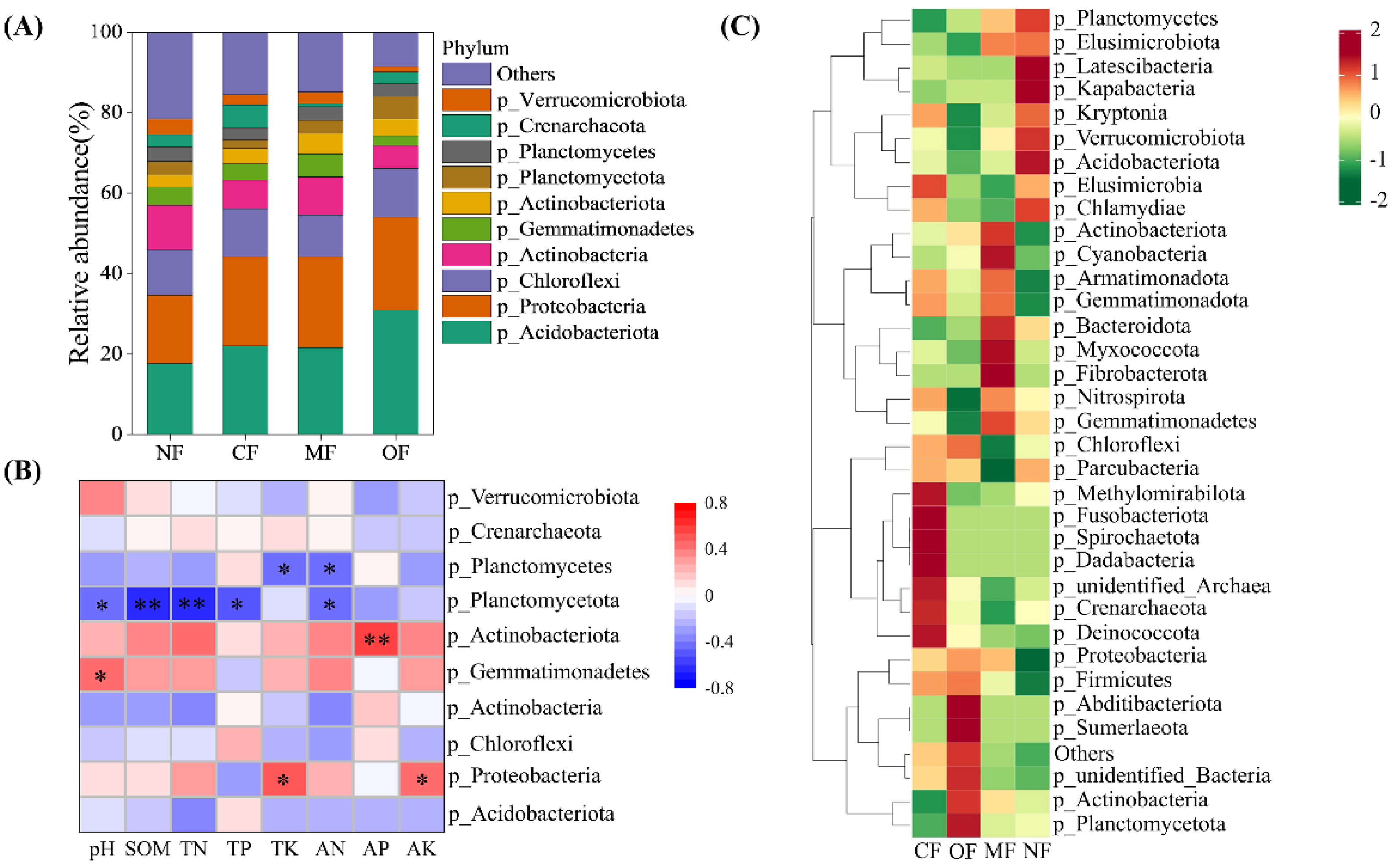

| Property | 0–20 cm | 20–40 cm |
|---|---|---|
| pH | 4.94 ± 0.37 | 4.91 ± 0.41 |
| TN (g kg−1) | 0.78 ± 0.02 | 0.61 ± 0.05 |
| TP (g kg−1) | 0.37 ± 0.15 | 0.34 ± 0.02 |
| TK (g kg−1) | 1.67 ± 0.17 | 1.60 ± 0.34 |
| SOM (g kg−1) | 10.30 ± 1.59 | 8.24 ± 1.28 |
| AN (mg kg−1) | 58.60 ± 7.74 | 90.4 ± 12.33 |
| AP (mg kg−1) | 10.40 ± 2.15 | 5.72 ± 0.39 |
| AK (mg kg−1) | 382.00 ± 32.48 | 300.00 ± 25.75 |
| Treatment | Description | Chemical N Amount (Kg Ha−1) | Organic N Amount (Kg ha−1) | OSR (%) |
|---|---|---|---|---|
| NF | No fertilization | 0 | 0 | — |
| CF | Conventional fertilization | 777 | 0 | — |
| MF | Microbial organic fertilizer + formula fertilizer + urea | 387 | 165 | 50 |
| OF | Special organic fertilizer + formula fertilizer +urea | 387 | 165 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, C.; Xiang, F.; Liu, H.; Zhou, L.; Li, W. The Prolonged Application of Organic Fertilizers Increases the Quality and Yield of Tea Crops. Plants 2025, 14, 1317. https://doi.org/10.3390/plants14091317
Dai C, Xiang F, Liu H, Zhou L, Li W. The Prolonged Application of Organic Fertilizers Increases the Quality and Yield of Tea Crops. Plants. 2025; 14(9):1317. https://doi.org/10.3390/plants14091317
Chicago/Turabian StyleDai, Cuiting, Fen Xiang, Hongyan Liu, Lingyun Zhou, and Wei Li. 2025. "The Prolonged Application of Organic Fertilizers Increases the Quality and Yield of Tea Crops" Plants 14, no. 9: 1317. https://doi.org/10.3390/plants14091317
APA StyleDai, C., Xiang, F., Liu, H., Zhou, L., & Li, W. (2025). The Prolonged Application of Organic Fertilizers Increases the Quality and Yield of Tea Crops. Plants, 14(9), 1317. https://doi.org/10.3390/plants14091317






