Application of Wine and Olive Oil Production Residues as Substrates for the Cultivation of Chrysanthemum morifolium Potted Plants
Abstract
1. Introduction
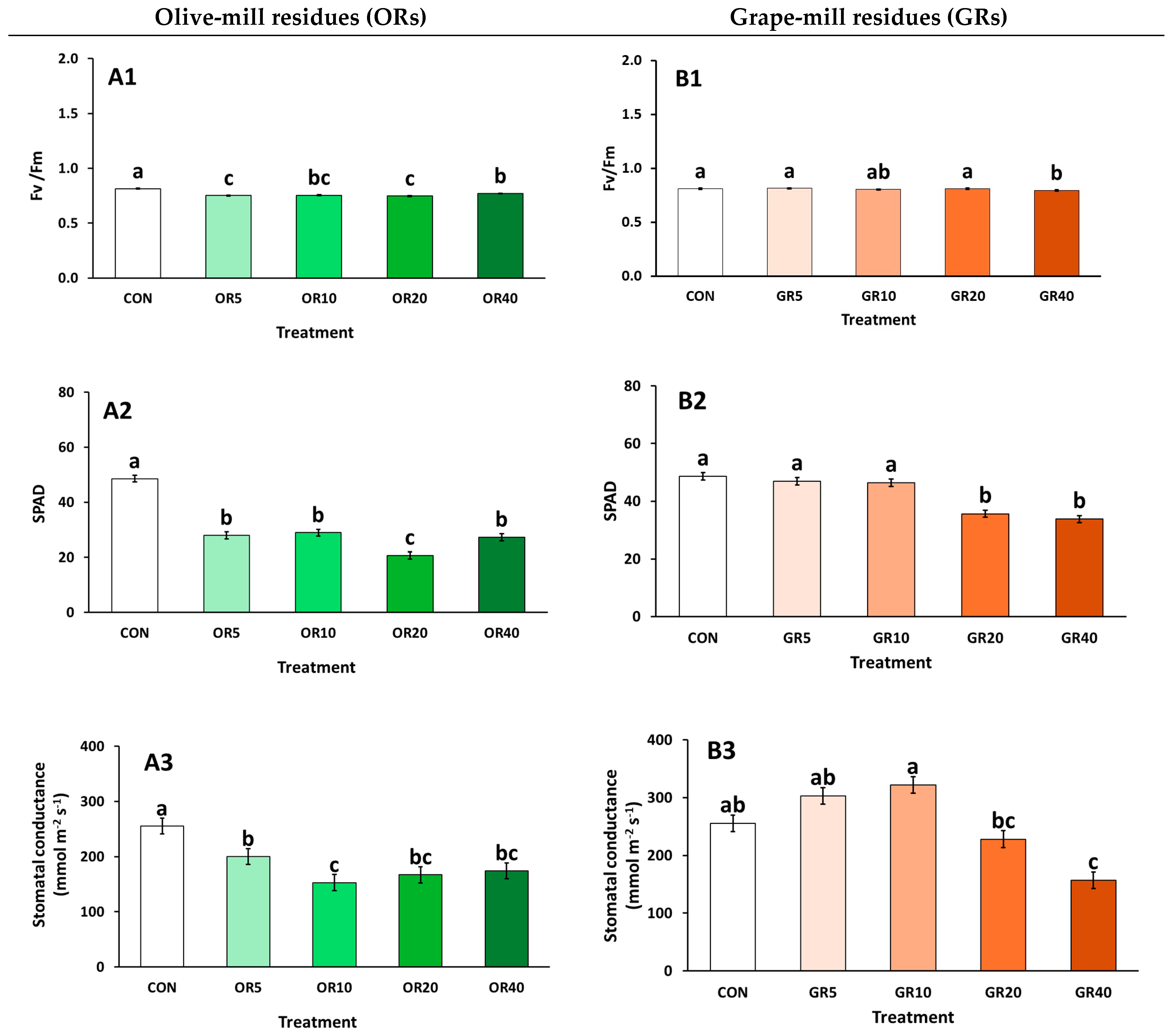
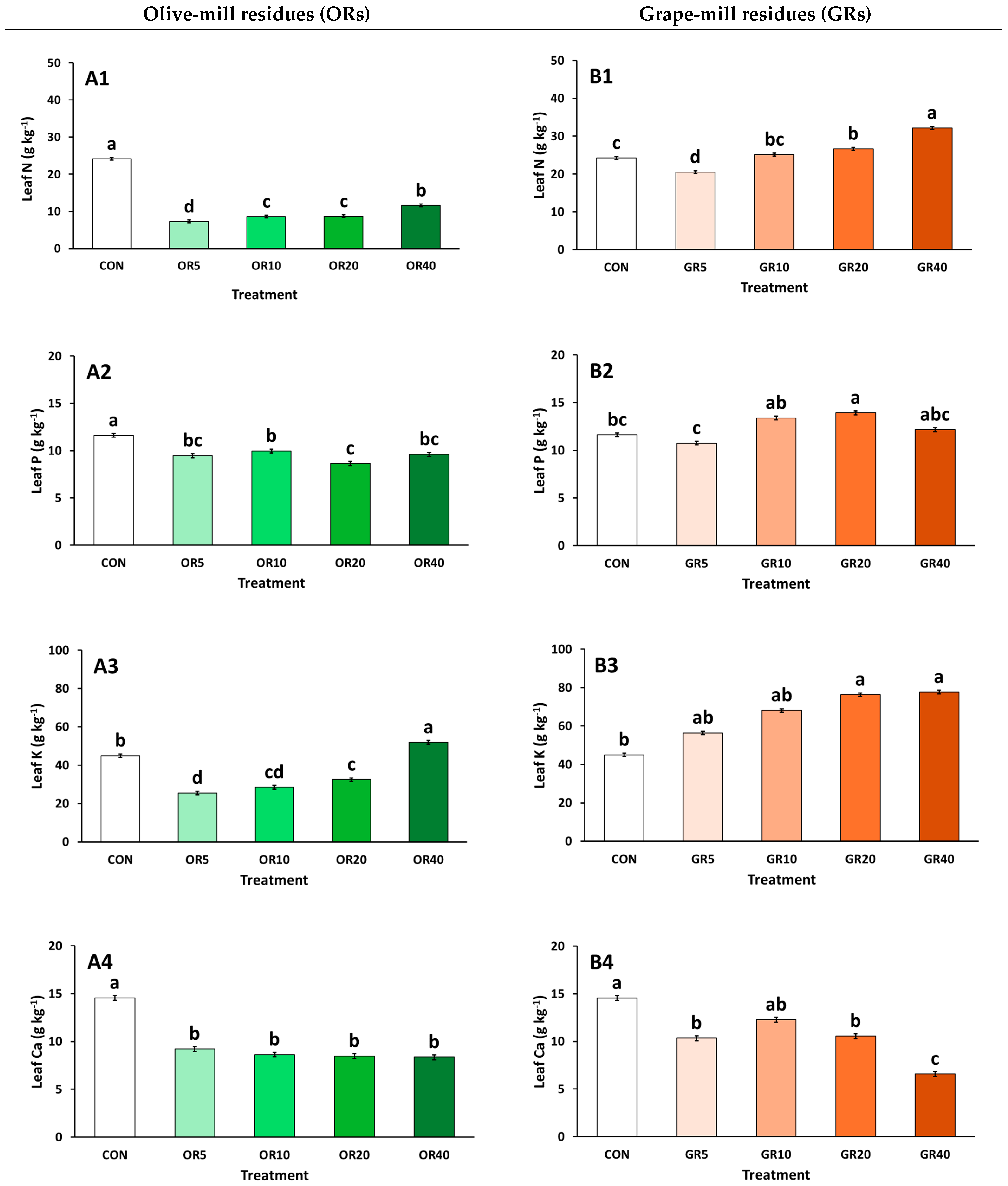
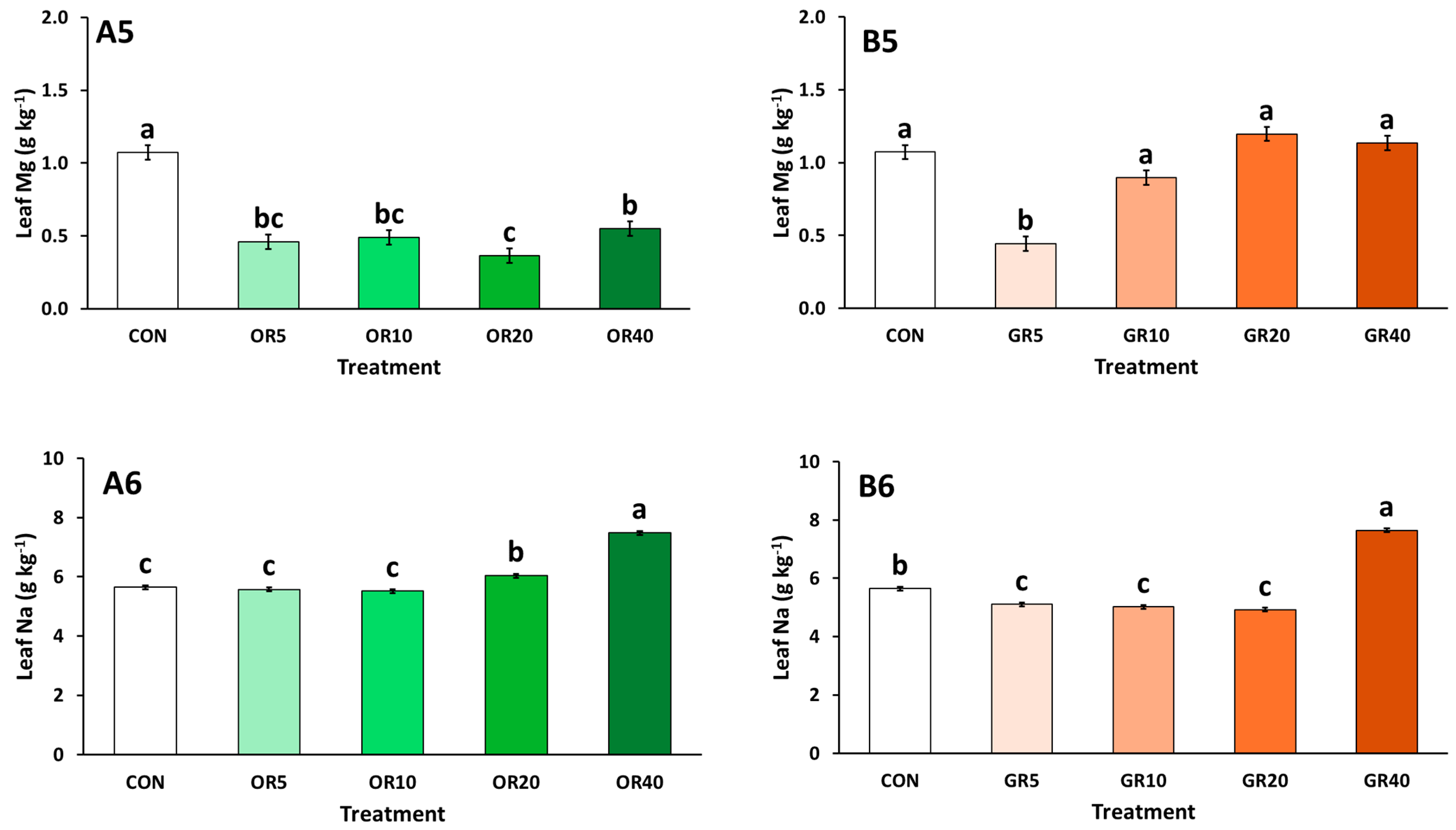
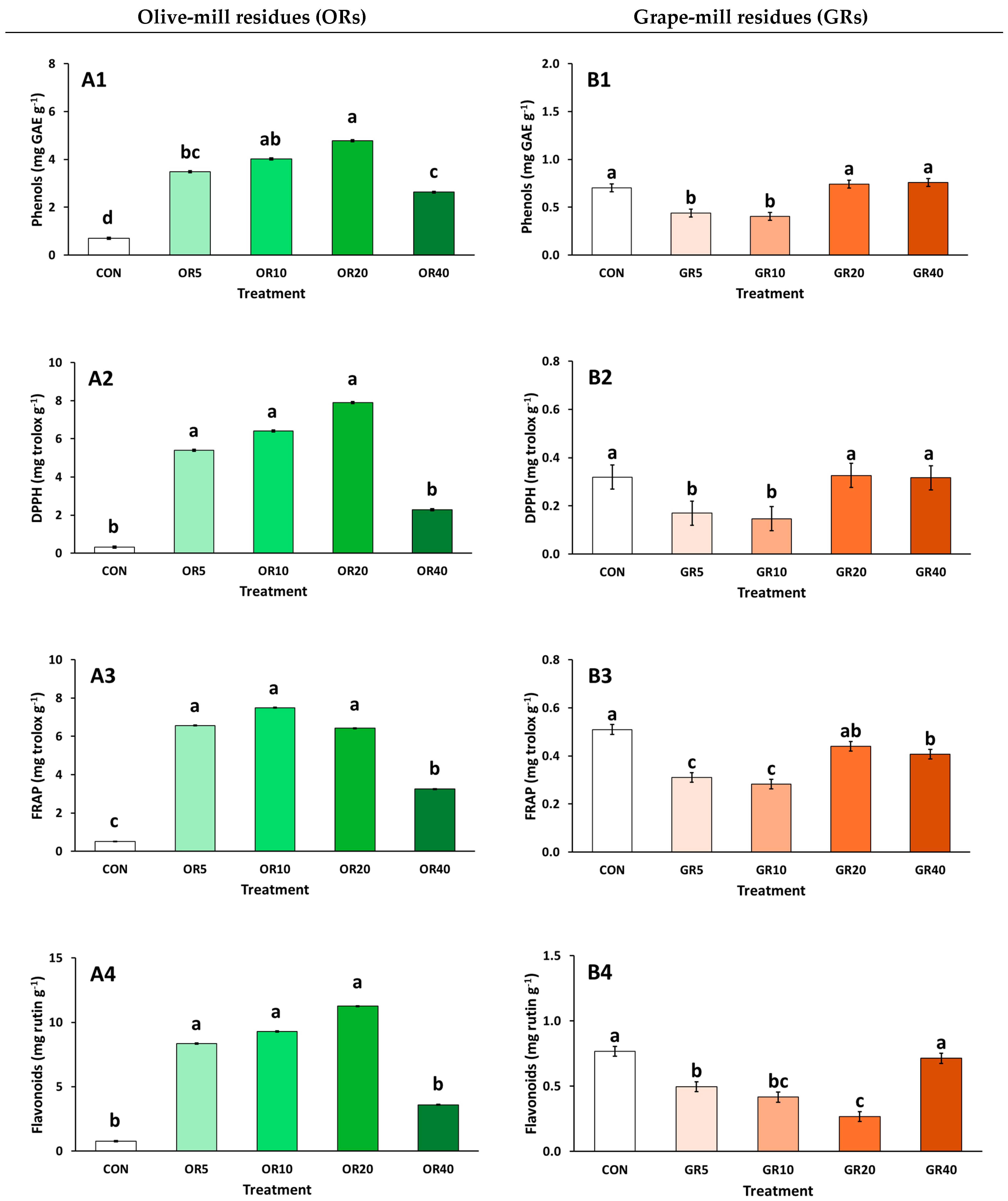
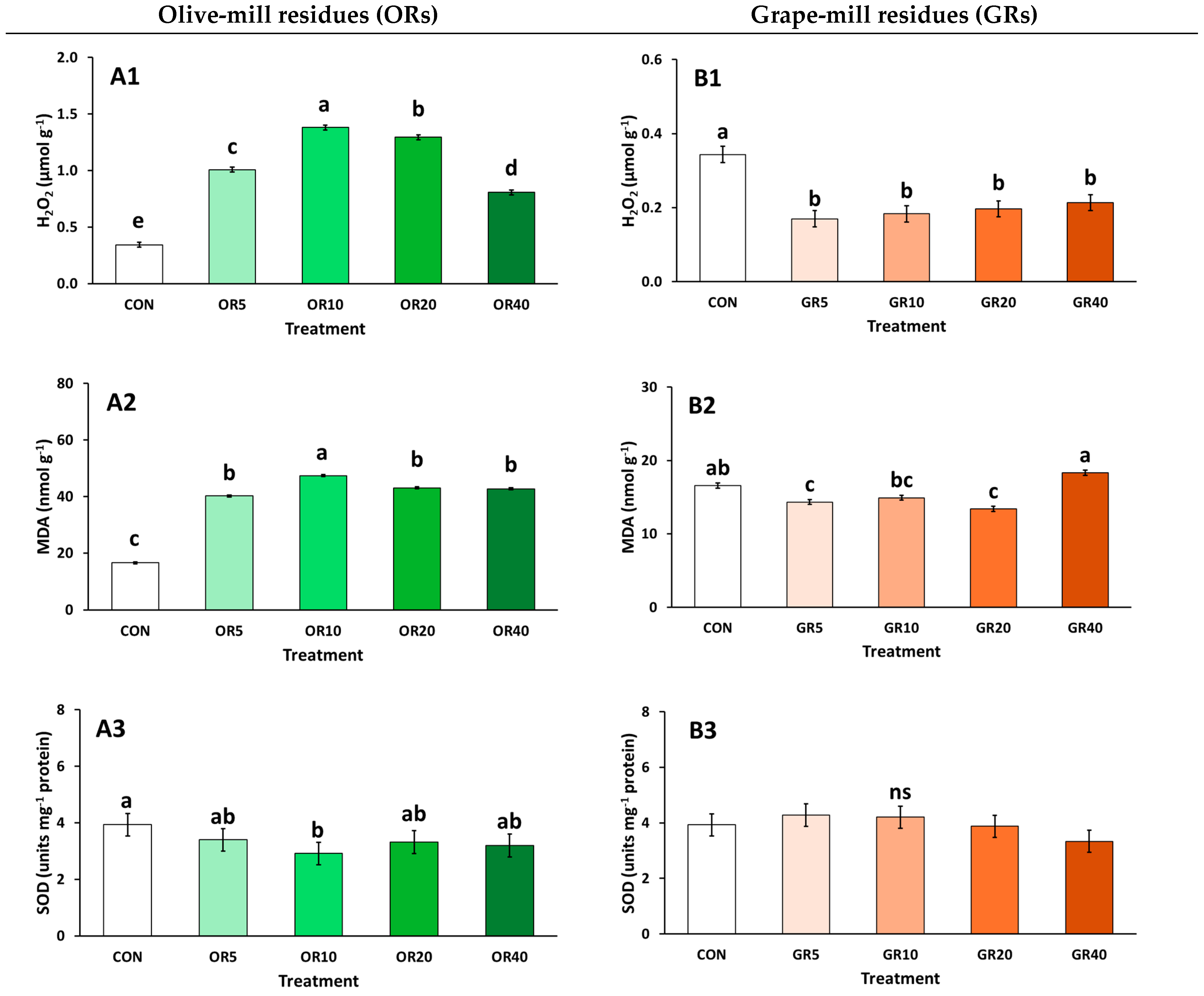
2. Results
3. Discussion
4. Materials and Methods
4.1. Preparation of Plant Material and Substrate Mixtures
4.2. Substrate Mixture Properties
4.3. Plant Growth, Physiology, and Mineral Analysis
4.4. Total Phenolics, Total Flavonoids, and Antioxidant Activity
4.5. Stress Indicators and Antioxidant Enzyme Activity
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gruda, N. Current and future perspective of growing media in Europe. Acta Hortic. 2012, 960, 37–43. [Google Scholar] [CrossRef]
- Leiber-Sauheitl, K.; Bohne, H.; Böttcher, J. First steps toward a test procedure to identify peat substitutes for growing media by means of chemical, physical and biological material characteristics. Horticulturae 2021, 7, 164. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, T.; Guo, J.; Zhong, J.; Li, D.; Shi, H.; Liu, C.; Xiang, R.; Sun, M. Effects of Substitute Substrate, Water, and Fertilizer Management on the Growth of Potted Chrysanthemums. Horticulturae 2024, 10, 138. [Google Scholar] [CrossRef]
- Glaser, B.; Asomah, A.A.A. Plant Growth and Chemical Properties of Commercial Biochar-versus Peat-Based Growing Media. Horticulturae 2022, 8, 339. [Google Scholar] [CrossRef]
- Hirschler, O.; Thrän, D. Peat Substitution in Horticulture: Interviews with German Growing Media Producers on the Transformation of the Resource Base. Horticulturae 2023, 9, 919. [Google Scholar] [CrossRef]
- Fascella, G. Growing substrates alternative to peat for ornamental plants. In Soilless Culture—Use of Substrates for the Production of Quality Horticultural Crops; Asaduzzaman, M., Ed.; InTech: Tokyo, Japan, 2015; ISBN 978-953-51-1739-1. [Google Scholar]
- Atzori, G.; Pane, C.; Zaccardelli, M.; Cacini, S.; Massa, D. The role of peat-free organic substrates in the sustainable management of soilless cultivations. Agronomy 2021, 11, 1236. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Allaire, S.E.; Akram, N.A.; Méndez, A.; Younis, A.; Peerzada, A.M.; Shaukat, N.; Wright, S.R. Challenges in organic component selection and biochar as an opportunity in potting substrates: A review. J. Plant Nutr. 2019, 42, 1386–1401. [Google Scholar] [CrossRef]
- Luo, J.; Fan, R.Q.; Wang, T.; Gao, Y.; Liu, L.Z.; Yan, S.H.; Zhang, Z.H. Evaluation of spent pig litter compost as a peat substitute in soilless growth media. Biol. Agric. Hortic. 2015, 31, 219–229. [Google Scholar] [CrossRef]
- Vandecasteele, B.; Hofkens, M.; De Zaeytijd, J.; Visser, R.; Melis, P. Towards environmentally sustainable growing media for strawberry cultivation: Effect of biochar and fertigation on circular use of nutrients. Agric. Water Manag. 2023, 284, 108361. [Google Scholar] [CrossRef]
- Verrillo, M.; Cozzolino, V.; Spaccini, R.; Piccolo, A. Humic substances from green compost increase bioactivity and antibacterial properties of essential oils in Basil leaves. Chem. Biol. Technol. Agric. 2021, 8, 28. [Google Scholar] [CrossRef]
- Vandecasteele, B.; Debode, J.; Willekens, K.; Van Delm, T. Recycling of P and K in circular horticulture through compost application in sustainable growing media for fertigated strawberry cultivation. Eur. J. Agron. 2018, 96, 131–145. [Google Scholar] [CrossRef]
- Picca, G.; Goñi-Urtiaga, A.; Gomez-Ruano, C.; Plaza, C.; Panettieri, M. Suitability of Co-Composted Biochar with Spent Coffee Grounds Substrate for Tomato (Solanum lycopersicum) Fruiting Stage. Horticulturae 2023, 9, 89. [Google Scholar] [CrossRef]
- Greco, C.; Comparetti, A.; Fascella, G.; Febo, P.; la Placa, G.; Saiano, F.; Mammano, M.M.; Orlando, S.; Laudicina, V.A. Effects of vermicompost, compost and digestate as commercial alternative peat-based substrates on qualitative parameters of Salvia officinalis. Agronomy 2021, 11, 98. [Google Scholar] [CrossRef]
- Peralbo-Molina, Á.; Luque deCastro, M.D. Potential of residues from the Mediterranean agriculture and agrifood industry. Trends Food Sci. Technol. 2013, 32, 16–24. [Google Scholar] [CrossRef]
- Al Afif, R.; Pfeifer, C. Biochemical methane potential of three-phase olive mill solid waste: Influence of temperature and supplemental enzymes. Carbon Resour. Convers. 2022, 5, 248–254. [Google Scholar] [CrossRef]
- Bolechowski, A.; Moral, R.; Bustamante, M.A.; Bartual, J.; Paredes, C.; Pérez-Murcia, M.D.; Carbonell-Barrachina, A.A. Winery-distillery composts as partial substitutes of traditional growing media: Effect on the volatile composition of thyme essential oils. Sci. Hortic. 2015, 193, 69–76. [Google Scholar] [CrossRef]
- Banias, G.; Achillas, C.; Vlachokostas, C.; Moussiopoulos, N.; Stefanou, M. Environmental impacts in the life cycle of olive oil: A literature review. J. Sci. Food Agric. 2017, 97, 1686–1697. [Google Scholar] [CrossRef]
- Roig, A.; Cayuela, M.L.; Sánchez-Monedero, M.A. An overview on olive mill wastes and their valorisation methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Muktadirul Bari Chowdhury, A.K.M.; Akratos, C.S.; Vayenas, D.V.; Pavlou, S. Olive mill waste composting: A review. Int. Biodeterior. Biodegrad. 2013, 85, 108–119. [Google Scholar] [CrossRef]
- Carmona, E.; Moreno, M.T.; Avilés, M.; Ordovas, J. Composting of wine industry wastes and their use as a substrate for growing soilless ornamental plants. Spanish J. Agric. Res. 2012, 10, 482–491. [Google Scholar] [CrossRef]
- del Pozo, C.; Rego, F.; Puy, N.; Bartrolí, J.; Fàbregas, E.; Yang, Y.; Bridgwater, A.V. The effect of reactor scale on biochars and pyrolysis liquids from slow pyrolysis of coffee silverskin, grape pomace and olive mill waste, in auger reactors. Waste Manag. 2022, 148, 106–116. [Google Scholar] [CrossRef]
- Bassan, A.; Bona, S.; Nicoletto, C.; Sambo, P.; Zanin, G. Rice hulls and anaerobic digestion residues as substrate components for potted production of geranium and rose. Agronomy 2020, 10, 950. [Google Scholar] [CrossRef]
- Ceglie, F.G.; Bustamante, M.A.; Ben Amara, M.; Tittarelli, F. The challenge of peat substitution in organic seedling production: Optimization of growing media formulation through mixture design and response surface analysis. PLoS ONE 2015, 10, e0128600. [Google Scholar] [CrossRef]
- Nocentini, M.; Panettieri, M.; García de Castro Barragán, J.M.; Mastrolonardo, G.; Knicker, H. Recycling pyrolyzed organic waste from plant nurseries, rice production and shrimp industry as peat substitute in potting substrates. J. Environ. Manag. 2021, 277, 111436. [Google Scholar] [CrossRef]
- Álvarez, M.L.; Gascó, G.; Plaza, C.; Paz-Ferreiro, J.; Méndez, A. Hydrochars from Biosolids and Urban Wastes as Substitute Materials for Peat. L. Degrad. Dev. 2017, 28, 2268–2276. [Google Scholar] [CrossRef]
- Bonaguro, J.E.; Coletto, L.; Zanin, G. Environmental and agronomic performance of fresh rice hulls used as growing medium component for Cyclamen persicum L. pot plants. J. Clean. Prod. 2017, 142, 2125–2132. [Google Scholar] [CrossRef]
- Prasad, R.; Lisiecka, J.; Antala, M.; Rastogi, A. Influence of different spent mushroom substrates on yield, morphological and photosynthetic parameters of strawberry (Fragaria × ananassa duch.). Agronomy 2021, 11, 2086. [Google Scholar] [CrossRef]
- Ronga, D.; Pane, C.; Zaccardelli, M.; Pecchioni, N. Use of Spent Coffee Ground Compost in Peat-Based Growing Media for the Production of Basil and Tomato Potting Plants. Commun. Soil Sci. Plant Anal. 2016, 47, 356–368. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Antoniou, O.; Xylia, P.; Petropoulos, S.; Tzortzakis, N. The use of spent coffee grounds in growing media for the production of Brassica seedlings in nurseries. Environ. Sci. Pollut. Res. 2021, 28, 24279–24290. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Hajisolomou, E.; Xylia, P.; Tzortzakis, N. Olive-mill and grape-mill waste as a substitute growing media component for unexploded vegetables production. Sustain. Chem. Pharm. 2023, 31, 100940. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Antoniou, O.; Tzionis, A.; Prasad, M.; Tzortzakis, N. Alternative soilless media using olive-mill and paper waste for growing ornamental plants. Environ. Sci. Pollut. Res. 2018, 25, 35915–35927. [Google Scholar] [CrossRef]
- Del Buono, D.; Said-Pullicino, D.; Proietti, P.; Nasini, L.; Gigliotti, G. Utilization of Olive Husks as Plant Growing Substrates: Phytotoxicity and Plant Biochemical Responses. Compost Sci. Util. 2011, 19, 52–60. [Google Scholar] [CrossRef]
- Warnita, A.N. Vina Growth response of two varieties chrysanthemum (Chrysanthemum sp.) on some media composition. Int. J. Adv. Sci. Eng. Inf. Technol. 2017, 7, 928–935. [Google Scholar] [CrossRef][Green Version]
- Bharti, A.; Prasanna, R.; Kumar, G.; Kumar, A.; Nain, L. Co-cultivation of cyanobacteria for raising nursery of chrysanthemum using a hydroponic system. J. Appl. Phycol. 2019, 31, 3625–3635. [Google Scholar] [CrossRef]
- Liu, S.; Liu, M.; Chen, S.; Ni, X.; Zhang, K.; Yue, L.; Zhou, Y. Rice Husks and Leaf Mold Used as Peat Substitutes to Improve the Morphological, Photosynthetic, and Biochemical Properties of Chrysanthemum (Chrysanthemum × morifolium). Sustain 2023, 15, 16137. [Google Scholar] [CrossRef]
- Vandecasteele, B.; Blindeman, L.; Amery, F.; Pieters, C.; Ommeslag, S.; Van Loo, K.; De Tender, C.; Debode, J. Grow—Store—Steam—Re-peat: Reuse of spent growing media for circular cultivation of Chrysanthemum. J. Clean. Prod. 2020, 276, 124128. [Google Scholar] [CrossRef]
- Ribeiro, H.M.; Romero, A.M.; Pereira, H.; Borges, P.; Cabral, F.; Vasconcelos, E. Evaluation of a compost obtained from forestry wastes and solid phase of pig slurry as a substrate for seedlings production. Bioresour. Technol. 2007, 98, 3294–3297. [Google Scholar] [CrossRef]
- Rinaldi, S.; De Lucia, B.; Salvati, L.; Rea, E. Understanding complexity in the response of ornamental rosemary to different substrates: A multivariate analysis. Sci. Hortic. 2014, 176, 218–224. [Google Scholar] [CrossRef]
- Ouzounidou, G.; Asfi, M.; Sotirakis, N.; Papadopoulou, P.; Gaitis, F. Olive mill wastewater triggered changes in physiology and nutritional quality of tomato (Lycopersicon esculentum Mill.) depending on growth substrate. J. Hazard. Mater. 2008, 158, 523–530. [Google Scholar] [CrossRef]
- Abad, M.; Noguera, P.; Burés, S. National inventory of organic wastes for use as growing media for ornamental potted plant production: Case study in Spain. Bioresour. Technol. 2001, 77, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Beckmann-Cavalcante, M.Z.; Pivetta, K.F.L.; Cavalcante, Í.H.L.; Cavalcante, L.F.; Bellingieri, P.A.; Campos, M.C.C. Condutividade elétrica da solução nutritiva para o cultivo do crisântemo em vaso. Rev. Bras. Ciência Solo 2010, 34, 747–756. [Google Scholar] [CrossRef]
- Noguera, P.; Abad, M.; Puchades, R.; Maquieira, A.; Noguera, V. Influence of particle size on physical and chemical properties of coconut coir dust as container medium. Commun. Soil Sci. Plant Anal. 2003, 34, 593–605. [Google Scholar] [CrossRef]
- Olejar, K.J.; Vandermeer, C.; Fedrizzi, B.; Kilmartin, P.A. A Horticultural Medium Established from the Rapid Removal of Phytotoxins from Winery Grape Marc. Horticulturae 2019, 5, 69. [Google Scholar] [CrossRef]
- Carlile, W.R.; Raviv, M.; Prasad, M. Organic Soilless Media Components. In Soilless Culture; Elsevier: Amsterdam, The Netherlands, 2019; pp. 303–378. [Google Scholar]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry—A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Medina, E.; Paredes, C.; Pérez-Murcia, M.D.; Bustamante, M.A.; Moral, R. Spent mushroom substrates as component of growing media for germination and growth of horticultural plants. Bioresour. Technol. 2009, 100, 4227–4232. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Xylia, P.; Tzortzakis, N. Oliviculture and Viticulture Crop Byproducts Use for Peat Partial Substitution for Carnation Production. Agronomy 2024, 14, 605. [Google Scholar] [CrossRef]
- Kelepesi, S.; Tzortzakis, N.G. Olive mill wastesA growing medium component for seedling and crop production of lettuce and chicory. Int. J. Veg. Sci. 2009, 15, 325–339. [Google Scholar] [CrossRef]
- Sofiadou, E.; Tzortzakis, N.G. Olive Mill Waste as a Substitute Growing Medium Component in Tomato Seedling and Crop Production. Int. J. Veg. Sci. 2012, 18, 272–283. [Google Scholar] [CrossRef]
- Xie, K.; Cakmak, I.; Wang, S.; Zhang, F.; Guo, S. Synergistic and antagonistic interactions between potassium and magnesium in higher plants. Crop J. 2021, 9, 249–256. [Google Scholar] [CrossRef]
- Papafotiou, M.; Kargas, G.; Lytra, I. Olive-mill waste compost as a growth medium component for foliage potted plants. HortScience 2005, 40, 1746–1750. [Google Scholar] [CrossRef]
- Bresson, J.; Vasseur, F.; Dauzat, M.; Koch, G.; Granier, C.; Vile, D. Quantifying spatial heterogeneity of chlorophyll fluorescence during plant growth and in response to water stress. Plant Methods 2015, 11, 23. [Google Scholar] [CrossRef]
- Fernández-Hernández, A.; Roig, A.; Serramiá, N.; Civantos, C.G.O.; Sánchez-Monedero, M.A. Application of compost of two-phase olive mill waste on olive grove: Effects on soil, olive fruit and olive oil quality. Waste Manag. 2014, 34, 1139–1147. [Google Scholar] [CrossRef]
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Novotný, M.; Šipka, M.; Miino, M.C.; Raček, J.; Chorazy, T.; Petreje, M.; Tošić, I.; Hlavínek, P.; Marković, M. Influence of different alternative organic substrates as fillings for green roofs on the quality of rainfall runoff. Sustain. Chem. Pharm. 2024, 38, 101465. [Google Scholar] [CrossRef]
- Kitajima, K.; Hogan, K.P. Increases of chlorophyll a/b ratios during acclimation of tropical woody seedlings to nitrogen limitation and high light. Plant Cell Environ. 2003, 26, 857–865. [Google Scholar] [CrossRef]
- Qu, S.; Li, H.; Zhang, X.; Gao, J.; Ma, R.; Ma, L.; Ma, J. Effects of Magnesium Imbalance on Root Growth and Nutrient Absorption in Different Genotypes of Vegetable Crops. Plants 2023, 12, 3518. [Google Scholar] [CrossRef]
- De Lucia, B.; Vecchietti, L.; Rinaldi, S.; Rivera, C.M.; Trinchera, A.; Rea, E. Effect of Peat-Reduced and Peat-Free Substrates on Rosemary Growth. J. Plant Nutr. 2013, 36, 863–876. [Google Scholar] [CrossRef]
- Regni, L.; Pezzolla, D.; Gigliotti, G.; Proietti, P. The sustainable reuse of compost from a new type of olive mill pomace in replacing peat for potted olive tree. Agron. Res. 2020, 18, 1444–1454. [Google Scholar]
- Araújo, M.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Phenolic compounds from olive mill wastes: Health effects, analytical approach and application as food antioxidants. Trends Food Sci. Technol. 2015, 45, 200–211. [Google Scholar] [CrossRef]
- Kotsou, M.; Mari, I.; Lasaridi, K.; Chatzipavlidis, I.; Balis, C.; Kyriacou, A. The effect of olive oil mill wastewater (OMW) on soil microbial communities and suppressiveness against Rhizoctonia solani. Appl. Soil Ecol. 2004, 26, 113–121. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Paredes, C.; Moral, R.; Agulló, E.; Pérez-Murcia, M.D.; Abad, M. Composts from distillery wastes as peat substitutes for transplant production. Resour. Conserv. Recycl. 2008, 52, 792–799. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.M.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Antoniou, O.; Athinodorou, F.; Vassiliou, R.; Papadaki, A.; Tzortzakis, N. Deployment of olive-stone waste as a substitute growing medium component for Brassica seedling production in nurseries. Environ. Sci. Pollut. Res. 2019, 26, 35461–35472. [Google Scholar] [CrossRef]
- Patsalou, M.; Chrysargyris, A.; Tzortzakis, N.; Koutinas, M. A biorefinery for conversion of citrus peel waste into essential oils, pectin, fertilizer and succinic acid via different fermentation strategies. Waste Manag. 2020, 113, 469–477. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Papakyriakou, E.; Petropoulos, S.A.S.A.; Tzortzakis, N. The combined and single effect of salinity and copper stress on growth and quality of Mentha spicata plants. J. Hazard. Mater. 2019, 368, 584–593. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- De Azevedo Neto, A.D.; Prisco, J.T.; Enéas-Filho, J.; De Abreu, C.E.B.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]

| CON | OR 5% | OR 10% | OR 20% | OR 40% | |
| pH | 6.44 ± 0.05 b | 6.69 ± 0.01 a | 6.47 ± 0.01 b | 6.51 ± 0.01 b | 6.74 ± 0.03 a |
| EC (μS cm−1) | 210.00 ± 16.26 d | 226.10 ± 6.93 d | 290.78 ± 15.54 c | 350.40 ± 24.65 b | 613.27 ± 2.98 a |
| Organic matter (%) | 94.29 ± 0.17 a | 93.63 ± 0.06 b | 94.87 ± 0.33 a | 94.57 ± 0.12 a | 94.66 ± 0.21 a |
| Organic C (%) | 54.70 ± 0.10 a | 54.31 ± 0.03 b | 55.03 ± 0.19 a | 54.85 ± 0.07 a | 54.91 ± 0.12 a |
| C/N ratio | 81.14 ± 1.27 a | 73.53 ± 2.71 b | 71.38 ± 0.24 b | 71.92 ± 1.63 b | 72.19 ± 2.55 b |
| N% | 0.68 ± 0.01 b | 0.74 ± 0.03 ab | 0.77 ± 0.00 a | 0.76 ± 0.02 a | 0.76 ± 0.03 a |
| N (g kg−1) | 6.75 ± 0.10 b | 7.41 ± 0.28 a | 7.71 ± 0.00 a | 7.64 ± 0.18 a | 7.63 ± 0.28 a |
| P (g kg−1) | 0.93 ± 0.01 a | 0.84 ± 0.01 b | 0.82 ± 0.03 b | 0.78 ± 0.01 b | 0.63 ± 0.03 c |
| K (g kg−1) | 1.51 ± 0.09 d | 2.41 ± 0.07 c | 2.74 ± 0.02 c | 3.43 ± 0.04 b | 4.01 ± 0.28 a |
| Ca (g kg−1) | 20.95 ± 0.37 a | 17.97 ± 0.42 b | 15.67 ± 0.15 c | 13.73 ± 0.29 d | 9.26 ± 0.27 e |
| Mg (g kg−1) | 2.02 ± 0.03 a | 1.71 ± 0.05 b | 1.56 ± 0.02 c | 1.40 ± 0.04 d | 0.96 ± 0.05 e |
| Na (g kg−1) | 0.34 ± 0.00 a | 0.32 ± 0.01 ab | 0.30 ± 0.01 b | 0.31 ± 0.00 b | 0.27 ± 0.01 c |
| Total porosity (% v/v) | 82.73 ± 0.96 a | 74.71 ± 0.19 b | 81.74 ± 3.06 ab | 75.84 ± 2.99 ab | 74.92 ± 2.69 b |
| Air-filled porosity (% v/v) | 12.88 ± 0.83 a | 8.93 ± 1.03 b | 11.43 ± 1.03 a | 6.61 ± 0.10 bc | 5.36 ± 0.21 c |
| Bulk density (g cm−3) | 0.18 ± 0.01 d | 0.18 ± 0.00 d | 0.22 ± 0.01 c | 0.24 ± 0.00 b | 0.35 ± 0.01 a |
| Container capacity (% v/v) | 69.85 ± 0.17 a | 65.78 ± 1.22 a | 70.32 ± 2.03 a | 69.24 ± 3.09 a | 69.55 ± 2.90 a |
| CON | GR 5% | GR 10% | GR 20% | GR 40% | |
| pH | 6.44 ± 0.05 e | 6.60 ± 0.06 d | 6.90 ± 0.05 c | 7.10 ± 0.05 b | 7.41 ± 0.01 a |
| EC (μS cm−1) | 210.00 ± 16.26 b | 230.90 ± 22.11 b | 265.59 ± 16.72 b | 351.65 ± 43.39 a | 389.05 ± 1.90 a |
| Organic matter (%) | 94.29 ± 0.17 a | 93.16 ± 0.24 b | 93.06 ± 0.16 b | 93.18 ± 0.25 b | 93.06 ± 0.31 b |
| Organic C (%) | 54.70 ± 0.10 a | 54.03 ± 0.14 b | 53.98 ± 0.09 b | 54.05 ± 0.15 b | 53.98 ± 0.18 b |
| C/N ratio | 81.14 ± 1.27 a | 60.18 ± 0.91 b | 51.78 ± 1.07 c | 36.52 ± 0.75 d | 29.54 ± 1.08 e |
| N% | 0.68 ± 0.01 e | 0.90 ± 0.01 d | 1.04 ± 0.02 c | 1.48 ± 0.03 b | 1.83 ± 0.07 a |
| N (g kg−1) | 6.75 ± 0.10 e | 8.98 ± 0.11 d | 10.43 ± 0.22 c | 14.81 ± 0.27 b | 18.33 ± 0.72 a |
| P (g kg−1) | 0.93 ± 0.01 d | 1.36 ± 0.05 c | 1.26 ± 0.01 c | 1.57 ± 0.09 b | 2.10 ± 0.06 a |
| K (g kg−1) | 1.51 ± 0.09 e | 3.56 ± 0.03 d | 4.57 ± 0.05 c | 5.79 ± 0.06 b | 6.76 ± 0.15 a |
| Ca (g kg−1) | 20.95 ± 0.37 a | 21.98 ± 0.65 a | 18.16 ± 0.59 b | 16.76 ± 0.61 b | 13.59 ± 0.26 c |
| Mg (g kg−1) | 2.02 ± 0.03 b | 2.22 ± 0.09 a | 1.83 ± 0.06 c | 1.78 ± 0.04 c | 1.54 ± 0.03 d |
| Na (g kg−1) | 0.34 ± 0.00 a | 0.34 ± 0.00 a | 0.31 ± 0.00 b | 0.27 ± 0.01 c | 0.21 ± 0.01 d |
| Total porosity (% v/v) | 82.73 ± 0.96 b | 79.60 ± 1.72 bc | 77.83 ± 1.56 bc | 75.88 ± 0.89 c | 87.96 ± 2.05 a |
| Air-filled porosity (% v/v) | 12.88 ± 0.83 b | 9.30 ± 0.82 c | 8.75 ± 0.10 c | 14.11 ± 0.31 ab | 15.03 ± 0.21 a |
| Bulk density (g cm−3) | 0.18 ± 0.01 c | 0.19 ± 0.00 c | 0.19 ± 0.00 c | 0.21 ± 0.01 b | 0.24 ± 0.01 a |
| Container capacity (% v/v) | 69.85 ± 0.17 a | 70.29 ± 0.89 a | 69.08 ± 1.67 a | 61.77 ± 0.59 b | 72.93 ± 2.25 a |
| CON | OR 5% | OR 10% | OR 20% | OR 40% | |
| pH | 6.49 ± 0.06 e | 6.74 ± 0.02 d | 6.97 ± 0.01 c | 7.28 ± 0.02 b | 7.43 ± 0.03 a |
| EC (μS cm−1) | 541.40 ± 22.82 b | 670.95 ± 48.93 a | 225.70 ± 33.37 d | 358.85 ± 2.68 c | 608.06 ± 9.95 ab |
| Organic matter (%) | 92.63 ± 0.30 a | 91.39 ± 0.30 ab | 94.13 ± 0.11 a | 88.03 ± 2.64 b | 95.21 ± 0.21 a |
| Organic C (%) | 53.73 ± 0.18 a | 53.01 ± 0.17 ab | 54.60 ± 0.06 a | 51.06 ± 1.53 b | 55.22 ± 0.12 a |
| C/N ratio | 88.29 ± 1.74 a | 76.71 ± 2.01 b | 75.72 ± 1.94 b | 66.28 ± 2.16 c | 62.29 ± 0.82 c |
| N% | 0.61 ± 0.01 d | 0.69 ± 0.02 c | 0.72 ± 0.02 bc | 0.77 ± 0.00 b | 0.89 ± 0.01 a |
| N (g kg−1) | 6.09 ± 0.14 d | 6.92 ± 0.20 c | 7.22 ± 0.19 c | 7.70 ± 0.02 b | 8.87 ± 0.13 a |
| P (g kg−1) | 0.58 ± 0.00 b | 1.43 ± 0.09 a | 0.51 ± 0.05 b | 0.69 ± 0.13 b | 0.62 ± 0.01 b |
| K (g kg−1) | 1.01 ± 0.01 e | 2.92 ± 0.04 b | 1.38 ± 0.10 d | 1.91 ± 0.01 c | 4.38 ± 0.07 a |
| Mg (g kg−1) | 2.38 ± 0.05 a | 2.34 ± 0.04 a | 1.96 ± 0.01 b | 1.35 ± 0.20 c | 1.20 ± 0.03 c |
| Ca (g kg−1) | 20.31 ± 0.51 a | 20.63 ± 0.59 a | 16.24 ± 0.13 b | 11.58 ± 1.84 c | 9.90 ± 0.21 c |
| Na (g kg−1) | 4.19 ± 0.06 a | 3.90 ± 0.05 b | 2.27 ± 0.09 c | 1.70 ± 0.11 d | 1.28 ± 0.03 e |
| CON | GR 5% | GR 10% | GR 20% | GR 40% | |
| pH | 6.49 ± 0.06 d | 6.77 ± 0.01 c | 7.32 ± 0.01 b | 7.40 ± 0.01 b | 7.53 ± 0.04 a |
| EC (μS cm−1) | 541.40 ± 22.82 ab | 633.70 ± 13.97 a | 314.05 ± 47.37 c | 461.75 ± 27.74 b | 488.06 ± 24.23 b |
| Organic matter (%) | 92.63 ± 0.30 a | 91.96 ± 0.21 b | 92.92 ± 0.05 a | 92.63 ± 0.16 a | 92.77 ± 0.17 a |
| Organic C (%) | 53.73 ± 0.18 a | 53.34 ± 0.12 b | 53.90 ± 0.03 a | 53.73 ± 0.09 a | 53.81 ± 0.10 a |
| C/N ratio | 88.29 ± 1.74 a | 72.09 ± 0.41 b | 55.21 ± 2.25 c | 42.59 ± 0.36 d | 32.97 ± 0.14 e |
| N% | 0.61 ± 0.01 e | 0.74 ± 0.01 d | 0.98 ± 0.04 c | 1.26 ± 0.01 b | 1.63 ± 0.01 a |
| N (g kg−1) | 6.09 ± 0.14 e | 7.40 ± 0.06 d | 9.80 ± 0.40 c | 12.62 ± 0.09 b | 16.32 ± 0.07 a |
| P (g kg−1) | 0.58 ± 0.00 d | 0.99 ± 0.12 bc | 0.82 ± 0.04 c | 1.20 ± 0.01 ab | 1.38 ± 0.09 a |
| K (g kg−1) | 1.01 ± 0.01 e | 2.08 ± 0.08 d | 2.44 ± 0.09 c | 3.91 ± 0.08 b | 6.13 ± 0.15 a |
| Mg (g kg−1) | 2.38 ± 0.05 ab | 2.51 ± 0.05 ab | 2.57 ± 0.19 a | 2.45 ± 0.16 ab | 2.16 ± 0.04 b |
| Ca (g kg−1) | 20.31 ± 0.51 a | 20.38 ± 0.11 a | 18.89 ± 0.26 a | 19.93 ± 1.00 a | 15.13 ± 0.62 b |
| Na (g kg−1) | 4.19 ± 0.06 a | 4.35 ± 0.12 a | 3.79 ± 0.04 b | 2.94 ± 0.05 c | 2.45 ± 0.02 d |
| CON | OR 5% | OR 10% | OR 20% | OR 40% | |
| Plant height (cm) | 30.27 ± 2.26 a | 23.17 ± 1.59 b | 19.42 ± 0.67 bc | 18.27 ± 0.56 c | 13.20 ± 0.76 d |
| Shoot diameter (mm) | 4.34 ± 0.18 a | 3.29 ± 0.09 b | 3.37 ± 0.16 b | 3.07 ± 0.13 b | 2.44 ± 0.17 c |
| Leaf number | 23.50 ± 3.58 a | 14.50 ± 0.56 b | 13.00 ± 0.86 b | 11.33 ± 0.56 b | 10.33 ± 0.80 b |
| Lateral shoot number | n.m. | n.m. | n.m. | n.m. | n.m. |
| Plant FW (g) | 25.18 ± 4.44 a | 6.70 ± 0.65 b | 4.83 ± 0.49 b | 3.98 ± 0.50 b | 3.48 ± 0.35 b |
| Leaf FW (g) | 8.77 ± 2.63 a | 3.94 ± 0.30 b | 2.77 ± 0.42 b | 3.16 ± 0.39 b | 2.66 ± 0.23 b |
| Shoot FW (g) | 9.17 ± 2.22 a | 2.39 ± 0.29 b | 1.63 ± 0.16 b | 1.45 ± 0.24 b | 1.02 ± 0.15 b |
| Flower FW (g) | 10.87 ± 2.76 a | 0.92 ± 0.14 b | 0.62 ± 0.18 b | 0.49 ± 0.19 b | 0.35 ± 0.09 b |
| Plant DW (g) | 4.70 ± 1.50 a | 1.88 ± 0.20 b | 1.28 ± 0.19 b | 1.32 ± 0.19 b | 0.83 ± 0.08 b |
| Leaf DW (g) | 0.99 ± 0.34 a | 0.90 ± 0.08 a | 0.62 ± 0.11 a | 0.72 ± 0.07 a | 0.45 ± 0.02 a |
| Shoot DW (g) | 2.32 ± 0.76 a | 0.83 ± 0.10 b | 0.57 ± 0.07 b | 0.52 ± 0.09 b | 0.33 ± 0.04 b |
| Flower DW (g) | 1.39 ± 0.40 a | 0.16 ± 0.02 b | 0.09 ± 0.02 b | 0.08 ± 0.03 b | 0.06 ± 0.01 b |
| Plant DM (%) | 15.52 ± 0.94 d | 25.45 ± 0.70 b | 27.40 ± 0.99 ab | 29.38 ± 1.62 a | 20.54 ± 0.62 c |
| Open flower number | 3.67 ± 1.17 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| Closed flower number | 6.33 ± 0.61 a | 3.50 ± 0.34 b | 2.83 ± 0.31 b | 1.33 ± 0.21 c | 1.17 ± 0.17 c |
| Total flower number | 10.00 ± 0.93 a | 3.50 ± 0.34 b | 2.83 ± 0.31 b | 1.33 ± 0.21 c | 1.17 ± 0.17 c |
| CON | GR 5% | GR 10% | GR 20% | GR 40% | |
| Plant height (cm) | 30.27 ± 2.26 ab | 34.88 ± 3.37 a | 37.48 ± 3.55 a | 25.24 ± 4.04 b | 14.75 ± 0.64 c |
| Shoot diameter (mm) | 4.34 ± 0.18 ab | 4.91 ± 0.37 a | 5.04 ± 0.39 a | 3.67 ± 0.17 bc | 3.06 ± 0.17 c |
| Leaf number | 23.50 ± 3.58 b | 23.17 ± 2.34 b | 35.80 ± 7.45 a | 21.33 ± 3.33 b | 11.50 ± 0.62 b |
| Lateral shoot number | 2.83 ± 0.70 bc | 6.50 ± 1.23 a | 5.60 ± 1.25 ab | 2.60 ± 0.68 bc | 2.00 ± 0.58 c |
| Plant FW (g) | 25.18 ± 4.44 b | 49.20 ± 10.78 a | 56.50 ± 16.31 a | 14.55 ± 1.95 b | 5.92 ± 0.80 b |
| Leaf FW (g) | 8.77 ± 2.63 b | 24.13 ± 1.94 a | 17.43 ± 2.97 a | 8.28 ± 1.51 b | 4.87 ± 0.34 b |
| Shoot FW (g) | 9.17 ± 2.22 b | 22.49 ± 2.01 a | 18.12 ± 2.97 a | 7.37 ± 1.81 bc | 1.23 ± 0.03 c |
| Flower FW (g) | 10.87 ± 2.76 ab | 19.07 ± 3.04 a | 15.78 ± 4.17 ab | 7.30 ± 2.03 bc | 0.40 ± 0.00 c |
| Plant DW (g) | 4.70 ± 1.50 bc | 11.67 ± 1.20 a | 8.32 ± 1.89 ab | 3.43 ± 0.81 c | 0.89 ± 0.07 c |
| Leaf DW (g) | 0.99 ± 0.34 c | 2.72 ± 0.22 a | 1.78 ± 0.32 b | 0.87 ± 0.13 c | 0.55 ± 0.06 c |
| Shoot DW (g) | 2.32 ± 0.76 bc | 6.31 ± 0.64 a | 4.48 ± 1.01 ab | 1.62 ± 0.43 c | 0.28 ± 0.01 c |
| Flower DW (g) | 1.39 ± 0.40 ab | 2.64 ± 0.40 a | 2.05 ± 0.55 ab | 0.94 ± 0.26 bc | 0.06 ± 0.01 c |
| Plant DM (%) | 15.52 ± 0.94 ab | 17.19 ± 0.44 a | 15.19 ± 0.58 ab | 14.46 ± 0.18 bc | 12.91 ± 1.02 c |
| Open flower number | 3.67 ± 1.17 a | 4.00 ± 1.44 a | 4.00 ± 2.05 a | 3.25 ± 2.25 a | 0.00 ± 0.00 b |
| Closed flower number | 6.33 ± 0.61 a | 8.33 ± 1.12 a | 9.33 ± 1.36 a | 7.33 ± 2.42 a | 1.17 ± 0.17 b |
| Total flower number | 10.00 ± 0.93 a | 12.33 ± 2.35 a | 13.33 ± 2.54 a | 9.50 ± 3.85 a | 1.17 ± 0.17 b |
| OR 100% | GR 100% | |
|---|---|---|
| pH | 6.57 | 7.19 |
| EC (μS cm−1) | 1006.60 | 767.05 |
| Organic matter (%) | 95.15 | 92.70 |
| Organic C (%) | 55.19 | 53.77 |
| C/N ratio | 69.00 | 26.20 |
| N% | 0.82 | 2.05 |
| N (g kg−1) | 8.19 | 20.53 |
| P (g kg−1) | 0.65 | 2.08 |
| K (g kg−1) | 6.23 | 9.86 |
| Ca (g kg−1) | 6.77 | 9.75 |
| Mg (g kg−1) | 0.76 | 1.24 |
| Na (g kg−1) | 0.28 | 0.13 |
| Total porosity (% v/v) | 80.40 | 99.59 |
| Air-filled porosity (% v/v) | 10.00 | 33.57 |
| Bulk density (g cm−3) | 0.34 | 0.50 |
| Container capacity (% v/v) | 70.40 | 66.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toumazou, G.; Prasad, M.; Chrysargyris, A. Application of Wine and Olive Oil Production Residues as Substrates for the Cultivation of Chrysanthemum morifolium Potted Plants. Plants 2025, 14, 1166. https://doi.org/10.3390/plants14081166
Toumazou G, Prasad M, Chrysargyris A. Application of Wine and Olive Oil Production Residues as Substrates for the Cultivation of Chrysanthemum morifolium Potted Plants. Plants. 2025; 14(8):1166. https://doi.org/10.3390/plants14081166
Chicago/Turabian StyleToumazou, Georgios, Munoo Prasad, and Antonios Chrysargyris. 2025. "Application of Wine and Olive Oil Production Residues as Substrates for the Cultivation of Chrysanthemum morifolium Potted Plants" Plants 14, no. 8: 1166. https://doi.org/10.3390/plants14081166
APA StyleToumazou, G., Prasad, M., & Chrysargyris, A. (2025). Application of Wine and Olive Oil Production Residues as Substrates for the Cultivation of Chrysanthemum morifolium Potted Plants. Plants, 14(8), 1166. https://doi.org/10.3390/plants14081166







