BcAS2 Regulates Leaf Adaxial Polarity Development in Non-Heading Chinese Cabbage by Directly Activating BcPHB Transcription
Abstract
1. Introduction
2. Results
2.1. Identification and Expression Analysis of AS2 in Related Species
2.2. Phenotypic Analysis of Leaf Polarity in BcAS2-Overexpressing Plants
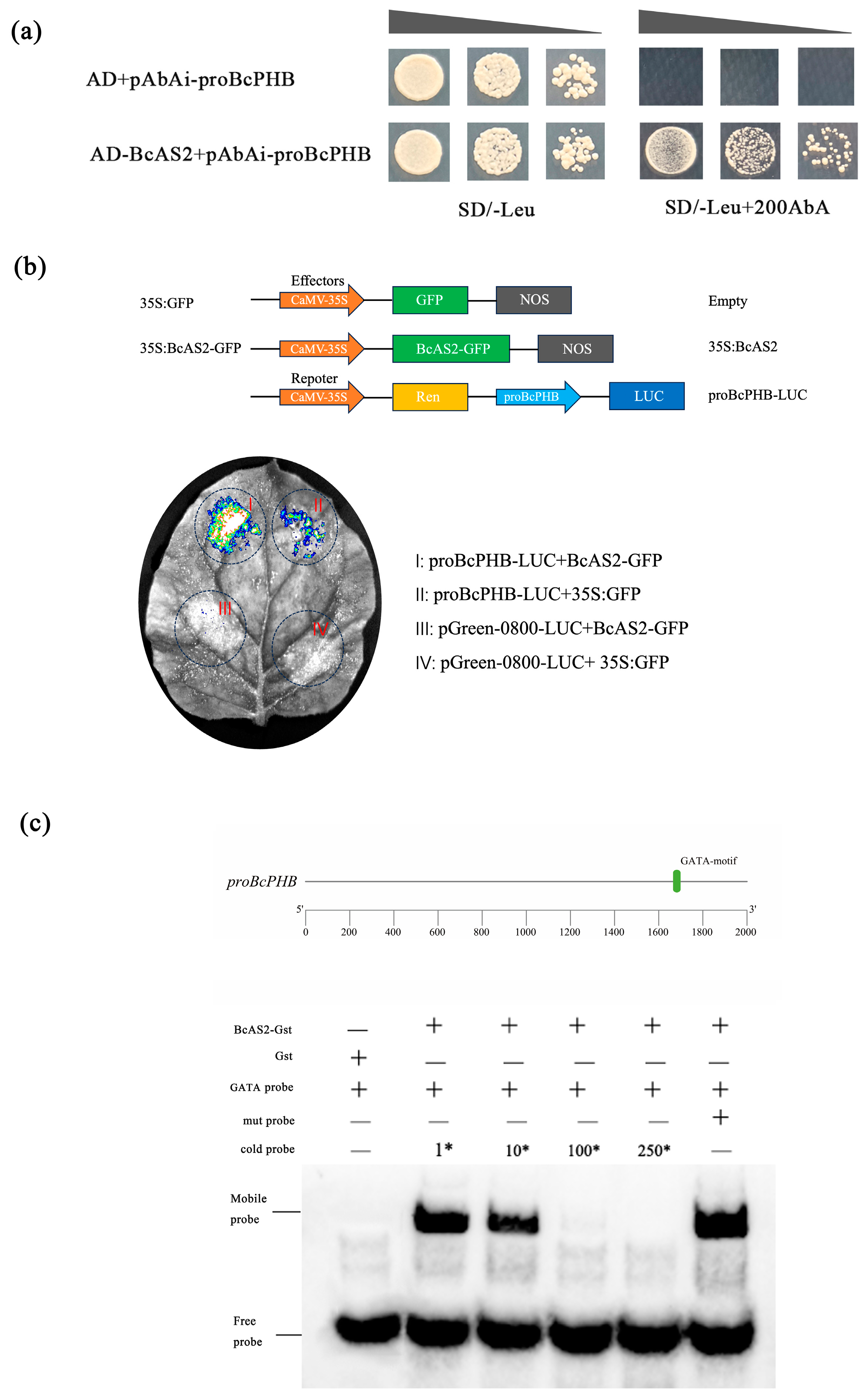
2.3. BcAS2 Directly Activates BcPHB Transcription
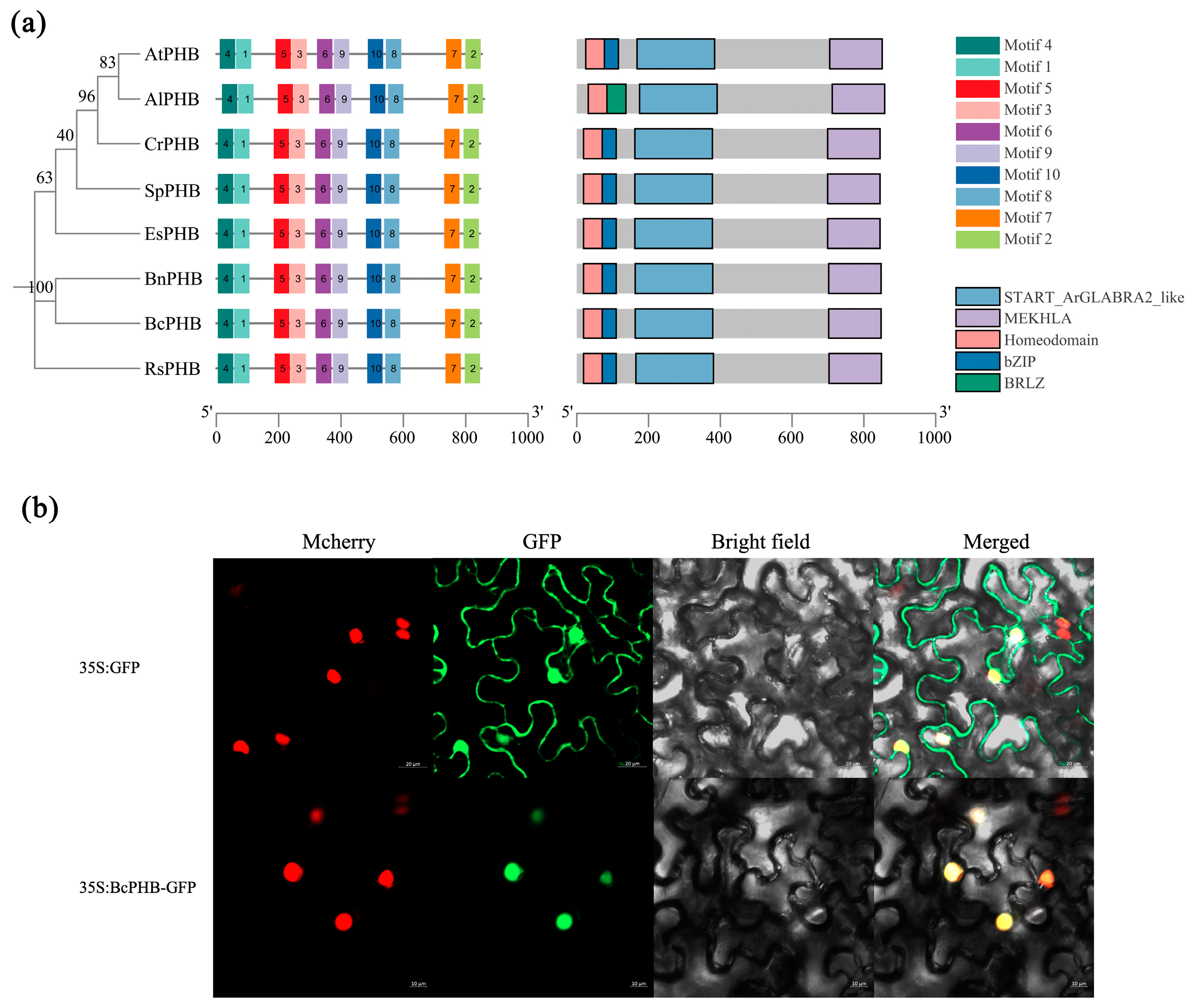
2.4. Characterization of BcPHB
2.5. Expression Pattern of BcPHB
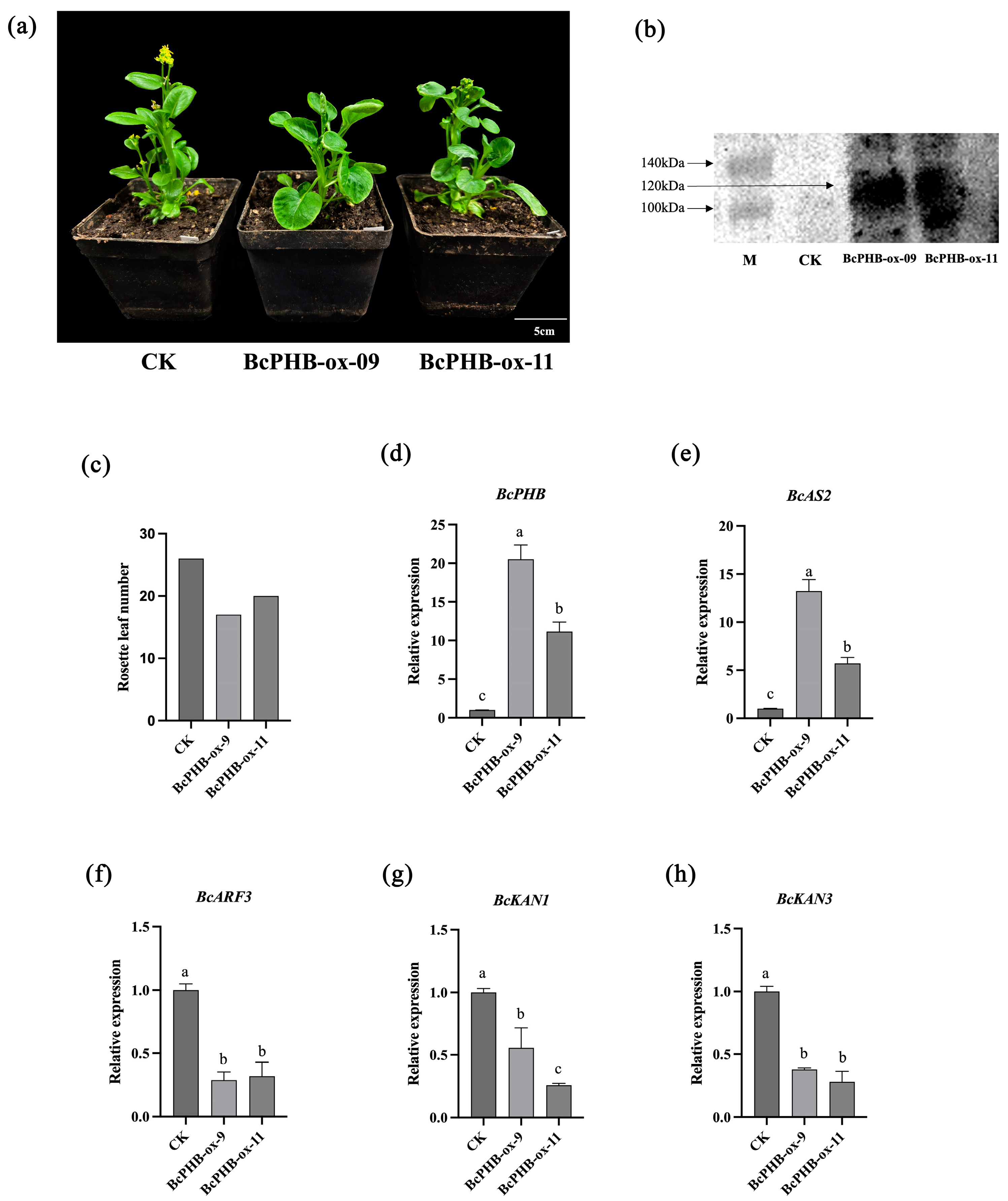
2.6. Phenotypic Analysis of Leaf Polarity in BcPHB-Overexpressing Plants
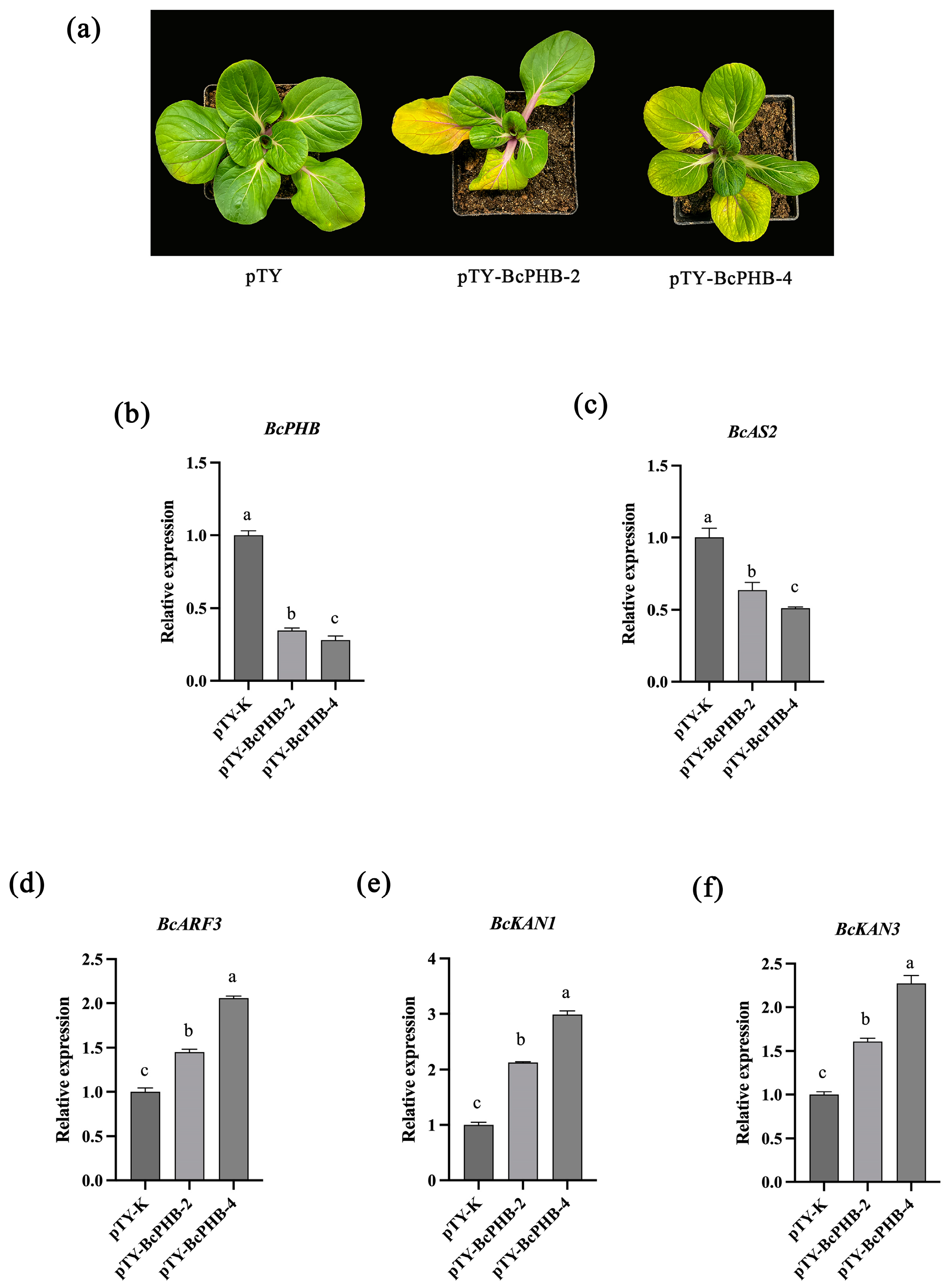
2.7. Phenotypic Analysis of BcPHB-Silenced Plants
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Cloning and Analysis of BcPHB
4.3. Subcellular Localization of BcPHB in Tobacco
4.4. Vector Construction and Transgenic Plant Generation
4.5. BcPHB Silencing via VIGS
4.6. RNA Extraction and qRT-PCR
4.7. Yeast One-Hybrid (Y1H) Assay
4.8. Dual-Luciferase Assay
4.9. Electrophoretic Mobility Shift Assay (EMSA)
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bowman, J.L.; Eshed, Y.; Baum, S.F. Establishment of polarity in angiosperm lateral organs. Trends Genet. 2002, 18, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.L. Axial patterning in leaves and other lateral organs. Curr. Opin. Genet. Dev. 2000, 10, 399–404. [Google Scholar] [CrossRef] [PubMed]
- ByrSne, M.; Timmermans, M.; Kidner, C.; Martienssen, R. Development of leaf shape. Curr. Opin. Plant Biol. 2001, 4, 38–43. [Google Scholar] [CrossRef]
- Hudson, A. Axioms and axes in leaf formation? Curr. Opin. Plant Biol. 1999, 2, 56–60. [Google Scholar] [CrossRef]
- Byrne, M.E.; Barley, R.; Curtis, M.; Arroyo, J.M.; Dunham, M.; Hudson, A.; Martienssen, R.A. Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 2000, 408, 967–971. [Google Scholar] [CrossRef]
- Douglas, S.J.; Chuck, G.; Dengler, R.E.; Pelecanda, L.; Riggs, C.D. KNAT1 and ERECTA regulate inflorescence architecture in Arabidopsis. Plant Cell 2002, 14, 547–558. [Google Scholar] [CrossRef]
- Venglat, S.P.; Dumonceaux, T.; Rozwadowski, K.; Parnell, L.; Babic, V.; Keller, W.; Martienssen, R.; Selvaraj, G.; Datla, R. The homeobox gene BREVIPEDICELLUS is a key regulator of inflorescence architecture in Arabidopsis. Proc. Natl. Acad. Sci. USA 2002, 99, 4730–4735. [Google Scholar] [CrossRef]
- Chien, J.C.; Sussex, I.M. Differential regulation of trichome formation on the adaxial and abaxial leaf surfaces by Gibberellins and photoperiod in Arabidopsis thaliana (L.) Heynh. Plant Physiol. 1996, 111, 1321–1328. [Google Scholar] [CrossRef]
- Medard, R. Initial dorsoventrality of the leaf primordium in manihot-esculenta. Can. J. Bot.-Rev. Can. Bot. 1988, 66, 2273–2284. [Google Scholar] [CrossRef]
- Scanlon, M.J. Developmental complexities of simple leaves. Curr. Opin. Plant Biol. 2000, 3, 31–36. [Google Scholar] [CrossRef]
- Semiarti, O.E.; Iwakawa, H.; Ueno, Y.; Tsukaya, H.; Machida, C.; Machida, Y. The asymmetric leaves2 gene of A. thaliana regulates the establishment of midvein as the longitudinal axis of leaf symmetry. Plant Cell Physiol. 2001, 42, s54. [Google Scholar]
- Matsumura, Y.; Iwakawa, H.; Machida, Y.; Machida, C. Characterization of genes in the ASYMMETRIC LEAVES2/LATERAL ORGAN BOUNDARIES (AS2/LOB) family in Arabidopsis thaliana, and functional and molecular comparisons between AS2 and other family members. Plant J. 2009, 58, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.F.; Wang, H.; Li, J.Q.; Huang, H.; Xu, L. Quantitative control of ASYMMETRIC LEAVES2 expression is critical for leaf axial patterning in Arabidopsis. J. Exp. Bot. 2013, 64, 4895–4905. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Takahashi, H.; Iwakawa, H.; Nakagawa, A.; Ishikawa, T.; Tanaka, H.; Matsumura, Y.; Pekker, I.; Eshed, Y.; Vial-Pradel, S.; et al. Dual regulation of ETTIN (ARF3) gene expression by AS1-AS2, which maintains the DNA methylation level, is involved in stabilization of leaf adaxial-abaxial partitioning in Arabidopsis. Development 2013, 140, 1958–1969. [Google Scholar] [CrossRef]
- Williams, L.; Carles, C.C.; Osmont, K.S.; Fletcher, J.C. A database analysis method identifies an endogenous trans-acting short-interfering RNA that targets the Arabidopsis ARF2, ARF3, and ARF4 genes. Proc. Natl. Acad. Sci. USA 2005, 102, 9703–9708. [Google Scholar] [CrossRef]
- McConnell, J.R.; Emery, J.; Eshed, Y.; Bao, N.; Bowman, J.; Barton, M.K. Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature 2001, 411, 709–713. [Google Scholar] [CrossRef]
- Prigge, M.J.; Otsuga, D.; Alonso, J.M.; Ecker, J.R.; Drews, G.N.; Clark, S.E. Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell 2005, 17, 61–76. [Google Scholar] [CrossRef]
- Eshed, Y.; Baum, S.F.; Bowman, J.L. Distinct mechanisms promote polarity establishment in carpels of Arabidopsis. Cell 1999, 99, 199–209. [Google Scholar] [CrossRef]
- Eshed, Y.; Baum, S.F.; Perea, J.V.; Bowman, J.L. Establishment of polarity in lateral organs of plants. Curr. Biol. 2001, 11, 1251–1260. [Google Scholar] [CrossRef]
- Emery, J.F.; Floyd, S.K.; Alvarez, J.; Eshed, Y.; Hawker, N.P.; Izhaki, A.; Baum, S.F.; Bowman, J.L. Radial patterning of Arabidopsis shoots by class IIIHD-ZIP and KANADI genes. Curr. Biol. 2003, 13, 1768–1774. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Dai, X.H.; Zhao, Y.D. Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev. 2006, 20, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Talbert, P.B.; Adler, H.T.; Parks, D.W.; Comai, L. The REVOLUTA gene is necessary for apical meristem development and for limiting cell divisions in the leaves and stems of Arabidopsis-thaliana. Development 1995, 121, 2723–2735. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jung, J.H.; Reyes, J.L.; Kim, Y.S.; Kim, S.Y.; Chung, K.S.; Kim, J.A.; Lee, M.; Lee, Y.; Kim, V.N.; et al. microRNA-directed cleavage of ATHB15 mRNA regulates vascular development in Arabidopsis inflorescence stems. Plant J. 2005, 42, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Itoh, J.I.; Hibara, K.I.; Sato, Y.; Nagato, Y. Developmental role and auxin responsiveness of class III homeodomain leucine zipper gene family members in rice. Plant Physiol. 2008, 147, 1960–1975. [Google Scholar] [CrossRef]
- Zhou, G.K.; Kubo, M.; Zhong, R.Q.; Demura, T.; Ye, Z.H. Overexpression of miR165 affects apical meristem formation, organ polarity establishment and vascular development in Arabidopsis. Plant Cell Physiol. 2007, 48, 391–404. [Google Scholar] [CrossRef]
- Izhaki, A.; Bowman, J.L. KANADI and class IIIHD-zip gene families regulate embryo patterning and modulate auxin flow during embryogenesis in Arabidopsis. Plant Cell 2007, 19, 495–508. [Google Scholar] [CrossRef]
- Otsuga, D.; DeGuzman, B.; Prigge, M.J.; Drews, G.N.; Clark, S.E. REVOLUTA regulates meristem initiation at lateral positions. Plant J. 2001, 25, 223–236. [Google Scholar] [CrossRef]
- Barton, M.K. Leaf polarity and meristem formation in Arabidopsis. Plant Biol. 2003, 2003, 3. [Google Scholar]
- Han, B.; Tao, Y.; Zhang, H.; Gu, C.; Liao, Y.; Peng, Y.; Zhang, H.; Xu, P.; Chen, X.; Wu, X. Identification and Gene Mapping of a Rolled Leaf Mutant rl(t) in Rice. Chin. J. Rice Sci. 2017, 31, 149–156. [Google Scholar] [CrossRef]
- Shi, Z.Y.; Wang, J.; Wan, X.S.; Shen, G.Z.; Wang, X.Q.; Zhang, J.L. Over-expression of rice OsAGO7 gene induces upward curling of the leaf blade that enhanced erect-leaf habit. Planta 2007, 226, 99–108. [Google Scholar] [CrossRef]
- Yang, M.; Huang, A.H.; Wen, R.L.; Tian, S.Y.; Mo, R.X.; Zhai, R.N.; Gong, X.; He, X.Y.; Li, F.Q.; Yang, X.H.; et al. Identification of the arl1 locus controlling leaf rolling and its application in maize breeding. Mol. Breed. 2025, 45, 18. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, G.F.; Ma, L.M.; Liu, T.K.; Zhang, C.W.; Xiao, D.; Zheng, H.K.; Chen, F.; Hou, X.L. A chromosome-level reference genome of non-heading Chinese cabbage Brassica campestris (syn. Brassica rapa) ssp. chinensis. Hortic. Res.-Engl. 2020, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Hou, H.L.; Zhang, Y.H.; Hou, X.L. Overexpression of a Pak Choi Gene, BcAS2, Causes Leaf Curvature in Arabidopsis thaliana. Genes 2021, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Araki, S.; Iwakawa, H.; Semiarti, E.; Ishikawa, T.; Tsukaya, H.; Machida, C.; Machida, Y. Analyses of ASYMMETRIC LEAVES2 in arabidopsis. Plant Cell Physiol. 2004, 45, S164. [Google Scholar]
- Iwakawa, H.; Takahashi, H.; Machida, Y.; Machida, C. Roles of ASYMMETRIC LEAVES2 (AS2) and Nucleolar Proteins in the Adaxial-Abaxial Polarity Specification at the Perinucleolar Region in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 7314. [Google Scholar] [CrossRef]
- Semiarti, E.; Ueno, Y.; Tsukaya, H.; Iwakawa, H.; Machida, C.; Machida, Y. The asymmetric leaves2 gene of Arabidopsis thaliana regulates formation of a symmetric lamina, establishment of venation and repression of meristem-related homeobox genes in leaves. Development 2001, 128, 1771–1783. [Google Scholar] [CrossRef]
- An, R.; Liu, X.Y.; Wang, R.; Wu, H.C.; Liang, S.; Shao, J.X.; Qi, Y.F.; An, L.J.; Yu, F. The Over-Expression of Two Transcription Factors, ABS5/bHLH30 and ABS7/MYB101, Leads to Upwardly Curly Leaves. PLoS ONE 2014, 9, e107637. [Google Scholar] [CrossRef]
- Ueno, Y.; Ishikawa, T.; Watanabe, K.; Terakura, S.; Iwakawa, H.; Okada, K.; Machida, C.; Machida, Y. Histone deacetylases and ASYMMETRIC LEAVES2 are involved in the establishment of polarity in leaves of Arabidopsis. Plant Cell 2007, 19, 445–457. [Google Scholar] [CrossRef]
- Lincoln, C.; Britton, J.H.; Estelle, M. Growth and development of the axr1 mutants of Arabidopsis. Plant Cell 1990, 2, 1071–1080. [Google Scholar] [CrossRef]
- Zhao, W.; Su, H.Y.; Song, J.; Zhao, X.Y.; Zhang, X.S. Ectopic expression of TaYAB1, a member of YABBY gene family in wheat, causes the partial abaxialization of the adaxial epidermises of leaves and arrests the development of shoot apical meristem in Arabidopsis. Plant Sci. 2006, 170, 364–371. [Google Scholar] [CrossRef]
- Kerstetter, R.A.; Bollman, K.; Taylor, R.A.; Bomblies, K.; Poethig, R.S. KANADI regulates organ polarity in Arabidopsis. Nature 2001, 411, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Eshed, Y.; Izhaki, A.; Baum, S.F.; Floyd, S.K.; Bowman, J.L. Asymmetric leaf development and blade expansion in Arabidopsis are mediated by KANADI and YABBY activities. Development 2004, 131, 2997–3006. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, J.; Ckurshumova, W.; Berleth, T. Auxin signaling in Arabidopsis leaf vascular development. Plant Physiol. 2003, 131, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Park, S.J.; Van Eck, J.; Lippman, Z.B. Control of inflorescence architecture in tomato by BTB/POZ transcriptional regulators. Genes Dev. 2016, 30, 2048–2061. [Google Scholar] [CrossRef]
- Du, F.; Guan, C.M.; Jiao, Y.L. Molecular Mechanisms of Leaf Morphogenesis. Mol. Plant. 2018, 11, 1117–1134. [Google Scholar] [CrossRef]
- Itoh, J.; Hibara, K.; Kojima, M.; Sakakibara, H.; Nagato, Y. Rice DECUSSATE controls phyllotaxy by affecting the cytokinin signaling pathway. Plant J. 2012, 72, 869–881. [Google Scholar] [CrossRef]
- Zhong, R.Q.; Ye, Z.H. IFL1, a gene regulating interfascicular fiber differentiation in Arabidopsis, encodes a homeodomain-leucine zipper protein. Plant Cell 1999, 11, 2139–2152. [Google Scholar] [CrossRef]
- Williams, L.; Grigg, S.P.; Xie, M.T.; Christensen, S.; Fletcher, J.C. Regulation of Arabidopsis shoot apical meristem and lateral organ formation by microRNA miR166g and its AtHD-ZIP target genes. Development 2005, 132, 3657–3668. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Zhang, W.X.; Ge, D.K.; Cao, H.X.; Liu, Y.; Fu, K.Y.; Feng, C.H.; Chen, W.T.; Song, C.W. Biomass-Based Leaf Curvilinear Model for Rapeseed (Brassica napus L.). In Proceedings of the 9th IFIP WG 5.14 International Conference on Computer and Computing Technologies in Agriculture (CCTA), Beijing, China, 27–30 September 2015; pp. 459–472. [Google Scholar]
- Guo, M.L.; Long, Y.; Xu, L.L.; Zhang, W.; Liu, T.K.; Zhang, C.W.; Hou, X.L.; Li, Y. CELL CYCLE SEITCH 52 regulates tillering by interacting with LATERAL SUPPRESSOR in non-heading Chinese cabbage. Plant Sci. 2021, 309, 110934. [Google Scholar] [CrossRef]
- Huang, F.; Liu, T.; Wang, J.; Hou, X. Isolation and Functional Characterization of a Floral Repressor, BcMAF1, From Pak-choi (Brassica rapa ssp. Chinensis). Planta 2018, 9, 290. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wu, J.Y.; Qi, W.L.; Coulter, J.A.; Fang, Y.; Li, X.C.; Liu, L.J.; Jin, J.J.; Niu, Z.X.; Yue, J.L.; et al. Screening and verification of reference genes for analysis of gene expression in winter rapeseed (Brassica rapa L.) under abiotic stress. PLoS ONE 2020, 15, e0236577. [Google Scholar] [CrossRef] [PubMed]
- Huiyu, W.; Zhubo, L.; Haibo, R.; Changwei, Z.; Dong, X.; Ying, L.; Xilin, H.; Tongkun, L. Regulatory interaction of BcWRKY33A and BcHSFA4A promotes salt tolerance in non-heading Chinese cabbage [Brassica campestris (syn. Brassica rapa) ssp. chinensis]. Hortic. Res. 2022, 9, uhac113. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.; Ding, Q.; He, Y.; Li, Y.; Gao, Z.; Li, E.; Hou, X. BcAS2 Regulates Leaf Adaxial Polarity Development in Non-Heading Chinese Cabbage by Directly Activating BcPHB Transcription. Plants 2025, 14, 1207. https://doi.org/10.3390/plants14081207
Jiang C, Ding Q, He Y, Li Y, Gao Z, Li E, Hou X. BcAS2 Regulates Leaf Adaxial Polarity Development in Non-Heading Chinese Cabbage by Directly Activating BcPHB Transcription. Plants. 2025; 14(8):1207. https://doi.org/10.3390/plants14081207
Chicago/Turabian StyleJiang, Cheng, Qiang Ding, Ying He, Yiran Li, Zhanyuan Gao, Entong Li, and Xilin Hou. 2025. "BcAS2 Regulates Leaf Adaxial Polarity Development in Non-Heading Chinese Cabbage by Directly Activating BcPHB Transcription" Plants 14, no. 8: 1207. https://doi.org/10.3390/plants14081207
APA StyleJiang, C., Ding, Q., He, Y., Li, Y., Gao, Z., Li, E., & Hou, X. (2025). BcAS2 Regulates Leaf Adaxial Polarity Development in Non-Heading Chinese Cabbage by Directly Activating BcPHB Transcription. Plants, 14(8), 1207. https://doi.org/10.3390/plants14081207






