Strategies to Improve γ-Aminobutyric Acid Biosynthesis in Rice via Optimal Conditions
Abstract
1. Introduction
2. Results and Analysis
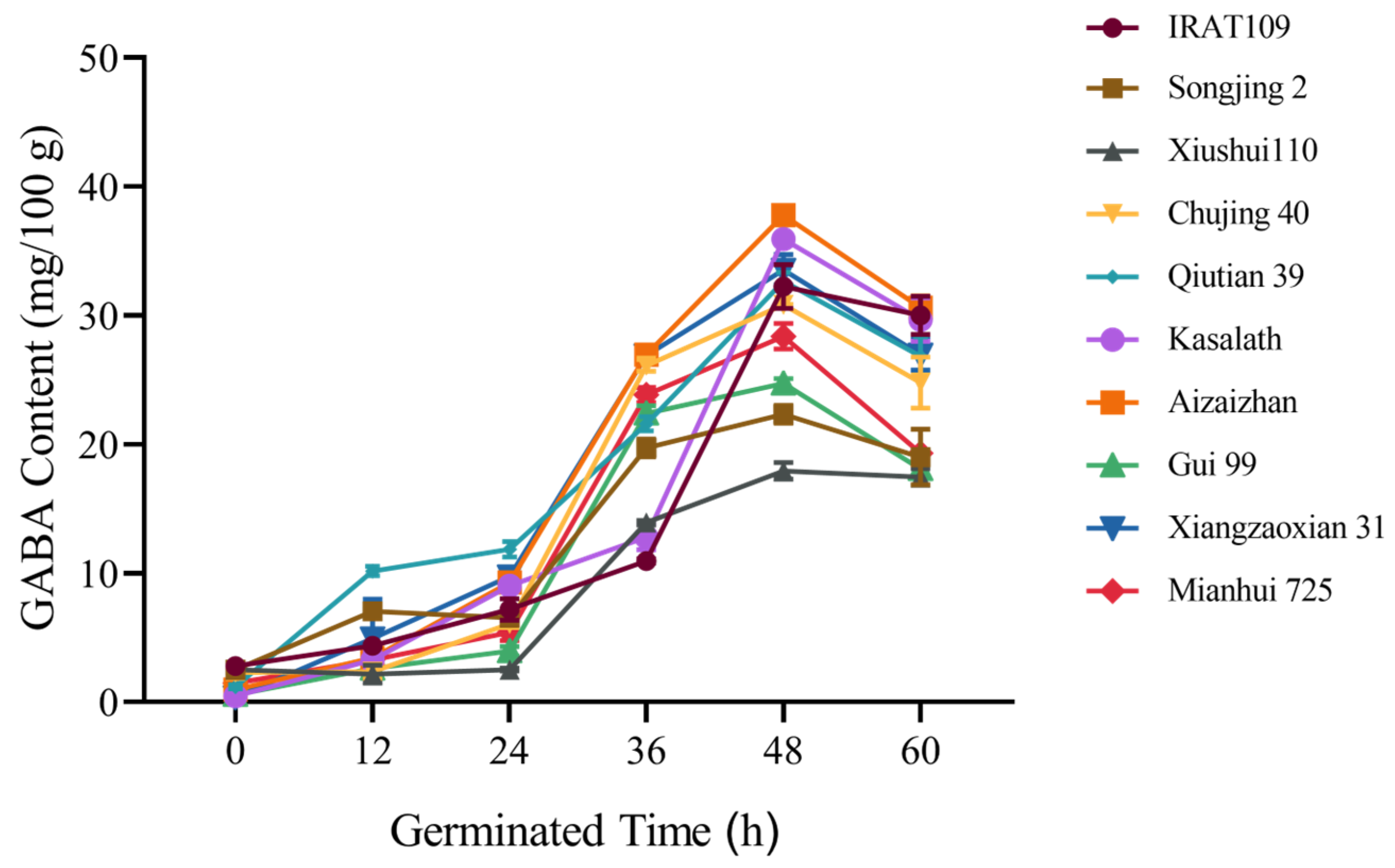
2.1. Effect of Germination Time on GABA Content
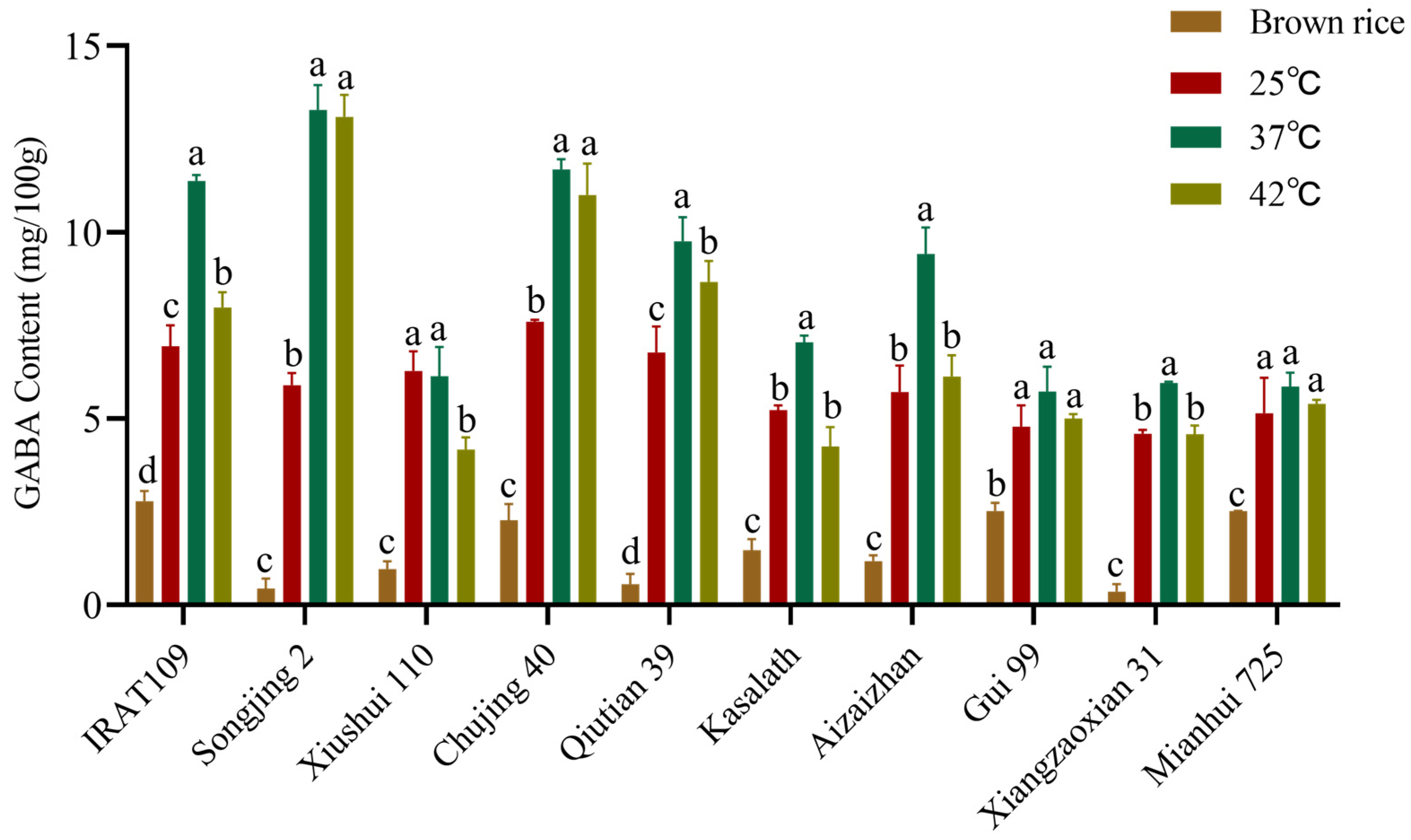
2.2. Effect of Germination Temperature on GABA Content
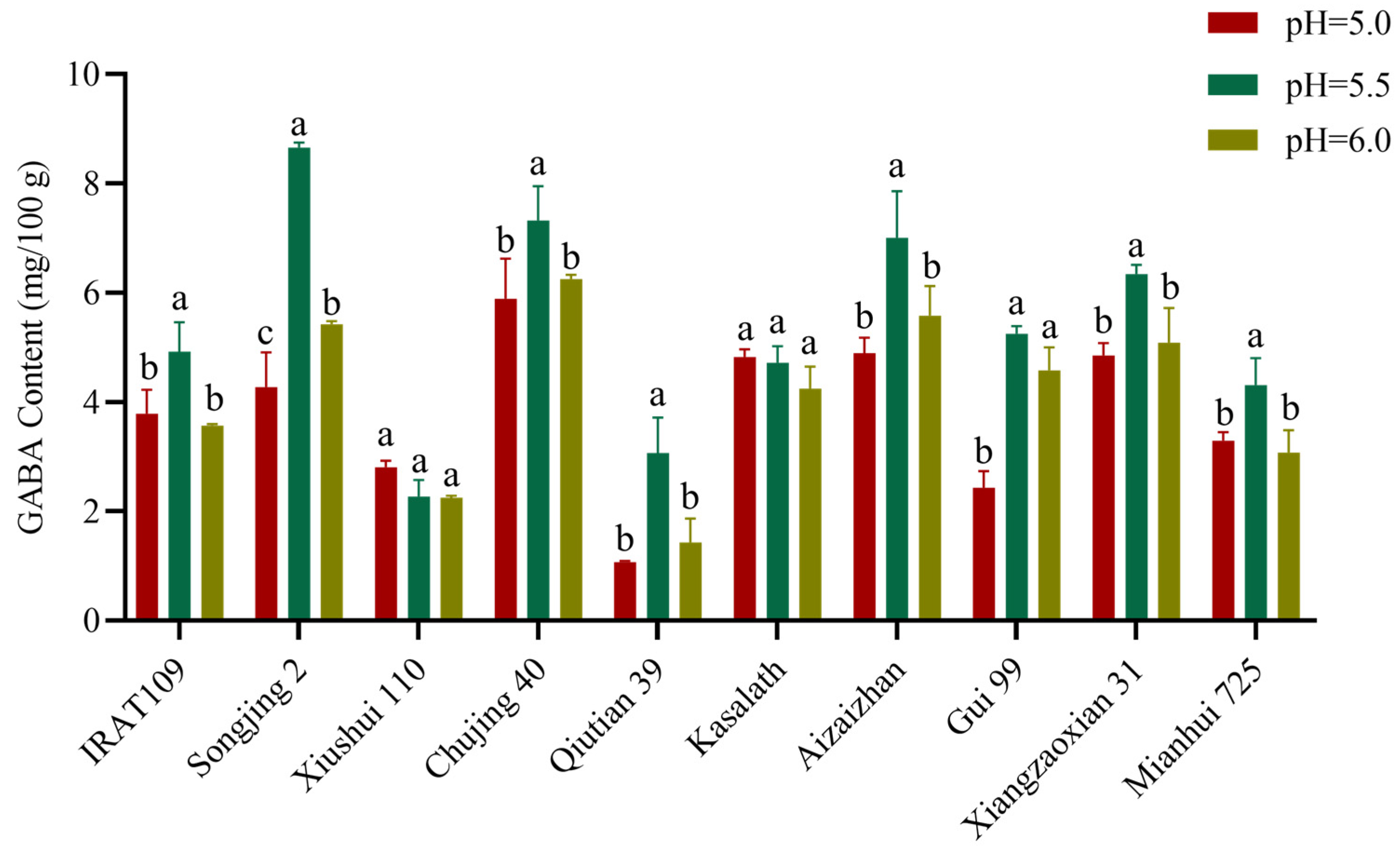
2.3. Effect of pH on GABA Content
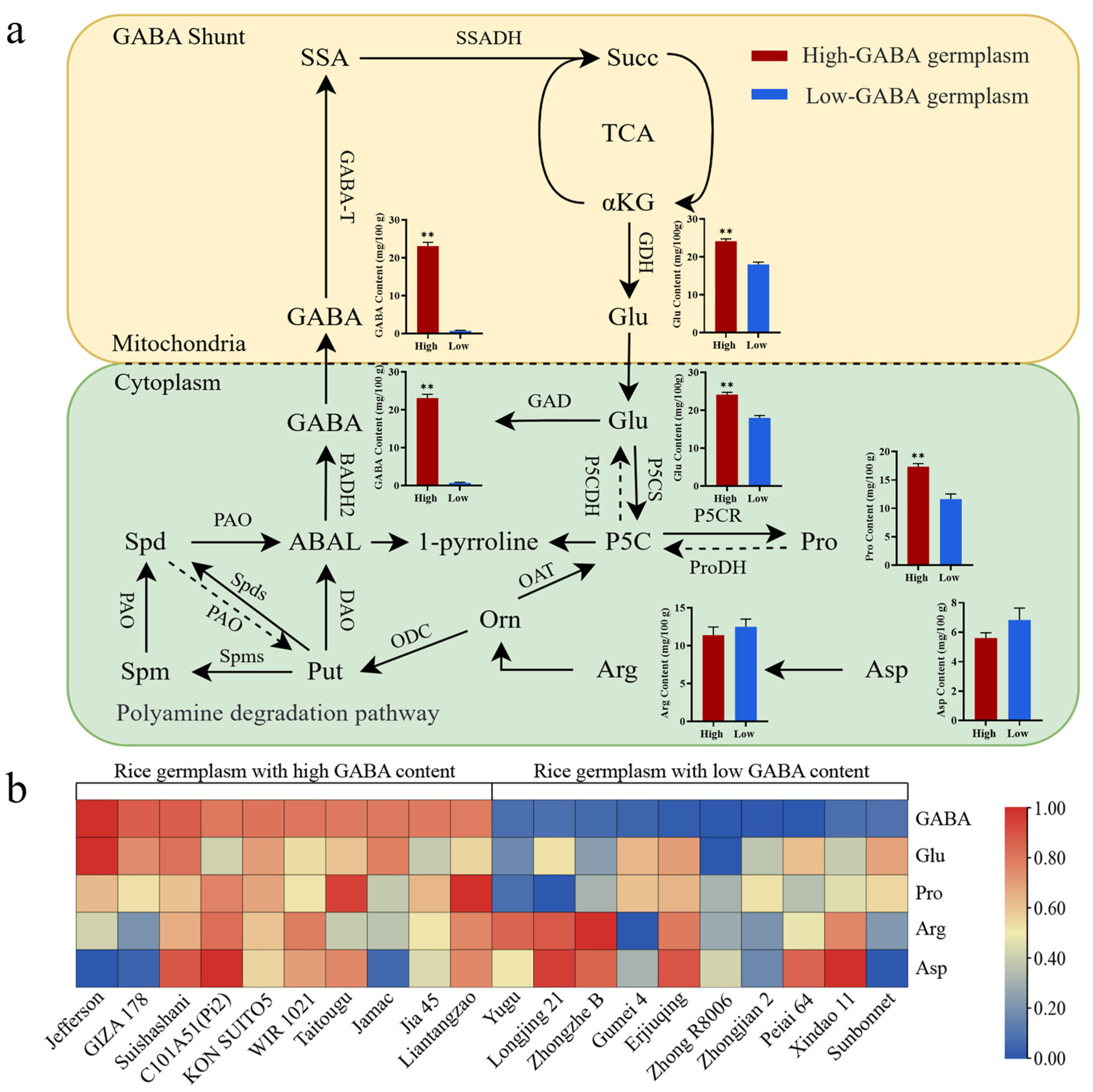
2.4. Analysis of the Difference of GABA Content Among Different Rice Germplasm Resources
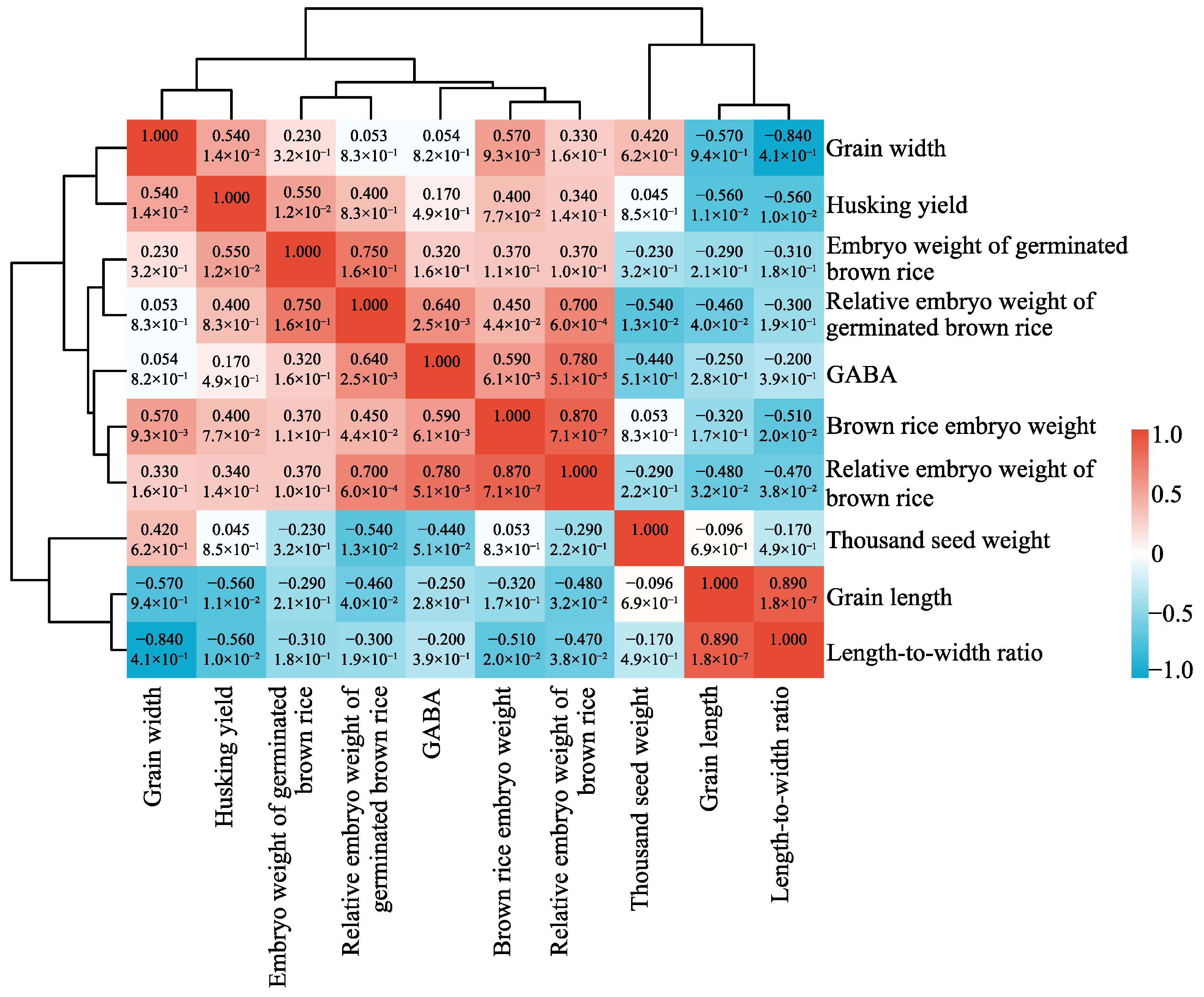
2.5. Correlation Analysis of GABA Content in Germinated Brown Rice with Brown Rice Morphology and Rice Embryo Traits
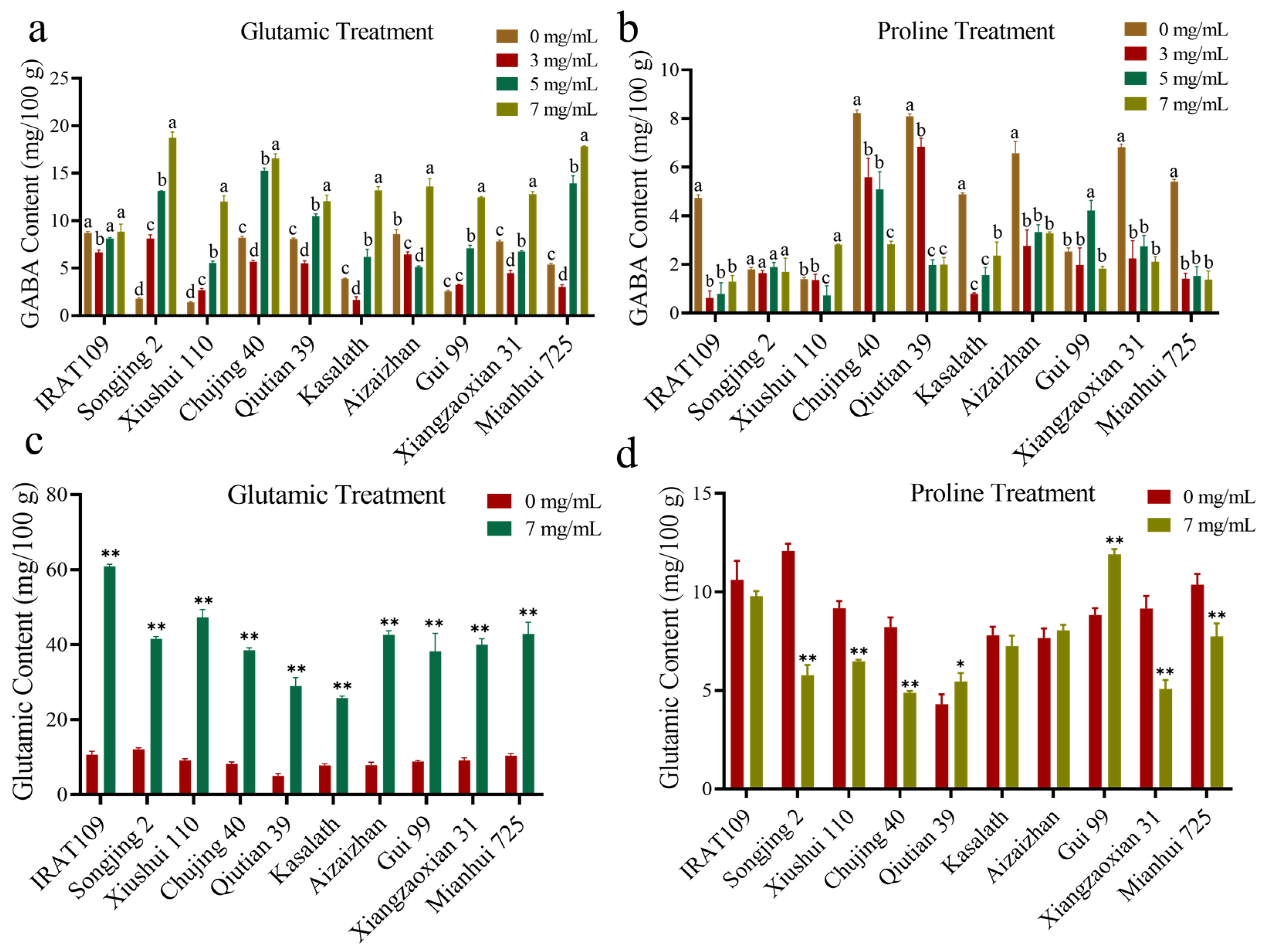
2.6. Relationship Between GABA Content and Amino Acid Content
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Germination Conditions and GABA Quantification
4.3. Determination of Grain Traits and Relative Embryo Weight
4.4. Determination of the Content of Amino Acids
4.4.1. Determination of Amino Acids in Rice
4.4.2. External Application of Different Concentrations of Amino Acids
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variety Name | Grain Length | Grain Width | Length-to-Width Ratio | GABA | Thousand-Seed Weight | Brown Rice Embryo Weight | Relative Embryo Weight of Brown Rice | Embryo Weight of Germinated Brown Rice | Relative Embryo Weight of Germinated Brown Rice | Husking Yield |
|---|---|---|---|---|---|---|---|---|---|---|
| Jefferson | 7.377 ± 0.210 | 2.245 ± 0.081 | 3.288 ± 0.091 | 39.240 | 21.159 ± 2.311 | 0.019 ± 0.002 | 0.032 ± 0.002 | 0.074 ± 0.003 | 0.094 ± 0.004 | 32.105 ± 3.210 |
| GIZA178 | 5.131 ± 0.150 | 2.319 ± 0.081 | 2.215 ± 0.113 | 25.820 | 17.551 ± 0.522 | 0.016 ± 0.001 | 0.033 ± 0.002 | 0.096 ± 0.002 | 0.147 ± 0.002 | 56.992 ± 4.111 |
| Suishashani | 5.918 ± 0.270 | 2.144 ± 0.084 | 2.762 ± 0.127 | 25.760 | 19.410 ± 1.702 | 0.022 ± 0.002 | 0.043 ± 0.003 | 0.073 ± 0.003 | 0.115 ± 0.005 | 32.59 ± 2.796 |
| C101A51(Pi2) | 5.266 ± 0.230 | 2.496 ± 0.103 | 2.112 ± 0.094 | 20.110 | 19.700 ± 0.433 | 0.018 ± 0.001 | 0.032 ± 0.001 | 0.076 ± 0.002 | 0.103 ± 0.002 | 59.638 ± 4.762 |
| KON SUIT05 | 6.163 ± 0.160 | 2.686 ± 0.083 | 2.296 ± 0.080 | 20.900 | 21.412 ± 1.171 | 0.025 ± 0.001 | 0.038 ± 0.002 | 0.090 ± 0.002 | 0.115 ± 0.003 | 66.631 ± 4.732 |
| WIR1021 | 6.297 ± 0.220 | 2.378 ± 0.074 | 2.650 ± 0.101 | 20.500 | 19.310 ± 1.420 | 0.026 ± 0.001 | 0.043 ± 0.001 | 0.133 ± 0.007 | 0.161 ± 0.009 | 49.359 ± 3.72 |
| Taitougu | 6.250 ± 0.270 | 1.961 ± 0.066 | 3.189 ± 0.156 | 19.960 | 16.750 ± 0.710 | 0.016 ± 0.001 | 0.030 ± 0.003 | 0.093 ± 0.009 | 0.143 ± 0.015 | 50.905 ± 4.350 |
| Jamac | 7.367 ± 0.200 | 2.677 ± 0.063 | 2.754 ± 0.091 | 19.900 | 21.487 ± 1.582 | 0.024 ± 0.002 | 0.032 ± 0.003 | 0.092 ± 0.004 | 0.099 ± 0.003 | 58.023 ± 3.240 |
| Jia 45 | 5.174 ± 0.100 | 2.837 ± 0.091 | 1.825 ± 0.067 | 19.740 | 22.593 ± 1.01 | 0.024 ± 0.002 | 0.035 ± 0.003 | 0.095 ± 0.002 | 0.106 ± 0.002 | 70.239 ± 4.604 |
| Liantangzao | 5.669 ± 0.220 | 2.659 ± 0.091 | 2.136 ± 0.130 | 19.450 | 21.044 ± 1.555 | 0.020 ± 0.001 | 0.033 ± 0.002 | 0.109 ± 0.008 | 0.136 ± 0.011 | 49.713 ± 4.823 |
| Yugu | 8.305 ± 0.260 | 2.008 ± 0.058 | 4.140 ± 0.175 | 0.950 | 21.809 ± 1.186 | 0.009 ± 0.001 | 0.012 ± 0.001 | 0.069 ± 0.009 | 0.076 ± 0.004 | 5.448 ± 0.980 |
| Longjing21 | 5.531 ± 0.220 | 2.753 ± 0.103 | 2.012 ± 0.102 | 0.930 | 25.310 ± 3.390 | 0.019 ± 0.002 | 0.028 ± 0.003 | 0.068 ± 0.003 | 0.079 ± 0.005 | 53.810 ± 3.651 |
| Zhongzhe B | 6.945 ± 0.230 | 2.284 ± 0.094 | 3.047 ± 0.180 | 0.830 | 23.287 ± 1.511 | 0.019 ± 0.001 | 0.026 ± 0.001 | 0.096 ± 0.002 | 0.101 ± 0.002 | 59.853 ± 3.990 |
| Gumei4 | 5.914 ± 0.160 | 2.809 ± 0.102 | 2.108 ± 0.092 | 0.730 | 21.443 ± 2.721 | 0.017 ± 0.001 | 0.024 ± 0.001 | 0.075 ± 0.001 | 0.077 ± 0.001 | 40.989 ± 4.780 |
| Erjiuqing | 5.492 ± 0.24 | 2.570 ± 0.073 | 2.138 ± 0.102 | 0.590 | 22.813 ± 0.511 | 0.015 ± 0.001 | 0.024 ± 0.002 | 0.090 ± 0.004 | 0.111 ± 0.003 | 53.857 ± 2.691 |
| ZhongR8006 | 7.500 ± 0.20 | 2.063 ± 0.042 | 3.636 ± 0.114 | 0.520 | 22.846 ± 1.271 | 0.009 ± 0.001 | 0.014 ± 0.003 | 0.077 ± 0.001 | 0.091 ± 0.001 | 34.511 ± 3.282 |
| Zhongjian2 | 9.607 ± 0.27 | 2.268 ± 0.082 | 4.277 ± 0.181 | 0.520 | 21.227 ± 0.45 | 0.011 ± 0.001 | 0.016 ± 0.001 | 0.057 ± 0.003 | 0.066 ± 0.004 | 12.881 ± 1.345 |
| Peiai64 | 6.569 ± 0.22 | 1.991 ± 0.068 | 3.302 ± 0.121 | 0.490 | 18.465 ± 1.37 | 0.014 ± 0.002 | 0.026 ± 0.003 | 0.060 ± 0.001 | 0.088 ± 0.002 | 43.909 ± 4.791 |
| Xindao11 | 5.620 ± 0.18 | 2.508 ± 0.101 | 2.243 ± 0.081 | 0.950 | 20.196 ± 0.946 | 0.017 ± 0.002 | 0.026 ± 0.002 | 0.094 ± 0.009 | 0.102 ± 0.022 | 74.154 ± 4.346 |
| Sunbonnet | 7.267 ± 0.31 | 2.251 ± 0.121 | 3.238 ± 0.231 | 1.000 | 18.319 ± 1.324 | 0.012 ± 0.001 | 0.017 ± 0.002 | 0.101 ± 0.005 | 0.113 ± 0.006 | 57.877 ± 3.482 |
References
- Ngo, D.H.; Vo, T.S. An updated review on pharmaceutical properties of Gamma-Aminobutyric Acid. Molecules 2019, 24, 2678. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jo, K.; Hong, K.B.; Han, S.H.; Suh, H. GABA and l-theanine mixture decreases sleep latency and improves NREM sleep. Pharm. Biol. 2019, 57, 65–73. [Google Scholar] [CrossRef]
- Wu, F.; Yang, N.; Touré, A.; Jin, Z.; Xu, X. Germinated brown rice and its role in human health. Crit. Rev. Food Sci. Nutr. 2013, 53, 451–463. [Google Scholar] [CrossRef]
- Tufail, T.; Ain, H.B.U.; Virk, M.S.; Ashraf, J.; Ahmed, Z.; Khalil, A.A.; Rasheed, A.; Xu, B. GABA (γ-aminobutyric acid) enrichment and detection methods in cereals: Unlocking sustainable health benefits. Food Chem. 2025, 464, 141750. [Google Scholar] [CrossRef]
- Chinma, C.E.; Adedeji, O.E.; Jolayemi, O.S.; Ezeocha, V.C.; Ilowefah, M.A.; Rosell, C.M.; Adebo, J.A.; Wilkin, J.D.; Adebo, O.A. Impact of germination on the techno-functional properties, nutritional composition, and health-promoting compounds of brown rice and its products: A review. J. Food Sci. 2024, 89, 8–32. [Google Scholar] [CrossRef]
- Hou, D.; Tang, J.; Feng, Q.; Niu, Z.; Shen, Q.; Wang, L.; Zhou, S. Gamma-aminobutyric acid (GABA): A comprehensive review of dietary sources, enrichment technologies, processing effects, health benefits, and its applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 8852–8874. [Google Scholar] [CrossRef] [PubMed]
- Saikusa, T.; Okada, T.; Murai, H.; Ohmori, M.; Mori, Y.; Horino, T.; Itou, M.; Onoda, A. The effect of defatting with organic solvent on accumulation of γ-aminobutyric acid (GABA) in the rice germ. J. Jpn. Soc. Food Sci. 2001, 43, 196–201. [Google Scholar] [CrossRef]
- Yao, S.; Zheng, L.; Zhao, S.; Xiong, S. Effect of germination conditions on γ-aminobutyric acid content in germinated brown rice. Trans. Chin. Soc. Agric. Eng. 2006, 12, 211–215. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Chen, J.; Xia, C.; Deng, J.; Li, Y.; Li, J.; Li, H.; Liu, P. Preparation process of rich GABA germinated red brown rice in Jinchuan. Food Nutr. China 2017, 23, 45–47. [Google Scholar] [CrossRef]
- Bai, H.; Ma, X.; Cao, G.; Liu, X.; Han, L. Differences in the contents of nutrients and functional components in different types of special rice germplasm. J. Plant Genet. Resour. 2017, 18, 1013–1022. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Wang, H.; Sun, L.; Zhang, Y.; Tian, L.; Yang, S.; Li, P. Screening of γ-aminobutyric acid-rich rice germplasm and its correlation with grain traits. J. Plant Genet. Resour. 2016, 17, 1116–1122. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, P.; Tang, S.; Zhao, H.; Wu, D. Comparative studies on major nutritional components of rice with a giant embryo and a normal embryo. J. Food Biochem. 2005, 29, 653–661. [Google Scholar] [CrossRef]
- Fait, A.; Fromm, H.; Walter, D.; Galili, G.; Fernie, A.R. Highway or byway: The metabolic role of the GABA shunt in plants. Trends Plant Sci. 2007, 13, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Thitinunsomboon, S.; Keeratipibul, S.; Boonsiriwit, A. Enhancing gamma-aminobutyric acid content in germinated brown rice by repeated treatment of soaking and incubation. Food Sci. Technol. Int. 2013, 19, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Podlešáková, K.; Ugena, L.; Spíchal, L.; Doležal, K.; Nuria, D.D. Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. New Biotechnol. 2018, 48, 4853–4865. [Google Scholar] [CrossRef]
- Yang, R.; Geng, C.; Gu, Z. Activation and tempering on γ-aminobutyric acid accumulation and distribution in brown rice. J. Food Process. Preserv. 2016, 40, 1364–1369. [Google Scholar] [CrossRef]
- Cataldo, P.G.; Villegas, J.M.; Giori, D.S.G.; Saavedra, L.; Hebert, E.M. Enhancement of γ-aminobutyric acid (GABA) production by Lactobacillus brevis CRL 2013 based on carbohydrate fermentation. Int. J. Food Microbiol. 2020, 16, 108792. [Google Scholar] [CrossRef]
- Stover, P.J.; Field, M.S. Vitamin B-6. Adv. Nutr. 2015, 6, 132–133. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, S.; Wang, L.; Pan, Q. Effects of germination and aeration treatment following segmented moisture conditioning on the γ-aminobutyric acid accumulation in germinated brown rice. Int. J. Agric. Biol. Eng. 2020, 13, 234–240. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, N.; Wang, S.; Liu, Y.; Lan, H. Effects of cyclic cellulase conditioning and germination treatment on the γ-aminobutyric acid content and the cooking and taste qualities of germinated brown rice. Food Chem. 2019, 289, 232–239. [Google Scholar] [CrossRef]
- Ohtsubo, K.; Suzuki, K.; Yasui, Y.; Kasumi, T. Bio-functional components in the processed pre-germinated brown rice by a twin-screw extruder. J. Food Compos. Anal. 2005, 18, 303–316. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, G.; Wang, Y.; Liu, G.; Chen, W. The variation characteristics of free amino acid content during the germination of different varieties of brown rice. In Proceedings of the Sixth Member Conference and Academic Seminar of Guangdong Food Society, Guangzhou, China, 30 March 2012. [Google Scholar]
- Wang, L.; Ding, G.; Li, L. Research progress of proline metabolism. J. Nat. Sci. Harbin Norm. Univ. 2010, 26, 84–89. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Y.; Gu, Z.; Li, B. Effects of exogenous monosodium glutamate and ascorbic acid on GABA accumulation in germinated brown rice. Food Chem. 2019, 289, 232–239. [Google Scholar] [CrossRef]
- Bauduin, S.; Latini, M.; Belleggia, I.; Migliore, M.; Biancucci, M.; Mattioli, R.; Francioso, A.; Mosca, L.; Funck, D.; Trovato, M. Interplay between proline metabolism and ROS in the fine tuning of root-meristem size in Arabidopsis. Plants 2022, 11, 1512. [Google Scholar] [CrossRef]
- Hayashi, F.; Ichino, T.; Osanai, M.; Wada, K. Oscillation and regulation of proline content by P5CS and ProDH gene expressions in the light/dark cycles in Arabidopsis thaliana L. Plant Cell Physiol. 2000, 41, 1096–1101. [Google Scholar] [CrossRef]
- Satoh, H.; Omura, T. New endosperm mutations induced by chemical mutagens in rice (Oryza sativa L.). Breed. Sci. 1981, 31, 316–326. [Google Scholar] [CrossRef]
- Maeda, H.; Nemoto, H.; Iida, S.; Ishii, T.; Nakagawa, N.; Hoshino, T.; Sakai, M.; Okamoto, M.; Shinoda, H.; Yoshida, T. A new rice variety with giant embryos, “Haiminori”. Breed. Sci. 2001, 51, 211–213. [Google Scholar] [CrossRef]
- Khwanchai, P.; Chinprahast, N.; Pichyangkura, R.; Chaiwanichsiri, S. Gamma-aminobutyric acid and Glu acid contents, and the GAD activity in germinated brown rice (Oryza sativa L.): Effect of rice cultivars. Food Sci. Biotechnol. 2014, 23, 373–379. [Google Scholar] [CrossRef]
- Wen, Q.; Zhao, H.; Shao, Y.; Hu, Y.; Qi, Y.; Wang, F.; Shen, J. Determination of γ-aminobutyric acid content and influencing factors of production of major edible mushroom fruiting bodies in China. Mycosystema 2023, 42, 231–243. [Google Scholar] [CrossRef]
- Ma, J.; Du, H.; Liu, H.; Sun, Z.; Ma, W.; Ma, R.; Tian, L.; Ma, T.; Li, P. Research progress on γ-aminobutyric acid in rice grains. Hybrid Rice 2022, 37, 6–14. [Google Scholar] [CrossRef]
- Fang, Q.; Lan, Q.; Wan, L.; Cheng, J. System optimization for rapid determination of γ-aminobutyric acid content in brown rice based on Berthelot colorimetric method. Food Nutr. China 2023, 29, 22–26. [Google Scholar] [CrossRef]
- Yu, Y.; Li, M.; Li, C.; Niu, M.; Dong, H.; Zhao, S.; Jia, C.; Xu, Y. Accelerated accumulation of γ-aminobutyric acid and modifications on its metabolic pathways in black rice grains by germination under cold stress. Foods 2023, 12, 1290. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J. Study on roughness test of rice yield. J. Chin. Inst. Food Sci. Technol. 2022, 6, 38–40. [Google Scholar] [CrossRef]
| Variety Name | Type | Content (mg/100 g) | Variety Name | Type | Content (mg/100 g) |
|---|---|---|---|---|---|
| Jefferson | Indica | 39.24 ± 3.87 | Sunbonnet | Indica | 1.00 ± 0.31 |
| GIZA 178 | Indica | 25.82 ± 1.48 | Yugu | Indica | 0.95 ± 0.12 |
| Suishashani | Japonica | 25.76 ± 4.89 | Xindao 11 | Japonica | 0.95 ± 0.34 |
| KON SUITO5 | Indica | 20.90 ± 2.57 | Longjing 21 | Japonica | 0.93 ± 0.21 |
| WIR 1021 | Indica | 20.50 ± 0.91 | Zhongzhe B | Indica | 0.83 ± 0.26 |
| C101A51 (Pi2) | Indica | 20.10 ± 0.93 | Gumei 4 | Indica | 0.73 ± 0.09 |
| Taitougu | Indica | 19.90 ± 1.48 | Erjiuqing | Indica | 0.59 ± 0.04 |
| Jamac | Indica | 19.90 ± 2.61 | Zhong R8006 | Indica | 0.52 ± 0.11 |
| Jia 45 | Japonica | 19.70 ± 4.15 | Zhongjian 2 | Indica | 0.52 ± 0.13 |
| Liantangzao | Indica | 19.40 ± 2.77 | Peiai 64 | Indica | 0.49 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, L.; Yang, Y.; Liu, X.; Zhao, H.; Zhang, Y.; Shen, L.; Zhu, L.; Hu, J.; Ren, D.; Zhang, Q.; et al. Strategies to Improve γ-Aminobutyric Acid Biosynthesis in Rice via Optimal Conditions. Plants 2025, 14, 1290. https://doi.org/10.3390/plants14091290
Shen L, Yang Y, Liu X, Zhao H, Zhang Y, Shen L, Zhu L, Hu J, Ren D, Zhang Q, et al. Strategies to Improve γ-Aminobutyric Acid Biosynthesis in Rice via Optimal Conditions. Plants. 2025; 14(9):1290. https://doi.org/10.3390/plants14091290
Chicago/Turabian StyleShen, Lixing, Yulu Yang, Xiong Liu, Huibo Zhao, Yanfang Zhang, Lan Shen, Li Zhu, Jiang Hu, Deyong Ren, Qiang Zhang, and et al. 2025. "Strategies to Improve γ-Aminobutyric Acid Biosynthesis in Rice via Optimal Conditions" Plants 14, no. 9: 1290. https://doi.org/10.3390/plants14091290
APA StyleShen, L., Yang, Y., Liu, X., Zhao, H., Zhang, Y., Shen, L., Zhu, L., Hu, J., Ren, D., Zhang, Q., Gao, Z., Dong, G., Li, Q., Qian, Q., Zeng, D., & Zhang, G. (2025). Strategies to Improve γ-Aminobutyric Acid Biosynthesis in Rice via Optimal Conditions. Plants, 14(9), 1290. https://doi.org/10.3390/plants14091290











