The First High-Throughput Sequencing-Based Study of Viruses Infecting Solanaceous Crops in Kosovo Reveals Multiple Infections in Peppers by Six Plant Viruses
Abstract
1. Introduction
2. Results
2.1. Determining the Viromes of Solanaceous Crops Grown in Kosovo Using HTS
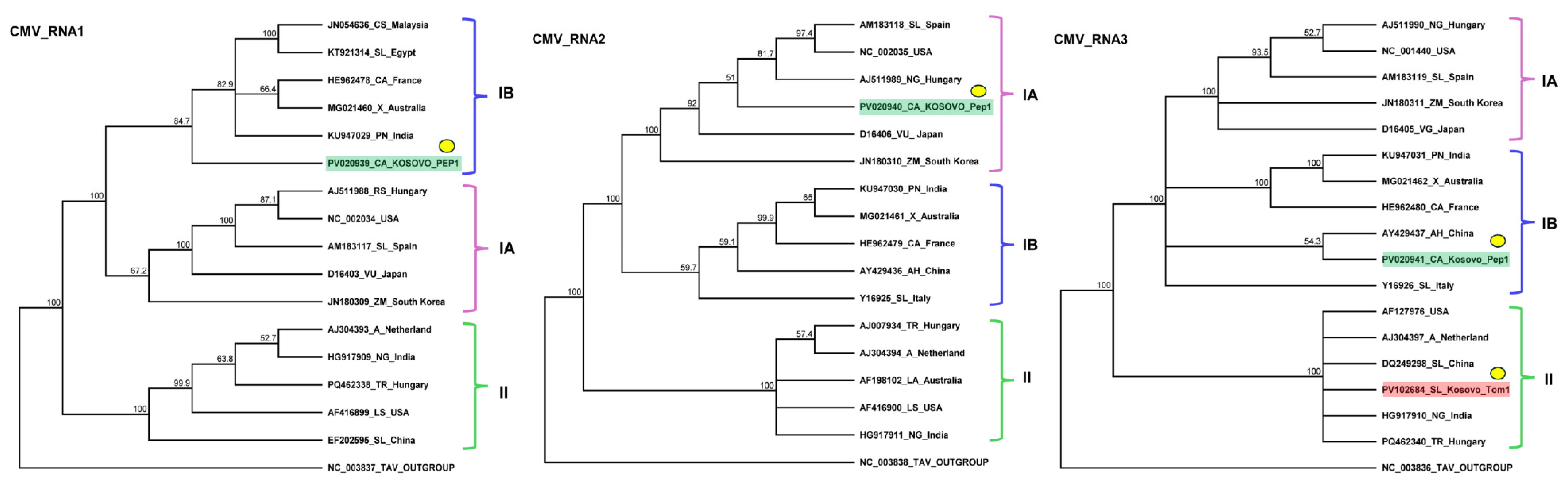
2.2. Identification of CMV Infection in Tomato and Pepper Samples
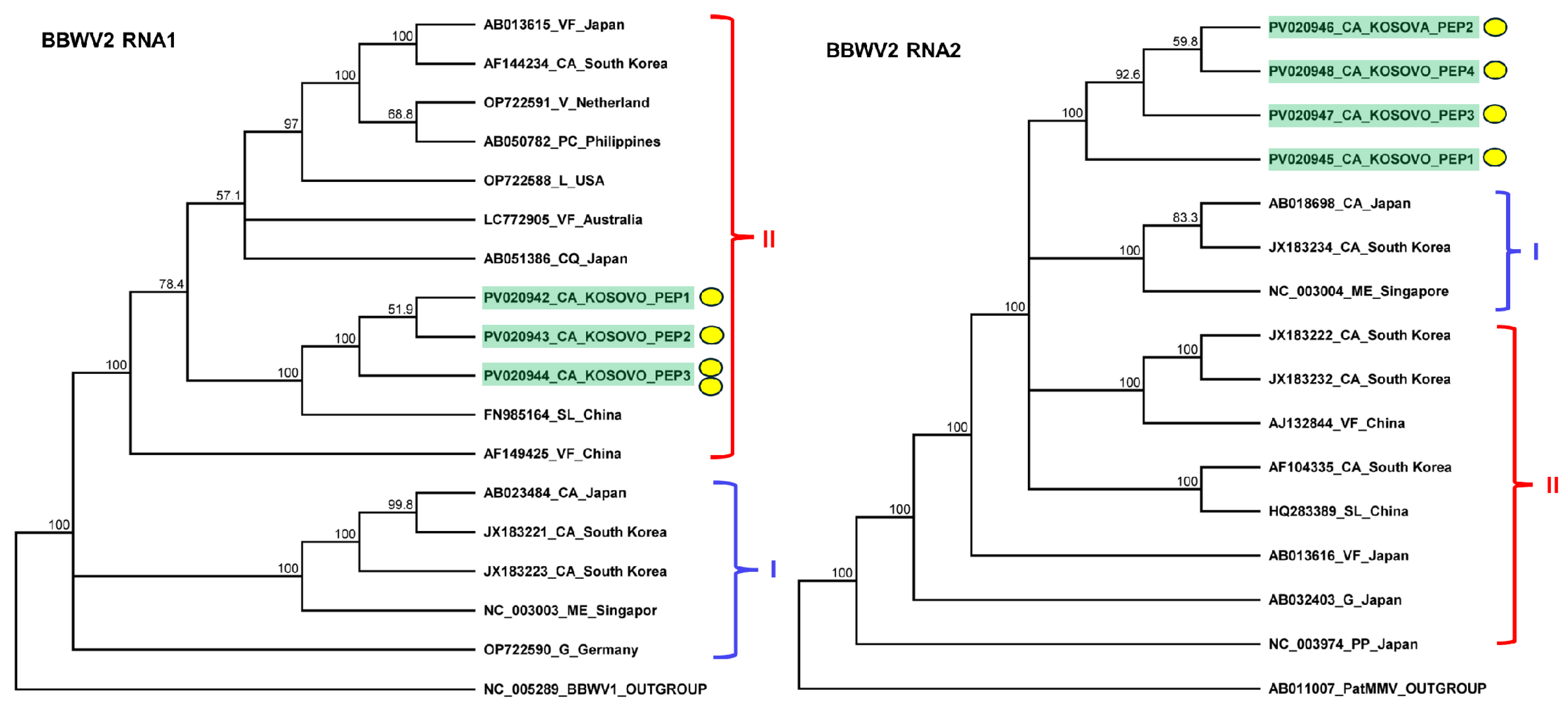
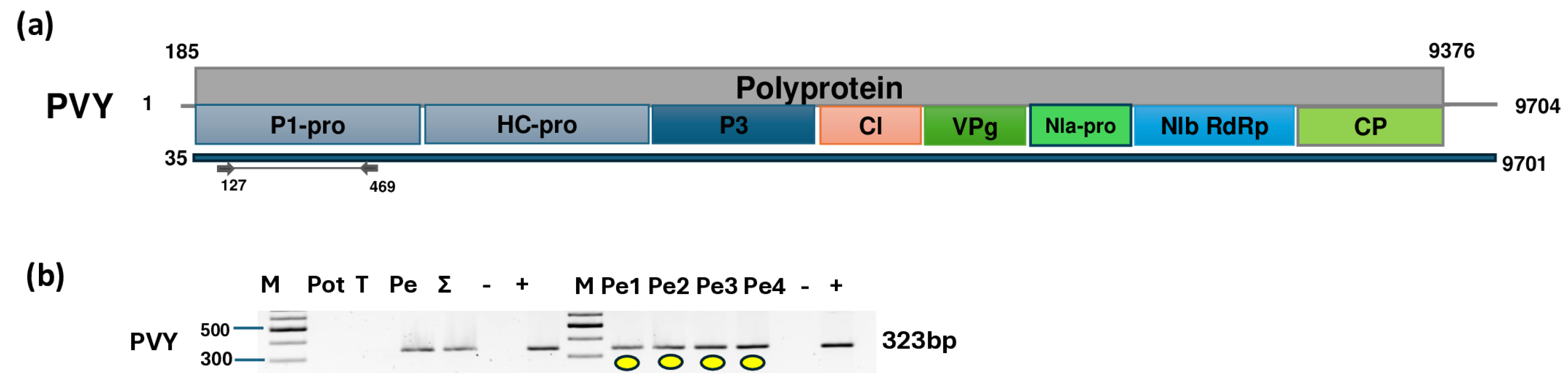
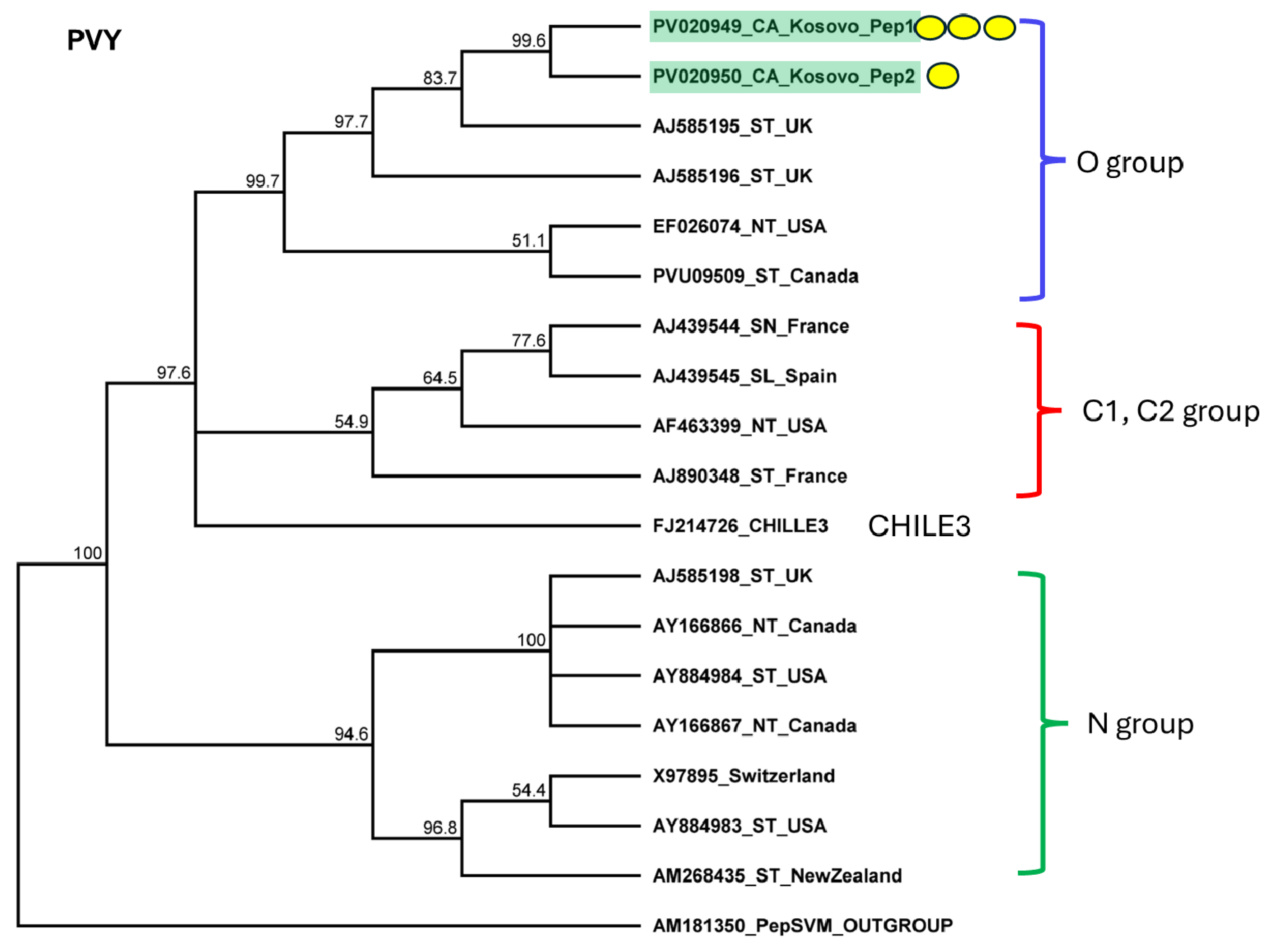
2.3. Pepper Plants Were Infected with BBWV2 and PVY
2.4. All Sampled Pepper Plants Were Infected with PCV2 and BPEV
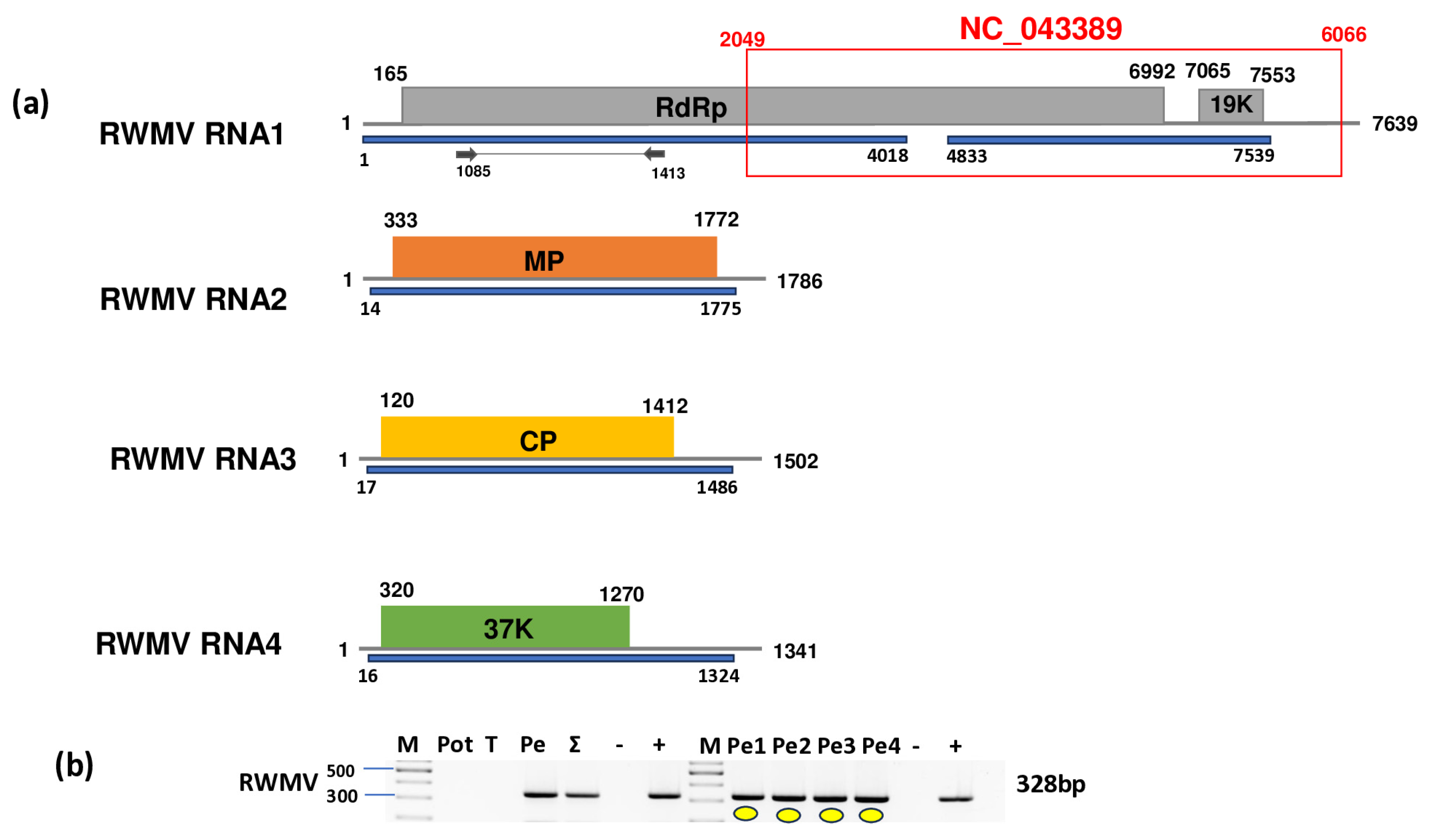
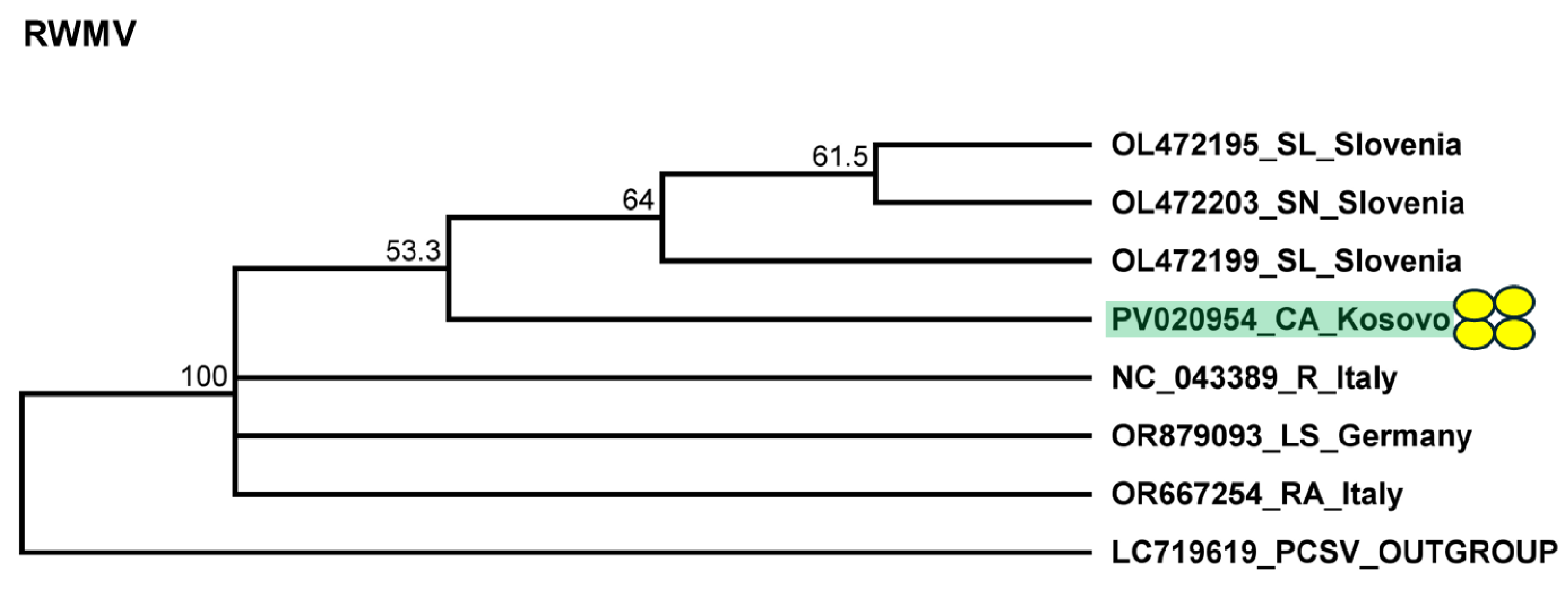
2.5. Pepper Plants Were Infected with Ranunculus White Mottle Virus
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Preparation
4.2. Sampling and Nucleic Acid Extraction
4.3. High-Throughput Sequencing
4.4. Bioinformatics Analysis of the HTS Results
4.5. Validation of HTS Using RT-PCR
4.6. Phylogenetic Analysis of the Detected Viral Strains
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodeva, R.; Kostova, D.; Chavdarov, P.; Mijatovic, M.; Merkuri, J.; Cara, M.; Pasev, G.; Stoyanova, Z.; Karov, I.; Mitrev, S. Pepper diseases in Balkan region. In Proceedings of the V Balkan Symposium on Vegetables and Potatoes 960, Tirana, Albania, 9–12 October 2011; pp. 365–370. [Google Scholar]
- Oreshkovikj, K.B.; Rusevski, R.; Kuzmanovska, B.; Jankulovska, M.; Popovski, Z.T. Occurrence of plant viruses on pepper cultivated in open fields in R. Macedonia and partial characterization of cucumber mosaic virus isolates. J. Plant Pathol. 2018, 100, 485–491. [Google Scholar] [CrossRef]
- Nikolić, D.; Vučurović, A.; Stanković, I.; Radović, N.; Zečević, K.; Bulajić, A.; Krstić, B. Viruses affecting tomato crops in Serbia. Eur. J. Plant Pathol. 2018, 152, 225–235. [Google Scholar] [CrossRef]
- Kwak, H.-R.; Kim, M.-K.; Nam, M.; Kim, J.-S.; Kim, K.-H.; Cha, B.; Choi, H.-S. Genetic compositions of Broad bean wilt virus 2 infecting red pepper in Korea. Plant Pathol. J. 2013, 29, 274. [Google Scholar] [CrossRef] [PubMed]
- Massart, S.; Chiumenti, M.; De Jonghe, K.; Glover, R.; Haegeman, A.; Koloniuk, I.; Kominek, P.; Kreuze, J.; Kutnjak, D.; Lotos, L. Virus detection by high-throughput sequencing of small RNAs: Large-scale performance testing of sequence analysis strategies. Phytopathology 2019, 109, 488–497. [Google Scholar] [CrossRef]
- Rivarez, M.P.S.; Pecman, A.; Bačnik, K.; Maksimović, O.; Vučurović, A.; Seljak, G.; Mehle, N.; Gutiérrez-Aguirre, I.; Ravnikar, M.; Kutnjak, D. In-depth study of tomato and weed viromes reveals undiscovered plant virus diversity in an agroecosystem. Microbiome 2023, 11, 60. [Google Scholar] [CrossRef]
- Tomašechová, J.; Predajňa, L.; Sihelská, N.; Kraic, J.; Mihálik, D.; Šoltys, K.; Glasa, M. First Report of Pepper Cryptic Virus 2 Infecting Pepper (Capsicum annum) in Slovakia. Plant Dis. 2020, 104, 1565. [Google Scholar] [CrossRef]
- Galipienso, L.; Elvira-González, L.; Velasco, L.; Herrera-Vásquez, J.; Rubio, L. Detection of Persistent Viruses by High-Throughput Sequencing in Tomato and Pepper from Panama: Phylogenetic and Evolutionary Studies. Plants 2021, 10, 2295. [Google Scholar] [CrossRef]
- Jo, Y.; Choi, H.; Lee, J.H.; Moh, S.H.; Cho, W.K. Viromes of 15 Pepper (Capsicum annuum L.) Cultivars. Int. J. Mol. Sci. 2022, 23, 10507. [Google Scholar] [CrossRef]
- Rivarez, M.P.S.; Kogej, Z.; Jakoš, N.; Pecman, A.; Seljak, G.; Vučurović, A.; Ravnikar, M.; Mehle, N.; Kutnjak, D. First Report of Ranunculus White Mottle Ophiovirus in Slovenia in Pepper with Yellow Leaf Curling Symptom and in Tomato. Plant Dis. 2022, 106, 2003. [Google Scholar] [CrossRef]
- Dias, N.P.; Hu, R.; Hale, F.A.; Hansen, Z.R.; Wszelaki, A.; Domier, L.L.; Hajimorad, M.R. Viromes of Field-Grown Tomatoes and Peppers in Tennessee Revealed by RNA Sequencing Followed by Bioinformatic Analysis. Plant Health Prog. 2023, 24, 207–213. [Google Scholar] [CrossRef]
- Minicka, J.; Taberska, A.; Borodynko-Filas, N.; Kaźmińska, K.; Bartoszewski, G.; Hasiów-Jaroszewska, B. Viruses infecting Capsicum crops in Poland and molecular characterization of newly detected bell pepper alphaendornavirus (BPEV). Crop Prot. 2024, 176, 106478. [Google Scholar] [CrossRef]
- Mochizuki, T.; Ohki, S.T. Cucumber mosaic virus: Viral genes as virulence determinants. Mol. Plant Pathol. 2012, 13, 217–225. [Google Scholar] [CrossRef]
- Ruiz, M.T.; Voinnet, O.; Baulcombe, D.C. Initiation and maintenance of virus-induced gene silencing. Plant Cell 1998, 10, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Trkulja, V.; Kovačić, D.; Ćurković, B.; Stanković, A.V.I.; Bulajić, A.; Krstić, B. First report of Cucumber mosaic virus on melon in Bosnia and Herzegovina. Plant Dis. 2013, 97, 1124. [Google Scholar] [CrossRef]
- Xanthis, C.; Maliogka, V.; Lecoq, H.; Dezbiez, C.; Tsvetkov, I.; Katis, N. First report of Cucumber mosaic virus infecting watermelon in Greece and Bulgaria. J. Plant Pathol. 2015, 97, 399. [Google Scholar]
- Zečević, K.; Stanković, I.; Petrović, B.; Krstić, B. Molecular characterization and differentiation of cucumber mosaic virus subgroups in Serbia by RT-PCR-RFLP. Arch. Biol. Sci. 2023, 75, 431–442. [Google Scholar] [CrossRef]
- Lim, K.; Whye, L.; Hung, M.; Chung, H.H. Cucumber mosaic virus: Global genome comparison and beyond. Malays. J. Microbiol. 2022, 18, 79. [Google Scholar]
- Roossinck, M.J. Cucumber mosaic virus, a model for RNA virus evolution. Mol. Plant Pathol. 2001, 2, 59–63. [Google Scholar] [CrossRef]
- Seo, J.-K.; Kwak, H.-R.; Choi, B.; Han, S.-J.; Kim, M.-K.; Choi, H.-S. Movement protein of broad bean wilt virus 2 serves as a determinant of symptom severity in pepper. Virus Res. 2017, 242, 141–145. [Google Scholar] [CrossRef]
- Nakamura, S.; Iwai, T.; Honkura, R. Complete nucleotide sequence and genome organization of Broad bean wilt virus 2. Jpn. J. Phytopathol. 1998, 64, 565–568. [Google Scholar] [CrossRef]
- Lee, U.; Hong, J.; Choi, J.; Kim, K.; Kim, Y.; Curtis, I.; Nam, H.; Lim, P. Broad bean wilt virus causes necrotic symptoms and generates defective RNAs in Capsicum annuum. Phytopathology 2000, 90, 1390–1395. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, R.M.; Ferriol, I.; Moreno, P.; Guerri, J.; Rubio, L. Genetic variation and evolutionary analysis of broad bean wilt virus 2. Arch. Virol. 2011, 156, 1445–1450. [Google Scholar] [CrossRef]
- Kwak, H.-R.; Kim, M.-K.; Lee, Y.-J.; Seo, J.-K.; Kim, J.-S.; Kim, K.-H.; Cha, B.; Choi, H.-S. Molecular characterization and variation of the broad bean wilt virus 2 isolates based on analyses of complete genome sequences. Plant Pathol. J. 2013, 29, 397. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scholthof, K.B.G.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P. Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef] [PubMed]
- Quenouille, J.; Vassilakos, N.; Moury, B. Potato virus Y: A major crop pathogen that has provided major insights into the evolution of viral pathogenicity. Mol. Plant Pathol. 2013, 14, 439–452. [Google Scholar] [CrossRef]
- Wylie, S.J.; Adams, M.; Chalam, C.; Kreuze, J.; López-Moya, J.J.; Ohshima, K.; Praveen, S.; Rabenstein, F.; Stenger, D.; Wang, A. ICTV virus taxonomy profile: Potyviridae. J. Gen. Virol. 2017, 98, 352–354. [Google Scholar] [CrossRef]
- Fanigliulo, A.; Comes, S.; Pacella, R.; Harrach, B.; Martin, D.; Crescenzi, A. Characterisation of Potato virus Y nnp strain inducing veinal necrosis in pepper: A naturally occurring recombinant strain of PVY. Arch. Virol. 2005, 150, 709–720. [Google Scholar] [CrossRef]
- Moodley, V.; Ibaba, J.D.; Naidoo, R.; Gubba, A. Full-genome analyses of a Potato Virus Y (PVY) isolate infecting pepper (Capsicum annuum L.) in the Republic of South Africa. Virus Genes 2014, 49, 466–476. [Google Scholar] [CrossRef]
- Sabanadzovic, S.; Valverde, R.A. Properties and detection of two cryptoviruses from pepper (Capsicum annuum). Virus Genes 2011, 43, 307–312. [Google Scholar] [CrossRef]
- Saritha, R.K.; Jain, P.; Baranwal, V.K.; Jain, R.K.; Srivastava, A.; Kalia, P. First record of Pepper cryptic virus 2 in chilli (Capsicum annuum) in India. VirusDisease 2016, 27, 327–328. [Google Scholar] [CrossRef]
- Sun, M.; Jing, C.; Wu, G.; Sun, X.; Liu, Y.; Qing, L. First report of pepper cryptic virus 2 infecting pepper in China. J. Plant Pathol. 2017, 99, 298. [Google Scholar]
- Okada, R.; Kiyota, E.; Sabanadzovic, S.; Moriyama, H.; Fukuhara, T.; Saha, P.; Roossinck, M.J.; Severin, A.; Valverde, R.A. Bell pepper endornavirus: Molecular and biological properties, and occurrence in the genus Capsicum. J. Gen. Virol. 2011, 92, 2664–2673. [Google Scholar] [CrossRef] [PubMed]
- Valverde, R.A.; Khalifa, M.E.; Okada, R.; Fukuhara, T.; Sabanadzovic, S.; Ictv Report, C. ICTV Virus Taxonomy Profile: Endornaviridae. J. Gen. Virol. 2019, 100, 1204–1205. [Google Scholar] [CrossRef]
- Sela, N.; Luria, N.; Dombrovsky, A. Genome assembly of bell pepper endornavirus from small RNA. J. Virol. 2012, 86, 7721. [Google Scholar] [CrossRef]
- Muñoz-Baena, L.; Marín-Montoya, M.; Gutiérrez, P.A. Genome sequencing of two Bell pepper endornavirus (BPEV) variants infecting Capsicum annuum in Colombia. Agron. Colomb. 2017, 35, 44–52. [Google Scholar] [CrossRef]
- Tomašechová, J.; Hančinský, R.; Predajňa, L.; Kraic, J.; Mihálik, D.; Šoltys, K.; Vávrová, S.; Böhmer, M.; Sabanadzovic, S.; Glasa, M. High-Throughput Sequencing Reveals Bell Pepper Endornavirus Infection in Pepper (Capsicum annum) in Slovakia and Enables Its Further Molecular Characterization. Plants 2019, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Abou Kubaa, R.; Saponari, M.; Heinoun, K.; Jreijiri, F.; Choueiri, E. First report of bell pepper endornavirus in pepper in Syria and Lebanon. J. Plant Pathol. 2024, 106, 781. [Google Scholar] [CrossRef]
- Vaira, A.; Milne, R.; Accotto, G.; Luisoni, E.; Masenga, V.; Lisa, V. Partial characterization of a new virus from ranunculus with a divided RNA genome and circular supercoiled thread-like particles. Arch. Virol. 1997, 142, 2131–2146. [Google Scholar] [CrossRef]
- Vaira, A.M.; Accotto, G.P.; Lisa, V.; Vecchiati, M.; Masenga, V.; Milne, R.G. Molecular diagnosis of ranunculus white mottle virus in two ornamental species. In Proceedings of the X International Symposium on Virus Diseases of Ornamental Plants, Annapolis, MD, USA, 27 May–1 June 2000; pp. 29–33. [Google Scholar]
- Gambley, C.; Persley, D.; Thomas, J.E. First record of Ranunculus white mottle virus from Australia. New Dis. Rep. 2019, 40, 13. [Google Scholar] [CrossRef]
- Gavrili, V.; Lotos, L.; Vasileiou, N.; Katis, N.; Maliogka, V. First report of ranunculus white mottle virus and lettuce ring necrosis virus in pepper in Greece. J. Plant Pathol. 2024, 106, 775–776. [Google Scholar] [CrossRef]
- García, M.L.; Bó, E.D.; da Graça, J.V.; Gago-Zachert, S.; Hammond, J.; Moreno, P.; Natsuaki, T.; Pallás, V.; Navarro, J.A.; Reyes, C.A.; et al. ICTV Virus Taxonomy Profile: Ophioviridae. J. Gen. Virol. 2017, 98, 1161–1162. [Google Scholar] [CrossRef] [PubMed]
- Palukaitis, P.; Roossinck, M.J.; Dietzgen, R.G.; Francki, R.I.B. Cucumber MOSAIC Virus. In Advances in Virus Research; Maramorosch, K., Murphy, F.A., Shatkin, A.J., Eds.; Academic Press: Cambridge, MA, USA, 1992; Volume 41, pp. 281–348. [Google Scholar]
- Milošević, D.; Ignjatov, M.; Nikolić, Z.; Gvozdanović-Varga, J.; Petrović, G.; Stanković, I.; Krstić, B. First Report of Cucumber mosaic virus causing chlorotic mottle on Pot Marigold (Calendula officinalis) in Serbia. Plant Dis. 2015, 99, 736. [Google Scholar] [CrossRef]
- Milošević, D.; Ignjatov, M.; Marjanović-Jeromela, A.; Nikolić, Z.; Tamindžić, G.; Miljaković, D.; Stanković, I. Presence and molecular characterization of cucumber mosaic virus on safflower in Serbia [Prisustvo i molekularna karakterizacija virusa mozaika krastavca u usevu šafranike u Srbiji]. Ratar. I Povrt. 2021, 57, 49–54. [Google Scholar] [CrossRef]
- Milošević, D.; Ignjatov, M.; Stanković, I.; Nikolić, Z.; Gvozdanović-Varga, J.; Krstić, B. Occurrence and diversity of viruses infecting pepper in Serbia. Acta Agric. Serbica 2018, 23, 141–155. [Google Scholar] [CrossRef]
- Maria Laura, G. Ophioviruses: State of the Art. In Viral Genomes; Maria Laura, G., Victor, R., Eds.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- White, J.L.; Kaper, J. A simple method for detection of viral satellite RNAs in small plant tissue samples. J. Virol. Methods 1989, 23, 83–93. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismajli, B.; Galbács, Z.N.; Takács, A.P.; Várallyay, É. The First High-Throughput Sequencing-Based Study of Viruses Infecting Solanaceous Crops in Kosovo Reveals Multiple Infections in Peppers by Six Plant Viruses. Plants 2025, 14, 1273. https://doi.org/10.3390/plants14091273
Ismajli B, Galbács ZN, Takács AP, Várallyay É. The First High-Throughput Sequencing-Based Study of Viruses Infecting Solanaceous Crops in Kosovo Reveals Multiple Infections in Peppers by Six Plant Viruses. Plants. 2025; 14(9):1273. https://doi.org/10.3390/plants14091273
Chicago/Turabian StyleIsmajli, Burim, Zsuzsanna N. Galbács, András Péter Takács, and Éva Várallyay. 2025. "The First High-Throughput Sequencing-Based Study of Viruses Infecting Solanaceous Crops in Kosovo Reveals Multiple Infections in Peppers by Six Plant Viruses" Plants 14, no. 9: 1273. https://doi.org/10.3390/plants14091273
APA StyleIsmajli, B., Galbács, Z. N., Takács, A. P., & Várallyay, É. (2025). The First High-Throughput Sequencing-Based Study of Viruses Infecting Solanaceous Crops in Kosovo Reveals Multiple Infections in Peppers by Six Plant Viruses. Plants, 14(9), 1273. https://doi.org/10.3390/plants14091273








