Silicon-Mediated Interactions Between Plant Antagonists
Abstract
1. Introduction
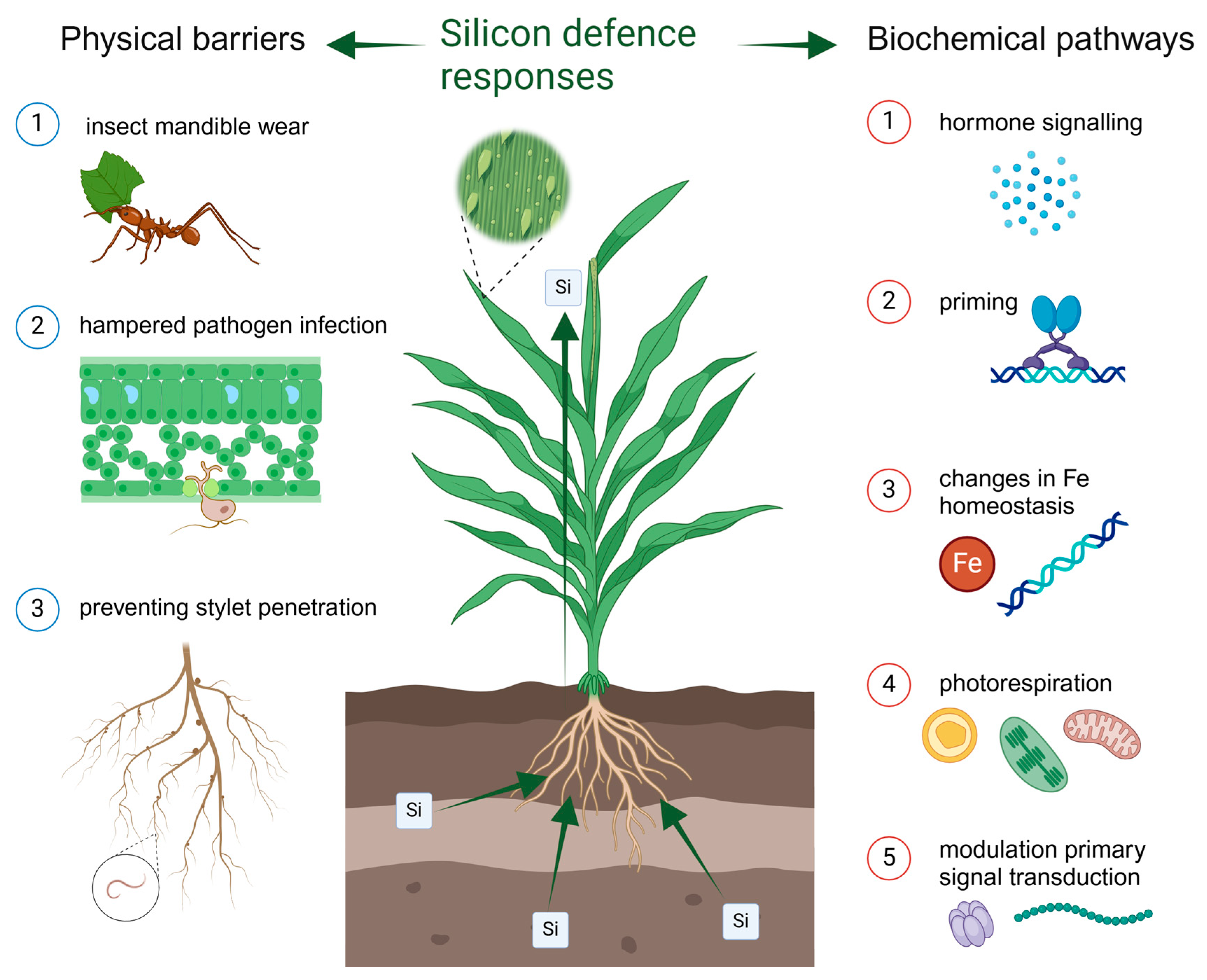
2. The Role of Plant Silicon in Response to Biotic Stresses
2.1. Insect Herbivores
2.2. Fungal Pathogens
2.3. Plant Parasitic Nematodes
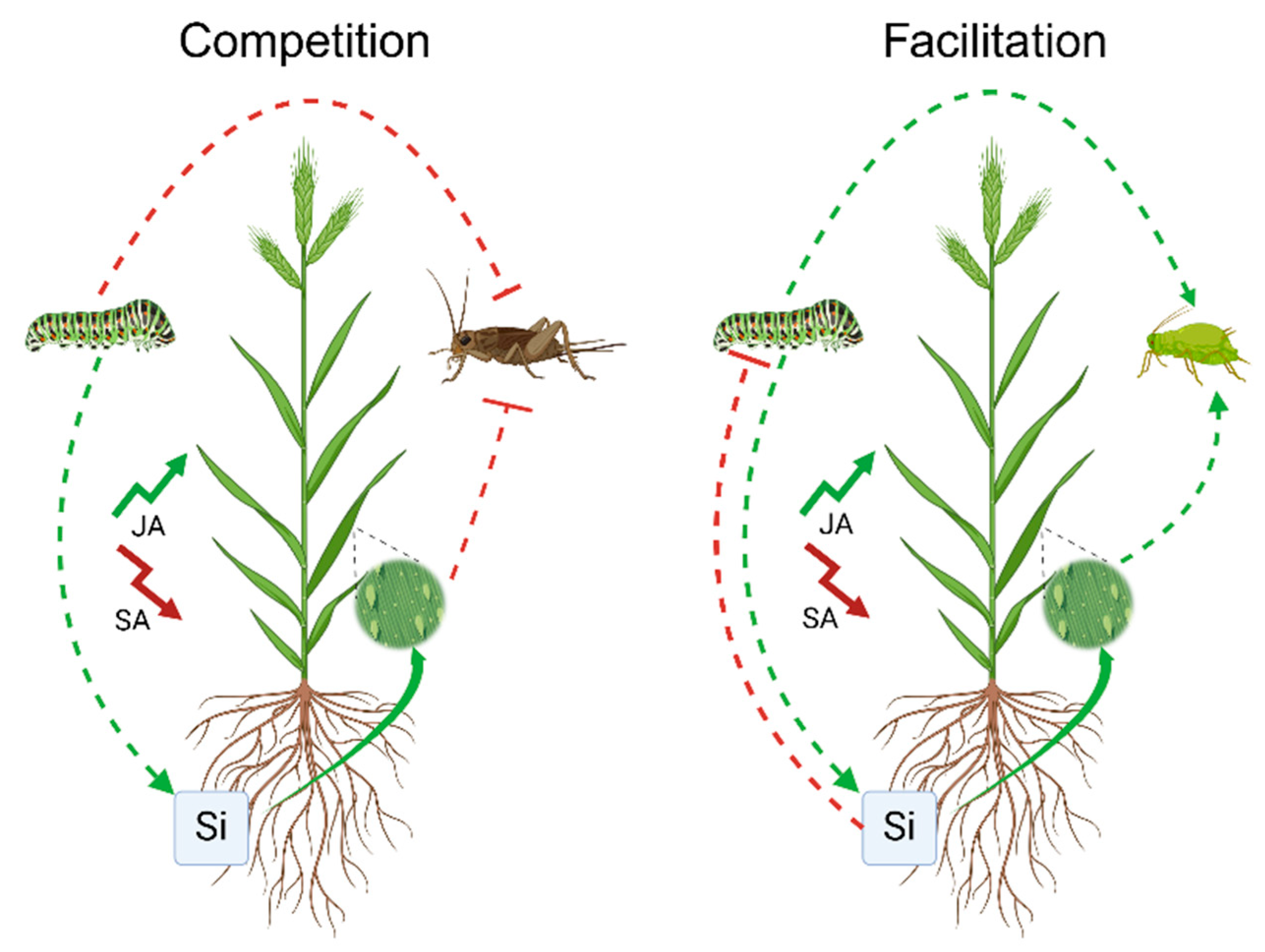
3. Plant-Mediated Interactions Between Antagonists
3.1. Facilitation
3.2. Competition
4. Silicon as a Mediator of Plant Antagonist Interactions
5. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Glossary
| Abbreviation | Definition |
| CAT | Catalase |
| ET | Ethylene |
| ETI | Effector-triggered immunity |
| ISR | Induced systemic resistance |
| JA | Jasmonic acid |
| MeJa | Methyl jasmonate |
| PAL | Phenylalanine ammonia lyase |
| POX | Peroxidase |
| PPN | Plant parasitic nematode |
| PPO | Polyphenol oxidase |
| PR | Pathogenesis related |
| RGR | Relative growth rate |
| RKN | Root knot nematode |
| ROS | Reactive oxygen species |
| SA | Salicylic acid |
| SAR | Systemic aquired resistance |
| Si | Silicon |
| SiO2 | Silicon dioxide, silica |
| Si(OH)4 | (ortho)silicic acid |
| TRV | Tobacco rattle virus |
References
- Schneider, U.A.; Havlík, P.; Schmid, E.; Valin, H.; Mosnier, A.; Obersteiner, M.; Böttcher, H.; Skalský, R.; Balkovič, J.; Sauer, T.; et al. Impacts of population growth, economic development, and technical change on global food production and consumption. Agric. Syst. 2011, 104, 204–215. [Google Scholar] [CrossRef]
- Oerke, E.-C.; Dehne, H.-W. Safeguarding production—Losses in major crops and the role of crop protection. Crop Prot. 2004, 23, 275–285. [Google Scholar] [CrossRef]
- Oerke, E.-C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Deutsch, C.A.; Tewksbury, J.J.; Tigchelaar, M.; Battisti, D.S.; Merrill, S.C.; Huey, R.B.; Naylor, R.L. Increase in crop losses to insect pests in a warming climate. Science 2018, 361, 916–919. [Google Scholar] [CrossRef]
- Van Bockhaven, J.; De Vleesschauwer, D.; Höfte, M. Towards establishing broad-spectrum disease resistance in plants: Silicon leads the way. J. Exp. Bot. 2013, 64, 1281–1293. [Google Scholar] [CrossRef]
- Zhan, L.P.; Peng, D.L.; Wang, X.L.; Kong, L.A.; Peng, H.; Liu, S.M.; Huang, W.K. Priming effect of root-applied silicon on the enhancement of induced resistance to the root-knot nematode Meloidogyne graminicola in rice. BMC Plant Biol. 2018, 18, 50. [Google Scholar] [CrossRef]
- Ode, P.J.; Johnson, S.N.; Moore, B.D. Atmospheric change and induced plant secondary metabolites—Are we reshaping the building blocks of multi-trophic interactions? Curr. Opin. Insect Sci. 2014, 5, 57–65. [Google Scholar] [CrossRef]
- Yu, J.; Yu, X.; Li, C.; Ayaz, M.; Abdulsalam, S.; Peng, D.; Huang, W. Silicon mediated plant immunity against nematodes: Summarizing the underline defence mechanisms in plant nematodes interaction. Int. J. Mol. Sci. 2022, 23, 14026. [Google Scholar] [CrossRef]
- Vicari, M.; Bazely, D.R. Do grasses fight back? The case for antherbivore defences. Trends Ecol. Evol. 1993, 8, 137–141. [Google Scholar] [CrossRef]
- Hall, C.R.; Waterman, J.M.; Vandegeer, R.K.; Hartley, S.E.; Johnson, S.N. The role of silicon in antiherbivore phytohormonal signalling. Front. Plant Sci. 2019, 10, 472867. [Google Scholar] [CrossRef]
- Thorne, S.J.; Hartley, S.E.; Maathuis, F.J. Is silicon a panacea for alleviating drought and salt stress in crops? Front. Plant Sci. 2020, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Thorne, S.J.; Stirnberg, P.M.; Hartley, S.E.; Maathuis, F.J. The ability of silicon fertilisation to alleviate salinity stress in rice is critically dependent on cultivar. Rice 2022, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Guével, M.H.; Menzies, J.G.; Bélanger, R.R. Effect of root and foliar applications of soluble silicon on powdery mildew control and growth of wheat plants. Eur. J. Plant Pathol. 2007, 119, 429–436. [Google Scholar] [CrossRef]
- Han, Y.-Q.; Wen, J.-H.; Peng, Z.-P.; Zhang, D.-Y.; Hou, M.-L. Effects of silicon amendment on the occurrence of rice insect pests and diseases in a field test. J. Integr. Agric. 2018, 17, 2172–2181. [Google Scholar] [CrossRef]
- McLarnon, E.; McQueen-Mason, S.; Lenk, I.; Hartley, S.E. Evidence for active uptake and deposition of Si-based defenses in tall fescue. Front. Plant Sci. 2017, 8, 1199. [Google Scholar] [CrossRef]
- Mitani-Ueno, N.; Ma, J.F. Linking transport system of silicon with its accumulation in different plant species. Soil Sci. Plant Nutr. 2021, 67, 10–17. [Google Scholar] [CrossRef]
- Clymans, W.; Struyf, E.; Govers, G.; Vandevenne, F.; Conley, D. Anthropogenic impact on amorphous silica pools in temperate soils. Biogeosciences 2011, 8, 2281–2293. [Google Scholar] [CrossRef]
- Sacala, E. Role of silicon in plant resistance to water stress. J. Elem. 2009, 14, 619–630. [Google Scholar] [CrossRef]
- Balakhnina, T.; Borkowska, A. Effects of silicon on plant resistance to environmental stresses. Int. Agrophysics 2013, 27, 225–232. [Google Scholar] [CrossRef]
- Wang, M.; Gao, L.; Dong, S.; Sun, Y.; Shen, Q.; Guo, S. Role of silicon on plant–pathogen interactions. Front. Plant Sci. 2017, 8, 701. [Google Scholar] [CrossRef]
- Alhousari, F.; Greger, M. Silicon and mechanisms of plant resistance to insect pests. Plants 2018, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, O.L.; Keeping, M.G.; Meyer, J.H. Silicon-augmented resistance of plants to herbivorous insects: A review. Ann. Appl. Biol. 2009, 155, 171–186. [Google Scholar] [CrossRef]
- Johnson, S.N.; Waterman, J.M.; Hartley, S.E.; Cooke, J.; Ryalls, J.M.; Lagisz, M.; Nakagawa, S. Plant silicon defences suppress herbivore performance, but mode of feeding is key. Ecol. Lett. 2024, 27, e14519. [Google Scholar] [CrossRef]
- Alvarenga, R.; Moraes, J.C.; Auad, A.M.; Coelho, M.; Nascimento, A.M. Induction of resistance of corn plants to Spodoptera frugiperda (JE Smith, 1797) (Lepidoptera: Noctuidae) by application of silicon and gibberellic acid. Bull. Entomol. Res. 2017, 107, 527–533. [Google Scholar] [CrossRef]
- Bakhat, H.F.; Bibi, N.; Zia, Z.; Abbas, S.; Hammad, H.M.; Fahad, S.; Saeed, S. Silicon mitigates biotic stresses in crop plants: A review. Crop Prot. 2018, 104, 21–34. [Google Scholar] [CrossRef]
- Correa, R.S.; Moraes, J.C.; Auad, A.M.; Carvalho, G.A. Silicon and acibenzolar-S-methyl as resistance inducers in cucumber, against the whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) biotype B. Neotrop. Entomol. 2005, 34, 429–433. [Google Scholar] [CrossRef]
- Ferreira, R.S.; Moraes, J.C. Silicon influence on resistance induction against Bemisia tabaci biotype B (Genn.) (Hemiptera: Aleyrodidae) and on vegetative development in two soybean cultivars. Neotrop. Entomol. 2011, 40, 495–500. [Google Scholar] [CrossRef]
- Gomes, F.B.; Moraes, J.C.D.; Santos, C.D.D.; Goussain, M.M. Resistance induction in wheat plants by silicon and aphids. Sci. Agric. 2005, 62, 547–551. [Google Scholar] [CrossRef]
- Han, Y.; Li, P.; Gong, S.; Yang, L.; Wen, L.; Hou, M. Defense responses in rice induced by silicon amendment against infestation by the leaf folder Cnaphalocrocis medinalis. PLoS ONE 2016, 11, e0153918. [Google Scholar] [CrossRef]
- Hunt, J.W.; Dean, A.P.; Webster, R.E.; Johnson, G.N.; Ennos, A.R. A novel mechanism by which silica defends grasses against herbivory. Ann. Bot. 2008, 102, 653–656. [Google Scholar] [CrossRef]
- Roy, S.; Mohammad, R.; Khamari, B.; Monalisa, S.P.; Swain, D.K. Silicon mediated defense response in rice plants against Brown Plant Hopper Nilaparvata lugens (Stål). Silicon 2023, 15, 7579–7591. [Google Scholar] [CrossRef]
- Johnson, S.N.; Rowe, R.C.; Hall, C.R. Silicon is an inducible and effective herbivore defence against Helicoverpa punctigera (Lepidoptera: Noctuidae) in soybean. Bull. Entomol. Res. 2020, 110, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Waterman, J.M.; Cibils-Stewart, X.; Cazzonelli, C.I.; Hartley, S.E.; Johnson, S.N. Short-term exposure to silicon rapidly enhances plant resistance to herbivory. Ecology 2021, 102, e03438. [Google Scholar] [CrossRef]
- Massey, F.P.; Ennos, A.R.; Hartley, S.E. Silica in grasses as a defence against insect herbivores: Contrasting effects on folivores and a phloem feeder. J. Anim. Ecol. 2006, 75, 595–603. [Google Scholar] [CrossRef]
- Johnson, S.N.; Rowe, R.C.; Hall, C.R. Aphid feeding induces phytohormonal cross-talk without affecting silicon defense against subsequent chewing herbivores. Plants 2020, 9, 1009. [Google Scholar] [CrossRef]
- Hartley, S.E.; Fitt, R.N.; McLarnon, E.L.; Wade, R.N. Defending the leaf surface: Intra-and inter-specific differences in silicon deposition in grasses in response to damage and silicon supply. Front. Plant Sci. 2015, 6, 35. [Google Scholar] [CrossRef]
- Islam, T.; Moore, B.D.; Johnson, S.N. Silicon fertilisation affects morphological and immune defences of an insect pest and enhances plant compensatory growth. J. Pest Sci. 2023, 96, 41–53. [Google Scholar] [CrossRef]
- Massey, F.P.; Hartley, S.E. Physical defences wear you down: Progressive and irreversible impacts of silica on insect herbivores. J. Anim. Ecol. 2009, 78, 281–291. [Google Scholar] [CrossRef]
- Kvedaras, O.L.; Byrne, M.J.; Coombes, N.E.; Keeping, M.G. Influence of plant silicon and sugarcane cultivar on mandibular wear in the stalk borer Eldana saccharina. Agric. For. Entomol. 2009, 11, 301–306. [Google Scholar] [CrossRef]
- Johnson, S.N.; Hartley, S.E.; Ryalls, J.M.; Frew, A.; Hall, C.R. Targeted plant defense: Silicon conserves hormonal defense signaling impacting chewing but not fluid-feeding herbivores. Ecology 2021, 102, e03250. [Google Scholar] [CrossRef]
- Hall, C.R.; Mikhael, M.; Hartley, S.E.; Johnson, S.N. Elevated atmospheric CO2 suppresses jasmonate and silicon-based defences without affecting herbivores. Funct. Ecol. 2020, 34, 993–1002. [Google Scholar] [CrossRef]
- Ye, M.; Song, Y.; Long, J.; Wang, R.; Baerson, S.R.; Pan, Z.; Zhu-Salzman, K.; Xie, J.; Cai, K.; Luo, S. Priming of jasmonate-mediated antiherbivore defense responses in rice by silicon. Proc. Natl. Acad. Sci. USA 2013, 110, E3631–E3639. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Khan, A.L.; Waqas, M.; Jeong, H.-J.; Kim, D.-H.; Shin, J.S.; Kim, J.-G.; Yeon, M.-H.; Lee, I.-J. Regulation of jasmonic acid biosynthesis by silicon application during physical injury to Oryza sativa L. J. Plant Res. 2014, 127, 525–532. [Google Scholar] [CrossRef]
- Fawe, A.; Abou-Zaid, M.; Menzies, J.G.; Bélanger, R.R. Silicon-mediated accumulation of flavonoid phytoalexins in cucumber. Phytopathology 1998, 88, 396–401. [Google Scholar] [CrossRef]
- Rodrigues, F.Á.; Jurick II, W.M.; Datnoff, L.E.; Jones, J.B.; Rollins, J.A. Silicon influences cytological and molecular events in compatible and incompatible rice-Magnaporthe grisea interactions. Physiol. Mol. Plant Pathol. 2005, 66, 144–159. [Google Scholar] [CrossRef]
- Zhang, G.; Cui, Y.; Ding, X.; Dai, Q. Stimulation of phenolic metabolism by silicon contributes to rice resistance to sheath blight. J. Plant Nutr. Soil Sci. 2013, 176, 118–124. [Google Scholar] [CrossRef]
- Hawerroth, C.; Araujo, L.; Bermúdez-Cardona, M.B.; Silveira, P.R.; Wordell Filho, J.A.; Rodrigues, F.A. Silicon-mediated maize resistance to macrospora leaf spot. Trop. Plant Pathol. 2019, 44, 192–196. [Google Scholar] [CrossRef]
- Bathoova, M.; Bokor, B.; Soukup, M.; Lux, A.; Martinka, M. Silicon-mediated cell wall modifications of sorghum root exodermis and suppression of invasion by fungus Alternaria alternata. Plant Pathol. 2018, 67, 1891–1900. [Google Scholar] [CrossRef]
- Gillman, J.H.; Zlesak, D.C.; Smith, J.A. Applications of Potassium Silicate Decrease Black Spot Infection in Rosa hybrida ‘Meipelta’ (Fuschia Meidiland™). HortScience 2003, 38, 1144–1147. [Google Scholar] [CrossRef]
- Arsenault-Labrecque, G.; Menzies, J.G.; Bélanger, R.R. Effect of silicon absorption on soybean resistance to Phakopsora pachyrhizi in different cultivars. Plant Dis. 2012, 96, 37–42. [Google Scholar] [CrossRef]
- Dallagnol, L.J.; Rodrigues, F.A.; DaMatta, F.M.; Mielli, M.V.; Pereira, S.C. Deficiency in silicon uptake affects cytological, physiological, and biochemical events in the rice–Bipolaris oryzae interaction. Phytopathology 2011, 101, 92–104. [Google Scholar] [CrossRef]
- Domiciano, G.P.; Rodrigues, F.A.; Guerra, A.; Vale, F.X. Infection process of Bipolaris sorokiniana on wheat leaves is affected by silicon. Trop. Plant Pathol. 2013, 38, 258–263. [Google Scholar] [CrossRef]
- Suriyaprabha, R.; Karunakaran, G.; Kavitha, K.; Yuvakkumar, R.; Rajendran, V.; Kannan, N. Application of silica nanoparticles in maize to enhance fungal resistance. IET Nanobiotechnology 2014, 8, 133–137. [Google Scholar] [CrossRef]
- Do Prado Mattos, A.; Dinelli, G.; Marotti, I.; Faedo, L.F.; Boff, M.I.C.; Boff, P. Effects of dynamised high dilutions and vegetal extract based on silicon on the growth and induction of resistance in tomato plants against Rhizoctonia solani. Biol. Agric. Hortic. 2025, 41, 13–34. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Yang, Y. Mechanisms of silicon-induced fungal disease resistance in plants. Plant Physiol. Biochem. 2021, 165, 200–206. [Google Scholar] [CrossRef]
- Heine, G.; Tikum, G.; Horst, W.J. The effect of silicon on the infection by and spread of Pythium aphanidermatum in single roots of tomato and bitter gourd. J. Exp. Bot. 2007, 58, 569–577. [Google Scholar] [CrossRef]
- Holz, T.M.; Dorneles, K.R.; Brunetto, A.E.; Segundo, J.B.M.; Delevatti, H.A.; Souza, G.M.; Dallagnol, L.J. Effect of silicon and fungicide on photosynthetic responses in barley leaves challenged by Bipolaris sorokiniana. Physiol. Mol. Plant Pathol. 2022, 120, 101849. [Google Scholar] [CrossRef]
- Puppe, D.; Sommer, M. Experiments, uptake mechanisms, and functioning of silicon foliar fertilization—A review focusing on maize, rice, and wheat. Adv. Agron. 2018, 152, 1–49. [Google Scholar]
- Leal, I.M.G.; Fontes, B.A.; Silva, L.C.; Quadros, L.P.; Picanco, B.B.M.; Castro, H.V.M.; Rodrigues, F.Á. Foliar application of nutrients and silicon for increasing soybean resistance against infection by Phakopsora pachyrhizi. Trop. Plant Pathol. 2025, 50, 15. [Google Scholar] [CrossRef]
- Sakr, N. The role of silicon (Si) in increasing plant resistance against fungal diseases. Hell. Plant Prot. J. 2016, 9, 1–15. [Google Scholar] [CrossRef]
- Chérif, M.; Menzies, J.G.; Benhamou, N.; Bélanger, R.R. Studies of silicon distribution in wounded and Pythium ultimum infected cucumber plants. Physiol. Mol. Plant Pathol. 1992, 41, 371–385. [Google Scholar] [CrossRef]
- Fauteux, F.; Chain, F.; Belzile, F.; Menzies, J.G.; Bélanger, R.R. The protective role of silicon in the Arabidopsis–powdery mildew pathosystem. Proc. Natl. Acad. Sci. USA 2006, 103, 17554–17559. [Google Scholar] [CrossRef]
- Rodgers-Gray, B.; Shaw, M. Effects of straw and silicon soil amendments on some foliar and stem-base diseases in pot-grown winter wheat. Plant Pathol. 2004, 53, 733–740. [Google Scholar] [CrossRef]
- Biere, A.; Goverse, A. Plant-mediated systemic interactions between pathogens, parasitic nematodes, and herbivores above-and belowground. Annu. Rev. Phytopathol. 2016, 54, 499–527. [Google Scholar] [CrossRef]
- Rajarammohan, S. Redefining plant-necrotroph interactions: The thin line between hemibiotrophs and necrotrophs. Front. Microbiol. 2021, 12, 673518. [Google Scholar] [CrossRef]
- Lata-Tenesaca, L.F.; Oliveira, M.J.B.; Barros, A.V.; Picanço, B.B.M.; Rodrigues, F.Á. Physiological and Biochemical Aspects of Silicon-Mediated Resistance in Maize against Maydis Leaf Blight. Plants 2024, 13, 531. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Bélanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef]
- Ahamad, L.; Siddiqui, Z.A. Effects of silicon dioxide, zinc oxide and titanium dioxide nanoparticles on Meloidogyne incognita, Alternaria dauci and Rhizoctonia solani disease complex of carrot. Exp. Parasitol. 2021, 230, 108176. [Google Scholar] [CrossRef]
- Ahmadi Mansourabad, M.; Kargar Bideh, A.; Abdollahi, M. Effects of some micronutrients and macronutrients on the root-knot nematode, Meloidogyne incognita, in greenhouse cucumber (Cucumis sativus cv. Negin). J. Crop Prot. 2016, 5, 507–517. [Google Scholar] [CrossRef]
- Santos, L.B.; de Souza Junior, J.P.; de Mello Prado, R.; Ferreira Junior, R.; de Souza, V.F.; dos Santos Sarah, M.M.; Soares, P.L.M. Silicon allows halving Cadusafos dose to control Meloidogyne incognita and increase cotton development. Silicon 2022, 14, 3809–3816. [Google Scholar] [CrossRef]
- El-Ashry, R.M.; El-Saadony, M.T.; El-Sobki, A.E.; El-Tahan, A.M.; Al-Otaibi, S.; El-Shehawi, A.M.; Saad, A.M.; Elshaer, N. Biological silicon nanoparticles maximize the efficiency of nematicides against biotic stress induced by Meloidogyne incognita in eggplant. Saudi J. Biol. Sci. 2022, 29, 920–932. [Google Scholar] [CrossRef]
- Qi, X.; Xue, X.; Su, G.; Han, Y.; Wang, Y.; Li, Y.; Jiang, Y. The physiological and biochemical role of silicon in enhancing the resistance of maize to root-lesion nematode. Plant Pathol. 2024, 73, 2112–2122. [Google Scholar] [CrossRef]
- Al Banna, L.; Salem, N.; Ghrair, A.M.; Habash, S.S. Impact of silicon carbide nanoparticles on hatching and survival of soil nematodes Caenorhabditis elegans and Meloidogyne incognita. Appl. Ecol. Environ. Res. 2018, 16, 2651–2662. [Google Scholar] [CrossRef]
- Silva, R.V.; Oliveira, R.D.; Ferreira, P.D.S.; Castro, D.B.; Rodrigues, F.Á. Effects of silicon on the penetration and reproduction events of Meloidogyne exigua on coffee roots. Bragantia 2015, 74, 196–199. [Google Scholar] [CrossRef]
- Dugui-Es, C.; Pedroche, N.; Villanueva, L.; Galeng, J.; De Waele, D. Management of root knot nematode, Meloidogyne incognita in cucumber (Cucumis sativus) using silicon. Commun. Agric. Appl. Biol. Sci. 2010, 75, 497–505. [Google Scholar]
- Khan, M.R.; Siddiqui, Z.A. Use of silicon dioxide nanoparticles for the management of Meloidogyne incognita, Pectobacterium betavasculorum and Rhizoctonia solani disease complex of beetroot (Beta vulgaris L.). Sci. Hortic. 2020, 265, 109211. [Google Scholar] [CrossRef]
- Udalova, Z.V.; Folmanis, G.E.; Fedotov, M.A.; Pelgunova, L.A.; Krysanov, E.Y.; Khasanov, F.K.; Zinovieva, S.V. Effects of silicon nanoparticles on photosynthetic pigments and biogenic elements in tomato plants infected with root-knot nematode Meloidogyne incognita. Dokl. Biochem. Biophys. Pleiades Publ. 2020, 495, 329–333. [Google Scholar] [CrossRef]
- Silva, R.V.; Oliveira, R.D.L.; Nascimento, K.J.T.; Rodrigues, F.A. Biochemical responses of coffee resistance against Meloidogyne exigua mediated by silicon. Plant Pathol. 2010, 59, 586–593. [Google Scholar] [CrossRef]
- De Bobadilla, M.F.; Vitiello, A.; Erb, M.; Poelman, E.H. Plant defense strategies against attack by multiple herbivores. Trends Plant Sci. 2022, 27, 528–535. [Google Scholar] [CrossRef]
- Van Griethuysen, P.A.; Redeker, K.R.; MacFarlane, S.A.; Neilson, R.; Hartley, S.E. Virus-induced changes in root volatiles attract soil nematode vectors to infected plants. New Phytol. 2024, 241, 2275–2286. [Google Scholar] [CrossRef]
- Willsey, T.; Chatterton, S.; Cárcamo, H. Interactions of root-feeding insects with fungal and oomycete plant pathogens. Front. Plant Sci. 2017, 8, 1764. [Google Scholar] [CrossRef]
- Islam, T.; Moore, B.D.; Johnson, S.N. Plant silicon defences reduce the performance of a chewing insect herbivore which benefits a contemporaneous sap-feeding insect. Ecol. Entomol. 2022, 47, 951–958. [Google Scholar] [CrossRef]
- Lazebnik, J.; Frago, E.; Dicke, M.; Van Loon, J.J. Phytohormone mediation of interactions between herbivores and plant pathogens. J. Chem. Ecol. 2014, 40, 730–741. [Google Scholar] [CrossRef]
- Stam, J.M.; Kroes, A.; Li, Y.; Gols, R.; van Loon, J.J.; Poelman, E.H.; Dicke, M. Plant interactions with multiple insect herbivores: From community to genes. Annu. Rev. Plant Biol. 2014, 65, 689–713. [Google Scholar] [CrossRef]
- Aerts, N.; Pereira Mendes, M.; Van Wees, S.C. Multiple levels of crosstalk in hormone networks regulating plant defense. Plant J. 2021, 105, 489–504. [Google Scholar] [CrossRef]
- Hoysted, G.A.; Lilley, C.J.; Field, K.J.; Dickinson, M.; Hartley, S.E.; Urwin, P.E. A plant-feeding nematode indirectly increases the fitness of an aphid. Front. Plant Sci. 2017, 8, 1897. [Google Scholar] [CrossRef]
- Wondafrash, M.; Van Dam, N.M.; Tytgat, T.O. Plant systemic induced responses mediate interactions between root parasitic nematodes and aboveground herbivorous insects. Front. Plant Sci. 2013, 4, 87. [Google Scholar] [CrossRef]
- Biru, F.N.; Cazzonelli, C.I.; Elbaum, R.; Johnson, S.N. Contrasting impacts of herbivore induction and elevated atmospheric CO2 on silicon defences and consequences for subsequent herbivores. Entomol. Exp. Appl. 2022, 170, 681–688. [Google Scholar] [CrossRef]
- Moreira, X.; Abdala-Roberts, L.; Castagneyrol, B. Interactions between plant defence signalling pathways: Evidence from bioassays with insect herbivores and plant pathogens. J. Ecol. 2018, 106, 2353–2364. [Google Scholar] [CrossRef]
- Manosalva, P.; Manohar, M.; Von Reuss, S.H.; Chen, S.; Koch, A.; Kaplan, F.; Choe, A.; Micikas, R.J.; Wang, X.; Kogel, K.-H. Conserved nematode signalling molecules elicit plant defenses and pathogen resistance. Nat. Commun. 2015, 6, 7795. [Google Scholar] [CrossRef]
- Guarneri, N.; Willig, J.J.; Sterken, M.G.; Zhou, W.; Hasan, M.S.; Sharon, L.; Grundler, F.M.; Willemsen, V.; Goverse, A.; Smant, G. Root architecture plasticity in response to endoparasitic cyst nematodes is mediated by damage signaling. New Phytol. 2023, 237, 807–822. [Google Scholar] [CrossRef]
- Kutyniok, M.; Müller, C. Plant-mediated interactions between shoot-feeding aphids and root-feeding nematodes depend on nitrate fertilization. Oecologia 2013, 173, 1367–1377. [Google Scholar] [CrossRef]
- Ripa, L.; Stevens, G.; Lewis, E. Two-way plant-mediated interactions between a plant parasitic nematode and a foliar herbivore arthropod. Rhizosphere 2023, 26, 100699. [Google Scholar] [CrossRef]
- Kohl, L.M. Foliar nematodes: A summary of biology and control with a compilation of host range. Plant Health Prog. 2011, 12, 23. [Google Scholar] [CrossRef]
- Jang, S.-W.; Kim, Y.; Khan, A.L.; Na, C.-I.; Lee, I.-J. Exogenous short-term silicon application regulates macro-nutrients, endogenous phytohormones, and protein expression in Oryza sativa L. BMC Plant Biol. 2018, 18, 4. [Google Scholar] [CrossRef]
- Hartley, S.E.; DeGabriel, J.L. The ecology of herbivore-induced silicon defences in grasses. Funct. Ecol. 2016, 30, 1311–1322. [Google Scholar] [CrossRef]
- Reynolds, J.J.; Lambin, X.; Massey, F.P.; Reidinger, S.; Sherratt, J.A.; Smith, M.J.; White, A.; Hartley, S.E. Delayed induced silica defences in grasses and their potential for destabilising herbivore population dynamics. Oecologia 2012, 170, 445–456. [Google Scholar] [CrossRef]
- Karban, R. The ecology and evolution of induced resistance against herbivores. Funct. Ecol. 2011, 25, 339–347. [Google Scholar] [CrossRef]
- Gershenzon, J.; Murtagh, G.J.; Croteau, R. Absence of rapid terpene turnover in several diverse species of terpene-accumulating plants. Oecologia 1993, 96, 583–592. [Google Scholar] [CrossRef]
- Stamp, N. Out of the quagmire of plant defense hypotheses. Q. Rev. Biol. 2003, 78, 23–55. [Google Scholar] [CrossRef]
- Thorne, S.J.; Maathuis, F.J.; Hartley, S.E. Induction of silicon defences in wheat landraces is local, not systemic, and driven by mobilization of soluble silicon to damaged leaves. J. Exp. Bot. 2023, 74, 5363–5373. [Google Scholar] [CrossRef]
- Vivancos, J.; Labbé, C.; Menzies, J.G.; Bélanger, R.R. Silicon-mediated resistance of Arabidopsis against powdery mildew involves mechanisms other than the salicylic acid (SA)-dependent defence pathway. Mol. Plant Pathol. 2015, 16, 572–582. [Google Scholar] [CrossRef]
- Massey, F.P.; Roland Ennos, A.; Hartley, S.E. Herbivore specific induction of silica-based plant defences. Oecologia 2007, 152, 677–683. [Google Scholar] [CrossRef]
- Cibils-Stewart, X.; Mace, W.J.; Popay, A.J.; Lattanzi, F.A.; Hartley, S.E.; Hall, C.R.; Powell, J.R.; Johnson, S.N. Interactions between silicon and alkaloid defences in endophyte-infected grasses and the consequences for a folivore. Funct. Ecol. 2022, 36, 249–261. [Google Scholar] [CrossRef]
- Cibils-Stewart, X.; Powell, J.R.; Popay, A.J.; Lattanzi, F.A.; Hartley, S.E.; Johnson, S.N. Reciprocal effects of silicon supply and endophytes on silicon accumulation and Epichloë colonization in grasses. Front. Plant Sci. 2020, 11, 593198. [Google Scholar] [CrossRef]
- Cibils-Stewart, X.; Putra, R.; Islam, T.; Fanna, D.; Wuhrer, R.; Mace, W.; Hartley, S.; Popay, A.; Johnson, S. Silicon and Epichloë-endophyte defences in a model temperate grass diminish feeding efficiency and immunity of an insect folivore. Funct. Ecol. 2023, 37, 3177–3192. [Google Scholar] [CrossRef]
- Guntzer, F.; Keller, C.; Meunier, J.-D. Benefits of plant silicon for crops: A review. Agron. Sustain. Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef]
- Kelland, M.E.; Wade, P.W.; Lewis, A.L.; Taylor, L.L.; Sarkar, B.; Andrews, M.G.; Lomas, M.R.; Cotton, T.A.; Kemp, S.J.; James, R.H. Increased yield and CO2 sequestration potential with the C4 cereal Sorghum bicolor cultivated in basaltic rock dust-amended agricultural soil. Glob. Change Biol. 2020, 26, 3658–3676. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denarié, M.-E.; Nielsen, U.N.; Hartley, S.E.; Johnson, S.N. Silicon-Mediated Interactions Between Plant Antagonists. Plants 2025, 14, 1204. https://doi.org/10.3390/plants14081204
Denarié M-E, Nielsen UN, Hartley SE, Johnson SN. Silicon-Mediated Interactions Between Plant Antagonists. Plants. 2025; 14(8):1204. https://doi.org/10.3390/plants14081204
Chicago/Turabian StyleDenarié, Marie-Emma, Uffe N. Nielsen, Susan E. Hartley, and Scott N. Johnson. 2025. "Silicon-Mediated Interactions Between Plant Antagonists" Plants 14, no. 8: 1204. https://doi.org/10.3390/plants14081204
APA StyleDenarié, M.-E., Nielsen, U. N., Hartley, S. E., & Johnson, S. N. (2025). Silicon-Mediated Interactions Between Plant Antagonists. Plants, 14(8), 1204. https://doi.org/10.3390/plants14081204