Phytochemical Analysis and Biological Activities of Wollemia nobilis W.G.Jones, K.D.Hill & J.M.Allen Leaves Collected in the Botanical Garden of Rome
Abstract
1. Introduction
2. Results and Discussion
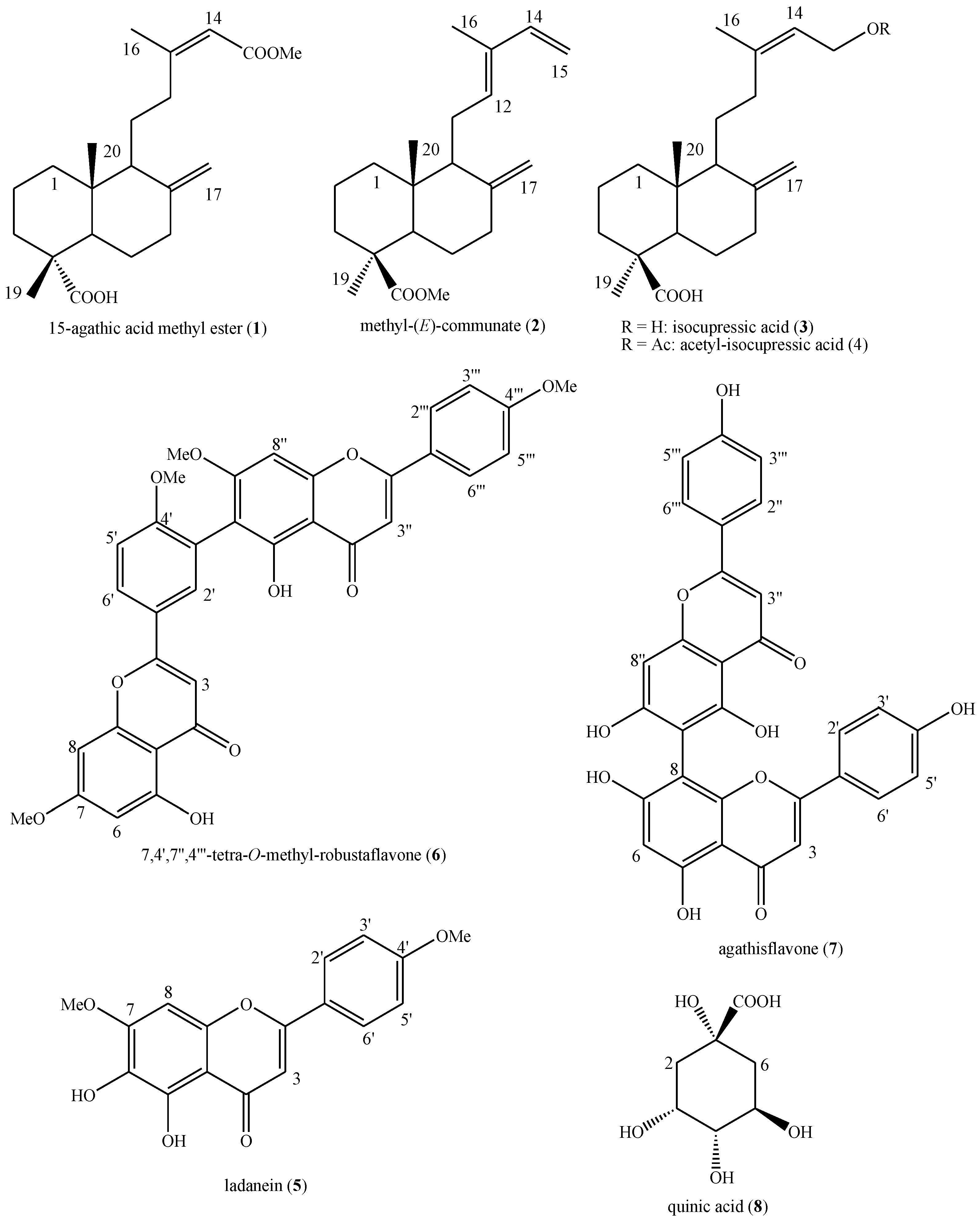
2.1. Phytochemical Analysis
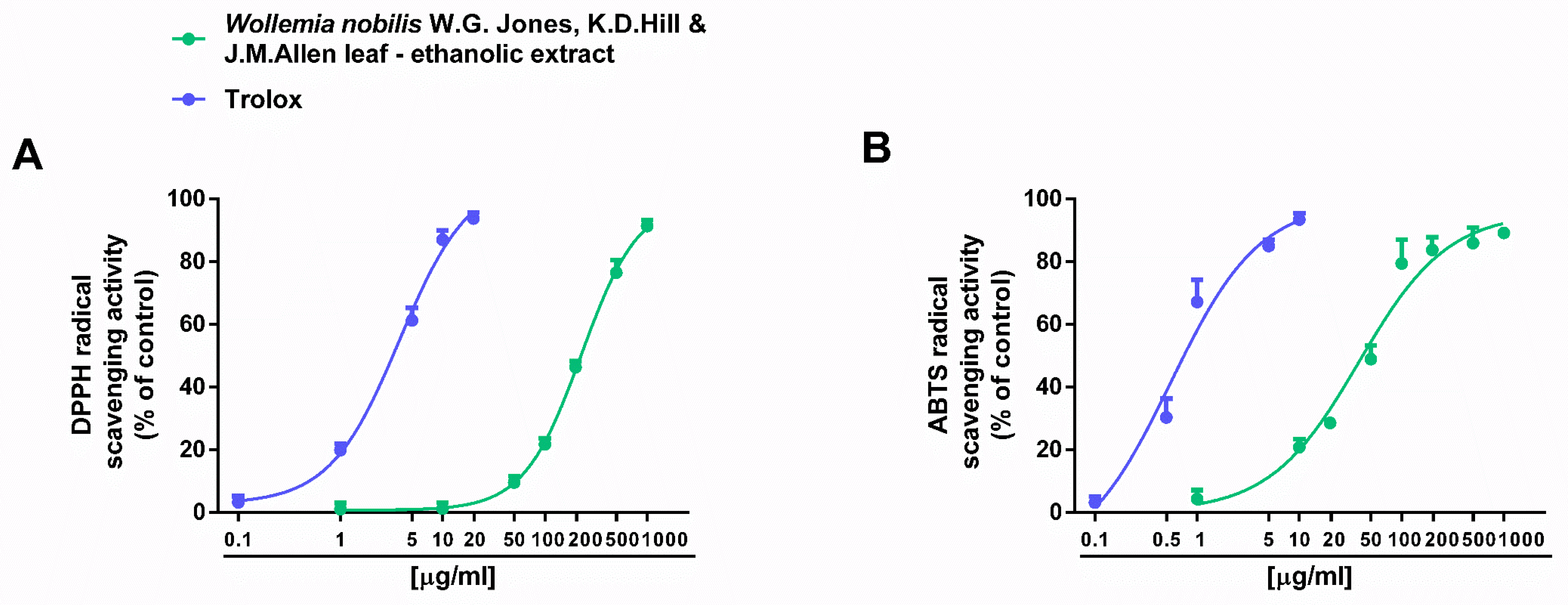
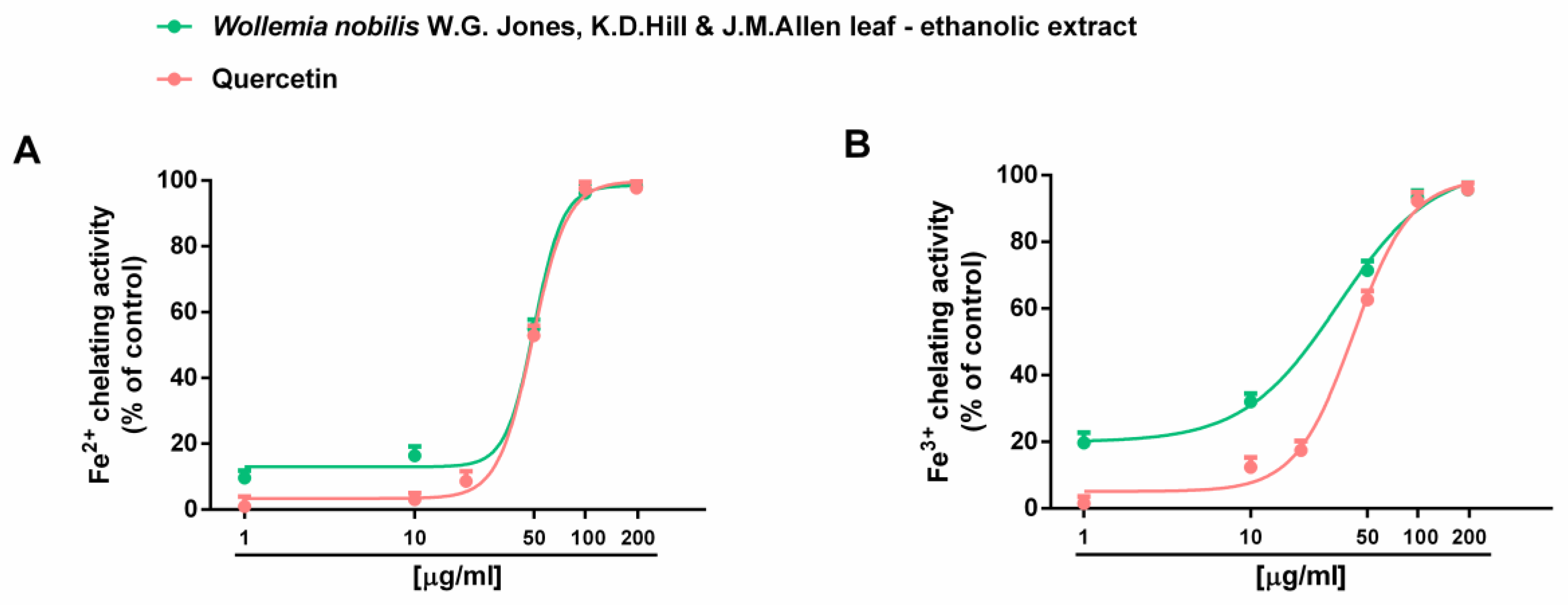
2.2. Antioxidant and Antiglycative Properties
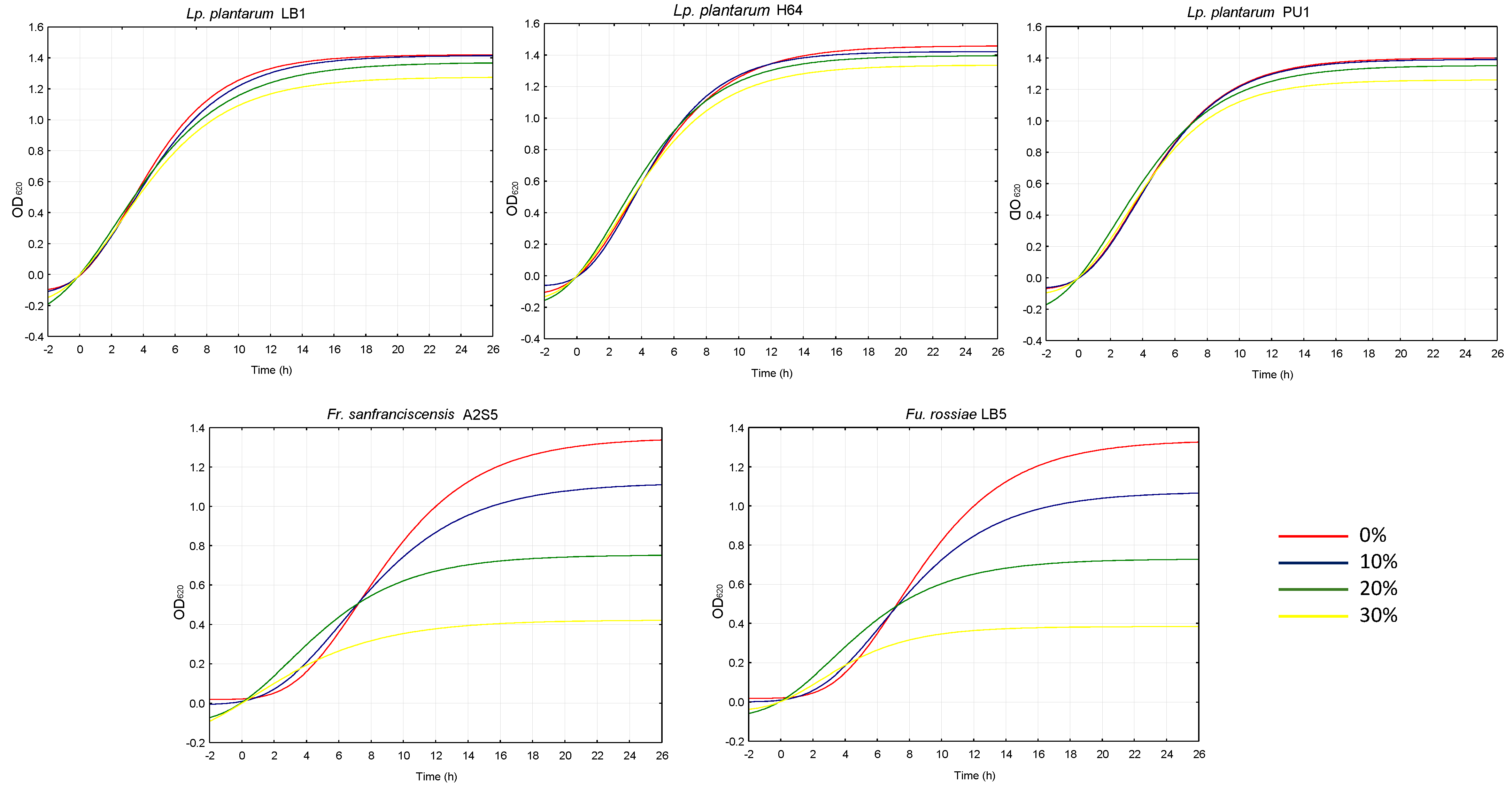
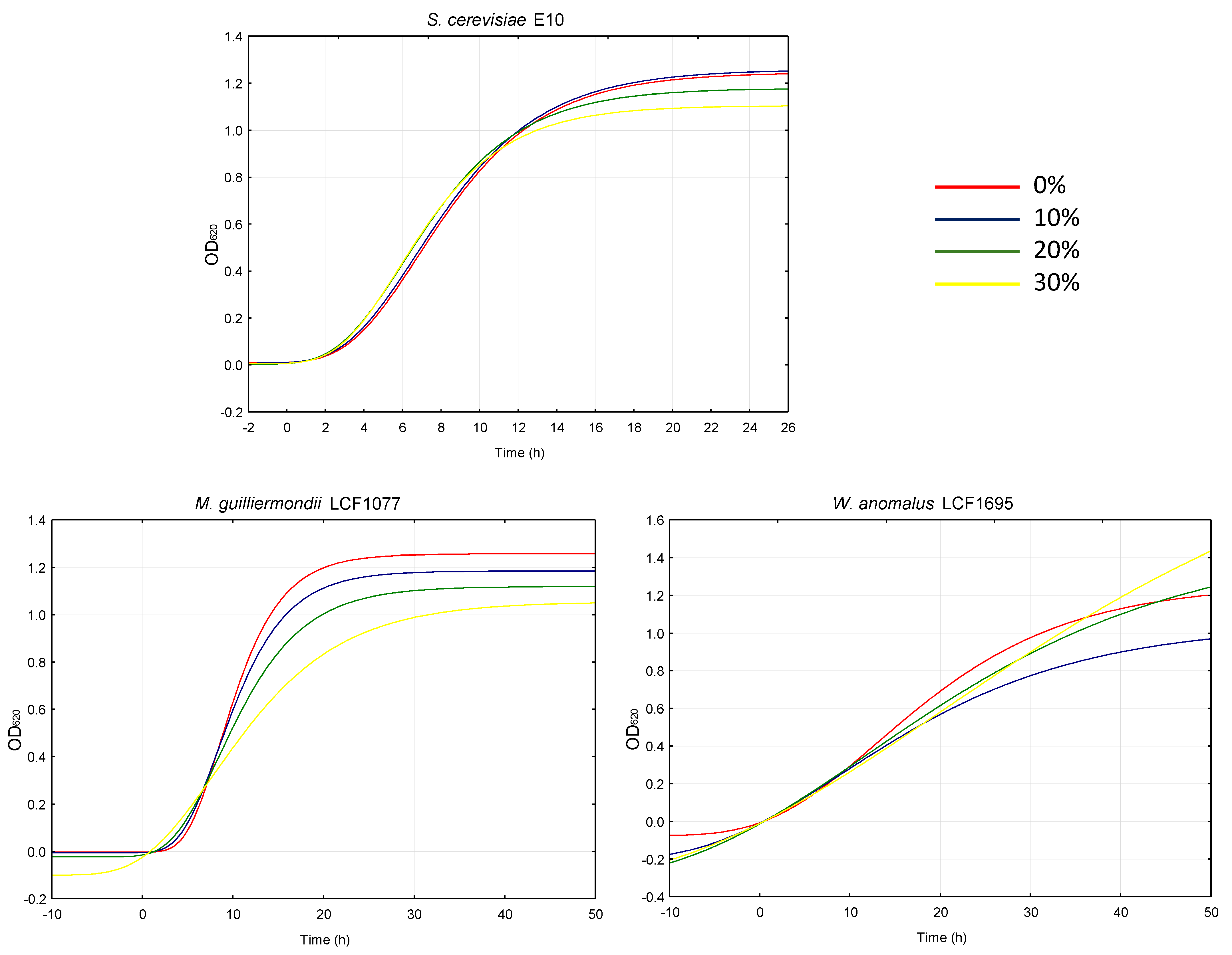
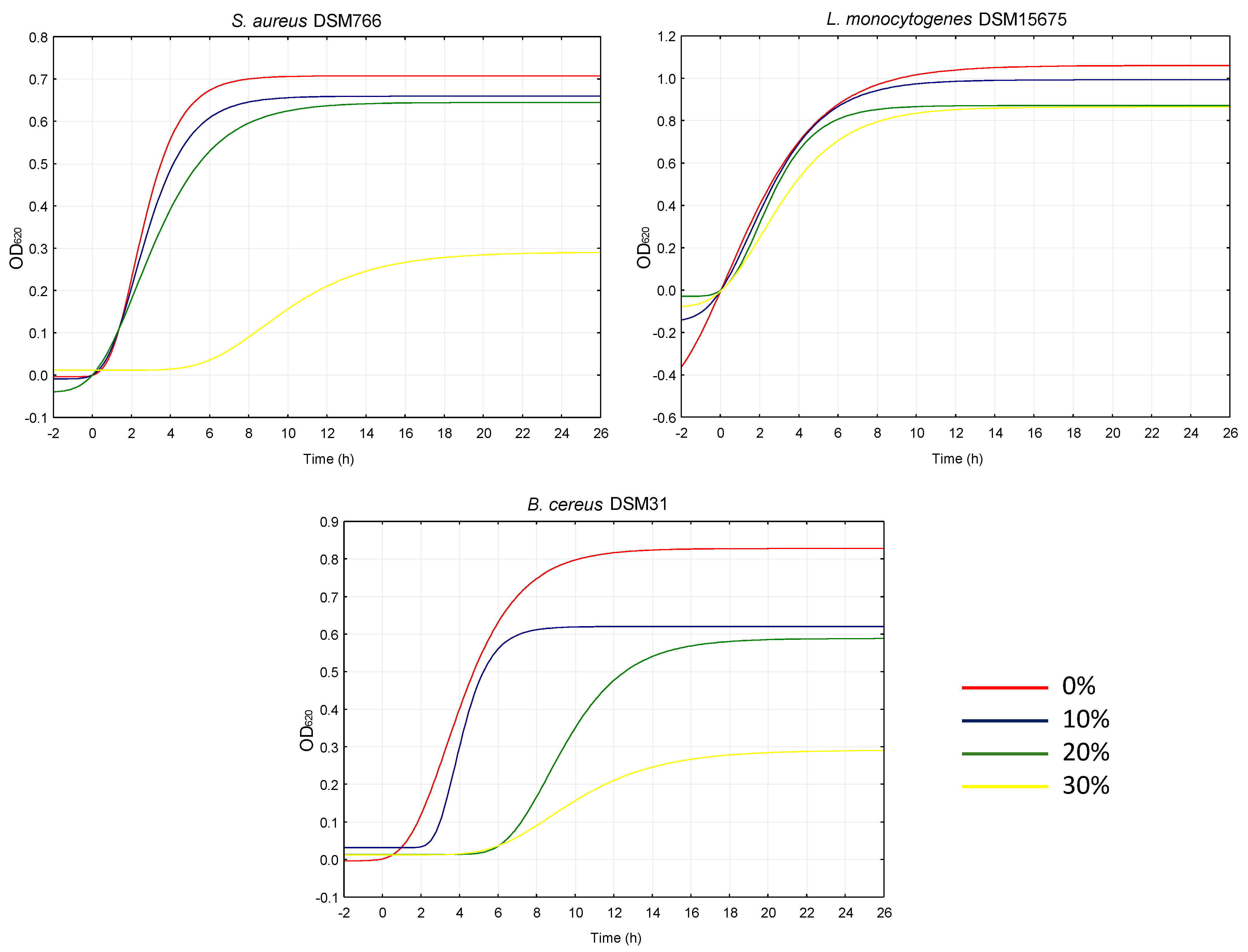
2.3. Antimicrobial Effect
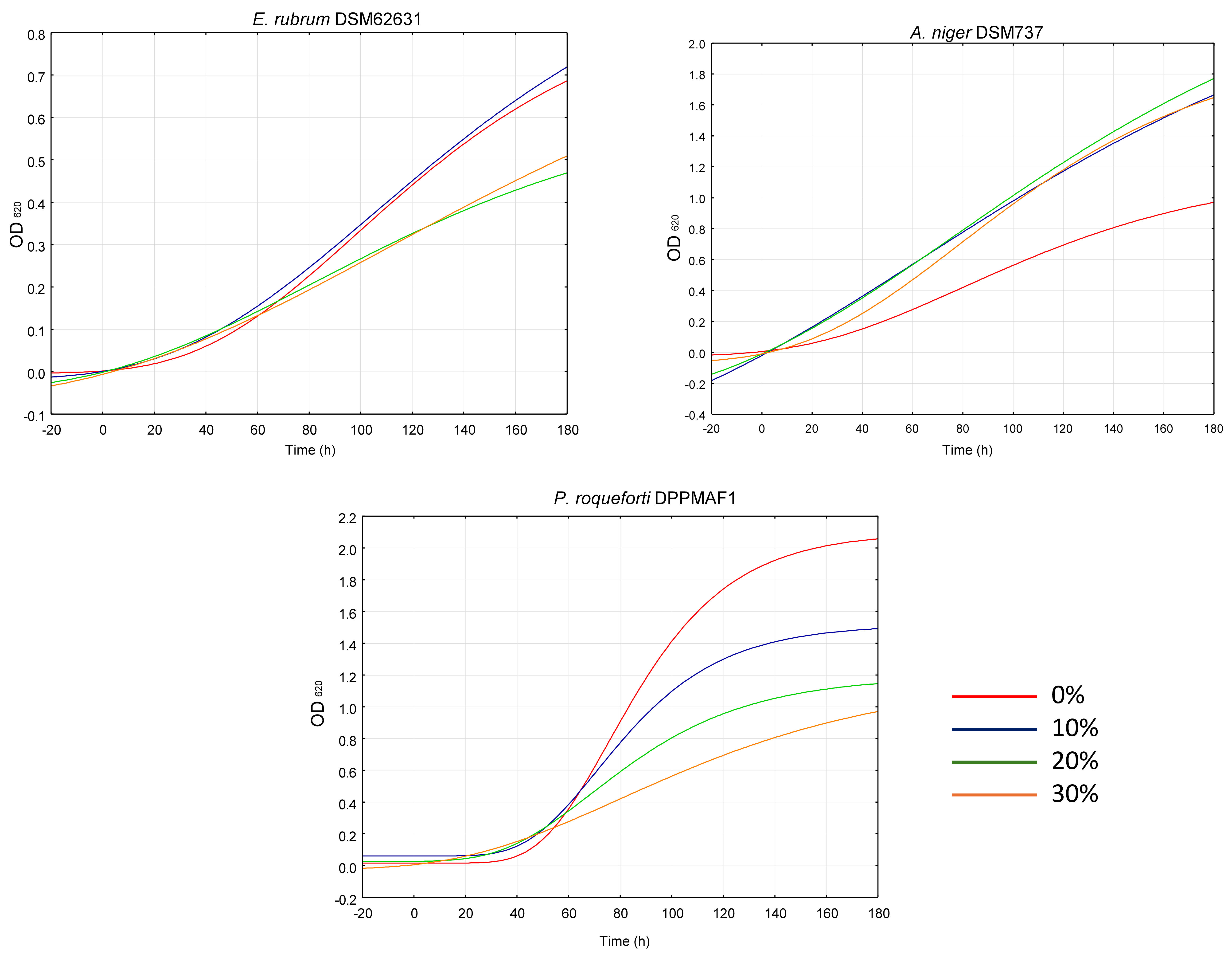
2.4. Antifungal and Inhibitory Effects on the Production of Aflatoxin B1 in Aspergillus flavus Link 3375
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals
3.3. Instrumentations
3.4. Extraction, Separation and Identification of the Metabolites
3.5. Spectroscopic Data of the Identified Compounds in W. nobilis Leaf Ethanol Extract
3.6. Spectrophotometric Determination of Total Polyphenols, Tannins and Flavonoids
3.7. Antioxidant Assays
3.7.1. Radical Scavenging Activity
3.7.2. Iron Chelating Activity
3.7.3. Ferric Reducing Activity
3.7.4. Antiglycative Activity
3.7.5. Statistical Analysis
3.8. Microorganisms and Cultivation
3.9. Antimicrobial Screening
3.10. Aspergillus flavus Growth and AFLAB1 Production and Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Frezza, C.; Venditti, A.; Rossi, G.; Serafini, I.; Pitorri, M.; Ciccóla, A.; Foddai, S.; Bianco, A.; Serafini, M. Phytochemical study on the leaves of Wollemia nobilis. Biochem. Syst. Ecol. 2017, 74, 63–66. [Google Scholar] [CrossRef]
- Frezza, C.; Venditti, A.; Scandurra, C.; Ciccòla, A.; Serafini, I.; Sciubba, F.; Foddai, S.; Franceschin, M.; Bianco, A.; Serafini, M. Phytochemical profile of Wollemia nobilis half-matured female cones and their potential ethnopharmacological and nutraceutical activities. J. Agric. Sci. Technol. A 2018, 8, 162–170. [Google Scholar]
- Venditti, A.; Frezza, C.; Sciubba, F.; Foddai, S.; Serafini, M.; Bianco, A. Terpenoids and more polar compounds from the male cones of Wollemia nobilis. Chem. Biodivers. 2017, 14, e1600332. [Google Scholar] [CrossRef]
- Venditti, A.; Frezza, C.; Rossi, G.; Serafini, I.; Ciccóla, A.; Sciubba, F.; Foddai, S.; Tomassini, L.; Bianco, A.; Serafini, M. A new byciclic monoterpene glucoside and a new biflavone from the male reproduction organs of Wollemia nobilis. Fitoterapia 2019, 133, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A.; Frezza, C.; Vincenti, F.; Brodella, A.; Sciubba, F.; Montesano, C.; Franceschin, M.; Sergi, M.; Foddai, S.; Di Cocco, M.E.; et al. A syn-ent-labdadiene derivative with a rare spiro-β-lactone function from the male cones of Wollemia nobilis. Phytochemistry 2019, 158, 91–95. [Google Scholar] [CrossRef]
- Venditti, A.; Frezza, C.; Rossi, G.; Sciubba, F.; Ornano, L.; De Vita, D.; Toniolo, C.; Tomassini, L.; Foddai, S.; Nicoletti, M.; et al. A new diterpene and other compounds from the unripe female cones of Wollemia nobilis. Nat. Prod. Res. 2021, 35, 3839–3849. [Google Scholar] [CrossRef] [PubMed]
- Frezza, C.; Sciubba, F.; De Vita, D.; Toniolo, C.; Foddai, S.; Tomassini, L.; Petrucci, R.; Bianco, A.; Serafini, M. Non-volatile compounds from Araucaria columnaris (G.Forst.) Hook leaves. Biochem. Syst. Ecol. 2022, 103, 104430. [Google Scholar] [CrossRef]
- Frezza, C.; Venditti, A.; De Vita, D.; Toniolo, C.; Franceschin, M.; Ventrone, A.; Tomassini, L.; Foddai, S.; Guiso, M.; Nicoletti, M.; et al. Phytochemistry, chemotaxonomy, and biological activities of the Araucariaceae family—A review. Plants 2020, 9, 888. [Google Scholar] [CrossRef]
- Andrade, A.W.L.; Machado, K.d.C.; Machado, K.d.C.; Figueiredo, D.D.R.; David, J.M.; Islam, M.T.; Uddin, S.J.; Shilpi, J.A.; Costa, J.P. In vitro antioxidant properties of the biflavonoid agathisflavone. Chem. Cent. J. 2018, 12, 75. [Google Scholar] [CrossRef]
- Rebai, O.; Belkhir, M.; Sanchez-Gomez, M.V.; Matute, C.; Fattouch, S.; Amri, M. Differential molecular targets for neuro-protective effect of chlorogenic acid and its related compounds against glutamate induced excitotoxicity and oxidative stress in rat cortical neurons. Neurochem. Res. 2017, 42, 3559–3572. [Google Scholar] [CrossRef]
- Farjam, M.H.; Rustaiyan, A.; Ezzatzadeh, E.; Jassbi, A.R. Labdane-type diterpene and two flavones from Salvia sharifii Rech. f. and Esfan. and their biological activities. Iran. J. Pharm. Res. 2013, 12, 395–400. [Google Scholar] [PubMed]
- Martin-Benlloch, X.; Novodomska, A.; Jacquemin, D.; Davioud-Charvet, E.; Elhabiri, M. Iron(III) coordination properties of ladanein, a flavone lead with a broad-spectrum antiviral activity. New J. Chem. 2018, 42, 8074–8087. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K. A novel HPLC-assisted method for investigation of the Fe2+-chelating activity of flavonoids and plant extracts. Molecules 2014, 19, 18296–18316. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, M.M.A.; Pieropan, F.; Oliveira, L.d.M.; Junior, M.C.d.S.; David, J.M.; David, J.P.; da Silva, V.D.A.; Souza, C.d.S.; Costa, S.L.; Butt, A.M. The flavonoid agathisflavone modulates the microglial neuroinflammatory response and enhances remyelination. Pharm. Res. 2020, 159, 104997. [Google Scholar] [CrossRef]
- Dos Santos, B.L.; Dos Santos, C.C.; Soares, J.R.P.; Da Silva, K.C.; De Oliveira, J.V.R.; Pereira, G.S.; de Araújo, F.M.; Costa, M.d.F.D.; David, J.M.; Da Silva, V.D.A.; et al. The flavonoid agathisflavone directs brain microglia/macrophages to a neuroprotective anti-inflammatory and antioxidant state via regulation of NLRP3 inflammasome. Pharmaceutics 2023, 15, 1410. [Google Scholar] [CrossRef]
- Dos Santos Souza, C.; Grangeiro, M.S.; Pereira, E.P.L.; dos Santos, C.C.; da Silva, A.B.; Sampaio, G.P.; Figueiredo, D.D.R.; David, J.M.; Da Silva, V.D.A.; Butt, A.M.; et al. Agathisflavone, a flavonoid derived from Poincianella pyramidalis (Tul.), enhances neuronal population and protects against glutamate excitotoxicity. Neurotoxicology 2018, 65, 85–97. [Google Scholar] [CrossRef]
- Velagapudi, R.; Ajileye, O.O.; Okorji, U.; Jain, P.; Aderogba, M.A.; Olajide, O.A. Agathisflavone isolated from Anacardium occidentale suppresses SIRT1-mediated neuroinflammation in BV2 microglia and neurotoxicity in APPSwe-transfected SH-SY5Y cells. Phytother. Res. 2018, 32, 1957–1966. [Google Scholar] [CrossRef]
- Alkhatib, R.; Joha, S.; Cheok, M.; Roumy, V.; Idziorek, T.; Preudhomme, C.; Quesnel, B.; Sahpaz, S.; Bailleul, F.; Hennebelle, T. Activity of ladanein on leukemia cell lines and its occurrence in Marrubium vulgare. Planta Med. 2010, 76, 86–87. [Google Scholar] [CrossRef]
- Muñoz, R.; Rivas, B.d.L.; Rodríguez, H.; Esteban-Torres, M.; Reverón, I.; Santamaría, L.; Landete, J.M.; Plaza-Vinuesa, L.; Sánchez-Arroyo, A.; Jiménez, N.; et al. Food phenolics and Lactiplantibacillus plantarum. Int. J. Food Microbiol. 2024, 412, 110555. [Google Scholar] [CrossRef]
- Parapouli, M.; Vasileiadi, A.; Afendra, A.-S.; Hatziloukas, E. Saccharomyces cerevisiae and its industrial applications. AIMS Microbiol. 2020, 6, 1–31. [Google Scholar] [CrossRef]
- Saha, P.; Rahman, F.I.; Hussain, F.; Rahman, S.M.A.; Rahman, M.M. Antimicrobial diterpenes: Recent development from natural sources. Front. Pharmacol. 2022, 12, 820312. [Google Scholar] [CrossRef] [PubMed]
- Lopes, I.; Campos, C.; Medeiros, R.; Cerqueira, F. Antimicrobial activity of dimeric flavonoids. Compounds 2024, 4, 214–229. [Google Scholar] [CrossRef]
- Kallio, H.; Ahtonen, S.; Sarimo, S.S. Effect of quinic acid on the growth of some wild yeasts and molds. J. Food Prot. 1985, 48, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Wu, Y.; Wang, X.; Liu, X.; Zhong, K.; Huang, Y.; Chen, Y.; Gao, H. In vitro and in vivo characterization of the antibacterial activity and membrane damage mechanism of quinic acid against Staphylococcus aureus. J. Food Saf. 2018, 38, e12416. [Google Scholar] [CrossRef]
- Aqil, F.; Zahin, M.; Ahmad, I.; Owais, M.; Khan, M.S.A.; Bansal, S.S.; Farooq, S. Antifungal activity of medicinal plant extracts and phytocompounds: A review. In Combating Fungal Infections: Problems and Remedy; Springer: Berlin/Heidelberg, Germany, 2010; pp. 449–484. [Google Scholar]
- Castano-Duque, L.; Lebar, M.D.; Mack, B.M.; Lohmar, J.M.; Carter-Wientjes, C. Investigating the impact of flavonoids on Aspergillus flavus: Insights into cell wall damage and biofilms. J. Fungi 2024, 10, 665. [Google Scholar] [CrossRef]
- Tian, F.; Chun, H.S. Natural products for preventing and controlling aflatoxin contamination of food. In Aflatoxin-Control, Analysis, Detection and Health Risks; Abdulra’uf, L., Ed.; Intech Open: London, UK, 2017; pp. 13–44. [Google Scholar]
- Ercan, L.; Doğru, M. Antioxidant and antimicrobial capacity of quinic acid. Bitlis Eren Univ. J. Sci. 2022, 11, 1018–1025. [Google Scholar] [CrossRef]
- Jones, W.; Hill, K.; Allen, J. Wollemia nobilis, a new living Australian genus and species in the Araucariaceae. Telopea 1995, 6, 173–176. [Google Scholar] [CrossRef]
- Sahu, B.; Bhardwaj, N.; Chatterjee, E.; Dey, B.; Tripathi, N.; Goel, B.; Kushwaha, M.; Kumar, B.; Singh, B.; Guru, S.K.; et al. LCMS-DNP based dereplication of Araucaria cunninghamii Mudie gum-resin: Identification of new cytotoxic labdane diterpene. Nat. Prod. Res. 2022, 36, 6207–6214. [Google Scholar] [CrossRef]
- Ndongo, J.T.; Issa, M.E.; Messi, A.N.; Mbing, J.N.; Cuendet, M.; Pegnyemb, D.E.; Bochet, C.G. Cytotoxic flavonoids and other constituents from the stem bark of Ochna schweinfurthiana. Nat. Prod. Res. 2015, 29, 1684–1687. [Google Scholar] [CrossRef]
- Aiello, F.; Malivindi, R.; Motta, M.F.; Crupi, P.; Nicoletti, R.; Benincasa, C.; Clodoveo, M.L.; Rago, V.; Spizzirri, U.G.; Restuccia, D. Synthesis and characterization of a biopolymer pectin/ethanolic extract from olive mill wastewater: In vitro safety and efficacy tests on skin wound healing. Int. J. Mol. Sci. 2023, 24, 15075. [Google Scholar] [CrossRef]
- Di Sotto, A.; Locatelli, M.; Macone, A.; Toniolo, C.; Cesa, C.; Carradori, S.; Eufemi, M.; Mazzanti, G.; Di Giacomo, S. Hypoglycemic, antiglycation, and cytoprotective properties of a phenol-rich extract from waste peel of Punica granatum L. var. Dente di Cavallo DC2. Molecules 2019, 27, 3103. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Durazzi, F.; Sarpietro, M.G.; Mazzanti, G. Antimutagenic and antioxidant activities of some bioflavours from wine. Food Chem. Toxicol. 2013, 60, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Nionelli, L.; Wang, Y.; Pontonio, E.; Immonen, M.; Rizzello, C.; Maina, H.; Katina, K.; Coda, R. Antifungal effect of bioprocessed surplus bread as ingredient for bread-making: Identification of active compounds and impact on shelf-life. Food Control 2020, 118, 107437. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Van’t Riet, K.J.A.E.M. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef]
- Frezza, C.; De Vita, D.; Venditti, A.; Baldani, C.; Giampaoli, O.; Sciubba, F.; Dal Bosco, C.; Franceschin, M.; Beccaccioli, M.; Reverberi, M.; et al. Phytochemical analysis and biological activities of the aerial parts of Odontites vulgaris Moench. Fitoterapia 2024, 175, 105936. [Google Scholar] [CrossRef]


| Classes of Compounds | µg/mg Extract (Mean ± SE) |
|---|---|
| Polyphenols (TAE) | 48.89 ± 5.51 |
| Tannins (TAE) | 33.72 ± 5.80 |
| Flavonoids (QE) | 210.10 ± 16.31 |
| Antioxidant Assay | Wollemia nobilis Leaf Extract | Positive Controls |
|---|---|---|
| IC50 (CL) μg/mL | ||
| DPPH· radical scavenger activity | 227.0 (180.8–284.9) | 3.8 (2.7–5.3) a |
| ABTS·+ radical scavenger activity | 39.7 (27.4–57.4) | 0.6 (0.3–1.0) a |
| Inhibition of AGE generation | 142.4 (82.3–235.3) | 33.7 (18.3–2.2) b |
| Ferrous ion chelating activity | 50.3 (47.3–53.6) | 49.1 (46.2–52.1) b |
| Ferric ion chelating activity | 33.4 (25.6–43.7) | 41.1 (36.3–43.4) b |
| Ferric ion reducing activity | nd | 7.0 (2.6–19.1) a |
| Pearson r (CL; R Square) | |||||
|---|---|---|---|---|---|
| DPPH· Scavenger Activity | ABTS·+ Scavenger Activity | Ferrous Ion Chelating Activity | Ferric Ion Chelating Activity | Inhibition of AGE Generation | |
| DPPH· scavenger activity | - | 0.81 * (0.15–0.99; 0.66) | 0.87 (0.31–0.99; 0.77) | 0.87 (0.93–0.99; 0.76) | 0.96 ** (0.68–0.99; 0.92) |
| ABTS·+ scavenger activity | 0.81 * (0.15–0.99; 0.66) | - | 0.98 ** (0.71–0.99; 0.96) | 0.99 ** (0.03–0.99; 0.98) | 0.94 ** (0.54–0.99; 0.88) |
| Ferrous ion chelating activity | 0.87 (0.31–0.99; 0.77) | 0.99 *** (0.90–0.99; 0.99) | - | 0.99 ** (0.81–0.99; 0.99) | 0.98 * (0.42–0.99; 0.97) |
| Ferric ion chelating activity | 0.87 (0.93–0.99; 0.76) | 0.99 ** (0.03–0.99; 0.98) | 0.99 *** (0.89–0.99; 0.99) | - | 0.95 (0.17–0.99; 0.89) |
| Inhibition of AGE generation | 0.96 ** (0.68–0.99; 0.92) | 0.94 ** (0.54–0.99; 0.88) | 0.98 * (0.42–0.99; 0.97) | 0.95 (0.17–0.99; 0.89) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frezza, C.; De Vita, D.; Giampaoli, O.; Beccaccioli, M.; Verni, M.; Conti, F.V.; Fonti, L.; Franceschin, M.; Sciubba, F.; Scintu, C.; et al. Phytochemical Analysis and Biological Activities of Wollemia nobilis W.G.Jones, K.D.Hill & J.M.Allen Leaves Collected in the Botanical Garden of Rome. Plants 2025, 14, 1244. https://doi.org/10.3390/plants14081244
Frezza C, De Vita D, Giampaoli O, Beccaccioli M, Verni M, Conti FV, Fonti L, Franceschin M, Sciubba F, Scintu C, et al. Phytochemical Analysis and Biological Activities of Wollemia nobilis W.G.Jones, K.D.Hill & J.M.Allen Leaves Collected in the Botanical Garden of Rome. Plants. 2025; 14(8):1244. https://doi.org/10.3390/plants14081244
Chicago/Turabian StyleFrezza, Claudio, Daniela De Vita, Ottavia Giampaoli, Marzia Beccaccioli, Michela Verni, Federica Violetta Conti, Laura Fonti, Marco Franceschin, Fabio Sciubba, Claudio Scintu, and et al. 2025. "Phytochemical Analysis and Biological Activities of Wollemia nobilis W.G.Jones, K.D.Hill & J.M.Allen Leaves Collected in the Botanical Garden of Rome" Plants 14, no. 8: 1244. https://doi.org/10.3390/plants14081244
APA StyleFrezza, C., De Vita, D., Giampaoli, O., Beccaccioli, M., Verni, M., Conti, F. V., Fonti, L., Franceschin, M., Sciubba, F., Scintu, C., Corsetti, L., Di Sotto, A., Rizzello, C. G., Reverberi, M., & Attorre, F. (2025). Phytochemical Analysis and Biological Activities of Wollemia nobilis W.G.Jones, K.D.Hill & J.M.Allen Leaves Collected in the Botanical Garden of Rome. Plants, 14(8), 1244. https://doi.org/10.3390/plants14081244












