Bridging Molecular Insights and Agronomic Innovations: Cutting-Edge Strategies for Overcoming Boron Deficiency in Sustainable Rapeseed Cultivation
Abstract
1. Introduction
2. Boron Deficiency in Rapeseed
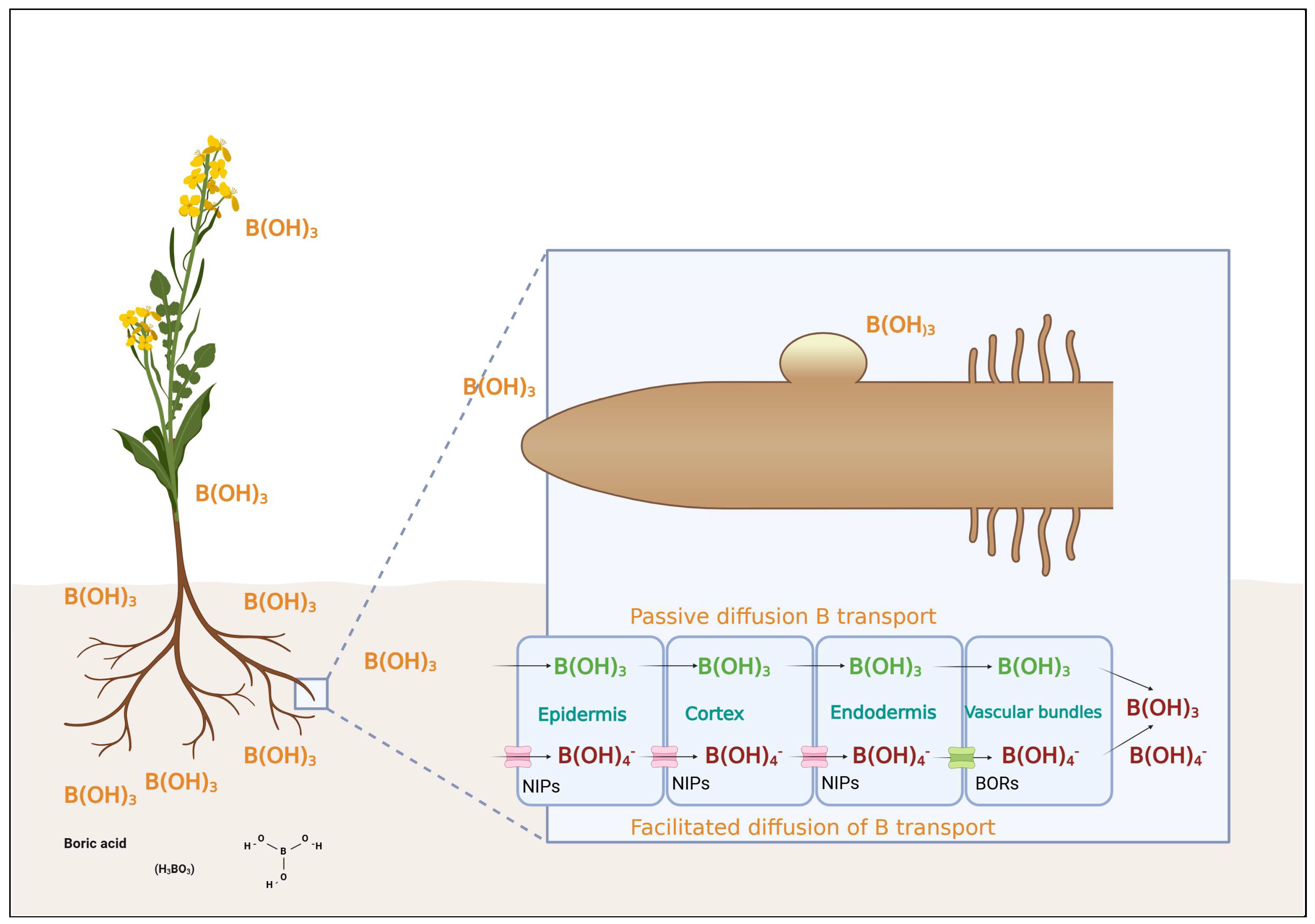
3. Mechanisms of Boron Uptake and Transport
3.1. Specialized Transport Proteins in Boron Transport
3.2. Aquaporins in Boron Transport
3.3. Genetic and Molecular Insights
3.4. Transporter Families and Mechanisms of Boron Deficiency Tolerance in Brassica Species
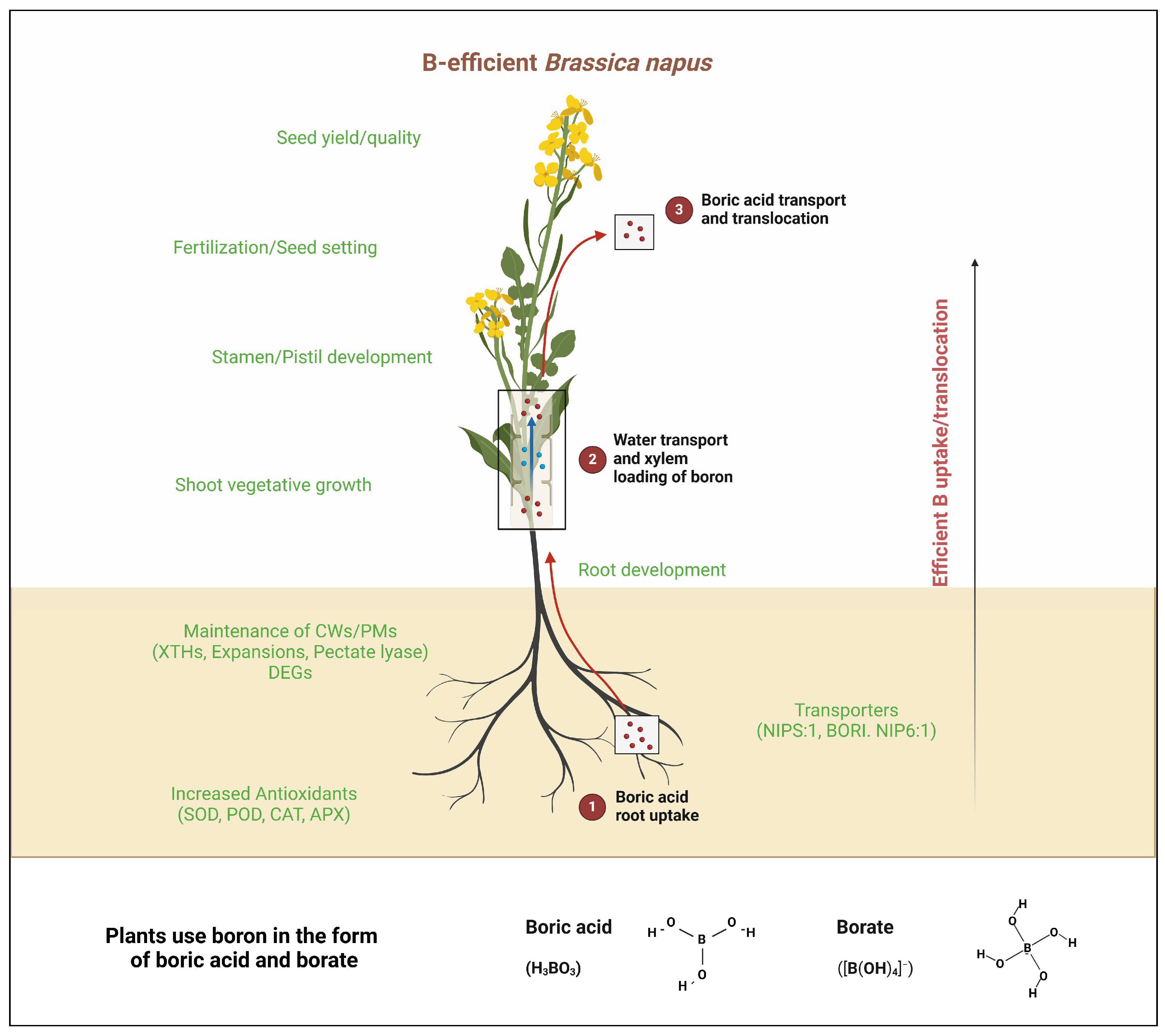
4. Implications of Boron Deficiency Tolerance in Rapeseed
4.1. Enhanced Crop Resilience and Yield Stability
4.2. Reduced Reliance on Chemical Amendments
4.3. Integration of Molecular and Agronomic Innovations
4.4. Sustainable Agricultural Practices
4.5. Economic and Market Benefits
5. Conclusions and Future Remarks
Funding
Conflicts of Interest
References
- Brdar-Jokanović, M. Boron toxicity and deficiency in agricultural plants. Int. J. Mol. Sci. 2020, 21, 1424. [Google Scholar] [CrossRef] [PubMed]
- Vera-Maldonado, P.; Aquea, F.; Reyes-Díaz, M.; Cárcamo-Fincheira, P.; Soto-Cerda, B.; Nunes-Nesi, A.; Inostroza-Blancheteau, C. Role of boron and its interaction with other elements in plants. Front. Plant Sci. 2024, 15, 1332459. [Google Scholar]
- Bolaños, L.; Abreu, I.; Bonilla, I.; Camacho-Cristóbal, J.J.; Reguera, M. What Can Boron Defic. Symptoms Tell Us About Its Funct. Regul? Plants 2023, 12, 777. [Google Scholar] [CrossRef]
- O’Neill, M.A.; Ishii, T.; Albersheim, P.; Darvill, A.G. Rhamnogalacturonan II: Structure and function of a borate cross-linked cell wall pectic polysaccharide. Annu. Rev. Plant Biol. 2004, 55, 109–139. [Google Scholar] [PubMed]
- Camacho-Cristóbal, J.J.; Navarro-Gochicoa, M.T.; Rexach, J.; González-Fontes, A.; Herrera-Rodríguez, M.B. Plant response to boron deficiency and boron use efficiency in crop plants. In Plant Micronutrient Use Efficiency; Elsevier: Amsterdam, The Netherlands, 2018; pp. 109–121. [Google Scholar]
- Takano, J.; Noguchi, K.; Yasumori, M.; Kobayashi, M.; Gajdos, Z.; Miwa, K.; Hayashi, H.; Yoneyama, T.; Fujiwara, T. Arabidopsis boron transporter for xylem loading. Nature 2002, 420, 337–340. [Google Scholar]
- Landi, M.; Margaritopoulou, T.; Papadakis, I.E.; Araniti, F. Boron toxicity in higher plants: An update. Planta 2019, 250, 1011–1032. [Google Scholar]
- Day, S.; Aasim, M. Role of boron in growth and development of plant: Deficiency and toxicity perspective. In Plant Micronutrients: Deficiency and Toxicity Management; Springer: Cham, Switzerland, 2020; pp. 435–453. [Google Scholar]
- Shorrocks, V.M. The occurrence and correction of boron deficiency. Plant Soil 1997, 193, 121–148. [Google Scholar]
- Smith, F. Interpretation of plant analysis: Concepts and principles. In Plant Analysis: An Interpretation Manual; CSIRO Publishing: Clayton, VIC, Australia, 1997. [Google Scholar]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic press: Cambridge, MA, USA, 2011. [Google Scholar]
- Dell, B.; Huang, L. Physiological response of plants to low boron. Plant Soil 1997, 193, 103–120. [Google Scholar]
- Huang, L.; Ye, Z.; Bell, R.W. The importance of sampling immature leaves for the diagnosis of boron deficiency in oilseed rape (Brassica napus cv. Eureka). Plant Soil 1996, 183, 187–198. [Google Scholar]
- Rashid, A.; Rafique, E.; Bughio, N. Diagnosing boron deficiency in rapeseed and mustard by plant analysis and soil testing. Commun. Soil Sci. Plant Anal. 1994, 25, 2883–2897. [Google Scholar]
- Bergmann, W. Nutritional Disorders of Plants: Visual and Analytical Diagnosis (English, French, Spanish); Gustav Fischer: Jena, Germany, 1992. [Google Scholar]
- Asad, A.; Bell, R.; Dell, B.; Huang, L. External boron requirements for canola (Brassica napus L.) in boron buffered solution culture. Ann. Bot. 1997, 80, 65–73. [Google Scholar]
- Zhang, D.; Zhao, H.; Shi, L.; Xu, F. Physiological and genetic responses to boron deficiency in Brassica napus: A review. Soil Sci. Plant Nutr. 2014, 60, 304–313. [Google Scholar]
- Wang, Z.; Wang, Z.; Shi, L.; Wang, L.; Xu, F. Proteomic alterations of Brassica napus root in response to boron deficiency. Plant Mol. Biol. 2010, 74, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hua, Y.; Wang, X.; Zhao, H.; Shi, L.; Xu, F. A high-density genetic map identifies a novel major QTL for boron efficiency in oilseed rape (Brassica napus L.). PLoS ONE 2014, 9, e112089. [Google Scholar]
- Hu, Q.; Hua, W.; Yin, Y.; Zhang, X.; Liu, L.; Shi, J.; Zhao, Y.; Qin, L.; Chen, C.; Wang, H. Rapeseed research and production in China. Crop J. 2017, 5, 127–135. [Google Scholar]
- Wang, K.; Yang, Y.; Bell, R.; Xue, J.; Ye, Z.; Wei, Y. Low risks of toxicity from boron fertiliser in oilseed rape–rice rotations in southeast China. Nutr. Cycl. Agroecosystems 1999, 54, 189–197. [Google Scholar]
- Fu, D.-H.; Jiang, L.-Y.; Mason, A.S.; Xiao, M.-L.; Zhu, L.-R.; Li, L.-Z.; Zhou, Q.-H.; Shen, C.-J.; Huang, C.-H. Research progress and strategies for multifunctional rapeseed: A case study of China. J. Integr. Agric. 2016, 15, 1673–1684. [Google Scholar]
- Li, F.; Guo, K.; Liao, X. Risk assessment of China rapeseed supply chain and policy suggestions. Int. J. Environ. Res. Public Health 2022, 20, 465. [Google Scholar] [CrossRef]
- Li, Q.; Wang, C. An evaluation of Chinese rapeseed production efficiency based on three-stage DEA and Malmquist index. Sustainability 2022, 14, 15822. [Google Scholar] [CrossRef]
- Brown, P.H.; Bellaloui, N.; Wimmer, M.; Bassil, E.S.; Ruiz, J.; Hu, H.; Pfeffer, H.; Dannel, F.; Römheld, V. Boron in plant biology. Plant Biol. 2002, 4, 205–223. [Google Scholar]
- Verwaaijen, B.; Alcock, T.D.; Spitzer, C.; Liu, Z.; Fiebig, A.; Bienert, M.D.; Bräutigam, A.; Bienert, G.P. The Brassica napus boron deficient inflorescence transcriptome resembles a wounding and infection response. Physiol. Plant. 2023, 175, e14088. [Google Scholar] [PubMed]
- Zhou, T.; Hua, Y.; Zhang, B.; Zhang, X.; Zhou, Y.; Shi, L.; Xu, F. Low-boron tolerance strategies involving pectin-mediated cell wall mechanical properties in Brassica napus. Plant Cell Physiol. 2017, 58, 1991–2005. [Google Scholar] [PubMed]
- Hua, Y.; Zhou, T.; Ding, G.; Yang, Q.; Shi, L.; Xu, F. Physiological, genomic and transcriptional diversity in responses to boron deficiency in rapeseed genotypes. J. Exp. Bot. 2016, 67, 5769–5784. [Google Scholar]
- Pommerrenig, B.; Junker, A.; Abreu, I.; Bieber, A.; Fuge, J.; Willner, E.; Bienert, M.D.; Altmann, T.; Bienert, G.P. Identification of rapeseed (Brassica napus) cultivars with a high tolerance to boron-deficient conditions. Front. Plant Sci. 2018, 9, 1142. [Google Scholar]
- Riaz, M.; Wu, X.; Yan, L.; Hussain, S.; Aziz, O.; Shah, A.; Jiang, C. Boron supply alleviates Al-induced inhibition of root elongation and physiological characteristics in rapeseed (Brassica napus L.). J. Plant Interact. 2018, 13, 270–276. [Google Scholar]
- Dinh, A.Q.; Naeem, A.; Mühling, K.H. Growth and distribution of boron in oilseed rape (Brassica napus L.) as affected by boron supply. Plants 2022, 11, 2746. [Google Scholar] [CrossRef]
- Wu, X.; Song, H.; Guan, C.; Zhang, Z. Boron mitigates cadmium toxicity to rapeseed (Brassica napus) shoots by relieving oxidative stress and enhancing cadmium chelation onto cell walls. Environ. Pollut. 2020, 263, 114546. [Google Scholar] [CrossRef]
- Wu, X.; Song, H.; Guan, C.; Zhang, Z. Boron alleviates cadmium toxicity in Brassica napus by promoting the chelation of cadmium onto the root cell wall components. Sci. Total Environ. 2020, 728, 138833. [Google Scholar]
- Yang, M.; Shi, L.; Xu, F.; Wang, Y. Effect of boron on dynamic change of seed yield and quality formation in developing seed of Brassica napus. J. Plant Nutr. 2009, 32, 785–797. [Google Scholar]
- Zeng, H.; Tang, B.; Yao, K.; Mo, T.; Mao, W.; Lu, B.; Li, Z. Effects of Different Boron Fertilizer Dosage on Yield and Nutrient Content of Rapeseed. Hans J. Agric. Sci. 2019, 9, 221–227. [Google Scholar]
- Wang, S.; Liu, L.; Zou, D.; Huang, Y.; Zhao, Z.; Ding, G.; Cai, H.; Wang, C.; Shi, L.; Xu, F. Vascular tissue-specific expression of BnaC4. BOR1; 1c, an efflux boron transporter gene, is regulated in response to boron availability for efficient boron acquisition in Brassica napus. Plant Soil 2021, 465, 171–184. [Google Scholar]
- Safdar, M.E.; Qamar, R.; Javed, A.; Nadeem, M.A.; Javeed, H.M.R.; Farooq, S.; Głowacka, A.; Michałek, S.; Alwahibi, M.S.; Elshikh, M.S. Combined application of boron and zinc improves seed and oil yields and oil quality of oilseed rape (Brassica napus L.). Agronomy 2023, 13, 2020. [Google Scholar] [CrossRef]
- Basumatary, A.; Chauhan, S.; Bhupenchandra, I.; Das, K.N.; Ozah, D.J. Impact of sulfur and boron fertilization on yield, quality of crop and nutrient use efficiencies in rapeseed in subtropical acidic soil of Assam, India. J. Plant Nutr. 2021, 44, 1779–1793. [Google Scholar]
- Varenyiova, M.; Ducsay, L. Effect of increasing doses of boron on oil production of oilseed rape (Brassica napus L.). Percipi 2007, 2008, 2009. [Google Scholar]
- Masood, S.; Zhao, X.Q.; Shen, R.F. Bacillus pumilus increases boron uptake and inhibits rapeseed growth under boron supply irrespective of phosphorus fertilization. AoB Plants 2019, 11, plz036. [Google Scholar]
- Barker, A.V.; Pilbeam, D.J. Handbook of Plant Nutrition; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Brown, P.H.; Shelp, B.J. Boron mobility in plants. Plant Soil 1997, 193, 85–101. [Google Scholar]
- Yoshinari, A.; Takano, J. Insights into the mechanisms underlying boron homeostasis in plants. Front. Plant Sci. 2017, 8, 1951. [Google Scholar]
- Onuh, A.F.; Miwa, K. Regulation, diversity and evolution of boron transporters in plants. Plant Cell Physiol. 2021, 62, 590–599. [Google Scholar]
- Takano, J.; Miwa, K.; Fujiwara, T. Boron transport mechanisms: Collaboration of channels and transporters. Trends Plant Sci. 2008, 13, 451–457. [Google Scholar]
- Miwa, K.; Fujiwara, T. Boron transport in plants: Co-ordinated regulation of transporters. Ann. Bot. 2010, 105, 1103–1108. [Google Scholar] [CrossRef]
- Hrmova, M.; Gilliham, M.; Tyerman, S.D. Plant transporters involved in combating boron toxicity: Beyond 3D structures. Biochem. Soc. Trans. 2020, 48, 1683–1696. [Google Scholar] [CrossRef]
- Miwa, K.; Aibara, I.; Fujiwara, T. Arabidopsis thaliana BOR4 is upregulated under high boron conditions and confers tolerance to high boron. Soil Sci. Plant Nutr. 2014, 60, 349–355. [Google Scholar] [CrossRef]
- Kohli, S.K.; Kaur, H.; Khanna, K.; Handa, N.; Bhardwaj, R.; Rinklebe, J.; Ahmad, P. Boron in plants: Uptake, deficiency and biological potential. Plant Growth Regul. 2023, 100, 267–282. [Google Scholar] [CrossRef]
- He, M.; Wang, S.; Zhang, C.; Liu, L.; Zhang, J.; Qiu, S.; Wang, H.; Yang, G.; Xue, S.; Shi, L. Genetic variation of BnaA3. NIP5; 1 expressing in the lateral root cap contributes to boron deficiency tolerance in Brassica napus. PLoS Genet. 2021, 17, e1009661. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Li, W.; Hua, Y.; King, G.J.; Xu, F.; Shi, L. Genome-wide identification and characterization of the aquaporin gene family and transcriptional responses to boron deficiency in Brassica napus. Front. Plant Sci. 2017, 8, 1336. [Google Scholar] [CrossRef] [PubMed]
- Diehn, T.A.; Bienert, M.D.; Pommerrenig, B.; Liu, Z.; Spitzer, C.; Bernhardt, N.; Fuge, J.; Bieber, A.; Richet, N.; Chaumont, F. Boron demanding tissues of Brassica napus express specific sets of functional Nodulin26-like Intrinsic Proteins and BOR 1 transporters. Plant J. 2019, 100, 68–82. [Google Scholar] [CrossRef]
- Song, G.; Li, X.; Munir, R.; Khan, A.R.; Azhar, W.; Khan, S.; Gan, Y. BnaA02. NIP6; 1a encodes a boron transporter required for plant development under boron deficiency in Brassica napus. Plant Physiol. Biochem. 2021, 161, 36–45. [Google Scholar] [CrossRef]
- Quiroga, G.; Erice, G.; Aroca, R.; Ruiz-Lozano, J.M. Elucidating the possible involvement of maize aquaporins in the plant boron transport and homeostasis mediated by Rhizophagus irregularis under drought stress conditions. Int. J. Mol. Sci. 2020, 21, 1748. [Google Scholar] [CrossRef]
- Bienert, G.P.; Schüssler, M.D.; Jahn, T.P. Metalloids: Essential, beneficial or toxic? Major intrinsic proteins sort it out. Trends Biochem. Sci. 2008, 33, 20–26. [Google Scholar] [CrossRef]
- Pommerrenig, B.; Diehn, T.A.; Bienert, G.P. Metalloido-porins: Essentiality of Nodulin 26-like intrinsic proteins in metalloid transport. Plant Sci. 2015, 238, 212–227. [Google Scholar] [CrossRef]
- Durbak, A.R.; Phillips, K.A.; Pike, S.; O’Neill, M.A.; Mares, J.; Gallavotti, A.; Malcomber, S.T.; Gassmann, W.; McSteen, P. Transport of boron by the tassel-less1 aquaporin is critical for vegetative and reproductive development in maize. Plant Cell 2014, 26, 2978–2995. [Google Scholar] [CrossRef] [PubMed]
- Porcel, R.; Bustamante, A.; Ros, R.; Serrano, R.; Mulet Salort, J.M. BvCOLD1: A novel aquaporin from sugar beet (Beta vulgaris L.) involved in boron homeostasis and abiotic stress. Plant Cell Environ. 2018, 41, 2844–2857. [Google Scholar] [PubMed]
- Xu, F.; Wang, Y.; Meng, J. Mapping boron efficiency gene(s) in Brassica napus using RFLP and AFLP markers. Plant Breed. 2001, 120, 319–324. [Google Scholar]
- Zhao, Z.; Wu, L.; Nian, F.; Ding, G.; Shi, T.; Zhang, D.; Shi, L.; Xu, F.; Meng, J. Dissecting quantitative trait loci for boron efficiency across multiple environments in Brassica napus. PLoS ONE 2012, 7, e45215. [Google Scholar] [CrossRef]
- Hua, Y.; Zhang, D.; Zhou, T.; He, M.; Ding, G.; Shi, L.; Xu, F. Transcriptomics-assisted quantitative trait locus fine mapping for the rapid identification of a nodulin 26-like intrinsic protein gene regulating boron efficiency in allotetraploid rapeseed. Plant Cell Environ. 2016, 39, 1601–1618. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, H.; He, M.; Zhao, Z.; Cai, H.; Ding, G.; Shi, L.; Xu, F. The boron transporter BnaC4. BOR1; 1c is critical for inflorescence development and fertility under boron limitation in Brassica napus. Plant Cell Environ. 2017, 40, 1819–1833. [Google Scholar]
- Xue, J.; Lin, M.; Bell, R.W.; Graham, R.D.; Yang, X.; Yang, Y. Differential response of oilseed rape (Brassica napus L.) cultivars to low boron supply. Plant Soil 1998, 204, 155–163. [Google Scholar]
- Liu, W.; Xu, F.; Ye, X.; Cai, H.; Shi, L.; Wang, S. BnaC4. BOR2 mediates boron uptake and translocation in Brassica napus under boron deficiency. Plant Cell Environ. 2024, 47, 3732–3748. [Google Scholar] [CrossRef]
- Feng, Y.; Cui, R.; Wang, S.; He, M.; Hua, Y.; Shi, L.; Ye, X.; Xu, F. Transcription factor BnaA9. WRKY47 contributes to the adaptation of Brassica napus to low boron stress by up-regulating the boric acid channel gene BnaA3. NIP5; 1. Plant Biotechnol. J. 2020, 18, 1241–1254. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Q.; He, M.; Wang, S.; Shi, L.; Xu, F. Molecular characterization of the genome-wide BOR transporter gene family and genetic analysis of BnaC04. BOR1; 1c in Brassica napus. BMC Plant Biol. 2018, 18, 193. [Google Scholar]
- He, M.; Zhang, C.; Chu, L.; Wang, S.; Shi, L.; Xu, F. Specific and multiple-target gene silencing reveals function diversity of BnaA2. NIP5; 1 and BnaA3. NIP5; 1 in Brassica napus. Plant Cell Environ. 2021, 44, 3184–3194. [Google Scholar] [PubMed]
- Hua, Y.; Pei, M.; Song, H.; Liu, Y.; Zhou, T.; Chao, H.; Yue, C.; Huang, J.; Qin, G.; Feng, Y. Boron confers salt tolerance through facilitating BnaA2. HKT1-mediated root xylem Na+ unloading in rapeseed (Brassica napus L.). Plant J. 2024, 120, 1326–1342. [Google Scholar] [PubMed]
- Kumar, K.; Mosa, K.A.; Chhikara, S.; Musante, C.; White, J.C.; Dhankher, O.P. Two rice plasma membrane intrinsic proteins, OsPIP2; 4 and OsPIP2; 7, are involved in transport and providing tolerance to boron toxicity. Planta 2014, 239, 187–198. [Google Scholar] [PubMed]
- Hanaoka, H.; Uraguchi, S.; Takano, J.; Tanaka, M.; Fujiwara, T. OsNIP3;1, a rice boric acid channel, regulates boron distribution and is essential for growth under boron-deficient conditions. Plant J. 2014, 78, 890–902. [Google Scholar]
- Ochiai, K.; Shimizu, A.; Okumoto, Y.; Fujiwara, T.; Matoh, T. Suppression of a NAC-like transcription factor gene improves boron-toxicity tolerance in rice. Plant Physiol. 2011, 156, 1457–1463. [Google Scholar]
- Rani, A.; Rajpoot, R. Insight of Oxidative Stress, Ultrastructural Transformations, and Elemental Variations in Rice Seedlings Exposed to Boron Toxicity: Unraveling the Role of Fe-SOD in Boron Tolerance. Asian J. Res. Biochem. 2023, 13, 26–42. [Google Scholar]
- Leaungthitikanchana, S.; Tanaka, M.; Lordkaew, S.; Jamjod, S.; Rerkasem, B.; Fujiwara, T. Comparison of BOR1-like gene expression in two genotypes with different boron efficiencies in commercial crop plants in Thailand. Soil Sci. Plant Nutr. 2014, 60, 333–340. [Google Scholar]
- Huai, Z.; Peng, L.; Wang, S.; Zhao, H.; Shi, L.; Xu, F. Identification and characterization of an Arabidopsis thaliana mutant lbt with high tolerance to boron deficiency. Front. Plant Sci. 2018, 9, 736. [Google Scholar]
- Zhang, C.; He, M.; Jiang, Z.; Liu, T.; Wang, C.; Wang, S.; Xu, F. STOP1 regulates the tolerance of Arabidopsis to low boron stress by directly activating NOD26-LIKE MAJOR INTRINSIC PROTEIN5; 1 expression. J. Exp. Bot. 2024, 75, erae038. [Google Scholar]
- Kato, Y.; Miwa, K.; Takano, J.; Wada, M.; Fujiwara, T. Highly boron deficiency-tolerant plants generated by enhanced expression of NIP5; 1, a boric acid channel. Plant Cell Physiol. 2009, 50, 58–66. [Google Scholar]
- Lv, Q.; Wang, L.; Wang, J.-Z.; Li, P.; Chen, Y.-L.; Du, J.; He, Y.-K.; Bao, F. SHB1/HY1 alleviates excess boron stress by increasing BOR4 expression level and maintaining boron homeostasis in Arabidopsis roots. Front. Plant Sci. 2017, 8, 790. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Li, L.; Ren, F.; Lu, P.; Wei, P.; Cai, J.; Xin, L.; Zhang, J.; Chen, J.; Wang, X. Overexpression of the tonoplast aquaporin AtTIP5; 1 conferred tolerance to boron toxicity in Arabidopsis. J. Genet. Genom. 2010, 37, 389–397. [Google Scholar]
- Sun, J.; Shi, L.; Zhang, C.; Xu, F. Cloning and characterization of boron transporters in Brassica napus. Mol. Biol. Rep. 2012, 39, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Takano, J.; Tanaka, M. Boron-sensing mechanisms involved in boron transport regulation in plants. J. Plant Nutr. Soil Sci. 2023, 1–8. [Google Scholar] [CrossRef]
- Zhou, T.; Hua, Y.; Huang, Y.; Ding, G.; Shi, L.; Xu, F. Physiological and transcriptional analyses reveal differential phytohormone responses to boron deficiency in Brassica napus genotypes. Front. Plant Sci. 2016, 7, 221. [Google Scholar]
- Giehl, R.F.; Gruber, B.D.; von Wirén, N. It’s time to make changes: Modulation of root system architecture by nutrient signals. J. Exp. Bot. 2014, 65, 769–778. [Google Scholar]
- Rerkasem, B.; Jamjod, S.; Pusadee, T. Productivity limiting impacts of boron deficiency, a review. Plant Soil 2020, 455, 23–40. [Google Scholar]
- Raboanatahiry, N.; Chao, H.; Dalin, H.; Pu, S.; Yan, W.; Yu, L.; Wang, B.; Li, M. QTL alignment for seed yield and yield related traits in Brassica napus. Front. Plant Sci. 2018, 9, 1127. [Google Scholar]
- Réthoré, E.; Ali, N.; Pluchon, S.; Hosseini, S.A. Silicon enhances Brassica napus tolerance to boron deficiency by the remobilisation of boron and by changing the expression of boron transporters. Plants 2023, 12, 2574. [Google Scholar] [CrossRef]
- Liang, Y.; Shen, Z. Interaction of silicon and boron in oilseed rape plants. J. Plant Nutr. 1994, 17, 415–425. [Google Scholar] [CrossRef]
- Rogalla, H.; Römheld, V. Effects of silicon on the availability of boron: Possible effects on the phenol pathway and on the redox status in Cucumis sativus L. In Boron in Plant and Animal Nutrition; Springer: Boston, MA, USA, 2002; pp. 205–211. [Google Scholar]
- Wangkheirakpam, M.; Singh, T.B.; Laishram, B.; Leitam, O.C.; Chanu, R.L.; Kalpana, A.; Jiten, W. Effect of salicylic acid and boron application on growth and yield of no-tilled rapeseed (Brassica campestris) under rainfed condition. Pharma Innov 2020, 9, 353–358. [Google Scholar]
- Zhao, Z.; Wang, S.; White, P.J.; Wang, Y.; Shi, L.; Xu, F. Boron and phosphorus act synergistically to modulate absorption and distribution of phosphorus and growth of Brassica napus. J. Agric. Food Chem. 2020, 68, 7830–7838. [Google Scholar] [PubMed]
- Gu, J.; Li, W.; Wang, S.; Zhang, X.; Coules, A.; Ding, G.; Xu, F.; Ren, J.; Lu, C.; Shi, L. Differential alternative splicing genes in response to boron deficiency in Brassica napus. Genes 2019, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Ozyigit, I.I.; Filiz, E.; Saracoglu, I.A.; Karadeniz, S. Exploration of two major boron transport genes BOR1 and NIP5; 1 in the genomes of different plants. Biotechnol. Biotechnol. Equip. 2020, 34, 455–468. [Google Scholar]
- Luo, J.; Liang, Z.; Wu, M.; Mei, L. Genome-wide identification of BOR genes in poplar and their roles in response to various environmental stimuli. Environ. Exp. Bot. 2019, 164, 101–113. [Google Scholar]
- Qin, S.; Liu, H.; Rengel, Z.; Gao, W.; Nie, Z.; Li, C.; Hou, M.; Cheng, J.; Zhao, P. Boron inhibits cadmium uptake in wheat (Triticum aestivum) by regulating gene expression. Plant Sci. 2020, 297, 110522. [Google Scholar]
- Dutta, A. Impact of improved technologies on productivity and profitability of rapeseed-mustard production at farm level in West Bengal, India. SAARC J. Agric. 2016, 14, 126–136. [Google Scholar]

| Scientific Name | Boron Concentration | Effects | Duration | References |
|---|---|---|---|---|
| Brassica napus L. | 0.25 to 1000 µM | Severe visible symptoms on leaves, root growth inhibition | 4 weeks | [30,31] |
| Brassica napus L. | 0.25 and 0.10 µM | Deformed morphology, lower viability, easily ruptured cell walls | - | [27] |
| Brassica napus L. | 2.5 and 25 µM | Alleviation of Al-induced root growth inhibition | - | [30] |
| Brassica napus L. | - | Increased root pectin content, decreased cellulose and hemicellulose under Cd stress | - | [32,33] |
| Brassica napus L. | Low B | Growth arrest, cell death, changes in cell wall pore size, oxidative burst | - | [3] |
| Brassica napus L. | High B | Increased seed yield, improved seed dry matter accumulation | Field plot trial | [34] |
| Brassica napus L. | Low B | Deformed cell morphology, lower viability, ruptured cell walls | - | [27] |
| Brassica napus L. | 7.5 kg/hm2 | Increased plant height, branch number, kernels per plant | 3 years | [35] |
| Brassica napus L. | Low B | Reduced seed yield, lower nitrogen use efficiency | 2 years | [36] |
| Brassica napus L. | 2 kg ha−1 | Improved seed yield, higher oil quality, lower erucic acid and glucosinolate contents | 2-year field study | [34,37] |
| Brassica napus L. | 1.5 kg B/ha | Increased seed and stover yields, improved oil content by 35.6% | 3 consecutive rabi seasons | [38] |
| Brassica napus L. | 200–800 g B ha−1 | Increased oil content in seeds by 3.96% | - | [39] |
| Brassica napus L. | - | Enhanced B uptake, inhibited growth under B supply | 6 weeks | [40] |
| Plant Name | Scientific Name | Gene | Functions | Effects | References |
|---|---|---|---|---|---|
| Rapeseed | Brassica napus | BnaA3.NIP5;1 | Encodes a boric acid channel, crucial for B uptake and root growth | Improves low-B tolerance, enhances seed yield | [50,67] |
| Rapeseed | Brassica napus | BnaA2.NIP5;1 | Essential for B uptake, expressed in root epidermis | Facilitates B translocation to shoots, supports normal growth | [50,67] |
| Rapeseed | Brassica napus | BnaA2.HKT1 | Functions as a Na+ transporter, involved in root xylem Na+ unloading | Enhances salt tolerance, supports growth under B deficiency and salinity | [68] |
| Rapeseed | Brassica napus | BnaA02.NIP6;1a | Boron transporter, localized in plasma membrane and cytoplasm | Required for plant development, prevents sterility under B deficiency | [53] |
| Rice | Oryza sativa L. | OsPIP2;4, OsPIP2;7 | Involved in B transport and tolerance | Increased B tolerance via efflux of excess B from roots and shoots | [69] |
| Rice | Oryza sativa L. | OsNIP3;1 | Boric acid channel, regulates B distribution | Essential for growth under B-deficient conditions | [70] |
| Rice | Oryza sativa L. | Os04g0477300 | Suppression improves B toxicity tolerance | Tolerance to B toxicity by abolishing transcript function | [71] |
| Rice | Oryza sativa L. | Fe-SOD | Antioxidative enzyme activity | Associated with B tolerance through increased expression | [72] |
| Rice | Oryza sativa L. | BOR1-like genes | Efflux-type B transporters | Correlated with B deficiency tolerance | [73] |
| Arabidopsis | Arabidopsis thaliana | AtWRKY47 | Regulates plant tolerance to boron toxicity by controlling B concentration in shoots. | Enhanced tolerance to B toxicity with better growth parameters. | [65] |
| Arabidopsis | Arabidopsis thaliana | LBT | Controls low-boron tolerance, independent of B uptake or transport. | Improved growth under B deficiency; controlled by a monogenic recessive gene. | [74] |
| Arabidopsis | Arabidopsis thaliana | STOP1 | Activates NIP5;1 expression to enhance B uptake by roots. | Increased tolerance to low-B stress and improved growth. | [75] |
| Arabidopsis | Arabidopsis thaliana | NIP5;1 | Boric acid channel for efficient B uptake. | Improved root elongation and fertility under B-limiting conditions. | [76] |
| Arabidopsis | Arabidopsis thaliana | SHB1/HY1 | Increases BOR4 expression to maintain boron homeostasis. | Alleviates excess boron stress and promotes root growth. | [77] |
| Arabidopsis | Arabidopsis thaliana | BOR4 | Efflux-type B transporter for high-B tolerance. | Upregulated under high B conditions, confers tolerance to high B. | [48] |
| Arabidopsis | Arabidopsis thaliana | AtTIP5;1 | Involved in B transport via vacuolar compartmentation. | Increased tolerance to high B toxicity with improved growth. | [78] |
| Arabidopsis | Arabidopsis thaliana | LBT | Controls low-boron tolerance, independent of B uptake or transport. | Improved growth under B deficiency; controlled by a monogenic recessive gene. | [74] |
| Scientific Name | Technique Name | Concentration | Effect | References |
|---|---|---|---|---|
| Brassica napus | Hydroponic | 0.1 µM B | Si improved growth by 34% in shoots and 49% in roots; increased B transporter expression | [85] |
| Brassica napus L. | Solution culture | 0.025, 0.5, and 5.0 µg B/mL | Si increased dry matter yield under B deficiency; enhanced B uptake and accumulation | [86] |
| Brassica napus | Not specified | - | Si increased the range between critical deficiency and toxicity concentration for B | [87] |
| Brassica napus | Co-application of N and B | 4.5 and 9 kg borax ha−1; 180 kg N ha−1 | Improved N uptake, NUE, seed yield, and N remobilization; yield increased by >40% under B deficiency | [36] |
| Brassica napus | Foliar application of B | 0.25% B | Highest growth and yield of rapeseed under no-tilled and rainfed conditions | [88] |
| Brassica napus | Silicon application | 0.1 µM B | Improved growth by 34% in shoots and 49% in roots under B deficiency; increased expression of B transporters | [85] |
| Brassica napus | Balanced B and P application | 4.5, 9, and 18 kg Na2B4O7·5H2O ha−1 | Enhanced seed yield and PUE; greater soil bacterial diversity with balanced B and P nutrition | [89] |
| Brassica napus | Sulfur and B fertilization | 1.5 kg B/ha | Highest seed and stover yields; improved oil and protein content; enhanced nutrient use efficiencies | [38] |
| Brassica napus | Transgenic lines | - | Improved low-B tolerance and seed yield through increased expression of BnaA3.NIP5;1 | [50] |
| Brassica napus | RNAi | - | BnaA3.NIP5;1 promotes root elongation under low-B conditions, important for seed production | [50] |
| Brassica napus | QTL fine mapping | - | Identification of a nodulin 26-like intrinsic protein gene regulating B efficiency | [28] |
| Brassica napus | Transcriptomics-assisted QTL-seq | - | Expedites identification of quantitative trait genes for B-deficiency response | [28] |
| Arabidopsis thaliana | Overexpression | - | Enhanced expression of NIP5;1 improves root elongation under B-limiting conditions | [76] |
| Brassica napus L. | Pectin-mediated cell wall analysis | 0.25 and 0.10 μM B | Low-B-tolerant genotype ‘QY10’ showed less cell wall deformation and higher viability compared to ‘W10’. | [27] |
| Brassica napus | Gene expression analysis | 0.25 and 0.10 μM B | ‘W10’ exhibited higher pectin concentrations and mRNA abundances of pectin biosynthesis-related genes. | [27] |
| Brassica napus | Soil substrate-based cultivation system | Below 0.1 mg B (kg soil)−1 | Identification of B-deficiency-tolerant cultivars CR2267, CR2280, and CR2285 | [29] |
| Brassica napus | Genetic variation analysis | - | Improved low-B tolerance through BnaA3.NIP5;1 gene expression | [67] |
| Brassica napus | Pectin-mediated cell wall analysis | 0.25 and 0.10 μM B | Differential tolerance due to pectin-endowed cell wall properties | [27] |
| Brassica napus | Alternative splicing analysis | - | Increased transcriptome diversity and tolerance in B-efficient cultivar QY10 | [90] |
| Brassica napus | Co-application of N and B | 4.5 and 9 kg borax ha−1 | Synergistic effect on seed yield and nitrogen use efficiency | [36] |
| Brassica napus | Transcriptomics-assisted QTL mapping | - | Identification of nodulin 26-like intrinsic protein gene for B efficiency | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riaz, M.; Rafiq, M.; Nawaz, H.H.; Miao, W. Bridging Molecular Insights and Agronomic Innovations: Cutting-Edge Strategies for Overcoming Boron Deficiency in Sustainable Rapeseed Cultivation. Plants 2025, 14, 995. https://doi.org/10.3390/plants14070995
Riaz M, Rafiq M, Nawaz HH, Miao W. Bridging Molecular Insights and Agronomic Innovations: Cutting-Edge Strategies for Overcoming Boron Deficiency in Sustainable Rapeseed Cultivation. Plants. 2025; 14(7):995. https://doi.org/10.3390/plants14070995
Chicago/Turabian StyleRiaz, Muhammad, Muhammad Rafiq, Hafiz Husnain Nawaz, and Weiguo Miao. 2025. "Bridging Molecular Insights and Agronomic Innovations: Cutting-Edge Strategies for Overcoming Boron Deficiency in Sustainable Rapeseed Cultivation" Plants 14, no. 7: 995. https://doi.org/10.3390/plants14070995
APA StyleRiaz, M., Rafiq, M., Nawaz, H. H., & Miao, W. (2025). Bridging Molecular Insights and Agronomic Innovations: Cutting-Edge Strategies for Overcoming Boron Deficiency in Sustainable Rapeseed Cultivation. Plants, 14(7), 995. https://doi.org/10.3390/plants14070995






