Declining Outcrossing Rates Inside Orchard Blocks of ‘Maluma’ and ‘Shepard’ Avocado (Persea americana Mill.) Trees: Effects on Fruit Yield and Quality
Abstract
1. Introduction
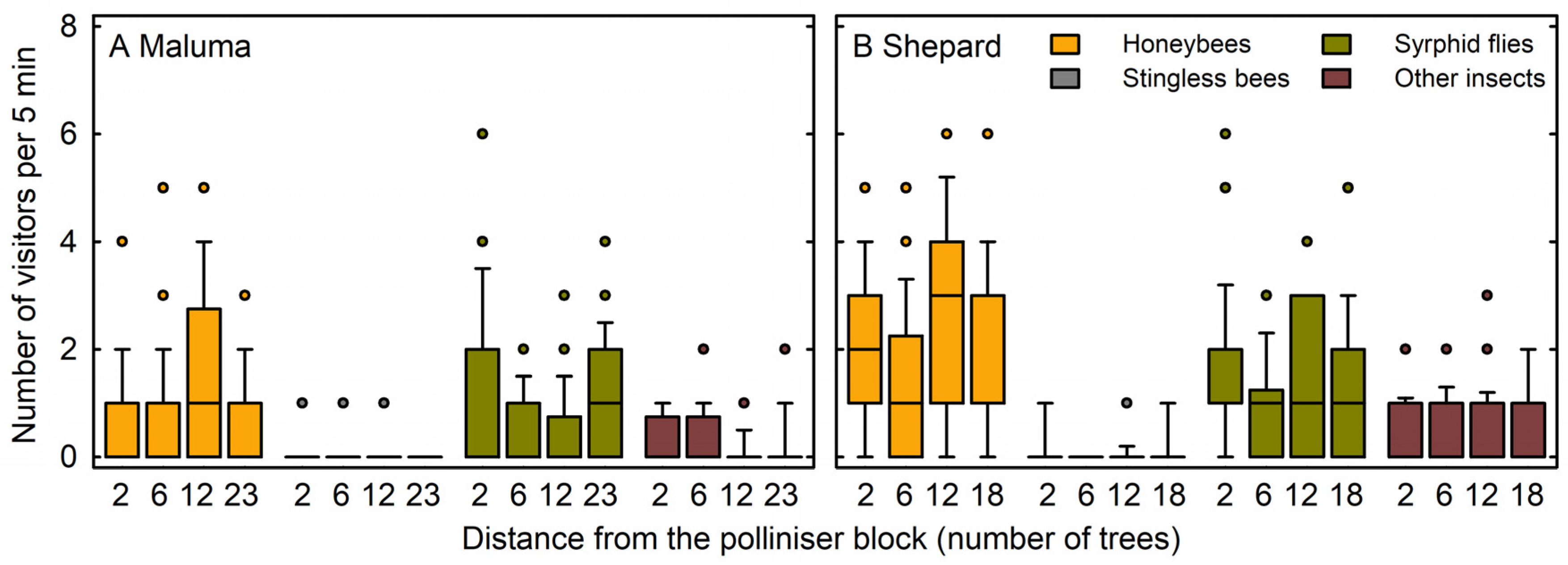
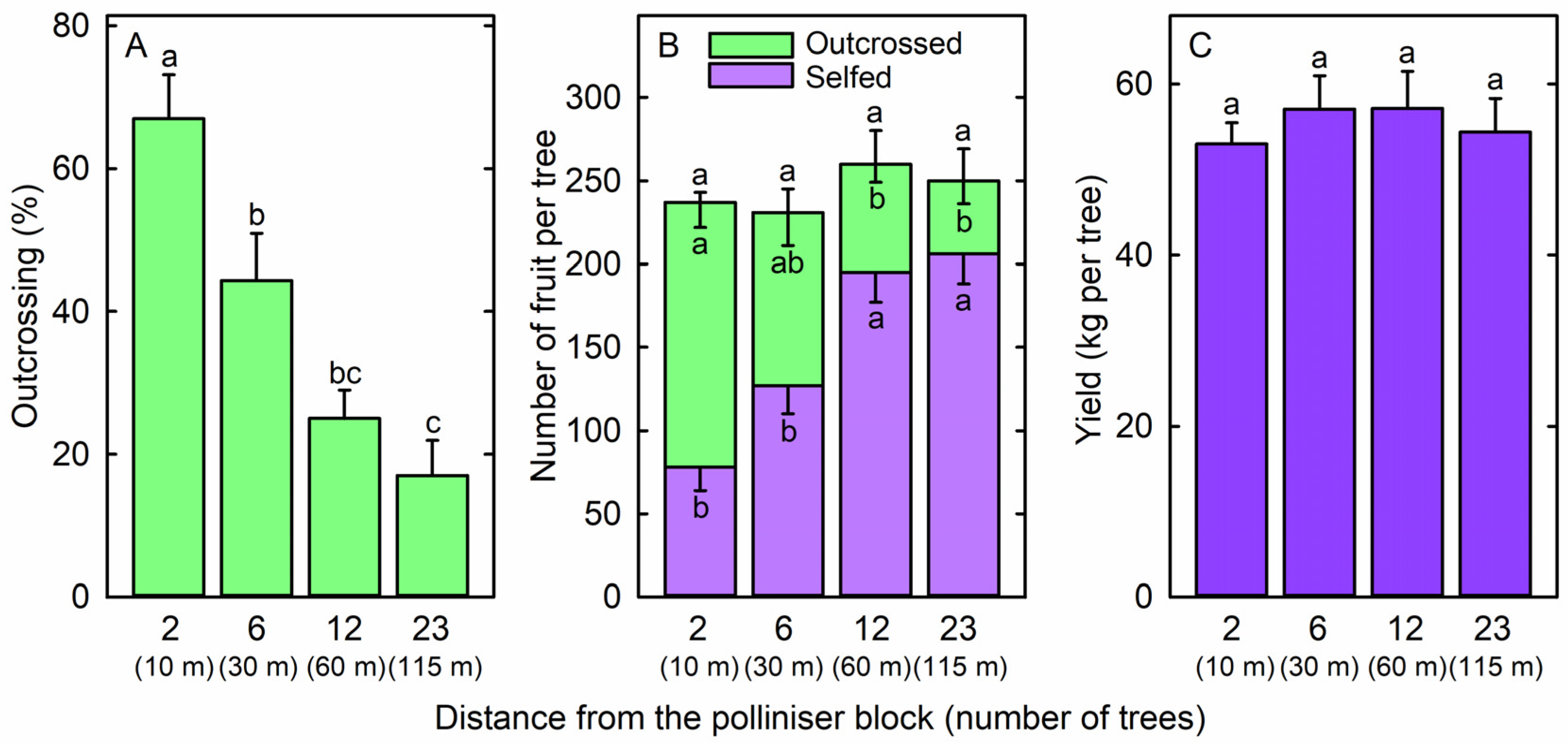
2. Results
3. Discussion
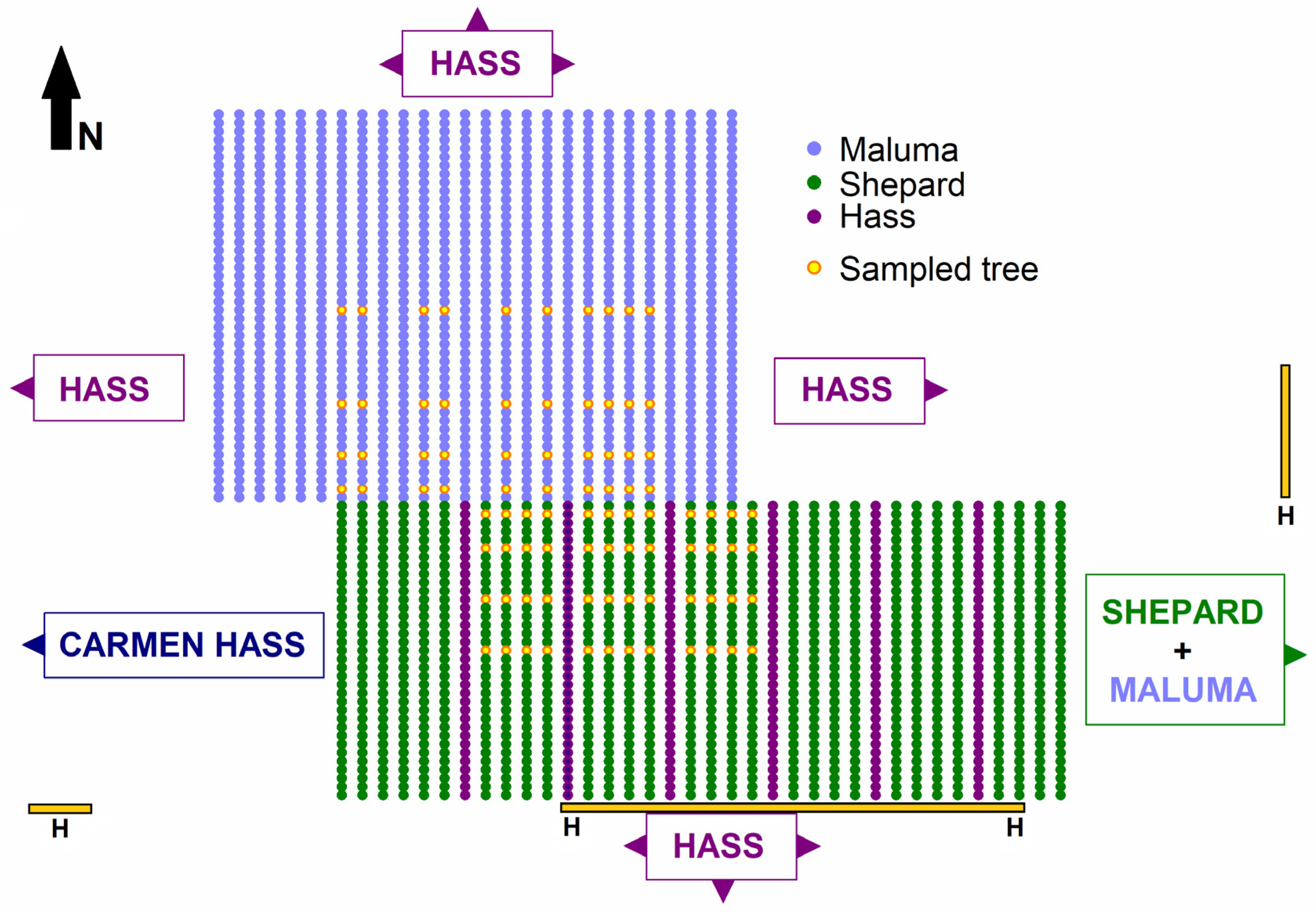
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef] [PubMed]
- FAO. World Food and Agriculture—Statistical Yearbook 2023; FAO: Rome, Italy, 2023. [Google Scholar]
- Ray, D.K.; Gerber, J.S.; MacDonald, G.K.; West, P.C. Climate variation explains a third of global crop yield variability. Nat. Commun. 2015, 6, 5989. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, Z.; Xu, J.; Chen, Y.; Jägermeyr, J.; Cao, J.; Luo, Y.; Cheng, F.; Zhuang, H.; Wu, H.; et al. Threat of low-frequency high-intensity floods to global cropland and crop yields. Nat. Sustain. 2024, 7, 994–1006. [Google Scholar] [CrossRef]
- Najafi, E.; Indrani, I.; Reza, K. Climate drives variability and joint variability of global crop yields. Sci. Total Environ. 2019, 662, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Wakatsuki, H.; Nelson, G.C. Evidence for and projection of multi-breadbasket failure caused by climate change. Curr. Opin. Environ. Sustain. 2022, 58, 101217. [Google Scholar] [CrossRef]
- Mueller, N.D.; Gerber, J.S.; Johnston, M.; Ray, D.K.; Ramankutty, N.; Foley, J.A. Closing yield gaps through nutrient and water management. Nature 2012, 490, 254–257. [Google Scholar] [CrossRef]
- Martre, P.; Dueri, S.; Guarin, J.R.; Ewert, F.; Webber, H.; Calderini, D.; Molero, G.; Reynolds, M.; Miralles, D.; Garcia, G.; et al. Global needs for nitrogen fertilizer to improve wheat yield under climate change. Nat. Plants 2024, 10, 1081–1090. [Google Scholar] [CrossRef]
- Guo, C.; Liu, X.; He, X. A global meta-analysis of crop yield and agricultural greenhouse gas emissions under nitrogen fertilizer application. Sci. Total Environ. 2022, 831, 154982. [Google Scholar] [CrossRef]
- Turo, K.J.; Reilly, J.R.; Fijen, T.P.M.; Magrach, A.; Winfree, R. Insufficient pollinator visitation often limits yield in crop systems worldwide. Nat. Ecol. Evol. 2024, 8, 1612–1622. [Google Scholar] [CrossRef]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef]
- Reilly, J.; Bartomeus, I.; Simpson, D.; Allen-Perkins, A.; Garibaldi, L.; Winfree, R. Wild insects and honey bees are equally important to crop yields in a global analysis. Glob. Ecol. Biogeogr. 2024, 33, e13843. [Google Scholar] [CrossRef]
- Aizen, M.A.; Harder, L.D. The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Curr. Biol. 2009, 19, 915–918. [Google Scholar] [CrossRef]
- Aizen, M.A.; Garibaldi, L.A.; Cunningham, S.A.; Klein, A.M. How much does agriculture depend on pollinators? Lessons from long-term trends in crop production. Ann. Bot. 2009, 103, 1579–1588. [Google Scholar] [CrossRef]
- Klein, A.-M.; Boreux, V.; Fornoff, F.; Mupepele, A.-C.; Pufal, G. Relevance of wild and managed bees for human well-being. Curr. Opin. Insect Sci. 2018, 26, 82–88. [Google Scholar] [CrossRef]
- Aizen, M.A.; Aguiar, S.; Biesmeijer, J.C.; Garibaldi, L.A.; Inouye, D.W.; Jung, C.; Martins, D.J.; Medel, R.; Morales, C.L.; Ngo, H.; et al. Global agricultural productivity is threatened by increasing pollinator dependence without a parallel increase in crop diversification. Glob. Change Biol. 2019, 25, 3516–3527. [Google Scholar] [CrossRef]
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Stefan Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Eilers, E.J.; Kremen, C.; Smith Greenleaf, S.; Garber, A.K.; Klein, A.-M. Contribution of pollinator-mediated crops to nutrients in the human food supply. PLoS ONE 2011, 6, e21363. [Google Scholar] [CrossRef]
- van der Neit, T.; Egan, P.A.; Schlüter, P.M. Evolutionarily inspired solutions to the crop pollination crisis. Trends Ecol. Evol. 2023, 38, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Siopa, C.; Carvalheiro, L.G.; Castro, H.; Loureiro, J.; Castro, S. Animal-pollinated crops and cultivars—A quantitative assessment of pollinator dependence values and evaluation of methodological approaches. J. Appl. Ecol. 2024, 61, 1279–1288. [Google Scholar] [CrossRef]
- Tscharntke, T.; Ocampo-Ariza, C.; Kämper, W. Pollinator, pollen, and cultivar identity drive crop quality. Trends Plant Sci. 2025, 30, 283–290. [Google Scholar] [CrossRef]
- Claessen, H.; Van de Poel, B.; Keulemans, W.; De Storme, N. A semi in vivo pollination technique to assess the level of gametophytic self incompatibility and pollen tube growth in pear (Pyrus communis L.). Plant Reprod. 2022, 35, 127–140. [Google Scholar] [CrossRef] [PubMed]
- López, M.E.; Ramírez, O.A.; Dubón, A.; Ribeiro, T.H.C.; Díaz, F.J.; Chalfun, A., Jr. Sexual compatibility in cacao clones drives arrangements in the field leading to high yield. Sci. Hortic. 2021, 287, 110276. [Google Scholar] [CrossRef]
- Ono, K.; Masui, K.; Tao, R. Artificial control of the Prunus self-incompatibility system using antisense oligonucleotides against pollen genes. Hortic. J. 2022, 91, 437–447. [Google Scholar] [CrossRef]
- Trueman, S.J.; Penter, M.G.; Malagodi-Braga, K.S.; Nichols, J.; De Silva, A.L.; Ramos, A.T.M.; Moriya, L.M.; Ogbourne, S.M.; Hawkes, D.; Peters, T.; et al. High outcrossing levels among global macadamia cultivars: Implications for nut quality, orchard designs and pollinator management. Horticulturae 2024, 10, 203. [Google Scholar] [CrossRef]
- Kämper, W.; Thorp, G.; Wirthensohn, M.; Brooks, P.; Trueman, S.J. Pollen paternity can affect kernel size and nutritional composition of self-incompatible and new self-compatible almond cultivars. Agronomy 2021, 11, 326. [Google Scholar] [CrossRef]
- Stern, R.A.; Gazit, S.; El-Batsri, R.; Degani, C. Pollen parent effect on outcrossing rate, yield, and fruit characteristics of ‘Floridian’ and ‘Mauritius’ lychee. J. Am. Soc. Hortic. Sci. 1993, 118, 109–114. [Google Scholar] [CrossRef]
- Wallace, H.M.; King, B.J.; Lee, L.S. Pollen flow and the effect on fruit size in an ‘Imperial’ mandarin orchard. HortScience 2002, 37, 84–86. [Google Scholar] [CrossRef]
- Pérez, V.; Herrero, M.; Hormaza, J.I. Self-fertility and preferential cross-fertilization in mango (Mangifera indica). Sci. Hortic. 2016, 213, 373–378. [Google Scholar] [CrossRef]
- Meland, M.; Frøynes, O.; Fotiric Akšić, M.; Pojskić, N.; Kalamujić Stroil, B.K.; Lasic, L.; Gasi, F. Identifying pollen donors and success rate of individual pollinizers in European plum (Prunus domestica L.) using microsatellite markers. Agronomy 2020, 10, 264. [Google Scholar] [CrossRef]
- Kämper, W.; Nichols, J.; Tran, T.D.; Burwell, C.J.; Byrnes, S.; Trueman, S.J. Flower visitors, levels of cross-fertilisation, and pollen-parent effects on fruit quality in mango orchards. Agronomy 2023, 13, 2568. [Google Scholar] [CrossRef]
- Trueman, S.J.; Nichols, J.; Burwell, C.J.; Kämper, W. Strategic selection of polliniser trees can improve fruit quality of lychee, a crop that exhibits mixed-mating. Basic Appl. Ecol. 2025, 83, 80–87. [Google Scholar] [CrossRef]
- Degani, C.; Goldring, A.; Gazit, S. Pollen parent effect on outcrossing rate in ‘Hass’ and ‘Fuerte’ avocado plots during fruit development. J. Am. Soc. Hortic. Sci. 1989, 114, 106–111. [Google Scholar] [CrossRef]
- Degani, C.; Stern, R.A.; El-Batsri, R.; Gazit, S. Pollen parent effect on the selective abscission of ‘Mauritius’ and ‘Floridian’ lychee fruitlets. J. Am. Soc. Hortic. Sci. 1995, 120, 523–526. [Google Scholar] [CrossRef]
- Degani, C.; El-Batsri, R.; Gazit, S. Outcrossing rate, yield, and selective fruit abscission in ‘Ettinger’ and ‘Ardith’ avocado plots. J. Am. Soc. Hortic. Sci. 1997, 122, 813–817. [Google Scholar] [CrossRef]
- Dag, A.; Eisenstein, D.; Gazit, S.; El-Batsri, R.; Degani, C. Effect of pollenizer distance and selective fruitlet abscission on outcrossing rate and yield in ‘Tommy Atkins’ mango. J. Am. Soc. Hortic. Sci. 1998, 123, 618–622. [Google Scholar] [CrossRef]
- Holanda-Neto, J.P.; Freitas, B.M.; Bueno, D.M.; Araújo, Z.B. Low seed/nut productivity in cashew (Anacardium occidentale): Effects of self-incompatibility and honeybee (Apis mellifera) foraging behavior. J. Hortic. Sci. Biotechnol. 2002, 77, 226–231. [Google Scholar] [CrossRef]
- Alcaraz, M.L.; Hormaza, J.I. Influence of physical distance between cultivars on yield, outcrossing rate and selective fruit drop in avocado (Persea americana, Lauraceae). Ann. Appl. Biol. 2011, 158, 354–361. [Google Scholar] [CrossRef]
- De Silva, A.L.; Kämper, W.; Ogbourne, S.M.; Nichols, J.; Royle, J.W.L.; Peters, T.; Hawkes, D.; Hosseini Bai, S.; Wallace, H.M.; Trueman, S.J. MassARRAY and SABER analyses of SNPs in embryo DNA reveal the abscission of self-fertilised progeny during fruit development of macadamia (Macadamia integrifolia Maiden & Betche). Int. J. Mol. Sci. 2024, 25, 6419. [Google Scholar] [CrossRef] [PubMed]
- Hapuarachchi, N.S.; Kämper, W.; Hosseini Bai, S.; Ogbourne, S.M.; Nichols, J.; Wallace, H.M.; Trueman, S.J. Selective retention of cross-fertilised fruitlets during premature fruit drop of Hass avocado. Horticulturae 2024, 10, 591. [Google Scholar] [CrossRef]
- Wallace, H.M.; Lee, L.S. Pollen source, fruit set and xenia in mandarins. J. Hortic. Sci. Biotechnol. 1999, 74, 82–86. [Google Scholar] [CrossRef]
- Papadakis, I.E.; Protopapadakis, E.E.; Therios, I.N. Yield and fruit quality of ‘Nova’ hybrid [Citrus clementina hort. ex Tanaka × (C. reticulata Blanco × C. paradisi Macfad)] and two Clementine varieties (C. clementina hort. ex Tanaka) as affected by self- and cross-pollination. Sci. Hortic. 2009, 121, 38–41. [Google Scholar] [CrossRef]
- Żurawicz, E. Cross-pollination increases the number of drupelets in the fruits of red raspberry (Rubus idaeus L.). Acta Hortic. 2016, 1133, 145–152. [Google Scholar] [CrossRef]
- Dung, C.D.; Wallace, H.M.; Bai, S.H.; Ogbourne, S.M.; Trueman, S.J. Biomass and mineral nutrient partitioning among self-pollinated and cross-pollinated fruit on the same strawberry plant. PLoS ONE 2022, 17, e0269485. [Google Scholar] [CrossRef] [PubMed]
- Nishio, S.; Takada, N.; Terakami, S.; Kato, H.; Inoue, H.; Takeuchi, Y.; Saito, T. Estimation of effective pollen dispersal distance for cross-pollination in chestnut orchards by microsatellite-based paternity analyses. Sci. Hortic. 2019, 250, 89–93. [Google Scholar] [CrossRef]
- Lucas-García, R.; Rosas-Guerrero, V.; Alemán-Figueroa, L.; Almazán-Núñez, R.C.; Violante-González, J.; Kuk-Dzul, J.G. Spatial proximity of ‘Ataulfo’ to ‘Haden’ cultivar increases mango yield and decreases incidence of nubbins. Agronomy 2021, 11, 450. [Google Scholar] [CrossRef]
- Raz, A.; Goldway, M.; Sapir, G.; Stern, R.A. “Hong Long” lychee (Litchi chinensis Sonn.) is the optimal pollinizer for the main lychee cultivars in Israel. Plants 2022, 11, 1996. [Google Scholar] [CrossRef]
- Trueman, S.J.; Kämper, W.; Nichols, J.; Ogbourne, S.M.; Hawkes, D.; Peters, T.; Hosseini Bai, S.; Wallace, H.M. Pollen limitation and xenia effects in a cultivated mass-flowering tree, Macadamia integrifolia (Proteaceae). Ann. Bot. 2022, 129, 135–146. [Google Scholar] [CrossRef]
- Brittain, C.; Kremen, C.; Garber, A.; Klein, A.-M. Pollination and plant resources change the nutritional quality of almonds for human health. PLoS ONE 2014, 9, e90082. [Google Scholar] [CrossRef]
- Doi, K.; Inoue, R.; Iwasaki, N. Seed weight mediates effects of pollen on berry weight, ripening, and anthocyanin content in highbush blueberry. Sci. Hortic. 2021, 288, 110313. [Google Scholar] [CrossRef]
- Liu, J.; Xu, J.; Wang, Y.; Li, K.; Zong, Y.; Yang, L.; Chen, W.; Liao, F.; Guo, W. The xenia effect promotes fruit quality and assists in optimizing cross combinations in ‘O’Neal’ and ‘Emerald’ blueberry. Horticulturae 2022, 8, 659. [Google Scholar] [CrossRef]
- Dung, C.D.; Wallace, H.M.; Hosseini Bai, S.; Ogbourne, S.M.; Trueman, S.J. Cross-pollination affects fruit colour, acidity, firmness and shelf life of self-compatible strawberry. PLoS ONE 2021, 16, 0256964. [Google Scholar] [CrossRef] [PubMed]
- Denney, J.O. Xenia includes metaxenia. HortScience 1992, 27, 722–728. [Google Scholar] [CrossRef]
- Stolp, L.J.; Kodali, D.R. Naturally occurring high-oleic oils: Avocado, macadamia, and olive oils. In High Oleic Oils. Development, Properties, and Uses; Flider, F.J., Ed.; Elsevier: London, UK, 2021; pp. 7–52. [Google Scholar]
- Dreher, M.L.; Cheng, F.W.; Ford, N.A. A comprehensive review of Hass avocado clinical trials, observational studies, and biological mechanisms. Nutrients 2021, 13, 4376. [Google Scholar] [CrossRef]
- Pacheco, L.S.; Li, Y.; Rimm, E.B.; Manson, J.E.; Sun, Q.; Rexrode, K.; Hu, F.B.; Guasch-Ferré, M. Avocado consumption and risk of cardiovascular disease in US adults. J. Am. Heart Assoc. 2022, 11, e024014. [Google Scholar] [CrossRef]
- Monge, A.; Stern, D.; Cortés-Valencia, A.; Catzín-Kuhlmann, A.; Lajous, M.; Denova-Gutiérrez, E. Avocado consumption is associated with a reduction in hypertension incidence in Mexican women. Br. J. Nutr. 2023, 129, 1976–1983. [Google Scholar] [CrossRef]
- FAOSTAT. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 18 December 2024).
- Dymond, K.; Celis-Diez, J.L.; Potts, S.G.; Howlett, B.G.; Willcox, B.K.; Garratt, M.P.D. The role of insect pollinators in avocado production: A global review. J. Appl. Entomol. 2021, 145, 369–383. [Google Scholar] [CrossRef]
- Stern, R.A.; Rozen, A.; Eshed, R.; Zviran, T.; Sisai, I.; Sherman, A.; Irihimovitch, V.; Sapir, G. Bumblebees (Bombus terrestris) improve ‘Hass’ avocado (Persea americana) pollination. Plants 2021, 10, 1372. [Google Scholar] [CrossRef] [PubMed]
- Hapuarachchi, N.S.; Kämper, W.; Wallace, H.M.; Hosseini Bai, S.; Ogbourne, S.M.; Nichols, J.; Trueman, S.J. Boron effects on fruit set, yield, quality and paternity of Hass avocado. Agronomy 2022, 12, 1479. [Google Scholar] [CrossRef]
- Sagwe, R.N.; Peters, M.K.; Dubois, T.; Steffan-Dewenter, I.; Latorff, H.M.G. Pollinator efficiency of avocado (Persea americana) flower insect visitors. Ecol. Solut. Evid. 2022, 3, e12178. [Google Scholar] [CrossRef]
- Degani, C.; Goldring, A.; Adato, I.; El-Batsri, R.; Gazit, S. Pollen parent effect on outcrossing rate, yield, and fruit characteristics of ‘Fuerte’ avocado. HortScience 1990, 25, 471–473. [Google Scholar] [CrossRef]
- Kobayashi, M.; Lin, J.-Z.; Davis, J.; Francis, L.; Clegg, M.T. Quantitative analysis of avocado outcrossing and yield in California using RAPD markers. Sci. Hortic. 2000, 86, 135–149. [Google Scholar] [CrossRef]
- Alcaraz, M.L.; Hormaza, J.I. Inadequate pollination is a key factor determining low fruit-to-flower ratios in avocado. Horticulturae 2024, 10, 140. [Google Scholar] [CrossRef]
- Kämper, W.; Ogbourne, S.M.; Hawkes, D.; Trueman, S.J. SNP markers reveal relationships between fruit paternity, fruit quality and distance from a cross-pollen source in avocado orchards. Sci. Rep. 2021, 11, 20043. [Google Scholar] [CrossRef]
- Trueman, S.J.; Nichols, J.; Farrar, M.B.; Wallace, H.M.; Hosseini Bai, S. Outcrossing rate and fruit yield of Hass avocado trees decline at increasing distance from a polliniser cultivar. Agronomy 2024, 14, 122. [Google Scholar] [CrossRef]
- Peña, J.F.; Carabalí, A. Effect of honey bee (Apis mellifera L.) density on pollination and fruit set of avocado (Persea americana Mill.) cv. Hass. J. Apic. Sci. 2018, 62, 5–14. [Google Scholar] [CrossRef]
- Alcaraz, M.L.; Hormaza, J.I. Fruit set in avocado: Pollen limitation, pollen load size, and selective fruit abortion. Agronomy 2021, 11, 1603. [Google Scholar] [CrossRef]
- Pattemore, D.E.; Evans, L.E.; McBrydie, H.M.; Dag, A.; Howlett, B.G.; Cutting, B.; Goodwin, R.M. Understanding pollination processes in avocado (Persea americana) orchards. Acta Hortic. 2020, 1299, 317–328. [Google Scholar] [CrossRef]
- Davenport, T. Avocado flowering. In Horticultural Reviews; Janick, J., Ed.; AVI Publishing: Westport, CN, USA, 1986; Volume 8, pp. 257–289. [Google Scholar]
- Scholefield, P.B. A scanning electron microscope study of flowers of avocado, litchi, macadamia and mango. Sci. Hortic. 1982, 16, 263–272. [Google Scholar] [CrossRef]
- Borrone, J.W.; Olano, C.T.; Kuhn, D.N.; Brown, J.S.; Schnell, R.J.; Violi, H.A. Outcrossing in Florida avocados as measured using microsatellite markers. J. Am. Soc. Hortic. Sci. 2008, 133, 255–261. [Google Scholar] [CrossRef]
- Stahl, P.; Lev Mirom, Y.; Stern, R.A.; Goldway, M. Comparing ‘Iriet’ and ‘Ettinger’ avocado cultivars as pollinators of ‘Hass’ using SNPs for paternal identification. Sci. Hortic. 2019, 248, 50–57. [Google Scholar] [CrossRef]
- Solares, E.; Morales-Cruz, A.; Figueroa Balderas, R.; Focht, E.; Ashworth, V.E.T.M.; Wyant, S.; Minio, A.; Cantu, D.; Arpaia, M.L.; Gaut, B.S. Insights into the domestication of avocado and potential genetic contributors to heterodichogamy. G3 Genes Genomes Gen. 2023, 13, jkac323. [Google Scholar] [CrossRef] [PubMed]
- Kron, P.; Husband, B.C.; Kevan, P.G.; Belaoussoff, S. Factors affecting pollen dispersal in high-density apple orchards. HortScience 2001, 36, 1039–1046. [Google Scholar] [CrossRef]
- Sánchez-Estrada, A.; Cuevas, J. Pollination strategies to improve fruit set in orchards of ‘Manzanillo’ olive in a nontraditional producing country, Mexico. HortTechnology 2019, 29, 258–264. [Google Scholar] [CrossRef]
- Carisio, L.; Díaz, S.S.; Ponso, S.; Manino, A.; Porporato, M. Effects of pollinizer density and apple tree position on pollination efficiency in cv. Gala. Sci. Hortic. 2020, 273, 109629. [Google Scholar] [CrossRef]
- Avocado Varieties. Available online: https://avocado.org.au/best-practice-resource/growing/varieties-and-pollinisers/ (accessed on 9 April 2025).
- Muñoz, A.E.; Plantegenest, M.; Amouroux, P.; Zaviezo, T. Native flower strips increase visitation by non-bee insects to avocado flowers and promote yield. Basic Appl. Ecol. 2021, 56, 369–378. [Google Scholar] [CrossRef]
- Pérez-Balam, J.; Quezada-Euán, J.J.G.; Alfaro-Bates, R.; Medina, S.; McKendrick, L.; Soro, A.; Paxton, R.J. The contribution of honey bees, flies and wasps to avocado (Persea americana) pollination in southern Mexico. J. Pollinat. Ecol. 2012, 8, 42–47. [Google Scholar] [CrossRef]
- Willcox, B.K.; Howlett, B.G.; Robson, A.J.; Cutting, B.; Evans, L.; Jesson, L.; Kirkland, L.; Jean-Meyzonnier, M.; Potdevin, V.; Saunders, M.E.; et al. Evaluating the taxa that provide shared pollination services across multiple crops and regions. Sci. Rep. 2019, 9, 13538. [Google Scholar] [CrossRef]
- Cook, D.F.; Voss, S.C.; Finch, J.T.D.; Rader, R.; Cook, J.M.; Spurr, C.J. The role of flies as pollinators of horticultural crops: An Australian case study with worldwide relevance. Insects 2020, 11, 341. [Google Scholar] [CrossRef]
- Cook, D.F.; Tufail, M.S.; Voss, S.C.; Deyl, R.A.; Howse, E.T.; Foley, J.; Norrish, B.; Delroy, N.; Shivananjappa, S.L. Blow flies (Diptera: Calliphoridae) ability to pollinate Hass avocado trees within paired tree enclosures. J. Appl. Entomol. 2023, 147, 577–591. [Google Scholar] [CrossRef]
- Evans, L.J.; Jesson, L.; Read, S.F.J.; Jochym, M.; Cutting, B.T.; Gayrard, T.; Jammes, M.A.S.; Roumier, R.; Howlett, B.G. Key factors influencing forager distribution across macadamia orchards differ among species of managed bees. Basic Appl. Ecol. 2021, 53, 74–85. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Martin, J.H. The size and distribution of the honey bee (Apis mellifera L.) cross-pollinating population on male-sterile sunflowers (Helianthus annuus L.). J. Apic. Res. 1993, 32, 135–142. [Google Scholar] [CrossRef]
- Ish-Am, G.; Eisikowitch, D. The behaviour of honey bees (Apis mellifera) visiting avocado (Persea americana) flowers and their contribution to its pollination. J. Apic. Res. 1993, 32, 175–186. [Google Scholar] [CrossRef]
- Dag, A.; Degani, C.; Gazit, S. In-hive pollen transfer in mango. Acta Hortic. 2001, 561, 61–65. [Google Scholar] [CrossRef]
- Karron, J.D.; Holmquist, K.G.; Flanagan, R.J.; Mitchell, R.J. Pollinator visitation patterns strongly influence among-flower variation in selfing rate. Ann. Bot. 2009, 103, 1379–1383. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Wilson, W.G.; Holmquist, K.G.; Karron, J.D. Influence of pollen transport dynamics on sire profiles and multiple paternity in flowering plants. PLoS ONE 2013, 8, e76312. [Google Scholar] [CrossRef]
- Hung, K.-L.J.; Fan, S.L.; Strang, C.G.; Park, M.G.; Thomson, J.D. Pollen carryover, pollinator movement, and spatial context impact the delivery of pollination services in apple orchards. Ecol. Appl. 2023, 33, e2917. [Google Scholar] [CrossRef]
- Ish-Am, G.; Eisikowitch, D. Mobility of honey bees (Apidae, Apis mellifera L.) during foraging in avocado orchards. Apidologie 1998, 29, 209–219. [Google Scholar] [CrossRef]
- Brittain, C.; Williams, N.; Kremen, C.; Klein, A.-M. Synergistic effects of non-Apis bees and honey bees for pollination services. Proc. R. Soc. B 2013, 280, 20122767. [Google Scholar] [CrossRef]
- Eeraerts, M.; Smagghe, G.; Meeus, I. Bumble bee abundance and richness improves honey bee pollination behaviour in sweet cherry. Basic Appl. Ecol. 2020, 43, 27–33. [Google Scholar] [CrossRef]
- Moreno-Ortega, G.; Pliego, C.; Sarmiento, D.; Barceló, A.; Martínez-Ferri, E. Yield and fruit quality of avocado trees under different regimes of water supply in the subtropical coast of Spain. Agric. Water Manag. 2019, 221, 192–201. [Google Scholar] [CrossRef]
- Durán Zuazo, V.H.; Lipan, L.; Cárceles Rodríguez, B.; Sendra, E.; Franco Tarifa, D.; Nemś, A.; Gálvez Ruiz, B.; Carbonell-Barachina, A.A.; García-Tejero, I.F. Impact of deficit irrigation on fruit yield and lipid profile of terraced avocado orchards. Agron. Sust. Dev. 2021, 41, 69. [Google Scholar] [CrossRef]
- Quispe-Rodriguez, J.; Paytan-Montañez, T.C.; Aliaga Barrera, I.N.; Saravia-Navarro, D. Ajuste osmótico y rendimiento de dos variedades de palta (Persea americana), Hass y Fuerte, con sistema de riego por goteo, en zona andina del Perú. Sci. Agropecu. 2024, 15, 225–234. [Google Scholar] [CrossRef]
- Campisi-Pinto, S.; Zheng, Y.; Rolshausen, P.E.; Crowley, D.E.; Faber, B.; Bender, G.; Bianchi, M.; Khuong, T.; Lovatt, C.L. Optimal nutrient concentration ranges of ‘Hass’ avocado cauliflower stage inflorescences—Potential diagnostic tool to optimize tree nutrient status and increase yield. HortScience 2017, 52, 1707–1715. [Google Scholar] [CrossRef]
- Morales-Payan, J.P. Avocado tree growth and fruit yield in response to nitrogen fertilization regimens. Acta Hortic. 2022, 1345, 435–437. [Google Scholar] [CrossRef]
- González-Vences, R.; Cajuste-Bontemps, L.; Sánchez-Escudero, J.; Gómez-Merion, F.C.; Trejo-Téllez, L.I. Efficient detection of nutrient deficiencies and development of corrections in avocado through the Compositional Nutrient Diagnosis (CND). Not. Bot. Horti Agrobot. Cluj-Napoca 2024, 52, 13118. [Google Scholar] [CrossRef]
- Scholefield, P.B.; Sedgley, M.; Alexander, D.M. Carbohydrate cycling in relation to shoot growth, floral initiation and development and yield in the avocado. Sci. Hortic. 1985, 25, 99–110. [Google Scholar] [CrossRef]
- Whiley, A.W.; Rasmussen, T.S.; Saranah, J.B.; Wolstenholme, B.N. Delayed harvest effects on yield, fruit size and starch cycling in avocado (Persea americana Mill.) in subtropical environments. II. The late-maturing cv. Hass. Sci. Hortic. 1996, 66, 35–49. [Google Scholar] [CrossRef]
- Alcaraz, M.L.; Hormaza, J.I.; Rodrigo, J. Pistil starch reserves at anthesis correlate with final flower fate in avocado (Persea americana). PLoS ONE 2013, 8, e78467. [Google Scholar] [CrossRef]
- Boldingh, H.L.; Alcaraz, M.L.; Thorp, T.G.; Minchin, P.E.H.; Gould, N.; Hormaza, K.I. Carbohydrate and boron content of styles of ‘Hass’ avocado (Persea americana Mill.) flowers at anthesis can affect final fruit set. Sci. Hortic. 2016, 198, 125–131. [Google Scholar] [CrossRef]
- Bashir, R.; Sharma, G.; Sharma, N. Studies on fruit set and fruit characteristics as affected by different pollinizers in apple (Malus × domestica Borkh.). Adv. Hortic. Sci. 2010, 24, 137–144. [Google Scholar]
- Fattahi, R.; Mohammadzedeh, M.; Khadivi-Khub, A. Influence of different pollen sources on nut and kernel characteristics of hazelnut. Sci. Hortic. 2014, 173, 15–19. [Google Scholar] [CrossRef]
- Deng, L.; Wang, T.; Hu, J.; Yang, X.; Yao, Y.; Jin, Z.; Huang, Z.; Sun, G.; Xiong, B.; Liao, L.; et al. Effects of pollen sources on fruit set and fruit characteristics of ‘Fengtangli’ plum (Prunus salicina Lindl.) based on microscopic and transcriptomic analysis. Int. J. Mol. Sci. 2022, 23, 12959. [Google Scholar] [CrossRef] [PubMed]
- Lovatt, C.J. Hass Avocado Nutrition Research in California; University of California: Riverside, CA, USA, 2013. [Google Scholar]
- Lovatt, C.; Zheng, Y.; Khuong, T.; Campisi-Pinto, S.; Crowley, D.; Rolshausen, P. Yield characteristics of ‘Hass’ avocado trees under California growing conditions. In Proceedings of the VIII World Avocado Congress, Lima, Peru, 13–18 September 2015. [Google Scholar]
- Avocados Australia. Avocados Australia Best Practice Resource–Packaging for Export; Avocados Australia: Rocklea, Australia, 2018. [Google Scholar]
- Whiley, A.W. Crop management. In The Avocado: Botany, Production and Uses; Whiley, A.W., Schaffer, B., Wolstenholme, B.N., Eds.; CABI Publishing: New York, NY, USA, 2002; pp. 231–253. [Google Scholar]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Devkota, K.; Ferreira, A.B.; Timberlake, T.P.; dos Santos, C.F. The impact of pollinator decline on global protein production: Implications for livestock and plant-based products. Glob. Ecol. Conserv. 2024, 50, e02815. [Google Scholar] [CrossRef]
- Dicks, L.V.; Breeze, T.D.; Ngo, H.T.; Senapathi, D.; An, J.; Aizen, M.A.; Basu, P.; Buchori, D.; Galetto, L.; Garibaldi, L.A.; et al. A global-scale expert assessment of drivers and risks associated with pollinator decline. Nat. Ecol. Evol. 2021, 5, 1453–1461. [Google Scholar] [CrossRef]
- Kämper, W.; Trueman, S.J.; Cooke, J.; Kasinadhuni, N.; Brunton, A.J.; Ogbourne, S.M. Single-nucleotide polymorphisms that uniquely identify cultivars of avocado (Persea americana). Appl. Plant Sci. 2021, 9, e11440. [Google Scholar] [CrossRef]

| Parameter | Mother Cultivar × Father Cultivar | |
|---|---|---|
| ‘Maluma’ × ‘Maluma’ (Selfed) | ‘Maluma’ × ‘Shepard’ (Outcrossed) | |
| Diameter (mm) | 66.6 ± 0.3 a | 68.6 ± 0.5 b |
| Length (mm) | 100.0 ± 0.6 a | 101.2 ± 0.9 b |
| Total fruit mass (g) | 215.7 ± 2.8 a | 233.8 ± 4.6 b |
| Seed mass (g) | 19.8 ± 0.5 a | 21.8 ± 0.7 b |
| Pericarp mass (g) | 196.0 ± 2.4 a | 212.1 ± 4.0 b |
| Seed/total mass (%) | 8.9 ± 0.1 a | 9.1 ± 0.2 a |
| Parameter | Mother Cultivar × Father Cultivar | ||
|---|---|---|---|
| ‘Shepard’ × ‘Shepard’ (Selfed) | ‘Shepard’ × ‘Maluma’ (Outcrossed) | ‘Shepard’ × ‘Hass’ (Outcrossed) | |
| Diameter (mm) | 63.9 ± 0.2 a | 63.6 ± 1.2 a | 65.3 ± 0.6 a |
| Length (mm) | 106.2 ± 0.4 a | 103.4 ± 1.8 a | 104.8 ± 1.1 a |
| Total fruit mass (g) | 202.8 ± 1.7 a | 195.4 ± 8.9 a | 213.1 ± 4.5 b |
| Seed mass (g) | 35.5 ± 0.5 a | 36.6 ± 2.5 a | 44.1 ± 1.3 b |
| Pericarp mass (g) | 167.3 ± 1.4 a | 158.8 ± 6.8 a | 169.0 ± 3.5 a |
| Seed/total mass (%) | 17.4 ± 0.2 a | 18.4 ± 0.7 a | 20.6 ± 0.4 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reese, M.A.; Wilson, R.S.; Nichols, J.; Trueman, S.J. Declining Outcrossing Rates Inside Orchard Blocks of ‘Maluma’ and ‘Shepard’ Avocado (Persea americana Mill.) Trees: Effects on Fruit Yield and Quality. Plants 2025, 14, 1218. https://doi.org/10.3390/plants14081218
Reese MA, Wilson RS, Nichols J, Trueman SJ. Declining Outcrossing Rates Inside Orchard Blocks of ‘Maluma’ and ‘Shepard’ Avocado (Persea americana Mill.) Trees: Effects on Fruit Yield and Quality. Plants. 2025; 14(8):1218. https://doi.org/10.3390/plants14081218
Chicago/Turabian StyleReese, Matthias A., Rachele S. Wilson, Joel Nichols, and Stephen J. Trueman. 2025. "Declining Outcrossing Rates Inside Orchard Blocks of ‘Maluma’ and ‘Shepard’ Avocado (Persea americana Mill.) Trees: Effects on Fruit Yield and Quality" Plants 14, no. 8: 1218. https://doi.org/10.3390/plants14081218
APA StyleReese, M. A., Wilson, R. S., Nichols, J., & Trueman, S. J. (2025). Declining Outcrossing Rates Inside Orchard Blocks of ‘Maluma’ and ‘Shepard’ Avocado (Persea americana Mill.) Trees: Effects on Fruit Yield and Quality. Plants, 14(8), 1218. https://doi.org/10.3390/plants14081218






