Selenium Alleviates Cadmium Toxicity in Pepper (Capsicum annuum L.) by Reducing Accumulation, Enhancing Stress Resistance, and Promoting Growth
Abstract
1. Introduction
2. Results
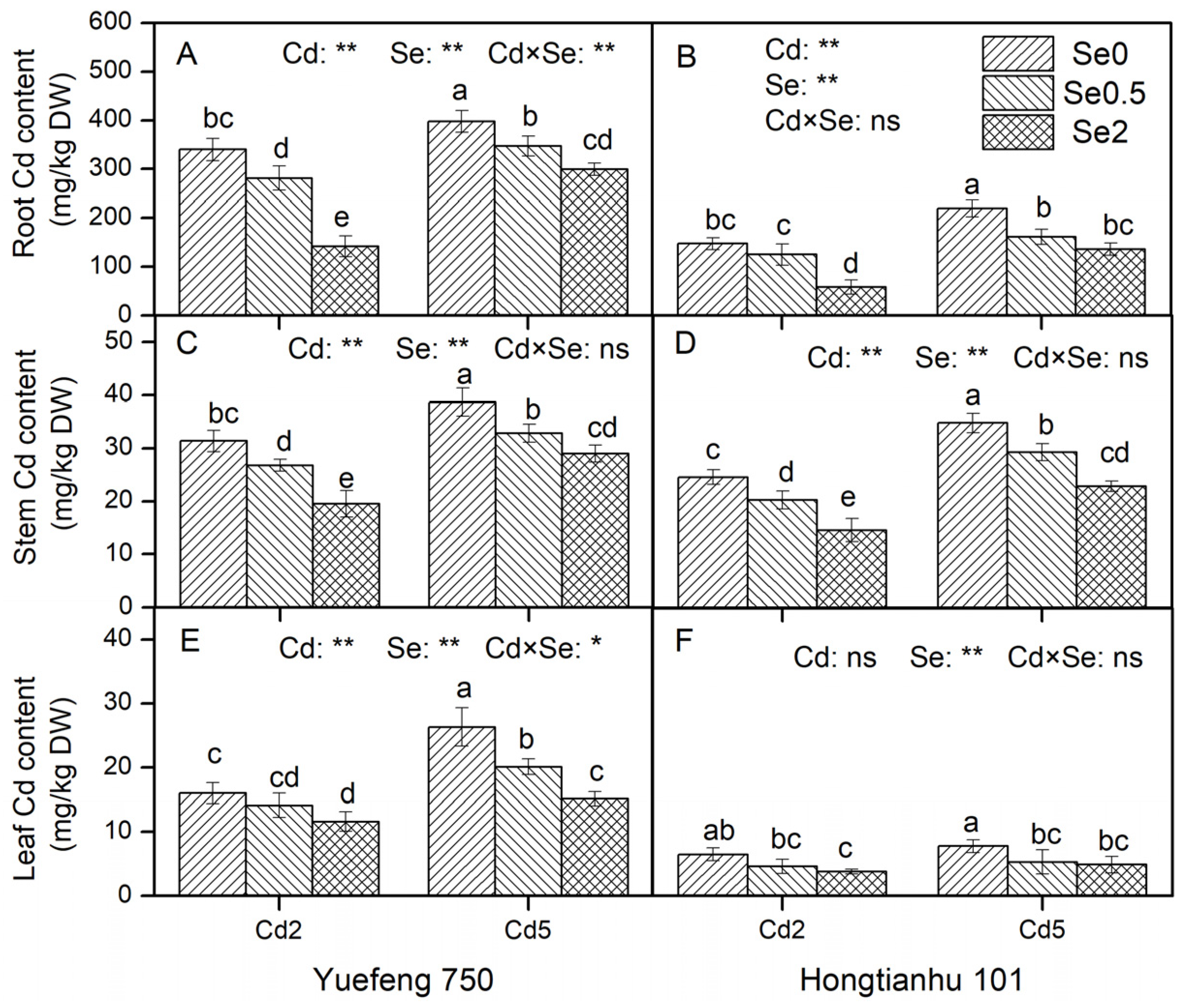
2.1. Characteristics of Cd and Se in Pepper Seedlings
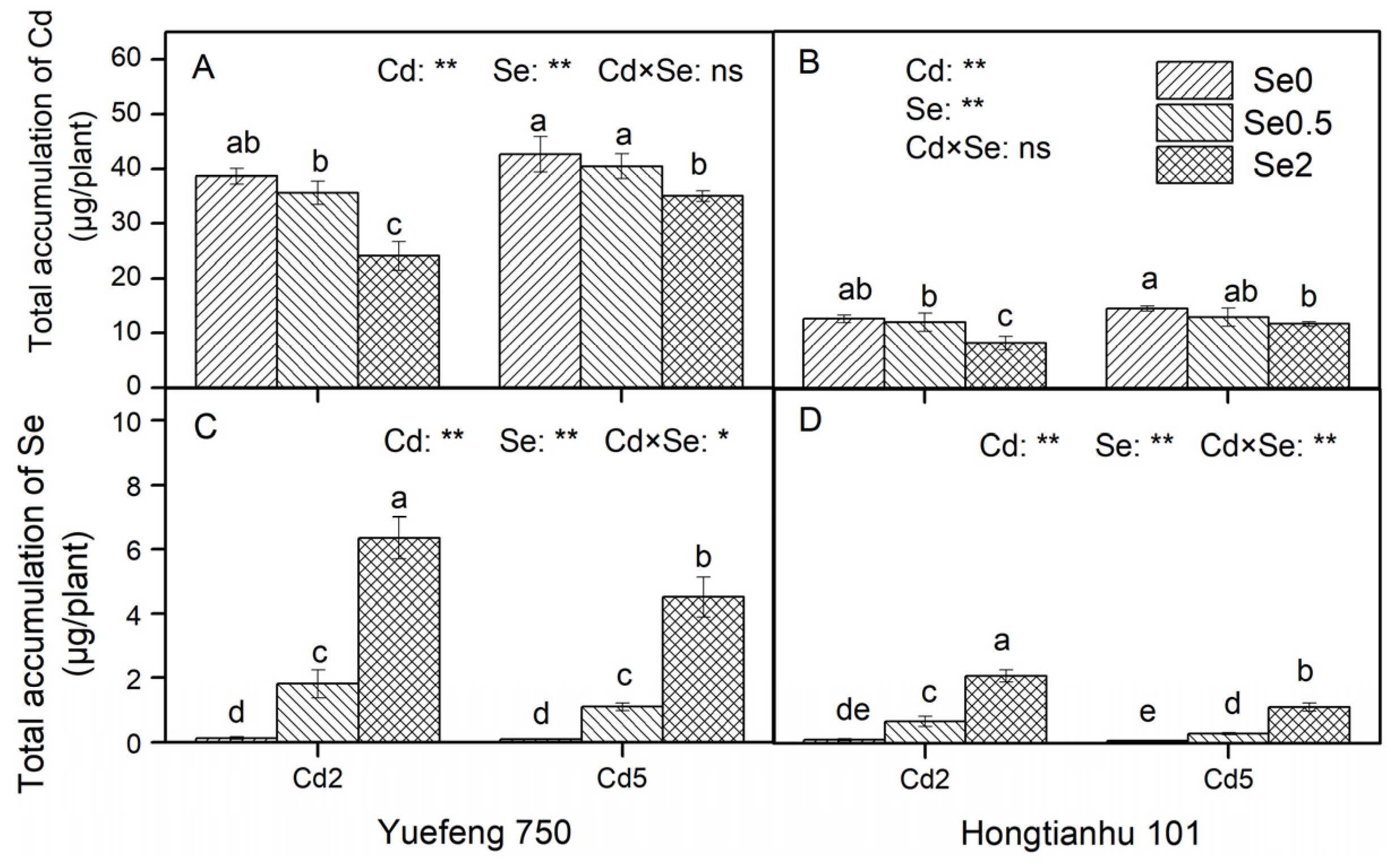
2.2. Total Accumulation of Cd and Se in Pepper Seedlings
2.3. Resistance Physiology
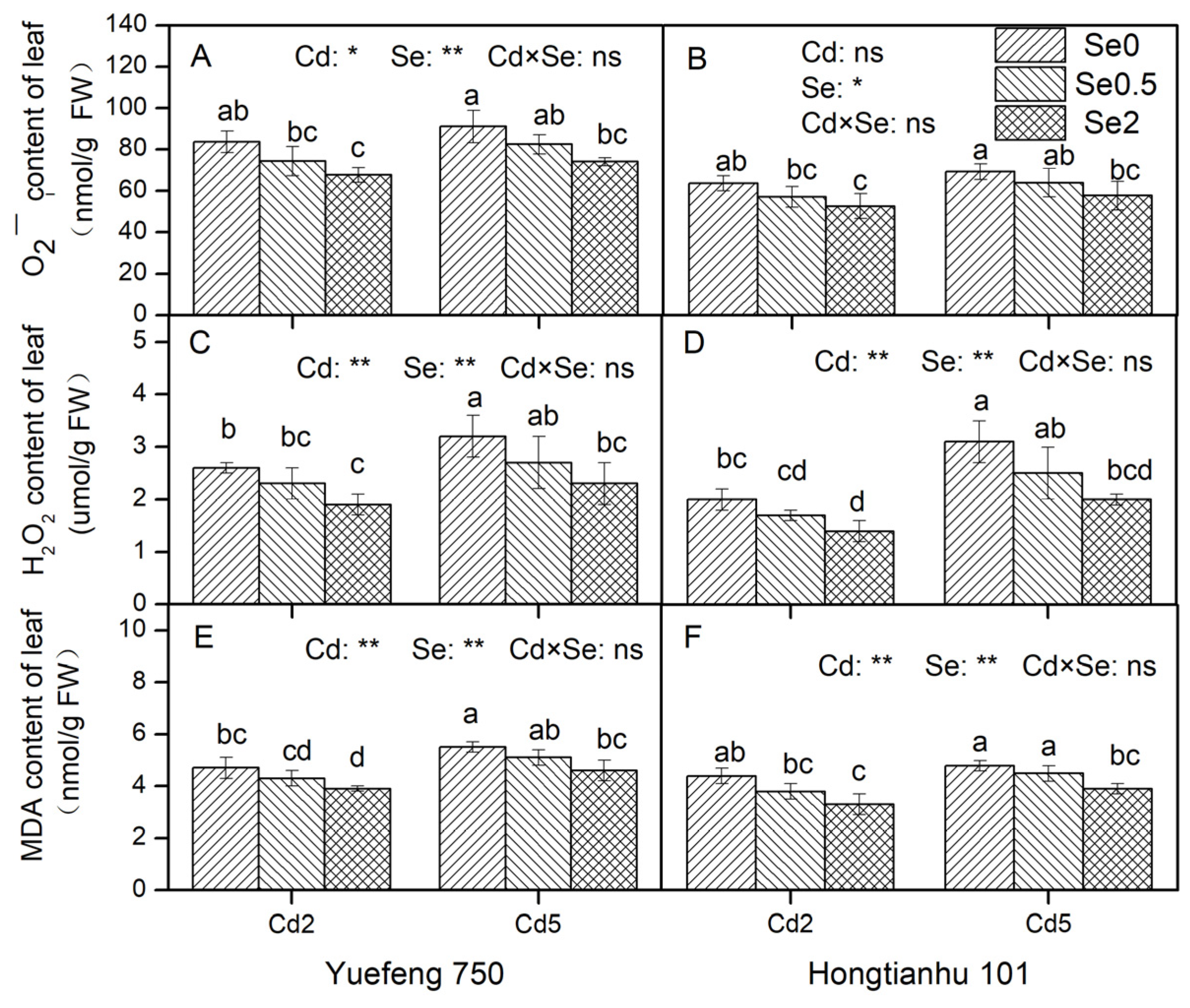
2.3.1. Analysis of O2−, H2O2, and MDA Contents
2.3.2. An Analysis of the Pro and ASA Contents
2.3.3. Analysis of Antioxidative Enzyme Activities: Superoxide Dismutase (SOD), Catalase (CAT), and Peroxidase (POD)
2.3.4. An Analysis of the ABA and ZR Contents and the IAAO Activity
2.4. Changes in the Characteristics of Roots
2.5. Changes in the Characteristics of Leaves
2.6. Plant Type
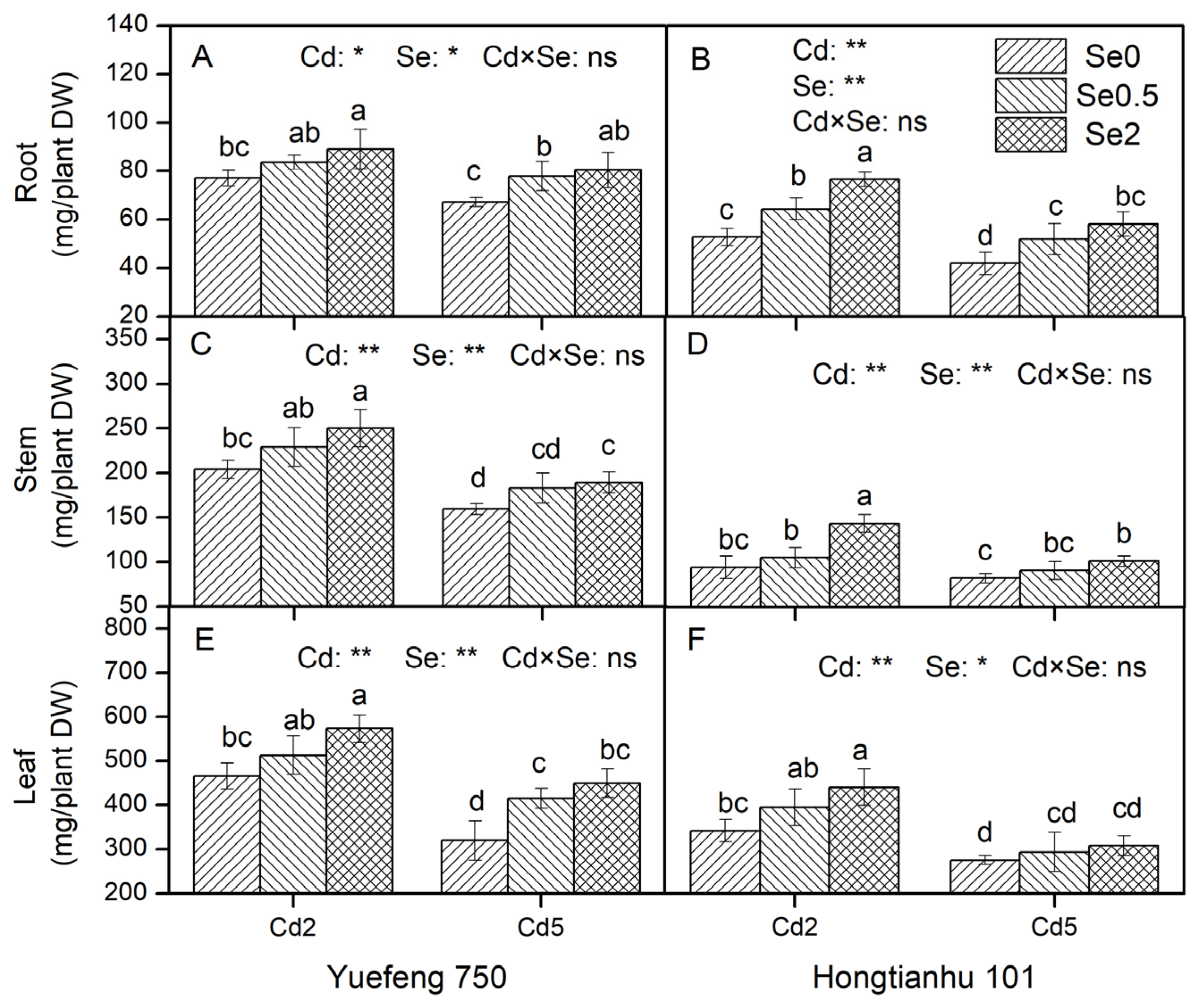
2.7. Biomass Production
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.2. Experimental Design
4.3. Plant Sampling and Analysis
4.3.1. Determination of Pepper Plant Type
4.3.2. Determination of Root Activity and Chloroplast Pigment Content
4.3.3. Dry Matter Production
4.3.4. Determination of the Total Se and Cd Contents in the Plants
4.3.5. Resistance Physiological-Related Indicators
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, B.; Lin, Q.; Hamid, Y.; Sanaullah, M.; Di, L.; Hashmi, M.; Khan, M.B.; He, Z.; Yang, X. Foliage application of selenium and silicon nanoparticles alleviates Cd and Pb toxicity in rice (Oryza sativa L.). Sci. Total Environ. 2020, 712, 136497. [Google Scholar] [CrossRef]
- China Ministry of Environmental Protection (CMEP); China Ministry of Land and Resources of (CMLR). Report on the National General Survey of Soil Contamination. Available online: https://www.mee.gov.cn/gkml/sthjbgw/qt/201404/t20140417_270670.htm (accessed on 17 April 2014).
- Lux, A.; Martinka, M.; Vaculík, M.; White, P.J. Root responses to cadmium in the rhizosphere: A review. J. Exp. Bot. 2011, 62, 21–37. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Zia-ur-Rehman, M.; Hannan, F.; Qayyum, M.F.; Hafeez, F.; Ok, Y.S. Cadmium stress in rice: Toxic effects, tolerance mechanisms, and management: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 17859–17879. [Google Scholar] [CrossRef]
- Dermont, G.; Bergeron, M.; Mercier, G.; Richer-Laflèche, M. Soil washing for metal removal: A review of physical/chemical technologies and field applications. J. Hazard. Mater. 2008, 152, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, L.; Liao, P.; Xiong, Q.; Deng, X.; Gao, H.; Wei, H.; Dai, Q.; Pan, X.; Zeng, Y. Effects of biochar on the dynamic immobilization of Cd and Cu and rice accumulation in soils with different acidity levels. J. Clean. Prod. 2022, 372, 133730. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, X.; Sun, X.; Tan, Q.; Tang, Y.; Nie, Z.; Hu, C. Xylem transport and gene expression play decisive roles in cadmium accumulation in shoots of two oilseed rape cultivars (Brassica napus). Chemosphere 2015, 119, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Liu, G.; Yousaf, B.; Ali, M.U.; Abbas, Q.; Munir, M.A.M.; Mian, M.M. Developmental selenium exposure and health risk in daily foodstuffs: A systematic review and meta-analysis. Ecotoxicol. Environ. Saf. 2018, 149, 291–306. [Google Scholar] [CrossRef]
- Shanker, K.; Mishra, S.; Srivastava, S.; Srivastava, R.; Dass, S.; Prakash, S.; Srivastava, M.M. Effect of Selenite and Selenate on Plant Uptake of Cadmium by Maize (Zea mays). Bull. Environ. Contam. Toxicol. 1996, 56, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Wang, B.; Ali, S.; Ghani, M.A.; Hayat, M.T.; Yang, C.; Xu, L.; Zhou, W.J. 5-Aminolevulinic Acid Ameliorates the Growth, Photosynthetic Gas Exchange Capacity, and Ultrastructural Changes Under Cadmium Stress in Brassica napus L. J. Plant Growth Regul. 2013, 32, 604–614. [Google Scholar] [CrossRef]
- Jia, H.; Song, Z.; Wu, F.; Ma, M.; Li, Y.; Han, D.; Yang, Y.; Zhang, S.; Cui, H. Low selenium increases the auxin concentration and enhances tolerance to low phosphorous stress in tobacco. Environ. Exp. Bot. 2018, 153, 127–134. [Google Scholar] [CrossRef]
- Lin, L.; Zhou, W.; Dai, H.; Cao, F.; Zhang, G.; Wu, F. Selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. J. Hazard. Mater. 2012, 235–236, 343–351. [Google Scholar] [CrossRef]
- Adeel, S.; Raza, M.; Iqbal, H.; Ahmad, R.; Sheraz, M.; Ahmed, M.; Azmi, U.; Naeem, M. Effects of Foliar Application of Selenium in Maize (Zea mays L.) under Cadmium Toxicity. Biol. Forum-Int. J. 2019, 11, 27–37. [Google Scholar]
- Alyemeni, M.N.; Ahanger, M.A.; Wijaya, L.; Alam, P.; Bhardwaj, R.; Ahmad, P. Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma 2018, 255, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Sardar, R.; Ahmed, S.; Shah, A.A.; Yasin, N.A. Selenium nanoparticles reduced cadmium uptake, regulated nutritional homeostasis and antioxidative system in Coriandrum sativum grown in cadmium toxic conditions. Chemosphere 2022, 287, 132332. [Google Scholar] [CrossRef]
- Zou, X.; Zhu, F. Origin, Evolution and Cultivation History of the Pepper. Acta Hortic. Sin. 2022, 49, 1371–1381. [Google Scholar] [CrossRef]
- Yang, J.; Guo, H.; Ma, Y.; Wang, L.; Wei, D.; Hua, L. Genotypic variations in the accumulation of Cd exhibited by different vegetables. J. Environ. Sci. 2010, 22, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wang, K.; Liu, Z.; Yu, Y.; Wang, Q.; Li, H. Effect of selenium on the subcellular distribution of cadmium and oxidative stress induced by cadmium in rice (Oryza sativa L.). Environ. Sci. Pollut. Res. Int. 2019, 26, 16220–16228. [Google Scholar] [CrossRef]
- Xie, Y.; Su, L.; He, Z.; Zhang, J.; Tang, Y. Selenium Inhibits Cadmium Absorption and Improves Yield and Quality of Cherry Tomato (Lycopersicon esculentum) Under Cadmium Stress. J. Soil Sci. Plant Nutr. 2021, 21, 1125–1133. [Google Scholar] [CrossRef]
- Wang, D.; Xia, X.; Wu, S.; Zheng, S.; Wang, G. The essentialness of glutathione reductase GorA for biosynthesis of Se(0)-nanoparticles and GSH for CdSe quantum dot formation in Pseudomonas stutzeri TS44. J. Hazard. Mater. 2019, 366, 301–310. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, C.; Wu, Z.; Liu, X.; Cai, M.; Jia, W.; Zhao, X. Selenium reduces cadmium accumulation in seed by increasing cadmium retention in root of oilseed rape (Brassica napus L.). Environ. Exp. Bot. 2019, 158, 161–170. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, C.; Wang, H.; Zhang, F. Effect of Si on the distribution of Cd in rice seedlings. Plant Soil 2005, 272, 53–60. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Taamalli, M.; Gevi, F.; Timperio, A.M.; Zolla, L.; Ghnaya, T. Cadmium stress responses in Brassica juncea: Hints from proteomics and metabolomics. J. Proteome research 2013, 12, 4979–4997. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Dong, Y.Y.; Feng, L.Y.; Deng, Z.L.; Xu, Q.; Tao, Q.; Wang, C.Q.; Chen, Y.E.; Yuan, M.; Yuan, S. Selenium Enhances Cadmium Accumulation Capability in Two Mustard Family Species—Brassica napus and B. juncea. Plants 2020, 9, 904. [Google Scholar] [CrossRef]
- Kumar, M.; Kumari, P.; Gupta, V.; Anisha, P.A.; Reddy, C.R.K.; Jha, B. Differential responses to cadmium induced oxidative stress in marine macroalga Ulva lactuca (Ulvales, Chlorophyta). BioMetals 2010, 23, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Hartikainen, H.; Xue, T.; Piironen, V. Selenium as an anti-oxidant and pro-oxidant in ryegrass. Plant Soil 2000, 225, 193–200. [Google Scholar] [CrossRef]
- Saidi, I.; Chtourou, Y.; Djebali, W. Selenium alleviates cadmium toxicity by preventing oxidative stress in sunflower (Helianthus annuus) seedlings. J. Plant Physiol. 2014, 171, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Abd-Allah, E.F.; Abeer, H.; Alqarawi, A.A. Mitigation of cadmium induced stress in tomato (Solanum lycopersicum L.) by selenium. Pak. J. Bot. 2016, 48, 953–961. [Google Scholar]
- Kermanian, H.; Namdjoyan, S.; Namdjoyan, S. Induction of phytochelatin and responses of antioxidants under cadmium stress in safflower (Carthamus tinctorius) seedlings. Turk. J. Bot. 2012, 23, 315–325. [Google Scholar] [CrossRef]
- Seppänen, M.; Turakainen, M.; Hartikainen, H. Selenium effects on oxidative stress in potato. Plant Sci. 2003, 165, 311–319. [Google Scholar] [CrossRef]
- Mendoza-Cózatl, D.; Loza-Tavera, H.; Hernández-Navarro, A.; Moreno-Sánchez, R. Sulfur assimilation and glutathione metabolism under cadmium stress in yeast, protists and plants. FEMS Microbiol. Rev. 2005, 29, 653–671. [Google Scholar] [CrossRef]
- Mohan, T.C.; Castrillo, G.; Navarro, C.; Zarco-Fernández, S.; Ramireddy, E.; Mateo, C.; Zamarreño, A.M.; Leyva, A. Cytokinin Determines Thiol-Mediated Arsenic Tolerance and Accumulation. Plant Physiol. 2016, 171, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How Plant Hormones Mediate Salt Stress Responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Wang, X.; Lu, W.; Zhao, Z.; Hao, W.; Du, R.; Li, Z.; Tang, Y.; Lin, L. Abscisic acid promotes selenium absorption, metabolism and toxicity via stress-related phytohormones regulation in Cyphomandra betacea Sendt. (Solanum betaceum Cav.). J. Hazard. Mater. 2024, 461, 132642. [Google Scholar] [CrossRef]
- Lehotai, N.; Kolbert, Z.; Peto, A.; Feigl, G.; Ördög, A.; Kumar, D.; Tari, I.; Erdei, L. Selenite-induced hormonal and signalling mechanisms during root growth of Arabidopsis thaliana L. J. Exp. Bot. 2012, 63, 5677–5687. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wei, Y.; Sun, S.; Wang, J.; Wang, W.; Han, D.; Shao, H.; Jia, H.; Fu, Y. Selenium Modulates the Level of Auxin to Alleviate the Toxicity of Cadmium in Tobacco. Int. J. Mol. Sci. 2019, 20, 3772. [Google Scholar] [CrossRef]
- Lee, S.H.; Ahsan, N.; Lee, K.W.; Kim, D.H.; Lee, D.G.; Kwak, S.S.; Kwon, S.Y.; Kim, T.H.; Lee, B.H. Simultaneous overexpression of both CuZn superoxide dismutase and ascorbate peroxidase in transgenic tall fescue plants confers increased tolerance to a wide range of abiotic stresses. J. Plant Physiol. 2007, 164, 1626–1638. [Google Scholar] [CrossRef] [PubMed]
- Pedrero, Z.; Madrid, Y.; Hartikainen, H.; Cámara, C. Protective effect of selenium in Broccoli (Brassica oleracea) plants subjected to cadmium exposure. J. Agric. Food Chem. 2008, 56, 266–271. [Google Scholar] [CrossRef]
- Zembala, M.; Filek, M.; Walas, S.; Mrowiec, H.; Kornaś, A.; Miszalski, Z.; Hartikainen, H. Effect of selenium on macro- and microelement distribution and physiological parameters of rape and wheat seedlings exposed to cadmium stress. Plant Soil 2010, 329, 457–468. [Google Scholar] [CrossRef]
- González-Chávez, O.; Alejo-Santiago, G.; Juárez-Rosete, C.R.; Arrieta-Ramos, B.G.J.J.o.P.N. Selenium concentration and method of application modulate growth and development of bell pepper (Capsicum annuum L.). J. Plant Nutr. 2024, 40, 2373–2388. [Google Scholar] [CrossRef]
- Huang, F.; Chen, L.; Zhou, Y.; Huang, J.; Wu, F.; Hu, Q.; Chang, N.; White, J.C.; Yang, W.; Fang, L. Exogenous selenium promotes cadmium reduction and selenium enrichment in rice: Evidence, mechanisms, and perspectives. J. Hazard. Mater. 2024, 476, 135043. [Google Scholar] [CrossRef]
- Sun, H.; Ha, J.; Liang, S.-X.; Kang, W.-J.J.C.I.S.S.; Analysis, P. Protective Role of Selenium on Garlic Growth under Cadmium Stress. Commun. Soil Sci. Plant Anal. 2010, 41, 1195–1204. [Google Scholar] [CrossRef]
- Ding, Y.; Feng, R.; Wang, R.; Guo, J.; Zheng, X. A dual effect of Se on Cd toxicity: Evidence from plant growth, root morphology and responses of the antioxidative systems of paddy rice. Plant Soil 2014, 375, 289–301. [Google Scholar] [CrossRef]
- Li, L.; Wang, S.; Wu, S.; Rao, S.; Li, L.; Cheng, S.; Cheng, H. Morphological and Physiological Indicators and Transcriptome Analyses Reveal the Mechanism of Selenium Multilevel Mitigation of Cadmium Damage in Brassica juncea. Plants 2023, 12, 1583. [Google Scholar] [CrossRef] [PubMed]
- Pennanen, A.H.; Xue, T.; Hartikainen, H.J.J.o.a.b. Protective role of selenium in plant subjected to severe UV irradiation stress. J. Appl. Bot. 2002, 76, 66–76. [Google Scholar]
- Feng, R.W.; Wei, C.Y.J.P. Antioxidative mechanisms on selenium accumulation in Pteris vittata L., a potential selenium phytoremediation plant. Plant Soil Environ. 2012, 58, 105–110. [Google Scholar] [CrossRef]
- Yamauchi, T.; Watanabe, K.; Fukazawa, A.; Mori, H.; Abe, F.; Kawaguchi, K.; Oyanagi, A.; Nakazono, M. Ethylene and reactive oxygen species are involved in root aerenchyma formation and adaptation of wheat seedlings to oxygen-deficient conditions. J. Exp. Bot. 2014, 65, 261–273. [Google Scholar] [CrossRef]

| Variety | Treatment | Leaf Area (cm2/Plant) | Chlorophyll a (mg/g FW) | Chlorophyll b (mg/g FW) | Carotenoids (mg/g FW) |
|---|---|---|---|---|---|
| Yuefeng 750 | Cd2 Se0 | 187.4 ± 13.1 bc | 1.91 ± 0.11 bc | 0.53 ± 0.04 bc | 0.42 ± 0.03 cd |
| Cd2 Se0.5 | 215.1 ± 16.3 ab | 2.06 ± 0.14 ab | 0.58 ± 0.07 ab | 0.44 ± 0.02 bc | |
| Cd2 Se2 | 228.6 ± 15.0 a | 2.27 ± 0.17 a | 0.63 ± 0.04 a | 0.51 ± 0.04 a | |
| Cd5 Se0 | 158.0 ± 9.3 c | 1.77 ± 0.11 c | 0.47 ± 0.03 c | 0.39 ± 0.02 d | |
| Cd5 Se0.5 | 186.1 ± 19.2 bc | 1.90 ± 0.07 bc | 0.52 ± 0.04 bc | 0.43 ± 0.03 bcd | |
| Cd5 Se2 | 204.7 ± 23.8 ab | 2.12 ± 0.11 ab | 0.59 ± 0.04 ab | 0.49 ± 0.03 ab | |
| Hongtianhu 101 | Cd2 Se0 | 143.8 ± 5.3 c | 2.09 ± 0.05 bc | 0.63 ± 0.03 bc | 0.44 ± 0.02 cd |
| Cd2 Se0.5 | 193.3 ± 13.0 b | 2.18 ± 0.15 ab | 0.68 ± 0.02 ab | 0.47 ± 0.02 bc | |
| Cd2 Se2 | 224.8 ± 15.6 a | 2.41 ± 0.17 a | 0.69 ± 0.05 a | 0.52 ± 0.03 a | |
| Cd5 Se0 | 118.7 ± 10.2 d | 1.87 ± 0.12 c | 0.61 ± 0.01 c | 0.41 ± 0.04 d | |
| Cd5 Se0.5 | 147.4 ± 11.7 c | 2.04 ± 0.10 bc | 0.65 ± 0.01 abc | 0.45 ± 0.02 bcd | |
| Cd5 Se2 | 159.6 ± 14.1 c | 2.17 ± 0.14 ab | 0.67 ± 0.05 ab | 0.50 ± 0.03 ab | |
| Statistical significance | |||||
| Variety (V) | ** | ** | ** | * | |
| Cd | ** | ** | * | * | |
| Se | ** | ** | ** | ** | |
| V × Cd | ns | ns | ns | ns | |
| V × Se | ns | ns | ns | ns | |
| Cd × Se | ns | ns | ns | ns | |
| V × Cd × Se | ns | ns | ns | ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.; Liu, J.; Liu, J.; Gao, Z.; Yang, Y.; Zhu, B.; Yao, F.; Ye, Q. Selenium Alleviates Cadmium Toxicity in Pepper (Capsicum annuum L.) by Reducing Accumulation, Enhancing Stress Resistance, and Promoting Growth. Plants 2025, 14, 1291. https://doi.org/10.3390/plants14091291
Cheng C, Liu J, Liu J, Gao Z, Yang Y, Zhu B, Yao F, Ye Q. Selenium Alleviates Cadmium Toxicity in Pepper (Capsicum annuum L.) by Reducing Accumulation, Enhancing Stress Resistance, and Promoting Growth. Plants. 2025; 14(9):1291. https://doi.org/10.3390/plants14091291
Chicago/Turabian StyleCheng, Chen, Jianxiu Liu, Jiahui Liu, Zhiqiang Gao, Yang Yang, Bo Zhu, Fengxian Yao, and Qing Ye. 2025. "Selenium Alleviates Cadmium Toxicity in Pepper (Capsicum annuum L.) by Reducing Accumulation, Enhancing Stress Resistance, and Promoting Growth" Plants 14, no. 9: 1291. https://doi.org/10.3390/plants14091291
APA StyleCheng, C., Liu, J., Liu, J., Gao, Z., Yang, Y., Zhu, B., Yao, F., & Ye, Q. (2025). Selenium Alleviates Cadmium Toxicity in Pepper (Capsicum annuum L.) by Reducing Accumulation, Enhancing Stress Resistance, and Promoting Growth. Plants, 14(9), 1291. https://doi.org/10.3390/plants14091291






