Silicon-Mediated Interactions Between Plant Antagonists
Abstract
:1. Introduction
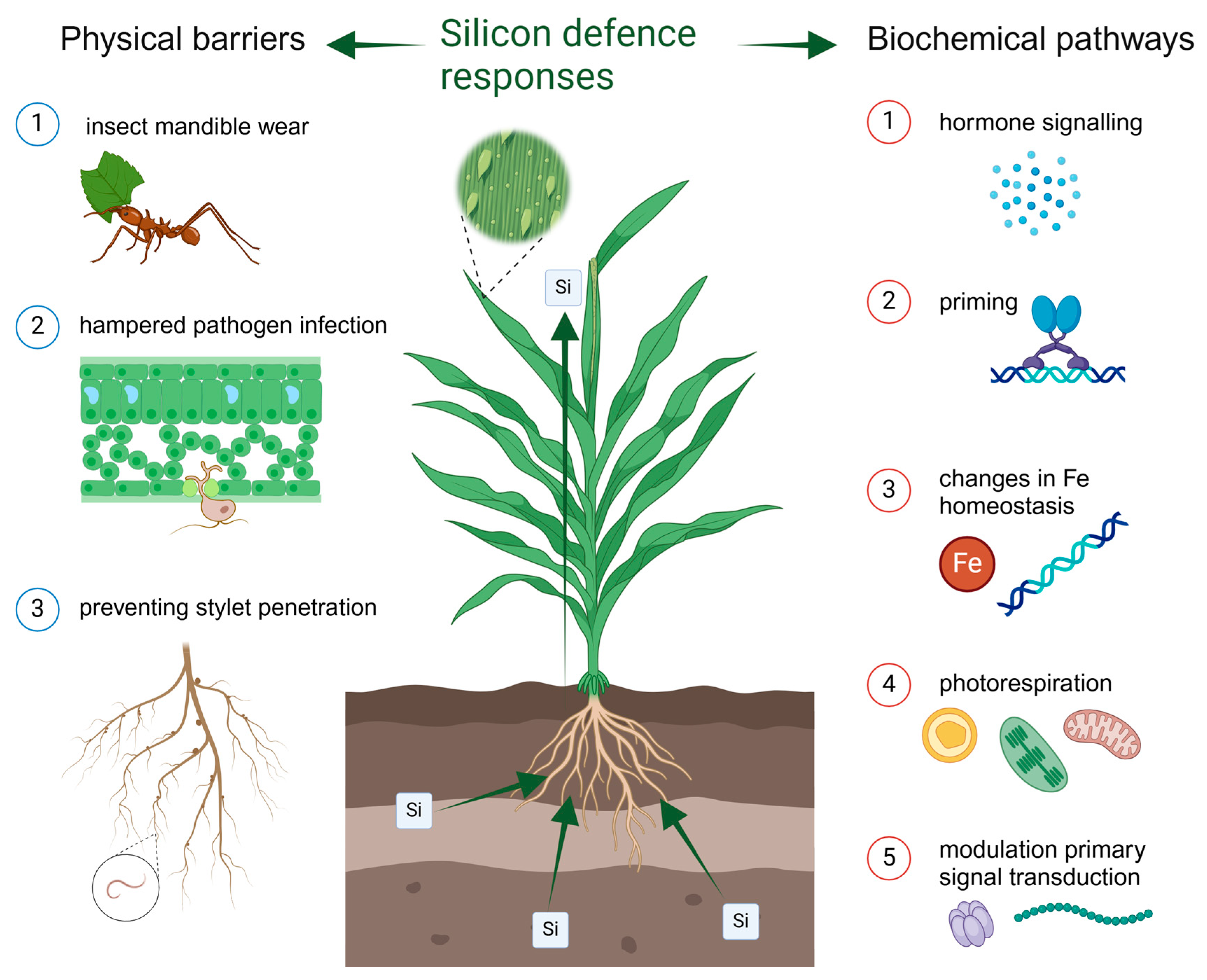
2. The Role of Plant Silicon in Response to Biotic Stresses
2.1. Insect Herbivores
2.2. Fungal Pathogens
2.3. Plant Parasitic Nematodes
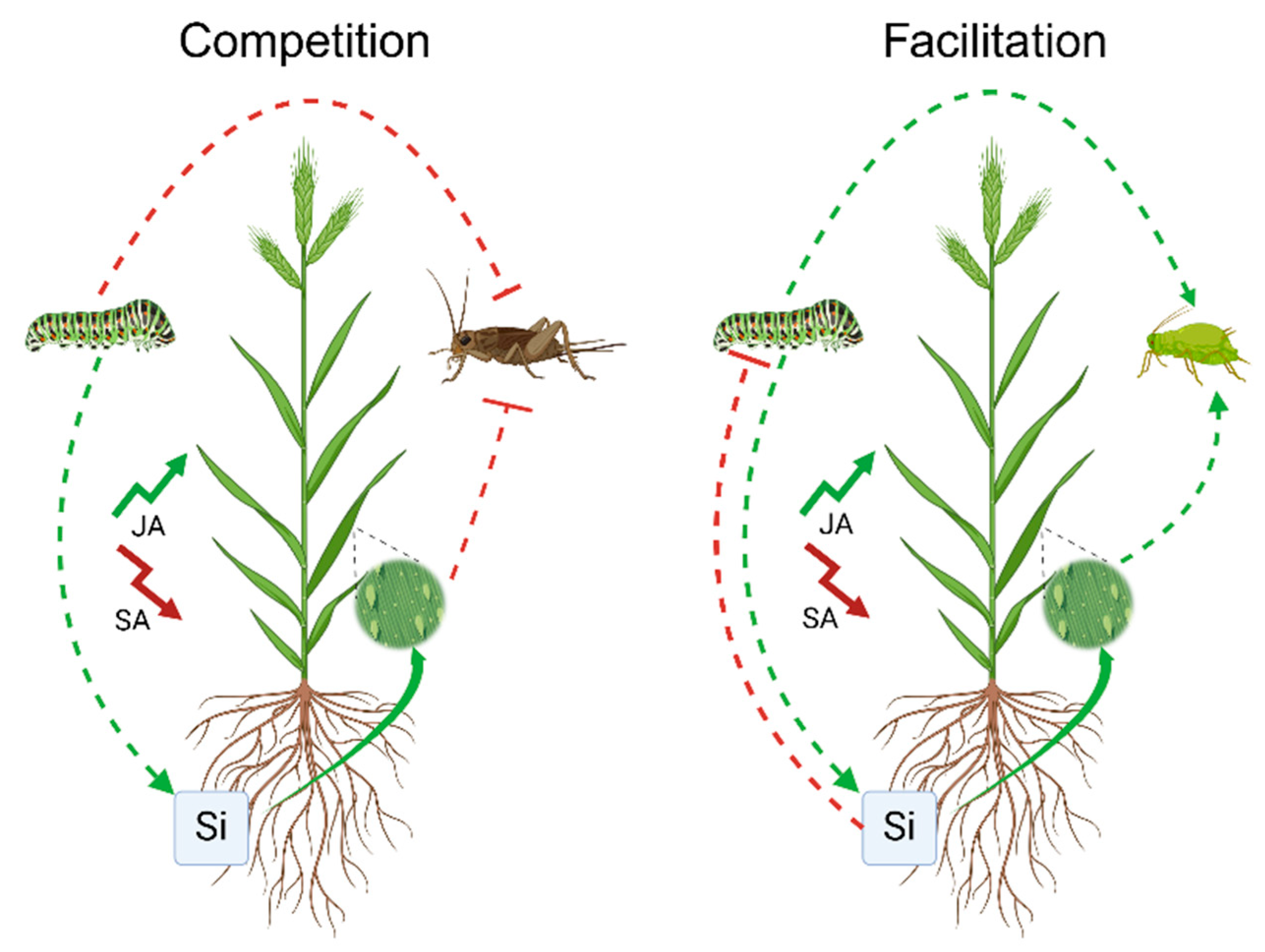
3. Plant-Mediated Interactions Between Antagonists
3.1. Facilitation
3.2. Competition
4. Silicon as a Mediator of Plant Antagonist Interactions
5. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Glossary
| Abbreviation | Definition |
| CAT | Catalase |
| ET | Ethylene |
| ETI | Effector-triggered immunity |
| ISR | Induced systemic resistance |
| JA | Jasmonic acid |
| MeJa | Methyl jasmonate |
| PAL | Phenylalanine ammonia lyase |
| POX | Peroxidase |
| PPN | Plant parasitic nematode |
| PPO | Polyphenol oxidase |
| PR | Pathogenesis related |
| RGR | Relative growth rate |
| RKN | Root knot nematode |
| ROS | Reactive oxygen species |
| SA | Salicylic acid |
| SAR | Systemic aquired resistance |
| Si | Silicon |
| SiO2 | Silicon dioxide, silica |
| Si(OH)4 | (ortho)silicic acid |
| TRV | Tobacco rattle virus |
References
- Schneider, U.A.; Havlík, P.; Schmid, E.; Valin, H.; Mosnier, A.; Obersteiner, M.; Böttcher, H.; Skalský, R.; Balkovič, J.; Sauer, T.; et al. Impacts of population growth, economic development, and technical change on global food production and consumption. Agric. Syst. 2011, 104, 204–215. [Google Scholar] [CrossRef]
- Oerke, E.-C.; Dehne, H.-W. Safeguarding production—Losses in major crops and the role of crop protection. Crop Prot. 2004, 23, 275–285. [Google Scholar] [CrossRef]
- Oerke, E.-C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Deutsch, C.A.; Tewksbury, J.J.; Tigchelaar, M.; Battisti, D.S.; Merrill, S.C.; Huey, R.B.; Naylor, R.L. Increase in crop losses to insect pests in a warming climate. Science 2018, 361, 916–919. [Google Scholar] [CrossRef]
- Van Bockhaven, J.; De Vleesschauwer, D.; Höfte, M. Towards establishing broad-spectrum disease resistance in plants: Silicon leads the way. J. Exp. Bot. 2013, 64, 1281–1293. [Google Scholar] [CrossRef]
- Zhan, L.P.; Peng, D.L.; Wang, X.L.; Kong, L.A.; Peng, H.; Liu, S.M.; Huang, W.K. Priming effect of root-applied silicon on the enhancement of induced resistance to the root-knot nematode Meloidogyne graminicola in rice. BMC Plant Biol. 2018, 18, 50. [Google Scholar] [CrossRef]
- Ode, P.J.; Johnson, S.N.; Moore, B.D. Atmospheric change and induced plant secondary metabolites—Are we reshaping the building blocks of multi-trophic interactions? Curr. Opin. Insect Sci. 2014, 5, 57–65. [Google Scholar] [CrossRef]
- Yu, J.; Yu, X.; Li, C.; Ayaz, M.; Abdulsalam, S.; Peng, D.; Huang, W. Silicon mediated plant immunity against nematodes: Summarizing the underline defence mechanisms in plant nematodes interaction. Int. J. Mol. Sci. 2022, 23, 14026. [Google Scholar] [CrossRef]
- Vicari, M.; Bazely, D.R. Do grasses fight back? The case for antherbivore defences. Trends Ecol. Evol. 1993, 8, 137–141. [Google Scholar] [CrossRef]
- Hall, C.R.; Waterman, J.M.; Vandegeer, R.K.; Hartley, S.E.; Johnson, S.N. The role of silicon in antiherbivore phytohormonal signalling. Front. Plant Sci. 2019, 10, 472867. [Google Scholar] [CrossRef]
- Thorne, S.J.; Hartley, S.E.; Maathuis, F.J. Is silicon a panacea for alleviating drought and salt stress in crops? Front. Plant Sci. 2020, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Thorne, S.J.; Stirnberg, P.M.; Hartley, S.E.; Maathuis, F.J. The ability of silicon fertilisation to alleviate salinity stress in rice is critically dependent on cultivar. Rice 2022, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Guével, M.H.; Menzies, J.G.; Bélanger, R.R. Effect of root and foliar applications of soluble silicon on powdery mildew control and growth of wheat plants. Eur. J. Plant Pathol. 2007, 119, 429–436. [Google Scholar] [CrossRef]
- Han, Y.-Q.; Wen, J.-H.; Peng, Z.-P.; Zhang, D.-Y.; Hou, M.-L. Effects of silicon amendment on the occurrence of rice insect pests and diseases in a field test. J. Integr. Agric. 2018, 17, 2172–2181. [Google Scholar] [CrossRef]
- McLarnon, E.; McQueen-Mason, S.; Lenk, I.; Hartley, S.E. Evidence for active uptake and deposition of Si-based defenses in tall fescue. Front. Plant Sci. 2017, 8, 1199. [Google Scholar] [CrossRef]
- Mitani-Ueno, N.; Ma, J.F. Linking transport system of silicon with its accumulation in different plant species. Soil Sci. Plant Nutr. 2021, 67, 10–17. [Google Scholar] [CrossRef]
- Clymans, W.; Struyf, E.; Govers, G.; Vandevenne, F.; Conley, D. Anthropogenic impact on amorphous silica pools in temperate soils. Biogeosciences 2011, 8, 2281–2293. [Google Scholar] [CrossRef]
- Sacala, E. Role of silicon in plant resistance to water stress. J. Elem. 2009, 14, 619–630. [Google Scholar] [CrossRef]
- Balakhnina, T.; Borkowska, A. Effects of silicon on plant resistance to environmental stresses. Int. Agrophysics 2013, 27, 225–232. [Google Scholar] [CrossRef]
- Wang, M.; Gao, L.; Dong, S.; Sun, Y.; Shen, Q.; Guo, S. Role of silicon on plant–pathogen interactions. Front. Plant Sci. 2017, 8, 701. [Google Scholar] [CrossRef]
- Alhousari, F.; Greger, M. Silicon and mechanisms of plant resistance to insect pests. Plants 2018, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, O.L.; Keeping, M.G.; Meyer, J.H. Silicon-augmented resistance of plants to herbivorous insects: A review. Ann. Appl. Biol. 2009, 155, 171–186. [Google Scholar] [CrossRef]
- Johnson, S.N.; Waterman, J.M.; Hartley, S.E.; Cooke, J.; Ryalls, J.M.; Lagisz, M.; Nakagawa, S. Plant silicon defences suppress herbivore performance, but mode of feeding is key. Ecol. Lett. 2024, 27, e14519. [Google Scholar] [CrossRef]
- Alvarenga, R.; Moraes, J.C.; Auad, A.M.; Coelho, M.; Nascimento, A.M. Induction of resistance of corn plants to Spodoptera frugiperda (JE Smith, 1797) (Lepidoptera: Noctuidae) by application of silicon and gibberellic acid. Bull. Entomol. Res. 2017, 107, 527–533. [Google Scholar] [CrossRef]
- Bakhat, H.F.; Bibi, N.; Zia, Z.; Abbas, S.; Hammad, H.M.; Fahad, S.; Saeed, S. Silicon mitigates biotic stresses in crop plants: A review. Crop Prot. 2018, 104, 21–34. [Google Scholar] [CrossRef]
- Correa, R.S.; Moraes, J.C.; Auad, A.M.; Carvalho, G.A. Silicon and acibenzolar-S-methyl as resistance inducers in cucumber, against the whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) biotype B. Neotrop. Entomol. 2005, 34, 429–433. [Google Scholar] [CrossRef]
- Ferreira, R.S.; Moraes, J.C. Silicon influence on resistance induction against Bemisia tabaci biotype B (Genn.) (Hemiptera: Aleyrodidae) and on vegetative development in two soybean cultivars. Neotrop. Entomol. 2011, 40, 495–500. [Google Scholar] [CrossRef]
- Gomes, F.B.; Moraes, J.C.D.; Santos, C.D.D.; Goussain, M.M. Resistance induction in wheat plants by silicon and aphids. Sci. Agric. 2005, 62, 547–551. [Google Scholar] [CrossRef]
- Han, Y.; Li, P.; Gong, S.; Yang, L.; Wen, L.; Hou, M. Defense responses in rice induced by silicon amendment against infestation by the leaf folder Cnaphalocrocis medinalis. PLoS ONE 2016, 11, e0153918. [Google Scholar] [CrossRef]
- Hunt, J.W.; Dean, A.P.; Webster, R.E.; Johnson, G.N.; Ennos, A.R. A novel mechanism by which silica defends grasses against herbivory. Ann. Bot. 2008, 102, 653–656. [Google Scholar] [CrossRef]
- Roy, S.; Mohammad, R.; Khamari, B.; Monalisa, S.P.; Swain, D.K. Silicon mediated defense response in rice plants against Brown Plant Hopper Nilaparvata lugens (Stål). Silicon 2023, 15, 7579–7591. [Google Scholar] [CrossRef]
- Johnson, S.N.; Rowe, R.C.; Hall, C.R. Silicon is an inducible and effective herbivore defence against Helicoverpa punctigera (Lepidoptera: Noctuidae) in soybean. Bull. Entomol. Res. 2020, 110, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Waterman, J.M.; Cibils-Stewart, X.; Cazzonelli, C.I.; Hartley, S.E.; Johnson, S.N. Short-term exposure to silicon rapidly enhances plant resistance to herbivory. Ecology 2021, 102, e03438. [Google Scholar] [CrossRef]
- Massey, F.P.; Ennos, A.R.; Hartley, S.E. Silica in grasses as a defence against insect herbivores: Contrasting effects on folivores and a phloem feeder. J. Anim. Ecol. 2006, 75, 595–603. [Google Scholar] [CrossRef]
- Johnson, S.N.; Rowe, R.C.; Hall, C.R. Aphid feeding induces phytohormonal cross-talk without affecting silicon defense against subsequent chewing herbivores. Plants 2020, 9, 1009. [Google Scholar] [CrossRef]
- Hartley, S.E.; Fitt, R.N.; McLarnon, E.L.; Wade, R.N. Defending the leaf surface: Intra-and inter-specific differences in silicon deposition in grasses in response to damage and silicon supply. Front. Plant Sci. 2015, 6, 35. [Google Scholar] [CrossRef]
- Islam, T.; Moore, B.D.; Johnson, S.N. Silicon fertilisation affects morphological and immune defences of an insect pest and enhances plant compensatory growth. J. Pest Sci. 2023, 96, 41–53. [Google Scholar] [CrossRef]
- Massey, F.P.; Hartley, S.E. Physical defences wear you down: Progressive and irreversible impacts of silica on insect herbivores. J. Anim. Ecol. 2009, 78, 281–291. [Google Scholar] [CrossRef]
- Kvedaras, O.L.; Byrne, M.J.; Coombes, N.E.; Keeping, M.G. Influence of plant silicon and sugarcane cultivar on mandibular wear in the stalk borer Eldana saccharina. Agric. For. Entomol. 2009, 11, 301–306. [Google Scholar] [CrossRef]
- Johnson, S.N.; Hartley, S.E.; Ryalls, J.M.; Frew, A.; Hall, C.R. Targeted plant defense: Silicon conserves hormonal defense signaling impacting chewing but not fluid-feeding herbivores. Ecology 2021, 102, e03250. [Google Scholar] [CrossRef]
- Hall, C.R.; Mikhael, M.; Hartley, S.E.; Johnson, S.N. Elevated atmospheric CO2 suppresses jasmonate and silicon-based defences without affecting herbivores. Funct. Ecol. 2020, 34, 993–1002. [Google Scholar] [CrossRef]
- Ye, M.; Song, Y.; Long, J.; Wang, R.; Baerson, S.R.; Pan, Z.; Zhu-Salzman, K.; Xie, J.; Cai, K.; Luo, S. Priming of jasmonate-mediated antiherbivore defense responses in rice by silicon. Proc. Natl. Acad. Sci. USA 2013, 110, E3631–E3639. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Khan, A.L.; Waqas, M.; Jeong, H.-J.; Kim, D.-H.; Shin, J.S.; Kim, J.-G.; Yeon, M.-H.; Lee, I.-J. Regulation of jasmonic acid biosynthesis by silicon application during physical injury to Oryza sativa L. J. Plant Res. 2014, 127, 525–532. [Google Scholar] [CrossRef]
- Fawe, A.; Abou-Zaid, M.; Menzies, J.G.; Bélanger, R.R. Silicon-mediated accumulation of flavonoid phytoalexins in cucumber. Phytopathology 1998, 88, 396–401. [Google Scholar] [CrossRef]
- Rodrigues, F.Á.; Jurick II, W.M.; Datnoff, L.E.; Jones, J.B.; Rollins, J.A. Silicon influences cytological and molecular events in compatible and incompatible rice-Magnaporthe grisea interactions. Physiol. Mol. Plant Pathol. 2005, 66, 144–159. [Google Scholar] [CrossRef]
- Zhang, G.; Cui, Y.; Ding, X.; Dai, Q. Stimulation of phenolic metabolism by silicon contributes to rice resistance to sheath blight. J. Plant Nutr. Soil Sci. 2013, 176, 118–124. [Google Scholar] [CrossRef]
- Hawerroth, C.; Araujo, L.; Bermúdez-Cardona, M.B.; Silveira, P.R.; Wordell Filho, J.A.; Rodrigues, F.A. Silicon-mediated maize resistance to macrospora leaf spot. Trop. Plant Pathol. 2019, 44, 192–196. [Google Scholar] [CrossRef]
- Bathoova, M.; Bokor, B.; Soukup, M.; Lux, A.; Martinka, M. Silicon-mediated cell wall modifications of sorghum root exodermis and suppression of invasion by fungus Alternaria alternata. Plant Pathol. 2018, 67, 1891–1900. [Google Scholar] [CrossRef]
- Gillman, J.H.; Zlesak, D.C.; Smith, J.A. Applications of Potassium Silicate Decrease Black Spot Infection in Rosa hybrida ‘Meipelta’ (Fuschia Meidiland™). HortScience 2003, 38, 1144–1147. [Google Scholar] [CrossRef]
- Arsenault-Labrecque, G.; Menzies, J.G.; Bélanger, R.R. Effect of silicon absorption on soybean resistance to Phakopsora pachyrhizi in different cultivars. Plant Dis. 2012, 96, 37–42. [Google Scholar] [CrossRef]
- Dallagnol, L.J.; Rodrigues, F.A.; DaMatta, F.M.; Mielli, M.V.; Pereira, S.C. Deficiency in silicon uptake affects cytological, physiological, and biochemical events in the rice–Bipolaris oryzae interaction. Phytopathology 2011, 101, 92–104. [Google Scholar] [CrossRef]
- Domiciano, G.P.; Rodrigues, F.A.; Guerra, A.; Vale, F.X. Infection process of Bipolaris sorokiniana on wheat leaves is affected by silicon. Trop. Plant Pathol. 2013, 38, 258–263. [Google Scholar] [CrossRef]
- Suriyaprabha, R.; Karunakaran, G.; Kavitha, K.; Yuvakkumar, R.; Rajendran, V.; Kannan, N. Application of silica nanoparticles in maize to enhance fungal resistance. IET Nanobiotechnology 2014, 8, 133–137. [Google Scholar] [CrossRef]
- Do Prado Mattos, A.; Dinelli, G.; Marotti, I.; Faedo, L.F.; Boff, M.I.C.; Boff, P. Effects of dynamised high dilutions and vegetal extract based on silicon on the growth and induction of resistance in tomato plants against Rhizoctonia solani. Biol. Agric. Hortic. 2025, 41, 13–34. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Yang, Y. Mechanisms of silicon-induced fungal disease resistance in plants. Plant Physiol. Biochem. 2021, 165, 200–206. [Google Scholar] [CrossRef]
- Heine, G.; Tikum, G.; Horst, W.J. The effect of silicon on the infection by and spread of Pythium aphanidermatum in single roots of tomato and bitter gourd. J. Exp. Bot. 2007, 58, 569–577. [Google Scholar] [CrossRef]
- Holz, T.M.; Dorneles, K.R.; Brunetto, A.E.; Segundo, J.B.M.; Delevatti, H.A.; Souza, G.M.; Dallagnol, L.J. Effect of silicon and fungicide on photosynthetic responses in barley leaves challenged by Bipolaris sorokiniana. Physiol. Mol. Plant Pathol. 2022, 120, 101849. [Google Scholar] [CrossRef]
- Puppe, D.; Sommer, M. Experiments, uptake mechanisms, and functioning of silicon foliar fertilization—A review focusing on maize, rice, and wheat. Adv. Agron. 2018, 152, 1–49. [Google Scholar]
- Leal, I.M.G.; Fontes, B.A.; Silva, L.C.; Quadros, L.P.; Picanco, B.B.M.; Castro, H.V.M.; Rodrigues, F.Á. Foliar application of nutrients and silicon for increasing soybean resistance against infection by Phakopsora pachyrhizi. Trop. Plant Pathol. 2025, 50, 15. [Google Scholar] [CrossRef]
- Sakr, N. The role of silicon (Si) in increasing plant resistance against fungal diseases. Hell. Plant Prot. J. 2016, 9, 1–15. [Google Scholar] [CrossRef]
- Chérif, M.; Menzies, J.G.; Benhamou, N.; Bélanger, R.R. Studies of silicon distribution in wounded and Pythium ultimum infected cucumber plants. Physiol. Mol. Plant Pathol. 1992, 41, 371–385. [Google Scholar] [CrossRef]
- Fauteux, F.; Chain, F.; Belzile, F.; Menzies, J.G.; Bélanger, R.R. The protective role of silicon in the Arabidopsis–powdery mildew pathosystem. Proc. Natl. Acad. Sci. USA 2006, 103, 17554–17559. [Google Scholar] [CrossRef]
- Rodgers-Gray, B.; Shaw, M. Effects of straw and silicon soil amendments on some foliar and stem-base diseases in pot-grown winter wheat. Plant Pathol. 2004, 53, 733–740. [Google Scholar] [CrossRef]
- Biere, A.; Goverse, A. Plant-mediated systemic interactions between pathogens, parasitic nematodes, and herbivores above-and belowground. Annu. Rev. Phytopathol. 2016, 54, 499–527. [Google Scholar] [CrossRef]
- Rajarammohan, S. Redefining plant-necrotroph interactions: The thin line between hemibiotrophs and necrotrophs. Front. Microbiol. 2021, 12, 673518. [Google Scholar] [CrossRef]
- Lata-Tenesaca, L.F.; Oliveira, M.J.B.; Barros, A.V.; Picanço, B.B.M.; Rodrigues, F.Á. Physiological and Biochemical Aspects of Silicon-Mediated Resistance in Maize against Maydis Leaf Blight. Plants 2024, 13, 531. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Bélanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef]
- Ahamad, L.; Siddiqui, Z.A. Effects of silicon dioxide, zinc oxide and titanium dioxide nanoparticles on Meloidogyne incognita, Alternaria dauci and Rhizoctonia solani disease complex of carrot. Exp. Parasitol. 2021, 230, 108176. [Google Scholar] [CrossRef]
- Ahmadi Mansourabad, M.; Kargar Bideh, A.; Abdollahi, M. Effects of some micronutrients and macronutrients on the root-knot nematode, Meloidogyne incognita, in greenhouse cucumber (Cucumis sativus cv. Negin). J. Crop Prot. 2016, 5, 507–517. [Google Scholar] [CrossRef]
- Santos, L.B.; de Souza Junior, J.P.; de Mello Prado, R.; Ferreira Junior, R.; de Souza, V.F.; dos Santos Sarah, M.M.; Soares, P.L.M. Silicon allows halving Cadusafos dose to control Meloidogyne incognita and increase cotton development. Silicon 2022, 14, 3809–3816. [Google Scholar] [CrossRef]
- El-Ashry, R.M.; El-Saadony, M.T.; El-Sobki, A.E.; El-Tahan, A.M.; Al-Otaibi, S.; El-Shehawi, A.M.; Saad, A.M.; Elshaer, N. Biological silicon nanoparticles maximize the efficiency of nematicides against biotic stress induced by Meloidogyne incognita in eggplant. Saudi J. Biol. Sci. 2022, 29, 920–932. [Google Scholar] [CrossRef]
- Qi, X.; Xue, X.; Su, G.; Han, Y.; Wang, Y.; Li, Y.; Jiang, Y. The physiological and biochemical role of silicon in enhancing the resistance of maize to root-lesion nematode. Plant Pathol. 2024, 73, 2112–2122. [Google Scholar] [CrossRef]
- Al Banna, L.; Salem, N.; Ghrair, A.M.; Habash, S.S. Impact of silicon carbide nanoparticles on hatching and survival of soil nematodes Caenorhabditis elegans and Meloidogyne incognita. Appl. Ecol. Environ. Res. 2018, 16, 2651–2662. [Google Scholar] [CrossRef]
- Silva, R.V.; Oliveira, R.D.; Ferreira, P.D.S.; Castro, D.B.; Rodrigues, F.Á. Effects of silicon on the penetration and reproduction events of Meloidogyne exigua on coffee roots. Bragantia 2015, 74, 196–199. [Google Scholar] [CrossRef]
- Dugui-Es, C.; Pedroche, N.; Villanueva, L.; Galeng, J.; De Waele, D. Management of root knot nematode, Meloidogyne incognita in cucumber (Cucumis sativus) using silicon. Commun. Agric. Appl. Biol. Sci. 2010, 75, 497–505. [Google Scholar]
- Khan, M.R.; Siddiqui, Z.A. Use of silicon dioxide nanoparticles for the management of Meloidogyne incognita, Pectobacterium betavasculorum and Rhizoctonia solani disease complex of beetroot (Beta vulgaris L.). Sci. Hortic. 2020, 265, 109211. [Google Scholar] [CrossRef]
- Udalova, Z.V.; Folmanis, G.E.; Fedotov, M.A.; Pelgunova, L.A.; Krysanov, E.Y.; Khasanov, F.K.; Zinovieva, S.V. Effects of silicon nanoparticles on photosynthetic pigments and biogenic elements in tomato plants infected with root-knot nematode Meloidogyne incognita. Dokl. Biochem. Biophys. Pleiades Publ. 2020, 495, 329–333. [Google Scholar] [CrossRef]
- Silva, R.V.; Oliveira, R.D.L.; Nascimento, K.J.T.; Rodrigues, F.A. Biochemical responses of coffee resistance against Meloidogyne exigua mediated by silicon. Plant Pathol. 2010, 59, 586–593. [Google Scholar] [CrossRef]
- De Bobadilla, M.F.; Vitiello, A.; Erb, M.; Poelman, E.H. Plant defense strategies against attack by multiple herbivores. Trends Plant Sci. 2022, 27, 528–535. [Google Scholar] [CrossRef]
- Van Griethuysen, P.A.; Redeker, K.R.; MacFarlane, S.A.; Neilson, R.; Hartley, S.E. Virus-induced changes in root volatiles attract soil nematode vectors to infected plants. New Phytol. 2024, 241, 2275–2286. [Google Scholar] [CrossRef]
- Willsey, T.; Chatterton, S.; Cárcamo, H. Interactions of root-feeding insects with fungal and oomycete plant pathogens. Front. Plant Sci. 2017, 8, 1764. [Google Scholar] [CrossRef]
- Islam, T.; Moore, B.D.; Johnson, S.N. Plant silicon defences reduce the performance of a chewing insect herbivore which benefits a contemporaneous sap-feeding insect. Ecol. Entomol. 2022, 47, 951–958. [Google Scholar] [CrossRef]
- Lazebnik, J.; Frago, E.; Dicke, M.; Van Loon, J.J. Phytohormone mediation of interactions between herbivores and plant pathogens. J. Chem. Ecol. 2014, 40, 730–741. [Google Scholar] [CrossRef]
- Stam, J.M.; Kroes, A.; Li, Y.; Gols, R.; van Loon, J.J.; Poelman, E.H.; Dicke, M. Plant interactions with multiple insect herbivores: From community to genes. Annu. Rev. Plant Biol. 2014, 65, 689–713. [Google Scholar] [CrossRef]
- Aerts, N.; Pereira Mendes, M.; Van Wees, S.C. Multiple levels of crosstalk in hormone networks regulating plant defense. Plant J. 2021, 105, 489–504. [Google Scholar] [CrossRef]
- Hoysted, G.A.; Lilley, C.J.; Field, K.J.; Dickinson, M.; Hartley, S.E.; Urwin, P.E. A plant-feeding nematode indirectly increases the fitness of an aphid. Front. Plant Sci. 2017, 8, 1897. [Google Scholar] [CrossRef]
- Wondafrash, M.; Van Dam, N.M.; Tytgat, T.O. Plant systemic induced responses mediate interactions between root parasitic nematodes and aboveground herbivorous insects. Front. Plant Sci. 2013, 4, 87. [Google Scholar] [CrossRef]
- Biru, F.N.; Cazzonelli, C.I.; Elbaum, R.; Johnson, S.N. Contrasting impacts of herbivore induction and elevated atmospheric CO2 on silicon defences and consequences for subsequent herbivores. Entomol. Exp. Appl. 2022, 170, 681–688. [Google Scholar] [CrossRef]
- Moreira, X.; Abdala-Roberts, L.; Castagneyrol, B. Interactions between plant defence signalling pathways: Evidence from bioassays with insect herbivores and plant pathogens. J. Ecol. 2018, 106, 2353–2364. [Google Scholar] [CrossRef]
- Manosalva, P.; Manohar, M.; Von Reuss, S.H.; Chen, S.; Koch, A.; Kaplan, F.; Choe, A.; Micikas, R.J.; Wang, X.; Kogel, K.-H. Conserved nematode signalling molecules elicit plant defenses and pathogen resistance. Nat. Commun. 2015, 6, 7795. [Google Scholar] [CrossRef]
- Guarneri, N.; Willig, J.J.; Sterken, M.G.; Zhou, W.; Hasan, M.S.; Sharon, L.; Grundler, F.M.; Willemsen, V.; Goverse, A.; Smant, G. Root architecture plasticity in response to endoparasitic cyst nematodes is mediated by damage signaling. New Phytol. 2023, 237, 807–822. [Google Scholar] [CrossRef]
- Kutyniok, M.; Müller, C. Plant-mediated interactions between shoot-feeding aphids and root-feeding nematodes depend on nitrate fertilization. Oecologia 2013, 173, 1367–1377. [Google Scholar] [CrossRef]
- Ripa, L.; Stevens, G.; Lewis, E. Two-way plant-mediated interactions between a plant parasitic nematode and a foliar herbivore arthropod. Rhizosphere 2023, 26, 100699. [Google Scholar] [CrossRef]
- Kohl, L.M. Foliar nematodes: A summary of biology and control with a compilation of host range. Plant Health Prog. 2011, 12, 23. [Google Scholar] [CrossRef]
- Jang, S.-W.; Kim, Y.; Khan, A.L.; Na, C.-I.; Lee, I.-J. Exogenous short-term silicon application regulates macro-nutrients, endogenous phytohormones, and protein expression in Oryza sativa L. BMC Plant Biol. 2018, 18, 4. [Google Scholar] [CrossRef]
- Hartley, S.E.; DeGabriel, J.L. The ecology of herbivore-induced silicon defences in grasses. Funct. Ecol. 2016, 30, 1311–1322. [Google Scholar] [CrossRef]
- Reynolds, J.J.; Lambin, X.; Massey, F.P.; Reidinger, S.; Sherratt, J.A.; Smith, M.J.; White, A.; Hartley, S.E. Delayed induced silica defences in grasses and their potential for destabilising herbivore population dynamics. Oecologia 2012, 170, 445–456. [Google Scholar] [CrossRef]
- Karban, R. The ecology and evolution of induced resistance against herbivores. Funct. Ecol. 2011, 25, 339–347. [Google Scholar] [CrossRef]
- Gershenzon, J.; Murtagh, G.J.; Croteau, R. Absence of rapid terpene turnover in several diverse species of terpene-accumulating plants. Oecologia 1993, 96, 583–592. [Google Scholar] [CrossRef]
- Stamp, N. Out of the quagmire of plant defense hypotheses. Q. Rev. Biol. 2003, 78, 23–55. [Google Scholar] [CrossRef]
- Thorne, S.J.; Maathuis, F.J.; Hartley, S.E. Induction of silicon defences in wheat landraces is local, not systemic, and driven by mobilization of soluble silicon to damaged leaves. J. Exp. Bot. 2023, 74, 5363–5373. [Google Scholar] [CrossRef]
- Vivancos, J.; Labbé, C.; Menzies, J.G.; Bélanger, R.R. Silicon-mediated resistance of Arabidopsis against powdery mildew involves mechanisms other than the salicylic acid (SA)-dependent defence pathway. Mol. Plant Pathol. 2015, 16, 572–582. [Google Scholar] [CrossRef]
- Massey, F.P.; Roland Ennos, A.; Hartley, S.E. Herbivore specific induction of silica-based plant defences. Oecologia 2007, 152, 677–683. [Google Scholar] [CrossRef]
- Cibils-Stewart, X.; Mace, W.J.; Popay, A.J.; Lattanzi, F.A.; Hartley, S.E.; Hall, C.R.; Powell, J.R.; Johnson, S.N. Interactions between silicon and alkaloid defences in endophyte-infected grasses and the consequences for a folivore. Funct. Ecol. 2022, 36, 249–261. [Google Scholar] [CrossRef]
- Cibils-Stewart, X.; Powell, J.R.; Popay, A.J.; Lattanzi, F.A.; Hartley, S.E.; Johnson, S.N. Reciprocal effects of silicon supply and endophytes on silicon accumulation and Epichloë colonization in grasses. Front. Plant Sci. 2020, 11, 593198. [Google Scholar] [CrossRef]
- Cibils-Stewart, X.; Putra, R.; Islam, T.; Fanna, D.; Wuhrer, R.; Mace, W.; Hartley, S.; Popay, A.; Johnson, S. Silicon and Epichloë-endophyte defences in a model temperate grass diminish feeding efficiency and immunity of an insect folivore. Funct. Ecol. 2023, 37, 3177–3192. [Google Scholar] [CrossRef]
- Guntzer, F.; Keller, C.; Meunier, J.-D. Benefits of plant silicon for crops: A review. Agron. Sustain. Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef]
- Kelland, M.E.; Wade, P.W.; Lewis, A.L.; Taylor, L.L.; Sarkar, B.; Andrews, M.G.; Lomas, M.R.; Cotton, T.A.; Kemp, S.J.; James, R.H. Increased yield and CO2 sequestration potential with the C4 cereal Sorghum bicolor cultivated in basaltic rock dust-amended agricultural soil. Glob. Change Biol. 2020, 26, 3658–3676. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denarié, M.-E.; Nielsen, U.N.; Hartley, S.E.; Johnson, S.N. Silicon-Mediated Interactions Between Plant Antagonists. Plants 2025, 14, 1204. https://doi.org/10.3390/plants14081204
Denarié M-E, Nielsen UN, Hartley SE, Johnson SN. Silicon-Mediated Interactions Between Plant Antagonists. Plants. 2025; 14(8):1204. https://doi.org/10.3390/plants14081204
Chicago/Turabian StyleDenarié, Marie-Emma, Uffe N. Nielsen, Susan E. Hartley, and Scott N. Johnson. 2025. "Silicon-Mediated Interactions Between Plant Antagonists" Plants 14, no. 8: 1204. https://doi.org/10.3390/plants14081204
APA StyleDenarié, M.-E., Nielsen, U. N., Hartley, S. E., & Johnson, S. N. (2025). Silicon-Mediated Interactions Between Plant Antagonists. Plants, 14(8), 1204. https://doi.org/10.3390/plants14081204