Promoting Effects of Piriformospora indica on the Growth and Development of Asparagus (Asparagus officinalis L.) Seedlings
Abstract
1. Introduction
2. Results
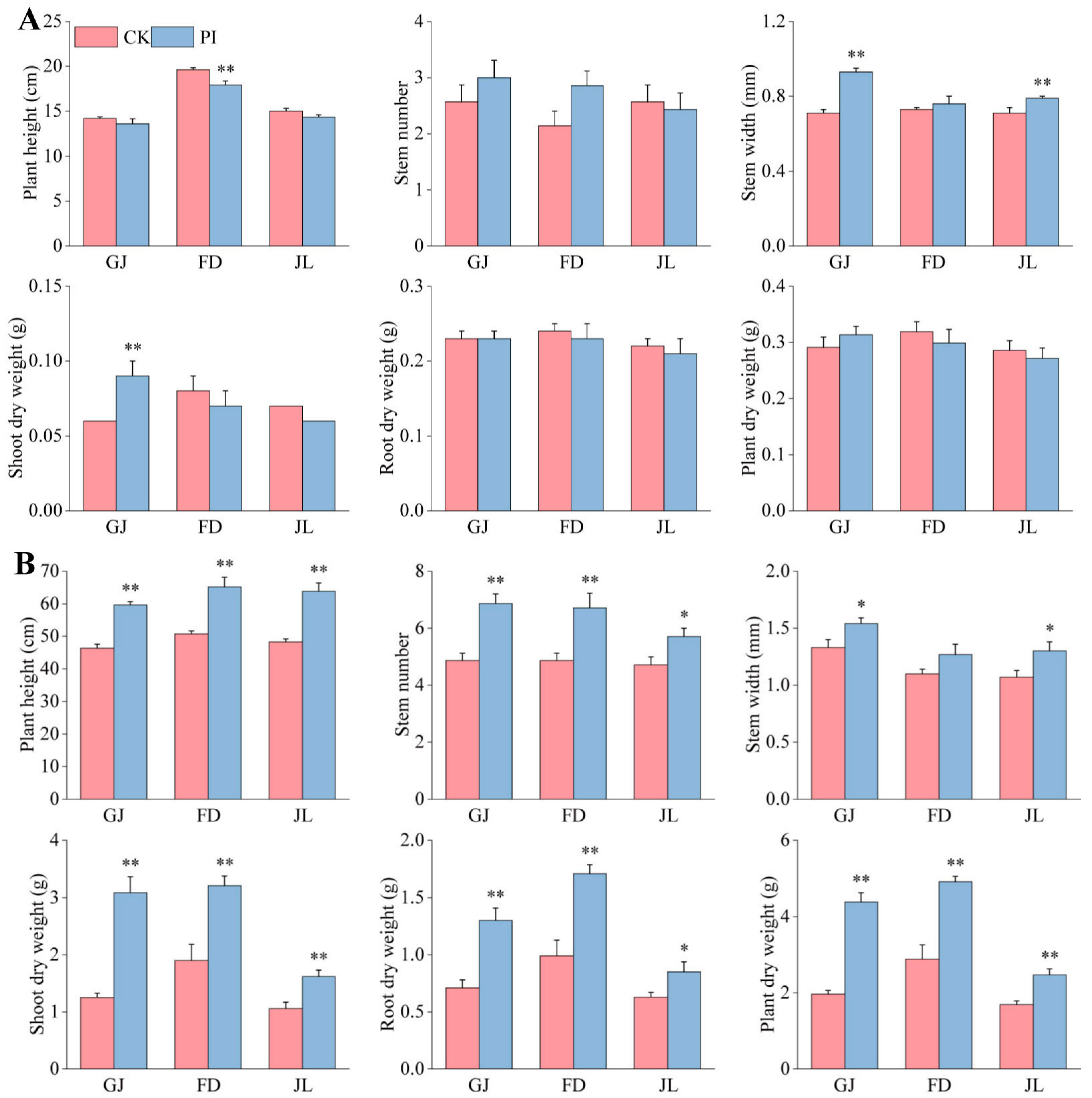
2.1. Effects of P. indica on the Growth and Development of Asparagus Seedlings
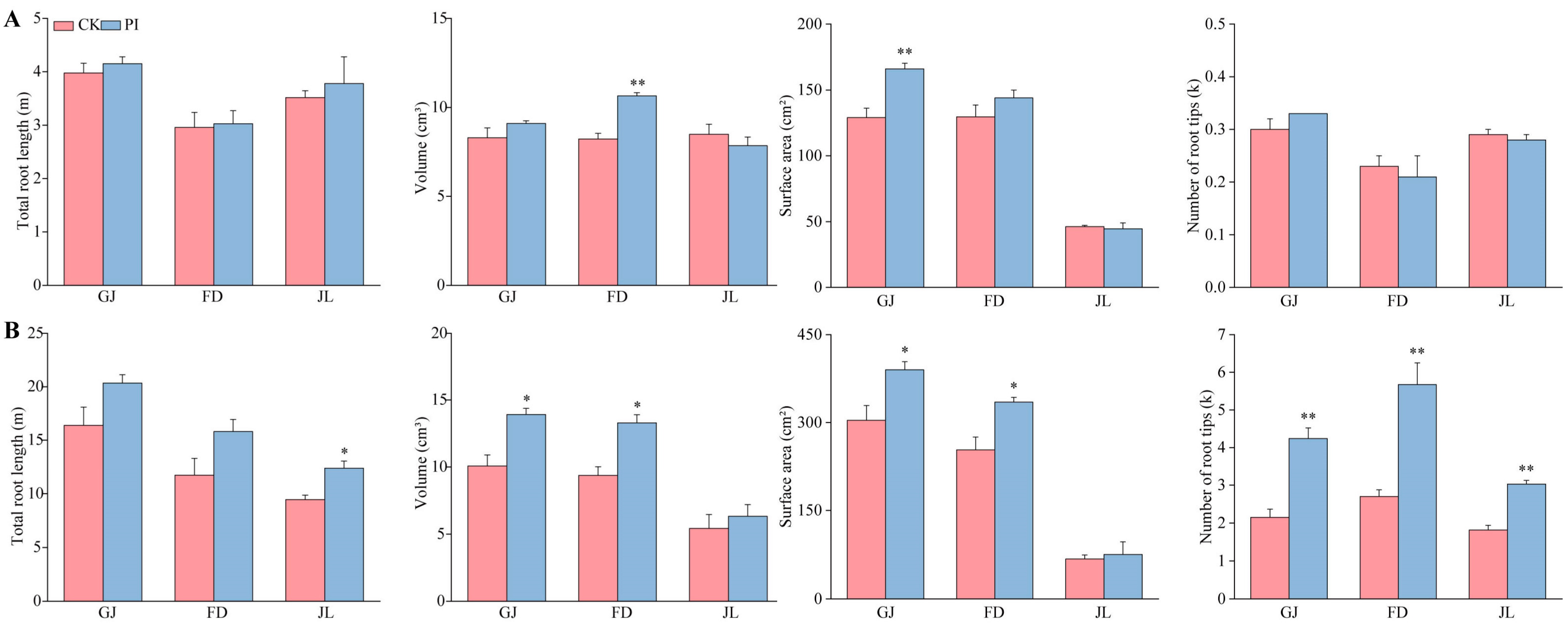
2.2. Influences of P. indica Colonization on Root Architecture Parameters of Asparagus Seedlings
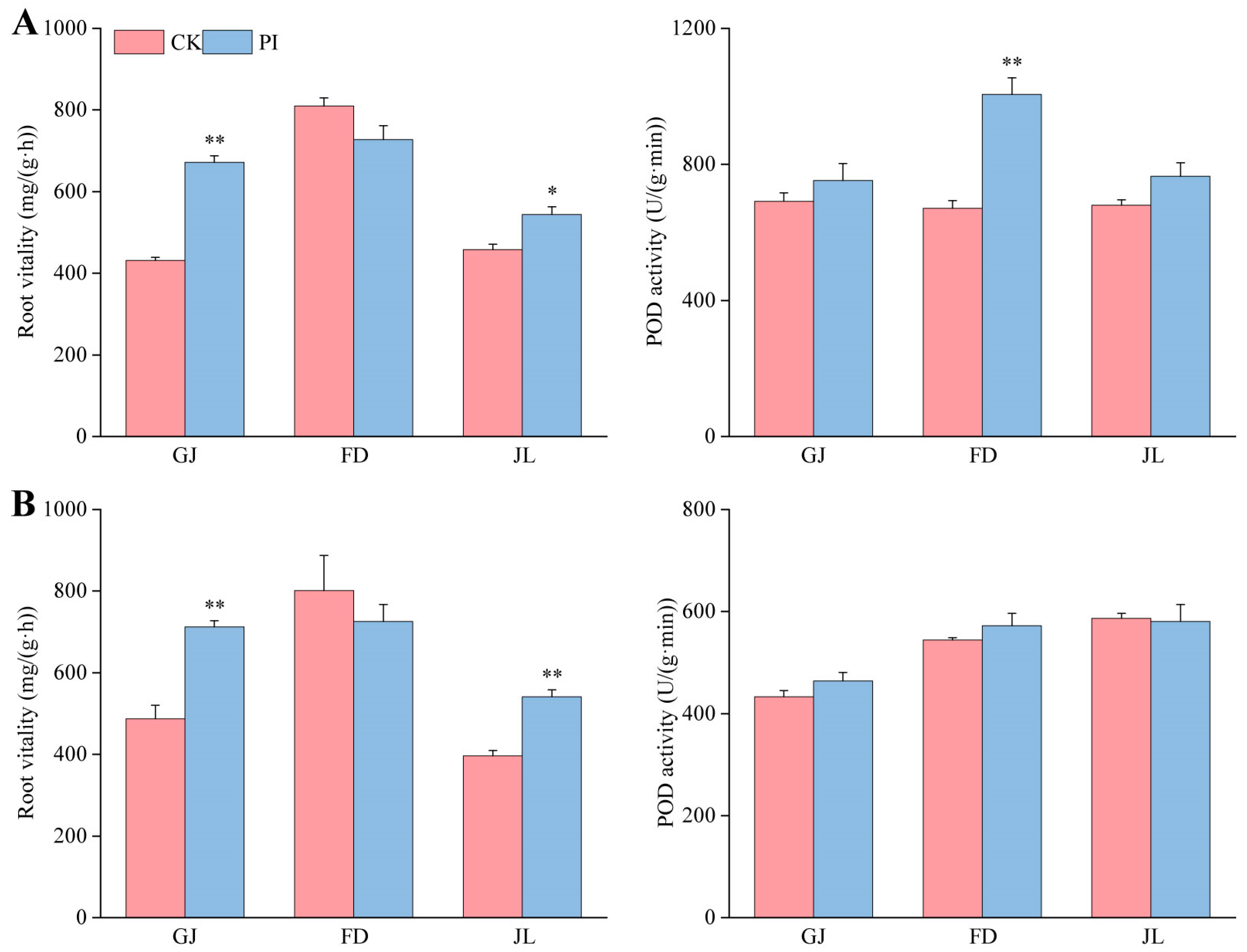
2.3. Effect of P. indica on Root Vitality and POD Activity of Asparagus Seedlings
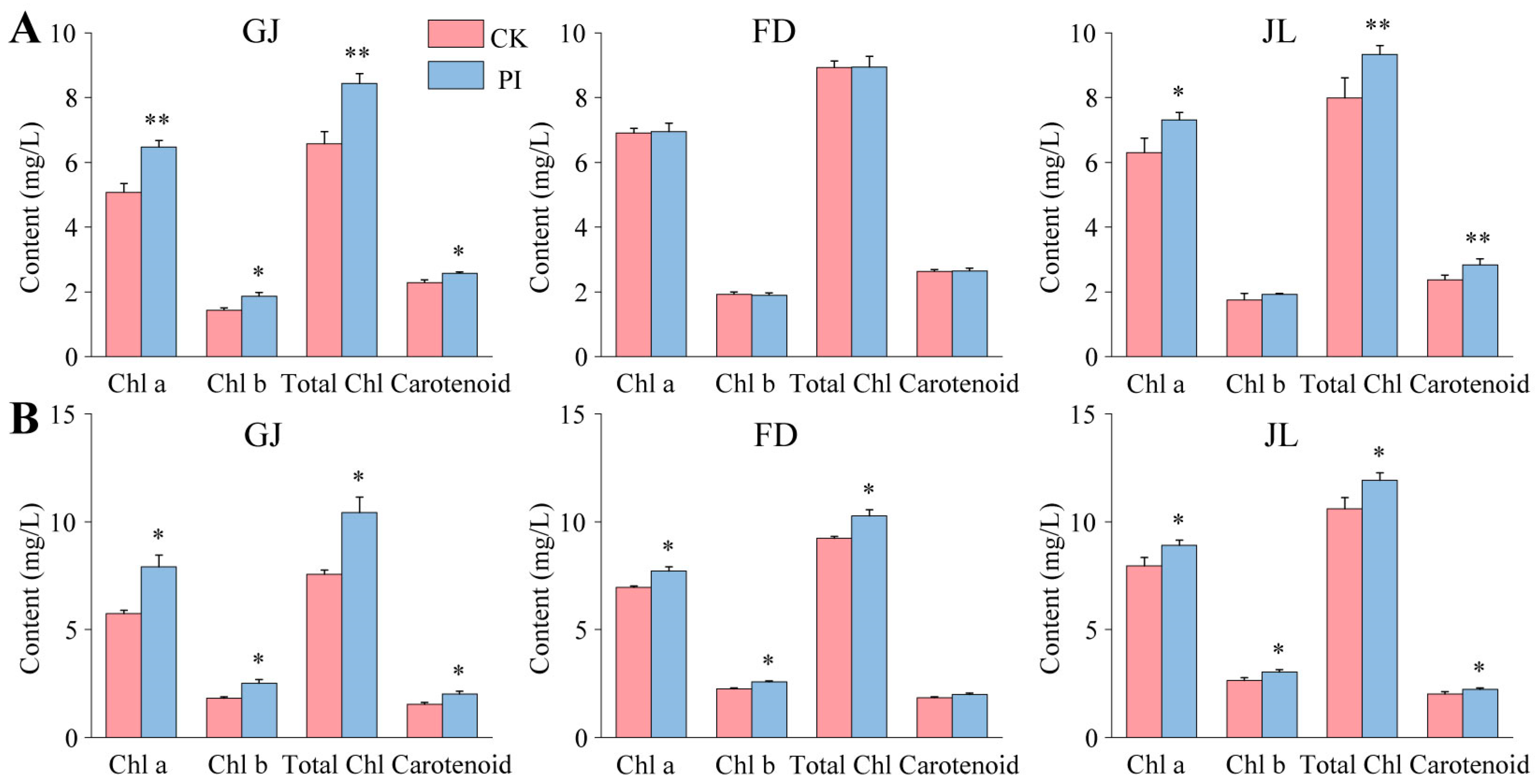
2.4. Effects of P. indica on Photosynthetic Pigment Accumulation in the Leaves of Asparagus Seedlings
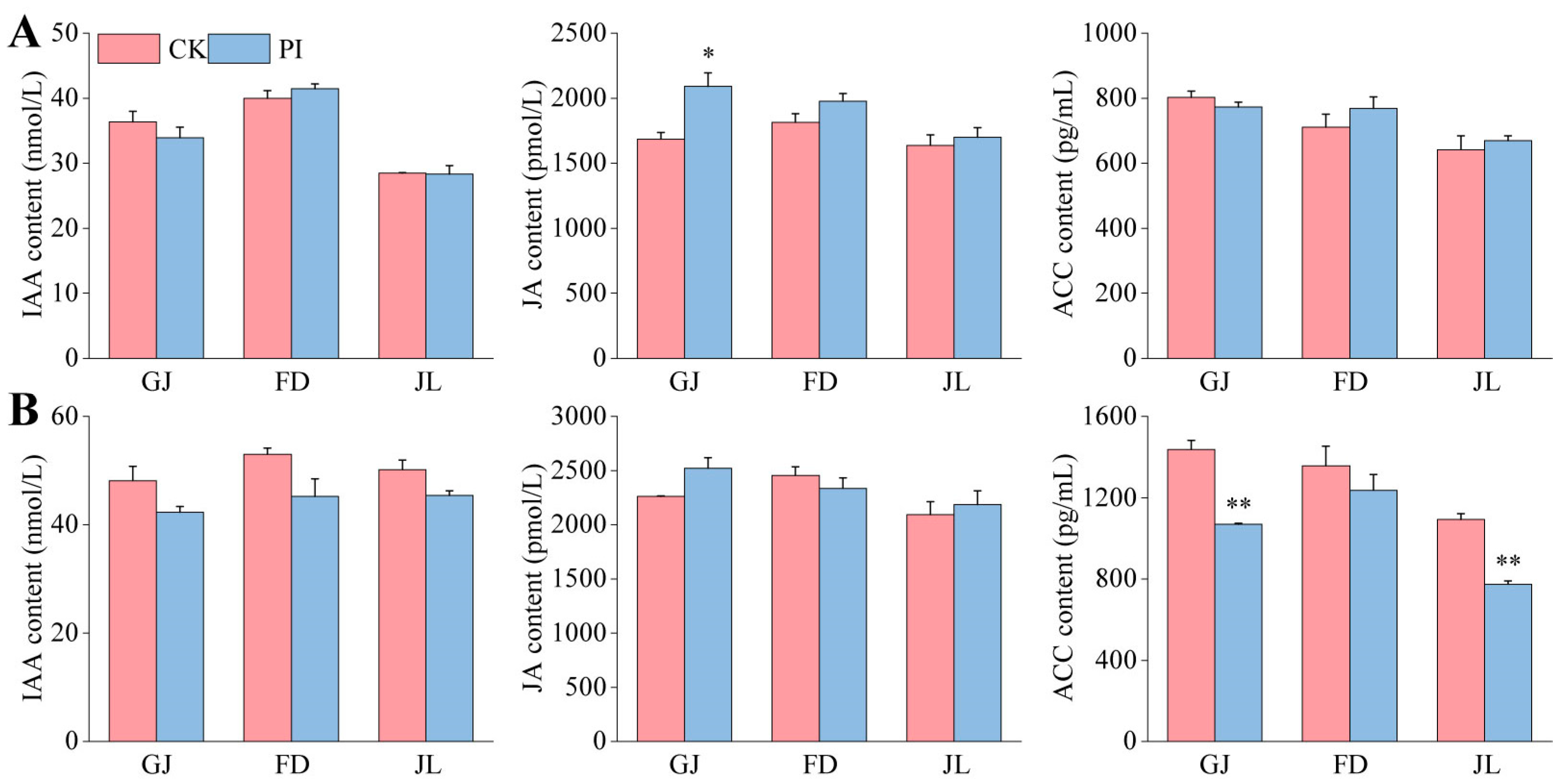
2.5. Effects of P. indica on IAA, JA, and ACC Contents in the Roots of Asparagus Seedlings
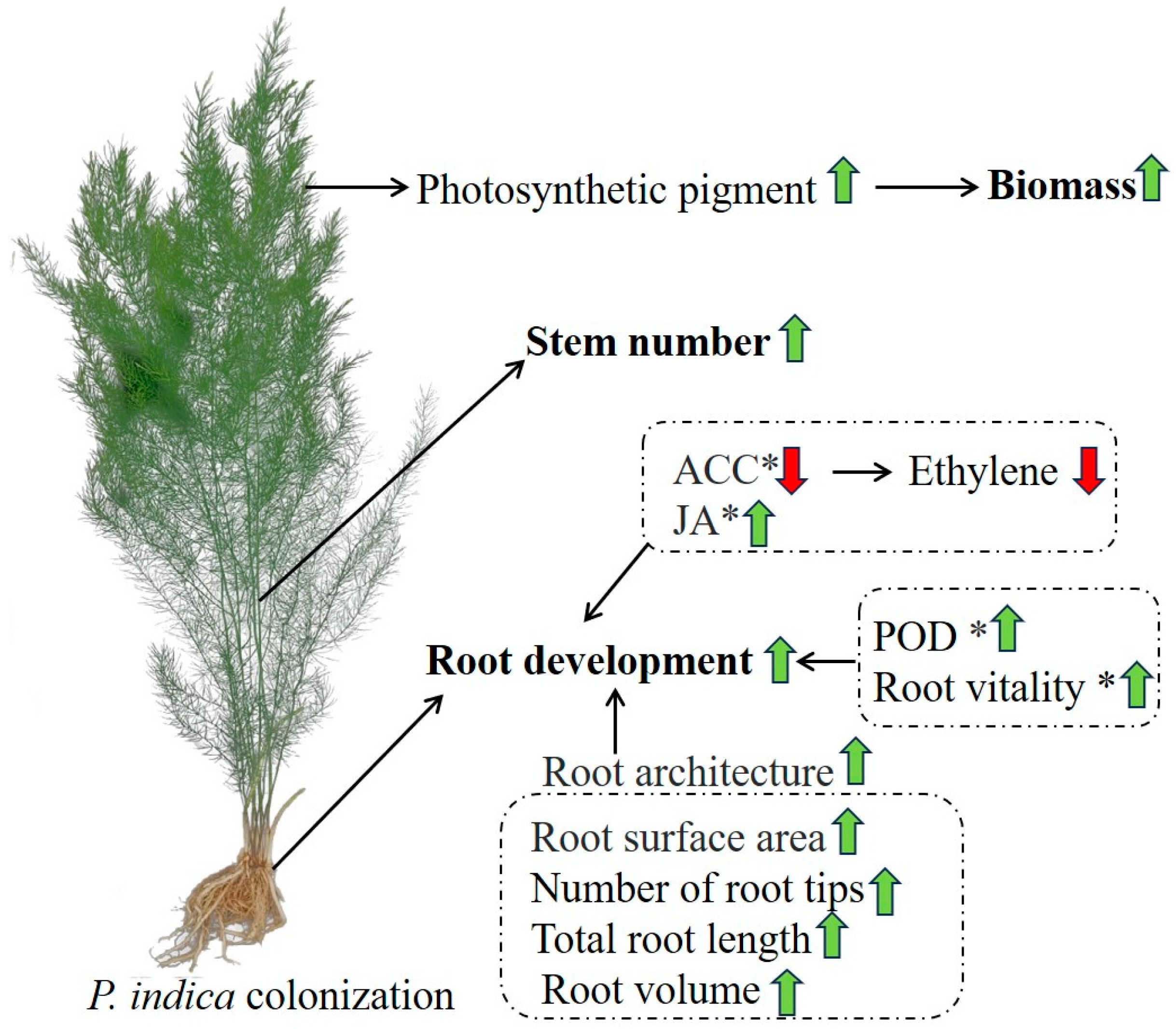
3. Discussion
3.1. P. indica Promotes the Growth of Asparagus Seedlings
3.2. P. indica Colonization Influences Phytohormone Accumulation in Asparagus Roots
4. Materials and Methods
4.1. Plant Materials and Fungal Inoculation
4.2. P. indica Colonization Observation
4.3. Determination of Plant Growth-Related Parameters
4.4. Determination of Root Architecture-Related Parameters
4.5. Determinations of Leaf Photosynthetic Pigment, Root Vitality, and Root POD Activity
4.6. Measurements of Phytohormone Contents in the Roots
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Verma, S.; Varma, A.; Rexer, K.-H.; Hassel, A.; Kost, G.; Sarbhoy, A.; Bisen, P.; Bütehorn, B.; Franken, P. Piriformospora indica, gen. et sp. nov., a new root-colonizing fungus. Mycologia 1998, 90, 896–903. [Google Scholar] [CrossRef]
- Franken, P.; Requena, N.; Bütehorn, B.; Krajinski, F.; Kuhn, G.; Lapopin, L.; Mann, P.; Rhody, D.; Stommel, M. Molecular analysis of the Arbuscular mycorrhiza symbiosis. Arch. Agron. Soil Sci. 2000, 45, 271–286. [Google Scholar] [CrossRef]
- Qu, P.; Zhang, Z.; Li, R.; Liu, R.; Zhang, Y.; Cheng, C. Insights into the rooting and growth-promoting effects of endophytic fungus Serendipita indica in blueberry (Vaccinium corymbosum). J. Plant Growth Regul. 2024, 1–12. [Google Scholar] [CrossRef]
- Yang, L.; Cao, J.-L.; Zou, Y.-N.; Wu, Q.-S.; Kuča, K. Piriformospora indica: A root endophytic fungus and its roles in plants. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1–13. [Google Scholar] [CrossRef]
- Mensah, R.A.; Li, D.; Liu, F.; Tian, N.; Sun, X.; Hao, X.; Lai, Z.; Cheng, C. Versatile Piriformospora indica and its potential applications in horticultural crops. Hortic. Plant J. 2020, 6, 111–121. [Google Scholar] [CrossRef]
- Ansari, M.W.; Trivedi, D.K.; Sahoo, R.K.; Gill, S.S.; Tuteja, N. A critical review on fungi mediated plant responses with special emphasis to Piriformospora indica on improved production and protection of crops. Plant Physiol. Biochem. 2013, 70, 403–410. [Google Scholar] [CrossRef]
- Hu, J.; Li, J.; Wang, H.; Sun, M.; Huang, C.; Wang, H. Analysis of growth dynamics in five different media and metabolic phenotypic characteristics of Piriformospora indica. Front. Microbiol. 2024, 14, 1301743. [Google Scholar] [CrossRef]
- Shao, A.; Yang, J.; Li, H.; Li, R.; Hu, Y.; Cheng, C. Piriformospora indica culture filtrate application adds brilliance to the promoting effects of facility warming on winter jujube fruit ripening. Food Chem. X 2024, 24, 101986. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Feng, Y.; Qi, F.; Hao, R. Research progress of Piriformospora indica in improving plant growth and stress resistance to plant. J. Fungi 2023, 9, 965. [Google Scholar] [CrossRef] [PubMed]
- Solanki, S.; Gupta, S.; Kapoor, R.; Varma, A. Chemically synthesized AgNPs and Piriformospora indica synergistically augment nutritional quality in black rice. J. Fungi 2023, 9, 611. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Wang, T.; Shrivastava, N.; Chen, Y.; Liu, X.; Sun, C.; Yin, Y.; Gao, Q.; Lou, B. Piriformospora indica promotes growth, seed yield and quality of Brassica napus L. Microbiol. Res. 2017, 199, 29–39. [Google Scholar] [CrossRef]
- Xu, L.; Wu, C.; Oelmüller, R.; Zhang, W. Role of phytohormones in Piriformospora indica-induced growth promotion and stress tolerance in plants: More questions than answers. Front. Microbiol. 2018, 9, 1646. [Google Scholar] [CrossRef]
- Cheng, C.; Li, D.; Wang, B.; Liao, B.; Qu, P.; Liu, W.; Zhang, Y.; Lü, P. Piriformospora indica colonization promotes the root growth of dimocarpus longan seedlings. Sci. Hortic. 2022, 301, 111137. [Google Scholar] [CrossRef]
- Wu, H.; Wang, B.; Hao, X.; Zhang, Y.; Wang, T.; Lu, Z.; Lai, Z.; Cheng, C. Piriformospora indica promotes the growth and enhances the root rot disease resistance of gerbera. Sci. Hortic. 2022, 297, 110946. [Google Scholar] [CrossRef]
- Ghaffari, M.R.; Mirzaei, M.; Ghabooli, M.; Khatabi, B.; Wu, Y.; Zabet-Moghaddam, M.; Mohammadi-Nejad, G.; Haynes, P.A.; Hajirezaei, M.R.; Sepehri, M.; et al. Root endophytic fungus Piriformospora indica improves drought stress adaptation in barley by metabolic and proteomic reprogramming. Environ. Exp. Bot. 2019, 157, 197–210. [Google Scholar] [CrossRef]
- Li, H.; Fu, S.; Zhu, J.; Gao, W.; Chen, L.; Li, X.; Zhang, S.; Zheng, S.; Zhang, H.; Liu, Y. Nitric oxide generated by Piriformospora indica-induced nitrate reductase promotes tobacco growth by regulating root architecture and ammonium and nitrate transporter gene expression. J. Plant Interact. 2022, 17, 861–872. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Xu, L.; Wang, A.; Huang, L.; Du, H.; Qiu, L.; Oelmüller, R. Drought stress responses in maize are diminished by Piriformospora indica. Plant Signal. Behav. 2018, 13, e1414121. [Google Scholar] [CrossRef]
- Mohan, S.L.; Beena, R.; Joy, M. An improvement in water stress tolerance in rice by altering morpho-physiological and biochemical mechanisms using root colonizing endophyte Piriformospora indica. Vegetos 2024, 38, 341–352. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Nong, C.; Lin, T.; Fang, L.; Feng, X.; Chen, Y.; Lin, Y.; Lai, Z.; Miao, L. Piriformospora indica enhances resistance to Fusarium wilt in strawberry by increasing the activity of superoxide dismutase, peroxidase, and catalase, while reducing the content of malondialdehyde in the roots. Horticulturae 2024, 10, 240. [Google Scholar] [CrossRef]
- Li, D.; Bodjrenou, D.M.; Zhang, S.; Wang, B.; Pan, H.; Yeh, K.-W.; Lai, Z.; Cheng, C. The endophytic fungus Piriformospora indica reprograms banana to cold resistance. Int. J. Mol. Sci. 2021, 22, 4973. [Google Scholar] [CrossRef]
- Kao, C.-W.; Bakshi, M.; Sherameti, I.; Dong, S.; Reichelt, M.; Oelmüller, R.; Yeh, K.-W. A Chinese cabbage (Brassica campetris Subsp. Chinensis) τ-type glutathione-s-transferase stimulates Arabidopsis development and primes against abiotic and biotic stress. Plant Mol. Biol. 2016, 92, 643–659. [Google Scholar] [CrossRef]
- Lin, P.-C.; Lilananda, I.; Shao, K.-H.; Wu, H.-Y.; Wang, S.-J. Role of auxin in the symbiotic relationship between Piriformospora indica and rice plants. Rhizosphere 2023, 25, 100632. [Google Scholar] [CrossRef]
- Yin, L.; Qu, P.; Wang, D.; Yan, S.; Gong, Q.; Yang, R.; Hu, Y.; Liu, N.; Cheng, C.; Wang, P.; et al. The influence of Piriformospora indica colonization on the root development and growth of Cerasus humilis cuttings. Plants 2024, 13, 1482. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, N.; Liu, H.; Li, Z.; Lu, L.; Wang, C. The bioactive compounds and biological functions of Asparagus officinalis L.—A review. J. Funct. Foods 2020, 65, 103727. [Google Scholar] [CrossRef]
- Olas, B. A review of the pro-health activity of Asparagus officinalis L. and its components. Foods 2024, 13, 288. [Google Scholar] [CrossRef]
- Goñi, I.; García-Alonso, A.; Alba, C.; Rodríguez, J.M.; Sánchez-Mata, M.C.; Guillén-Bejarano, R.; Redondo-Cuenca, A. Composition and functional properties of the edible spear and by-products from Asparagus officinalis L. and their potential prebiotic effect. Foods 2024, 13, 1154. [Google Scholar] [CrossRef] [PubMed]
- Viera-Alcaide, I.; Hamdi, A.; Guillén-Bejarano, R.; Rodríguez-Arcos, R.; Espejo-Calvo, J.A.; Jiménez-Araujo, A. Asparagus roots: From an agricultural by-product to a valuable source of fructans. Foods 2022, 11, 652. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Liu, H.; Zhang, S.; Wang, B.; Liu, Y.; Zhang, X.; Zhang, M. Polysaccharide of Asparagus cochinchinensis (Lour.) Merr: Preliminary characterization and antitumor activity in vivo. Food Biosci. 2023, 56, 103387. [Google Scholar] [CrossRef]
- Gao, R.; Li, G.; Liu, P.; Gao, L.; Bi, J.; Jiang, Y.; Liu, H.; Wang, Y. The quality evaluation of 30 Asparagus officinalis L. varieties. Food Sci. Nutr. 2024, 12, 2908–2916. [Google Scholar] [CrossRef]
- Yu, E.; He, C. Effects of AMF inoculation on growth and nutrient quality of Asparagus plant under protected cultivation. In Proceedings of the Acta Horticulturae, Nanchang, China, 16–18 October 2013; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2020; pp. 145–148. [Google Scholar]
- Conversa, G.; Miedico, O.; Chiaravalle, A.E.; Elia, A. Heavy metal contents in green spears of asparagus (Asparagus officinalis L.) grown in southern Italy: Variability among farms, genotypes and effect of soil mycorrhizal inoculation. Sci. Hortic. 2019, 256, 108559. [Google Scholar] [CrossRef]
- Deshmukh, S.; Hückelhoven, R.; Schäfer, P.; Imani, J.; Sharma, M.; Weiss, M.; Waller, F.; Kogel, K.-H. The root endophytic fungus Piriformospora indica requires host cell death for proliferation during mutualistic symbiosis with barley. Proc. Natl. Acad. Sci. USA 2006, 103, 18450–18457. [Google Scholar] [CrossRef]
- Sabeem, M.; Abdul Aziz, M.; Mullath, S.K.; Brini, F.; Rouached, H.; Masmoudi, K. Enhancing growth and salinity stress tolerance of date palm using Piriformospora indica. Front. Plant Sci. 2022, 13, 1037273. [Google Scholar] [CrossRef]
- Yan, C.; Muhammad Rizwan, H.; Liang, D.; Reichelt, M.; Mithöfer, A.; Scholz, S.S.; Oelmüller, R.; Chen, F. The effect of the root-colonizing Piriformospora indica on passion fruit (Passiflora edulis) development: Initial defense shifts to fitness benefits and higher fruit quality. Food Chem. 2021, 359, 129671. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kuo, Y.-W.; Lin, K.-H.; Huang, W.; Deng, C.; Yeh, K.-W.; Chen, S.-P. Piriformospora indica colonization increases the growth, development, and herbivory resistance of sweet potato (Ipomoea batatas L.). Plant Cell Rep. 2021, 40, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-F.; Xiong, J.; Zhou, H.-M.; Chen, C.-M.; Lin, F.-Z.; Xu, X.-M.; Oelmüller, R.; Xu, W.-F.; Yeh, K.-W. Growth promotion and disease resistance induced in Anthurium colonized by the beneficial root endophyte Piriformospora indica. BMC Plant Biol. 2019, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, X.; Zhang, Z.; Li, C.; Xing, Y.; Luo, Y.; Li, D.; Ma, Z.; Cai, H. Symbiotic system establishment between Piriformospora indica and Glycine max and its effects on the antioxidant activity and ion-transporter-related gene expression in soybean under salt stress. Int. J. Mol. Sci. 2022, 23, 14961. [Google Scholar] [CrossRef]
- Shahabivand, S.; Parvaneh, A.; Aliloo, A.A. Root endophytic fungus Piriformospora indica affected growth, cadmium partitioning and chlorophyll fluorescence of sunflower under cadmium toxicity. Ecotoxicol. Environ. Saf. 2017, 145, 496–502. [Google Scholar] [CrossRef]
- Lakehal, A.; Bellini, C. Control of adventitious root formation: Insights into synergistic and antagonistic hormonal interactions. Physiol. Plant. 2019, 165, 90–100. [Google Scholar] [CrossRef]
- Vadassery, J.; Ritter, C.; Venus, Y.; Camehl, I.; Varma, A.; Shahollari, B.; Novák, O.; Strnad, M.; Ludwig-Müller, J.; Oelmüller, R. The role of auxins and cytokinins in the mutualistic interaction between Arabidopsis and Piriformospora indica. Mol. Plant-Microbe Interact. 2008, 21, 1371–1383. [Google Scholar] [CrossRef]
- Sun, T.; Tan, W.; Yang, Y.; Mu, H. AM fungi and Piriformospora indica improve plant growth of Pinus elliottii seedlings. Phyton 2021, 90, 171–178. [Google Scholar] [CrossRef]
- Khatabi, B.; Schäfer, P. Ethylene in mutualistic symbioses. Plant Signal. Behav. 2012, 7, 1634–1638. [Google Scholar] [CrossRef]
- Le, J.; Vandenbussche, F.; Van Der Straeten, D.; Verbelen, J.-P. In the early response of Arabidopsis roots to ethylene, cell elongation is up- and down-regulated and uncoupled from differentiation. Plant Physiol. 2001, 125, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Vanderstraeten, L.; Depaepe, T.; Bertrand, S.; Van Der Straeten, D. The ethylene precursor ACC affects early vegetative development independently of ethylene signaling. Front. Plant Sci. 2019, 10, 1591. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, P.; Pfiffi, S.; Voll, L.M.; Zajic, D.; Chandler, P.M.; Waller, F.; Scholz, U.; Pons-Kühnemann, J.; Sonnewald, S.; Sonnewald, U.; et al. Manipulation of plant innate immunity and gibberellin as factor of compatibility in the mutualistic association of barley roots with Piriformospora indica. Plant J. 2009, 59, 461–474. [Google Scholar] [CrossRef]
- Khatabi, B.; Molitor, A.; Lindermayr, C.; Pfiffi, S.; Durner, J.; Von Wettstein, D.; Kogel, K.-H.; Schäfer, P. Ethylene supports colonization of plant roots by the mutualistic fungus Piriformospora indica. PLoS ONE 2012, 7, e35502. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Li, D.; Qi, Q.; Sun, X.; Anue, M.R.; David, B.M.; Zhang, Y.; Hao, X.; Zhang, Z.; Lai, Z. The root endophytic fungus Serendipita indica improves resistance of banana to Fusarium oxysporum f. sp. cubense tropical race 4. Eur. J. Plant Pathol. 2020, 156, 87–100. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Wang, Y.; Song, H.; Luo, C.; Cheng, C.; Mao, L. Promoting Effects of Piriformospora indica on the Growth and Development of Asparagus (Asparagus officinalis L.) Seedlings. Plants 2025, 14, 1232. https://doi.org/10.3390/plants14081232
Zhao J, Wang Y, Song H, Luo C, Cheng C, Mao L. Promoting Effects of Piriformospora indica on the Growth and Development of Asparagus (Asparagus officinalis L.) Seedlings. Plants. 2025; 14(8):1232. https://doi.org/10.3390/plants14081232
Chicago/Turabian StyleZhao, Jing, Ying Wang, Huixia Song, Chao Luo, Chunzhen Cheng, and Liping Mao. 2025. "Promoting Effects of Piriformospora indica on the Growth and Development of Asparagus (Asparagus officinalis L.) Seedlings" Plants 14, no. 8: 1232. https://doi.org/10.3390/plants14081232
APA StyleZhao, J., Wang, Y., Song, H., Luo, C., Cheng, C., & Mao, L. (2025). Promoting Effects of Piriformospora indica on the Growth and Development of Asparagus (Asparagus officinalis L.) Seedlings. Plants, 14(8), 1232. https://doi.org/10.3390/plants14081232







