Abstract
In ornamental plants, one of the most complex life processes, i.e., flowering, is regulated by interaction between the microbiota, hormones, and genes. Flowering plays an integral role in overall development and is quintessential for reproduction. Considering its importance, this review explores the complex mechanisms that determine the induction of flowering, highlighting the relationship between hormonal and genetic networks as well as the growing significance of the microbiome. Important genes involved in genetic control include FT, SOC1, and LFY. These genes react to environmental stimuli like photoperiod and vernalization. Auxins, cytokinin, and gibberellins are only a few hormone pathways important for floral growth and timing. The importance of plant–microbe interactions has been emphasized by current research, which shows that the microbiome affects flowering through processes like hormone production and availability of food. A comprehensive understanding of flowering induction is possible by integrating results from microbiota, hormones, and genetics studies, which may improve the breeding and culture of ornamental plants. For researchers to understand the complexity of flowering in ornamental plants and develop unique breeding strategies and improved floral qualities, it is critical to use interdisciplinary approaches, as this comprehensive investigation demonstrates.
1. Introduction
1.1. Brief Overview of the Importance of Flowering in Ornamental Plants
Ornamental plants are the most diverse products in horticulture and belong to a rapidly developing industry [1]. They consist of a vast and diverse range of whole plants or plant parts primarily cultivated for decorative purposes [2]. Nowadays, the global ornamental plant industry is characterized by significant growth in both production and consumption, contributing to globalization and international trade [3]. Major markets have become increasingly interconnected, leading to greater seasonality and variability in the supply and demand for plants and flowers, which has contributed to enhanced price volatility [3]. Europe is one of the major markets for ornamentals and is expected to have one of the best growth rates in both production and consumption over the next ten years [4]. Demand for ornamental plants in Europe has shown a significant increase in purchases and price premiums across both the institutional and private sectors [5].
Ornamental plants provide apparent delight through their colorful and differently shaped flowers, fruits, and leaves, making ’beauty’ a major ornamentation trait [6]. Plants play a significant role in various sectors because of their importance and versatility. Multifaceted uses of ornamental plants include floristry, landscaping, gardening, potted plants, and cut flowers [7]. Flowers alone have played a significant role in human society. Their multifaceted applications in medicine, culinary arts, psychological well-being, and mental health have been well-noted. Studies demonstrate various functions of flowers, including their potential for food production and agriculture, pharmaceutical applications, health benefits, and environmental remediation [8]. Flowers have medicinal properties that help reduce anxiety, headaches, and memory loss. They also help to maintain physical health by treating digestive disorders and stomach ulcers [9]. Flowers are employed in the culinary arts to enhance the visual appeal of dishes and provide different dining experiences [10]. Flowers benefit mood and emotional wellness, as evidenced by a study demonstrating that examining photos of flowers makes one feel cheerful. This reflects the significance of ornamental flowers for enhancing general happiness and well-being [11].
Additionally, edible flowers are considered a diverse and vital source of essential vitamins, antioxidants, and medicinal pigments. They also have the potential to be used as nutraceuticals for supplemental health products, as shown in Figure 1 [12]. The demand for edible flowers has increased for several reasons, including growing knowledge of their beneficial effects and nutritional value [13]. Edible flowers contain numerous bioactive compounds, each with particular health benefits, such as vitamins, carotenoids, flavonoids, and phenolics [13]. The increased demand for functional food items and the shift in eating habits towards a healthier diet have also contributed to the increasing popularity of edible flowers [14]. Furthermore, the development of special horticulture products, such as edible flowers, has made it easier to expand diets and meet the demand of consumers for useful and healthy foods, which has spurred the development of this particular market sector [15].

Figure 1.
Different roles and applications of ornamental plants.
1.2. Introduction to the Genetic and Hormonal Networks Regulating Flowering
Plants flower due to complex hormonal and genetic processes that combine internal and external stimuli to control the transition from vegetative to reproductive stages. Several studies have explored these systems, such as the Arabidopsis thaliana Flowering Transition Gene Regulatory Network (FT-GRN), which combines intrinsic and extrinsic cues to trigger shoot apical meristem (SAM) phase modifications [16]. Research on Arabidopsis thaliana has shown the crucial functions performed by important genes, such as blooming Flowering Locus C (FLC) and Flowering Locus T (FT), in the regulatory system that controls the blooming process [17]. Moreover, microRNAs like miR156 and miR172 are part of regulatory circuits that control how environmental factors like CO2 levels affect the flowering time [18]. Gene-centric GWAS approaches have also highlighted the significance of gene interactions in controlling flowering time, as demonstrated by gene–phenotype association studies that have identified many genes associated with flowering time [19]. These genetic networks control blooming time and show intricate interactions between multiple genes and environmental stimuli, highlighting the flexibility and complexity of the mechanisms controlling flowering time in plants.
On the other hand, hormones like gibberellins (GA), auxin, cytokinins, abscisic acid (ABA), and ethylene also play important roles in controlling blooming [20]. Studying species like Rosa rugosa showed how phytohormones, starch, and sugars function during the changing process from vegetative to floral state, demonstrating the intricate gene-to-floral interaction process [21]. Plant hormones are typically essential to regulating orchid flowering [22]. Auxin is a morphogen [23] that serves as a signal for the specification of plant tissues based on its concentration gradient [24]. In Dendrobium and Phalaenopsis orchids, applying 6-benzylaminopurine (BA), a synthetic cytokinin, promotes flowering. This effect is mitigated by auxin. Exogenous BA is introduced into Phalaenopsis and Doritaenopsis plants to induce an early flowering time [25]. The limitation of Petunia hybrida flower development triggered by high temperatures can be alleviated by injecting GAs [26]. When GA (GA3) and BA are applied together, flowering has a noticeable effect [27]. GA also regulates essential processes like blooming timing and stem elongation [28]. Bud break and blooming period are both regulated by ABA [29]. It is hypothesized that strigolactones interact with GA, ethylene, auxins, and cytokinins to influence blooming [30]. ABA controls bud dormancy via the photoperiodic pathway [31]. It also inhibits glucanase activity while increasing the expression of Callose Synthase 1 (CALS1). This results in using dormancy sphincters that close intercellular channels (plasmodesmata) by inhibiting the flow of growth-promoting stimuli that cause dormancy [32]. The transcriptome of Arundina graminifolia included several homologs of CALS. The expression was significantly upregulated in the first stage of flower bud emergence. Furthermore, in the initial phases of bud development, the ABA-responsive ABFs showed significant expression levels, demonstrating that ABA influences the temporal regulation of bud growth [33]. Studies on the level of ABA in different Phalaenopsis tissues have shown that the dormant axillary buds contain significant amounts of free ABA [34]. Moreover, ABA inhibits the development of floral spikes when applied externally to the stem of Phalaenopsis, suggesting that ABA has an inhibitory effect on the transition of orchid flowers [34]. However, additional thorough research studies will be required to understand the role of ABA and other key hormones in regulating orchid flowering.
1.3. Microbial Diversity and Its Impact on Floral Biology and Pollination
Several scientific studies contribute to the potentially rising appeal of floral microbiomes [35]. Searching for rare yeast strains has demonstrated that flowers and the insects pollinating them are important biodiversity sources [36]. Nectar microcosms have been employed in community ecology to investigate factors influencing community assembly [37]. Within the field of conservation biology, there has been a notable decline in pollinators, alongside a growing acknowledgment of the significance of pathogens. Consequently, this has resulted in categorizing flowers as key sites for transmitting crucial pollinator pathogens [38]. Biocontrol compounds produced from floral surfaces have demonstrated significance in plant pathology for addressing floral diseases and enhancing orchard sustainability in pathogen management [39]. All these different studies combined are the demonstration of significance of floral microbiota as indispensable model for research into evolutionary and ecological processes.
Fungi and bacteria are already present while a bloom is just starting to develop. Both the nectar inside closed flowers and the flower buds individually may contain recognizable bacterial and fungal species before anthesis, the stage at which the flower opens [40]. Microbial communities that can be detected and cultured are present in the petals of newly opened flowers [41]. Within the grasses, the ovaries are said to contain filamentous fungi, which can easily be isolated for further identification [42]. Such fungi can also be found in the pollen of forbs [43] early in the growth of flowers. Secondly, the number of microorganisms tends to increase as time progresses on individual blossoms. The most evident illustrations arise from the analysis of microorganisms that dwell on the pistil’s surface or are found in the nectar of the flower. An exponential increase was observed in a number of bacterial and fungal colonies in Datura wrightii once the blooming process started. Similar observations were recorded for Agave palmeri, which displayed minimal bacterial and fungal presence of microbial community in the nectar prior to blooming [40].
Mimulus aurantiacus, whose flowers are pollinated by hummingbirds, had a reported presence of yeast in one-fifth of flowers that were one day old. This number significantly increased as the flowers aged [44]. Likewise, there was a rise in both the frequency and quantity of bacteria found in the nectar of Epilobium canum [45]. The presence of animals visiting flowers leads to changes in the microbial communities found within the flowers. Despite the potential existence of microbes on flowers that show no signs of visitation, a significant amount of studies suggest that animal displays play a crucial part in transmitting the microbiota to and between floral parts. In 1884, Boutroux observed a higher prevalence of fungi (yeast) in flowers that were pollinated by bees [46]. Recent studies have shown that ascomycete flower yeasts are absent when bees and large-bodied pollinators are absent. Conversely, these yeasts are found in high quantities in the nectar of flowers imposed by pollinators [47]. Specific pollinator species can sometimes be implicated in flower dispersion [36]. In Mimulus aurantiacus, a plant pollinated by hummingbirds, flowers are more prone to the presence of bacteria in the nectar [45]. Nitidulid beetles vector particular species of large-spored ascomycete yeasts [48], and ants, florivores, and other non-mutualistic floral visitors have been shown to disperse flower-colonizing bacteria and yeasts [49].
Apart from large insects and animals, small insects are also important vectors when it comes to the distribution of microbes residing in the nectar. For example, the microorganism that resides in flower-feeding thrips consists of Rosenbergiella and Pantoea [50]. More commonly, these microbes are present in parenthesis flowers [40] and pollen [51]. The evidence of dependency on the distribution of microbiota through animal vectors is more common in studies related to the microbiome of nectars. However, it does not necessarily mean that animal vectors do not contribute to microbial dispersion in other parts of the flowers. Bumble bees, for instance, are known to contribute to the dispersal of bacteria within stamens and petals of flower [52]. Other parts of flower-like corolla and leaves of the plants in close vicinity of the flowers have been reported for the presence of microbe-rich bee feces [38]. The presence of microbes in various floral parts suggests that microbes indeed play a significant role in flower development, either directly or indirectly. The underlying mechanisms, however, remain unexplored and require further investigation. Lu et al. (2018) recently investigated a network where root exudates influence rhizosphere microbes, which produce IAA (indole-3-acetic acid) and induce the nitrogen cycle, which further promotes flowering [53]. This creates a positive feedback loop where increased growth boosts exudation, further affecting flowering time (Figure 2).
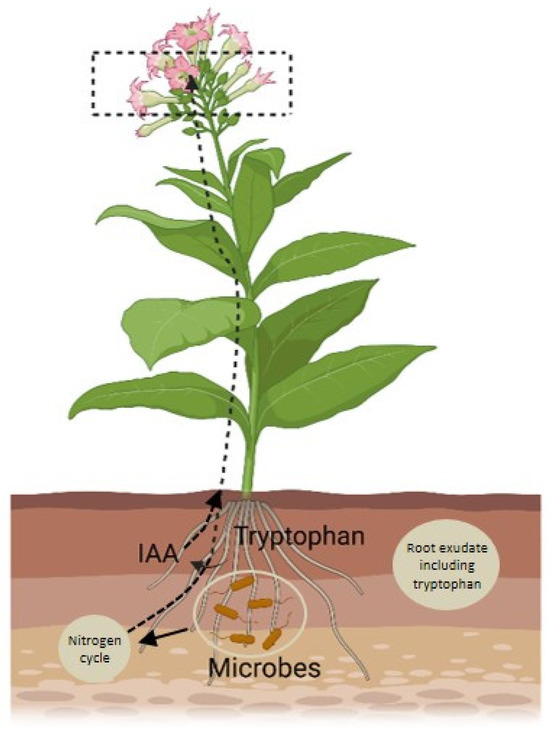
Figure 2.
Schematic diagram illustrates the role of the rhizospheric microbiome in promoting floral development. The microbiome converts tryptophan into IAA (indole-3-acetic acid) and enhances the nitrogen cycle, both of which regulate floral development. The figure is adapted from the study reported by [53].
2. Genetic Regulation of Flowering
2.1. Overview of Key Genes Involved in Flowering Time Regulation
For plants to successfully reproduce, blossoming at the right time is essential. It is controlled by a complex interaction between genetic pathways and environmental signals [54]. Several blooming processes are coordinated by genes such as Leafy (LFY), blooming Locus T (FT), and Suppressor of Overexpression of CO1 (SOC1) [55]. FT genes play an important part in the regulation of time required for flowering in plants by interacting with various transcriptional regulators. These genes are members of the Phosphatidylethanolamine Binding Protein (PEBP) family and play a vital role in plant development [56]. Several species of plants that have FT genes are thoroughly investigated, suggesting their significance in stimulating inflorescence development and flowering [57]. The regulation of FT family genes in temperate grasses is influenced by the monocot-specific gene Indeterminate1 (BdID1), which affects the transition to flowering [58]. Different plant species have specific SOC1 genes involved in flowering control. For instance, Chrysanthemum morifolium cv. Jimba expresses CmSOC1, which induces early flowering under short-day conditions by upregulating floral identity genes like LFY and APETALA1 [59]. Three SOC1 paralogs, MtSOC1a, MtSOC1b, and MtSOC1c, in Medicago truncatula provide overlapping roles in the transition from dormant to flowering. Triple mutants exhibited a phenotype of non-flowering [60]. BrcSOC1, found in pak choi, is overexpressed in Arabidopsis thaliana and causes a significant increase in flowering by positively impacting genes downstream, such as AGAMOUS-LIKE 24 and LFY [61].
LFY genes are associated with flowering regulation in various plants, such as DlLFY in Dimocarpus longan [62] PmLFY-like in Prunus mume [63], and MsLFY in Medicago sativa [64] play crucial roles in regulating the timing of flowering in plants. These genes encode transcription factors that control floral growth and meristem differentiation. Overexpression of the LFY gene causes Arabidopsis thaliana to bloom and undergo morphological changes, suggesting that the gene functions similarly across all plant species. Through interactions with other proteins, including ZFP4 and TFL1, LFY genes influence flowering processes. The floral repressors MADS Affecting Flowering 4 (MAF4) and MAF5, as well as the LFY gene, interact to regulate floral transition in Arabidopsis thaliana. LDL1 and LDL2 are essential in this interaction [65]. These interactions demonstrate the complex relationship of regulators that influence plant flowering processes.
2.2. Discussion of Major Flowering Pathways (Photoperiod, Vernalization, Autonomous Pathways)
Both photoperiod and vernalization are known to affect flowering mechanisms in different plants. Arabidopsis thaliana flower in the spring or early summer when the days grow longer because of the plant’s resilient photoperiod sensitivity. Mutants with a reduced response to day duration were used to isolate mutations interacting with these responses [66]. These mutants were divided into two classes: those that flower under long days but not short ones and those that flower under short days but flower earlier than wild-type plants. Circadian rhythms are generally affected by some of the mutations that occur in early flowering during short days. In addition, about 6% of genes expressed properly are controlled by the circadian clock [67]. These circadian rhythms are generally affected by those types of mutations responsible for reducing response to day length. This, in turn, leads to early flowering in short days. These mutations generally impact genes like Early Flowering 3 (ELF3), Late Elongated Hypocotyl (LHY), Timing of Chlorophyll a/b Binding Protein 1 (TOC1), and the Circadian Clock Associated 1 (CCA1). Because the circadian clock is also involved in regulating the expression of these genes, mRNAs for LHY and CCA1 only build up in the morning, and ELF3 and TOC1 only in the evening. The central mechanisms that produce circadian rhythms in plants may include LHY, CCA1, and TOC1. The expression pattern and sequence of CCA1 and LHY are comparable, as described in Figure 3 [68], and have some genetic redundancy [69]. Circadian rhythms cycle more quickly, and plants blossom earlier in short days in the LHY, CCA1 double mutant or the TOC1 single mutant than in wild-type plants [70]. It was suggested that LHY, CCA1, and TOC1 function together in a transcriptional feedback loop whereby the only expressed TOC1 at night promotes the expression of LHY/CCA1 at dawn, suppressing the expression of TOC1 [69]. The circadian rhythms were reported to stop when ELF3 mutants were exposed to prolonged photoperiods or continuous light [71], signifying the role of photoperiod in blossoming. Squamosa Promoter-Binding (SPB) genes, influenced by the circadian clock, play an important part in regulating the shift between vegetative growth and flowering. Additionally, miR156 and miR172 are involved in controlling some SPB genes during the flowering process [72].
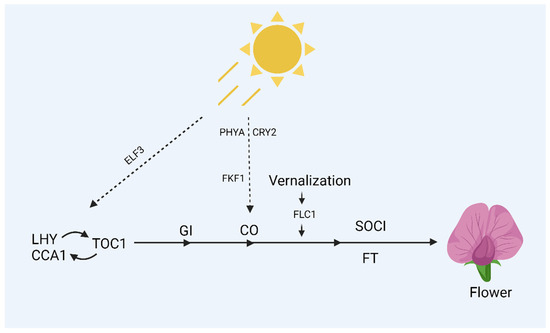
Figure 3.
A molecule hierarchy regulating flowering in response to photoperiod. Arrows represent promotive effects between genes, while perpendicular lines represent repressive effects.
Apart from photoperiod, vernalization also plays a critical role in the transition from the vegetative stage to flowering. Vernalization is the process of prolonged contact with the winter’s cold to promote the blossoming of plants [73]. The expression of a gene that codes for flowering repression is reduced in plants during vernalization. On the other hand, prolonged exposure to cold causes epigenetic modifications in the winter that last until the spring, which speeds up the next year’s flowering [73,74]. According to a study, the primary components involved in blooming time regulation by vernalization are FLC and FRIGIDA (FRI). Both FLC and FRI reduce flowers. By upregulating FLC expression, FRI negatively affects the length of blooming time [75]. The FRI gene is functional in winter-annual Arabidopsis thaliana plants. At the same time, it has been eliminated genomically in summer-annual plants [76], resulting in summer-annual plants blossoming earlier. Plants express high levels of FLC before vernalization to inhibit flowering. Winter cold exposure over an extended period suppresses the production of FLC, which releases SOC1 and FT [77]. For the following spring’s floral transition, consistent suppression of FLC through vernalization is essential. FLC expressions are suppressed during vernalization due to epigenetic control. Polycomb Group Proteins (PcG) regulate epigenetic modifications that underline the repression of FLC through vernalization [78]. Histone H3 Lysine 27 Trimethylation (H3K27me3) develops during vernalization on enrichment of the Polycomb Repressive Complex 2 (PRC2) complex at FLC chromatin [79]. H3K27me3 increases at FLC because of VAL1 and VAL2′s direct interaction with the PRC2 complex to bring PHD-PRC2 to FLC. The FLC locus produces noncoding RNAs that support FLC silencing in addition to the PHD2-PRC2 complex [80].
2.3. Summary of Current Understanding of Genetic Regulation in Ornamental Plants
Recent investigations of genetic control in ornamentals focus on four areas: genomics, gene editing, molecular breeding, and polymerization for the improvement of some ornamentation traits (such as scent, color, flower shape, etc.) [81]. The molecular mechanisms of flowering in the ornamental species, where floral and fragrance phenotypes are under strict genetic control, are associated with flower development pathways, gene expression profiles, and the chemistry behind the scent aromas that can be modified utilizing biotechnological approaches [82]. Genetic engineering and genome-editing techniques have been widely used in the genetic manipulation of ornamental plants for trait improvement, including transgenic technology to produce blue flowers and CRISPR/Cas9-mediated precise gene editing [83]. In addition, special promoters are necessary for their expression in transgenic plants and overall improvements in the discovery of new types of cultivars [83]. Understanding disease resistance’s genetic and epigenetic regulation is important to help improve their genetic resources and develop resilient plant varieties through breeding and transgenics [84].
3. Hormonal Regulation of Flowering
3.1. Role of Key Hormones in Flowering
The flower indicates a change in the developmental stage of the plant from the vegetative to the reproductive stage [85]. Plant hormones, such as GA, ABA, cytokinin, ethylene, and auxin, have been identified as regulators of various developmental processes, including seed germination, plant growth, senescence, and flowering. The precise involvement of plant hormones in controlling the flowering process has long been a subject of debate [86] however, the literature suggests that endogenous hormones play an active role in flower bud differentiation [87] and the development of flower organs [88]. Auxin was previously noted as a crucial regulator of floral growth, but until recently, its function in flower development was unclear [89]. Auxins may impact floral initiation by inhibiting stem cells’ pluripotency or stimulating the genes that generate floral organ primordia [90]. Auxins significantly regulate flowers and floral organs [89]. Development of ovule and stamen in Hyacinthus from explants of perianth is influenced by different ratios of auxin and cytokinin [91]. ABA hormone in plants serves an important function in flowering. Several studies involving ABA mutants suggest a detrimental impact on Arabidopsis flowering [92]. Saffron has been reported to be negatively impacted by ABA in terms of induction of flowering [93].
Apart from auxin and ABA, GA has also been reported to play a critical role in flowering. It has been demonstrated that GA regulates all flowering stages, including floral growth and induction [94]. GA has been positively correlated with the induction of flowering in numerous species of plants, such as Arabidopsis, Nicotiana, and radish (Raphanus sativus) [86]. GA regulates the initiation and development of the flowers, which is critical for the fertility of males and females [95]. GA-deficient mutants in Solanum lycopersicum and Arabidopsis thaliana displayed abnormalities in development of stamen [96]. On the other hand, extreme GA deficiency resulted in sterility in female flowers [97]. The application of GAs and GA9 (a precursor of GA) helps in restoring the normal development of flowers. GA is also known for its role in corolla development in Glechoma hederacea [98]. Hu [99] identified stamens or flower receptacles as two potential sites for bioactive GA synthesis in Arabidopsis thaliana flowers and suggested that GAs are transported from these organs to promote petal growth. GA-deficit mutants produced short stamen and short filaments. The capability of self-pollination was also compromised in these plants [100]. In coordination with JA, GA also regulates late stamen development. In contrast, GA alone mediated early anther development [101].
In addition to model plants, the role of GA in the process of flowering has been investigated in various geophytes, including Tulipa gesneriana, Anemone coronaria L., Zantedeschia aethiopica, Allium sativum, Genus Lilium, Saffron, Paeonia and Hyacinthus, etc. [102]. Within the genus Zantedeschia, GA stimulates the initiation and development of floral growth, with the number of flower clusters contingent upon the specific dosages and duration of GA application [103]. Various studies on saffron have further identified that GA plays a contrary role in the control of flowering [93]. GA may decrease floral initiation, as reported in a study that investigated the signaling mechanism of hormones during the induction of flowering and its subsequent developmental stages [104]. GA is important in elongation of the pollen tube and germination of pollen [105]. The study also revealed non-germination of pollen in mutant varieties that could not produce GA. Upon supplementation with exogenous GA, the same plants resulted in germination of pollen [105]. A study revealed up to a seven-fold increase in GA content of the pollens during the growth of pollen tubes. However, this may only be limited to certain species. In plant species like Lilium and Petunia hybrid, the GA content was 200-fold more in the pollen when compared to GA content of the ovarian tissue [106]. Treatment of conscious and hermaphroditic lines in Cucumis sativus with exogenous GA3 supplementation promotes male tendency [107]. In an experiment on female Cucumis sativus lines, it was reported that consistent supplementation of GA3 could prevent the continuous female phase [108]. On the other hand, experiments on Momordica charantia L. revealed improved fruiting and female flower induction upon exposure to low concentrations of GA3 [109]. From the above discussion, it may be proposed that plant hormones play unique roles that vary from species to species at various phases of the development of flowers.
3.2. Interaction Between Hormones and Genetic Pathways in Flowering Regulation
A complex interplay between hormonal signals and genetic pathways governs the regulation of the blooming process, ensuring that flowers bloom at the optimal time to maximize reproductive success [110]. In Arabidopsis thaliana, auxins have been demonstrated to control the expression of the genes APETALA 1 (AP1) and LFY during floral initiation [90]. Auxin has been reported to promote flowering in strawberries (Fragaria ananassa). This is achieved through the expression of FveARF4, which is controlled by auxin. The expressed FveARF4 binds to the regulators of the floral meristem recognition genes FULL (FUL) and APETALA1 (AP1) and triggers their expression, which in turn promotes blossoming in strawberries [111]. In addition to controlling plant flowering, ARF is also involved in controlling floral abscission. During the early stages of rose development, RhARF7 regulates petal abscission [112]. The local distribution of auxins can be modulated by certain transcription factors. This is achieved through the regulation of gene expression in genes like YUC and PIN [113]. YUCCA1 and PIN1 genes could be detected in the developing flower head in Asteraceae flower head formation studies [24]. Auxin (ARF3) was discovered to regulate the AGAMOUS and APETALA2 genes of flower organs passing through the developing stage [114]. Apart from auxins, cytokinins are considered to play an active part in the induction of flowering of several plant species. BAP has been reported to trigger the paralogs of FT genes to promote blooming in Arabidopsis thaliana [115]. In roses (Rosa indica), cytokinin stimulates flowering growth [116]. Cytokinin is also involved in strawberry flowering, where high levels of cytokinin are produced and transported to axillary buds. The auxin production thereby is inhibited in axillary buds due to high concentrations of cytokinins, which results in overcoming the dormancy [117,118]. Cytokinin also promotes flowering in saffron. This is achieved by cytokinin-modulated increases in LFY expression [119]. The function of APETALA1 in developing floral meristems in Arabidopsis thaliana is further controlled by the cytokinin pathway [120]. In short-day conditions, the GA pathway supports the activation of the SOC1 gene, which encourages flowering; however, this pathway does not affect the regulation of other flowering-related genes, such as FLC and FT [121]. At the same time, another study by [122] suggested reduced levels of GA2ox, an inhibitor of the GA pathway, and increased GA accumulations, indicating a beneficial role for GA in stimulating blooming. ABA regulates FT gene transcription through GI, CO, and SOC1 expressions. However, its specific mechanism of action to regulate flowering is unclear [123].
3.3. Hormonal Crosstalk and Its Impact on Flowering Induction
Plant hormones, i.e., GA, ABA, auxins, cytokinin, and ethylene, have been abundantly reported in a number of studies to take part in the regulation of several developmental processes; however, a significant research gap exists when it comes to the mechanism of regulation of the process of flowering. In plants like Raphanus sativus, Nicotiana, and Arabidopsis thaliana, GA has been linked to the induction of flowering [124]. On the other hand, in citrus plants, Vitis spp., and apples (Malus pumila), GA has been reported to have an inhibitory effect on flower induction [125]. Besides the model plants, species like saffron, lilies, Zantedeschia, tulips, garlic, Hyacinthus, lilium, and Anemone, etc., have also been explored for the potential of GA in regulating flowering [102]. Promotion of initiation and development of flowers in Zantedeschia and the inflorescence number is regulated upon exposure to GA. However, the exact nature of the effect depends upon exposure time and dosage [103]. Varieties of Arabidopsis thaliana that need low temperatures for flower initiation have been reported to induce flowers even at high temperatures when supplemented with exogenous GA [126]. The contradictory impact of GA in terms of flower induction in saffron has also been indicated in the literature. Renau-Morata et al. (2021) [93] suggested inhibitory role of GA in flower initiation and development; however, in another study, evidence suggests that the downregulation of a GA-pathway inhibitor like GA2ox promotes flower induction [122]. It is therefore pertinent to further explore the exact role of GA in terms of regulation of processes involving initiation of flowering and its subsequent development.
Along with GA, another essential plant hormone, i.e., ABA, also impacts flowering in plants. In Arabidopsis species, ABA mutant varieties have displayed a negative impact in terms of flowering [92]; however, the evidence is not enough to elucidate a particular mode of action when it comes to the regulation of flowering upon exposure to ABA. Studies depict that ABA is responsible for regulating the transcription of FT gene by controlling the expression pattern of SOC1, GI, and CO [123]. Renau-Morata et al. (2021) [93] suggested the inhibitory role of ABA in flowering induction in saffron. Cytokinins on the other hand, have been reported to positively influence the induction of flowering in a number of plants, including Arabidopsis thaliana [120]. A similar positive role of cytokinin in flower induction was also reported in a study on rose plants [116] and strawberry [117]. Cytokinins are also known to regulate the functioning of APETALA1 in order to establish floral meristems in Arabidopsis [120]. The auxin-to-cytokinin ratio plays a significant role in the ovule and stamen development from explants of parianth in Hyacinthus [91]. In a nutshell, it can be suggested that the role of plant hormones in various developmental stages of flowering is highly species-specific.
3.4. Recent Advances in Hormone Research and Their Implications for Ornamental Plant Breeding
Ornamental plants are an essential part of the horticultural industry, contributing significantly to the economy by producing and selling flowers, shrubs, and other decorative [127]. A primary area of interest for researchers has been breeding these plants to improve desired characteristics, including color, size, aroma, and stress tolerance [128]. Since hormones are essential for controlling a plant’s growth and development, new directions in hormone research have been explored in breeding ornamental plants [129]. Recent research has delved deeper into the hormonal pathways and their interactions within plants [130]. The development of synthetic hormones and growth regulators has provided new tools for breeders to influence plant growth and development [131]. Compounds that mimic natural hormones can induce rooting, flowering, and fruiting in ornamental plants, enhancing their esthetic appeal and market value [132]. By manipulating hormone pathways, breeders can enhance traits such as flower size, color, and longevity. For example, modifying ethylene sensitivity in ornamental flowers can delay senescence, resulting in longer-lasting blooms [133]. According to Chandler [134], plants can become more visually appealing by enhancing flower symmetry and branching patterns through hormonal regulation. Hormones are crucial in plant responses to abiotic stresses such as drought, salinity, and temperature. Advances in hormone research have led to ornamental plants with enhanced stress tolerance [135]. For instance, overexpressing genes involved in ABA biosynthesis can improve drought tolerance, making ornamental plants more resilient in various climates [136].
Hormones are integral to in vitro propagation techniques and are widely used in the ornamental plant industry for mass production [137]. Understanding the hormonal requirements for tissue culture has enabled the efficient propagation of numerous ornamental species, ensuring uniformity and quality in the final product [137]. Hormonal research has facilitated overcoming hybridization barriers in ornamental plant breeding [138]. For example, cytokinins can promote cell division and development in hybrid embryos, increasing the success rate of interspecific crosses [139]. Advanced breeding programs now incorporate hormone treatments to synchronize flowering times, enhancing the efficiency of hybridization efforts. Studies on ethylene, a hormone associated with fruit ripening and flower senescence, have led to the development of ethylene-resistant varieties. These varieties exhibit prolonged flower life, a trait that is highly desirable for cut and potted plants [140]. Research on GA has enabled the creation of dwarf varieties of ornamental plants [141]. These compact plants are popular in urban gardening and landscape design due to their manageable size and reduced maintenance needs [142]. Advances in auxin research have improved the rooting efficiency of cuttings, a common propagation method in the ornamental plant industry. Enhanced rooting leads to higher propagation success rates and better plant establishment [143].
4. The Microbiome and Flowering Induction
4.1. Introduction to the Plant Microbiome and Its Importance in Plant Health and Development
The interaction between plants and the wide variety of microbes contributing to their microbiome is essential to their growth and development, as shown in Table 1. Microorganisms play significant functions in these symbiotic relationships, assisting plants by increasing nutrient intake, stimulating hormones, and improving stress tolerance [144]. While it impacts plant health through interactions between microbes and plants, the rhizosphere, the area around plant roots, is particularly important for these interactions [145]. Additionally, plant microbiomes may contain plant growth-promoting bacteria (PGPB), which promote plant development and function as biocontrol agents to neutralize phytopathogens, improving crop fitness in agriculture [146]. For example, endophytes colonize various crop parts and benefit the hosts, rhizobia fix nitrogen for legumes, and mycorrhizae assist in nutrient absorption [147]. Sustainable agriculture depends upon comprehending and utilizing these complex plant–microbe interactions, illustrating the need for further research and useful applications in agricultural biotechnology [144].

Table 1.
Beneficial microbes’ role in flowering growth.
4.2. Evidence Suggesting a Role of the Microbiome in Flowering Induction
Studies involving the exploration of plant–microbe interaction have demonstrated the critical function of the microbiome in flowering induction. According to studies, when flowers are exposed to phytopathogens like Erwinia amylovora, their microbiome, especially the stigma, exhibits dynamic changes that influence the development of disease [163]. Furthermore, it has been found that nectar-dwelling bacteria, including Acinetobacter, take advantage of pollen nutrition by stimulating pollen germination and bursting, suggesting a direct microbial influence on pollen physiology and possibly on plant reproduction [164]. In addition, recent research discovered that insect-vectored bacteria can genetically modify plant cells to produce complex structures like galls. This demonstrates how microorganisms can control plant development to produce unique interactions between insects and plants [165]. Studies have demonstrated that the microbial communities in the rhizosphere can control the time of flowering by influencing the availability of nitrogen and the generation of phytohormones [53]. Moreover, plant-associated microbial communities, particularly floral microorganisms, can change the aroma of plants and flowers, thus changing insect reactions and possibly pollination [166].
4.3. Mechanisms by Which Microbiota May Influence Flowering Time (e.g., Hormone Production, Nutrient Availability)
Microbiota can influence flowering time through multiple mechanisms. Research has demonstrated that rhizosphere microbial communities can regulate the timing of flowering by modulating nutrient availability and hormone signaling pathways, which affect the gene expression associated with flowering time [53]. For example, studies on plants like Arabis alpina have shown that root microbiota plays a crucial role in determining flowering time, with factors such as soil type and the duration of plant residence in the soil being significant determinants of root microbiota variation [167]. Additionally, soil microbial communities have been identified as key influencers of flowering time, with different soil microbiota altering selection patterns and contributing to the phenotypic plasticity of flowering time in plants like Boechera stricta [168]. These interactions between microbes and plants impact various phenological transitions, highlighting the significant role of microbiota in nutrient acquisition, phytohormone signaling, and gene expression related to flowering time.
5. Interconnection Between Endogenous and External Factors
5.1. Integration of Findings from Endogenous and External Factors Studies to Provide a Holistic Understanding of Flowering Induction
This review paper thoroughly explains flowering induction by investigating microbiome, hormones, and genetics studies. Genetically, blooming is regulated by a network of genes that react to various internal and external stimuli. Important genes such as SOC1, blooming LOCUS T (FT), and CO flowers are primarily responsible for the photoperiodic regulation of blooming [169]. Meanwhile, CO, a crucial regulator, promotes FT expression in response to long-day conditions. Ultimately, the FT protein proceeds from the leaves to the shoot’s apical meristem, interacting with SOC1 to initiate the flowering process [170]. FLC, a floral regulator gene, is epigenetically inhibited by FT and SOC1 triggering, resulting in vernalization, which promotes flowering [171]. Plants perform a complex process of induction of blooming, which is regulated by hormone signals, genetic pathways, and environmental factors. The photoperiodic, vernalization, autonomous, and GA pathways are among the important genetic pathways connected to this process that have been identified through research on Arabidopsis thaliana and other plant species [172]. Furthermore, hormone regulation significantly influences blooming control, even though this pathway has not historically been considered important. Hormonal pathways may potentially mediate stress signals [173]. The formation of flower buds in pears has been associated with regulating the circadian clock by specific genes, such as ELF3, demonstrating the genetic foundation of blooming [174]. Specific microbial populations can produce plant hormones or hormone-like substances that affect flowering. For instance, rhizobacteria that generate the IAA might impact blooming indirectly by influencing the hormonal balance of the plant to promote root growth [175]. Integration of information obtained from genes, hormones, and microbiome is essential for controlling blooming. Hormonal signals can influence the genetic pathways that regulate flowering, and the microbiome can potentially impact these processes [176].
5.2. Implications for Ornamental Plant Breeding and Cultivation Practices
In recent years, there has been a major change in the breeding and cultivation of ornamental plants, with an increase in the application of biotechnological technologies such as genome editing and genetic engineering to enhance particular traits like unique flowers [177]. By methods like somatic embryogenesis and organogenesis, in vitro cultures are essential for propagating ornamentals, providing solutions to the lack of plant materials, and boosting diversity [137]. Sustainability is a significant problem in the production of ornamentals. It aims to lessen adverse environmental effects by altering cultivation practices such as integrated fertilizer management and the use of recyclable materials [178]. Genetic engineering and interspecific hybridization are techniques used to address abiotic stress tolerance in ornamental crops, leading to the development of high-quality, stress-tolerant ornamental varieties [179]. For breeders seeking to commercialize genetically modified or gene-edited ornamental plants, challenges exist despite the developments, including the need for international harmonization in genetic modification regulations and regulatory challenges [180].
5.3. Future Directions for Research in Understanding the Complex Networks Underlying Flowering in Ornamental Plants
Analyzing gene co-expression networks to identify the genetic mechanisms influencing floral variety may be the next step in investigating the intricate networks behind the flowering of ornamental plants [181]. Examining the regulatory networks associated with flower development, particularly in species like Chrysanthemum morifolium, can provide insight into the mechanisms controlling the determination of floral morphology [182]. More understanding of the many gene interactions influencing important agronomic characteristics might be realized with further investigations into the molecular basis of photoperiodic flowering regulation, as demonstrated in soybeans, using sophisticated network inference techniques like CausNet [183]. Combining transcriptome data with network analysis techniques might enable further studies to identify the important genes, regulatory relationships, and modules that control the blossoming processes of ornamental plants. This allows for breeding and genetic modification strategies to be more specific.
6. Summary and Future Prospects
The regulation of flowering in plants is a multifaceted process governed by intricate interactions between endogenous and external factors, as well as environmental cues such as vernalization and photoperiodism. Genetic pathways, including photoperiod and vernalization pathways, play a pivotal role in transitioning plants from vegetative to reproductive stages. Key genes, such as FT, FLC, LFY, and SOC1, coordinate responses to these external signals, with vernalization epigenetically repressing flowering inhibitors like FLC to facilitate bloom induction in response to prolonged cold exposure. Similarly, photoperiodism, regulated by circadian-controlled genes like SPB and downstream effectors, aligns flowering with seasonal and day-length changes to maximize reproductive success. Hormones such as GA, auxins, and cytokinins modulate flowering by influencing meristem size, organ differentiation, and developmental timing. Hormonal crosstalk is crucial in integrating genetic signals with environmental stimuli, while the microbiome plays an emerging role by producing hormones like IAA and enhancing nutrient availability, further influencing flowering time and quality. Despite recent advancements, gaps remain in our understanding of how these systems interact holistically. For instance, the precise molecular and epigenetic mechanisms integrating photoperiod and vernalization pathways with hormonal and microbiome inputs are not fully elucidated. The summary of this study is illustrated in Figure 4. Future research should use high-resolution multi-omics approaches to unravel the integration of vernalization and photoperiod pathways with endogenous (genetic) and external reasons (biotic and abiotic stress) interactions. Investigating microbiome contributions to flowering regulation under diverse conditions can promote sustainable practices. Advanced biotechnological tools like CRISPR-Cas9 can tailor flowering traits and overcome seasonal limitations.
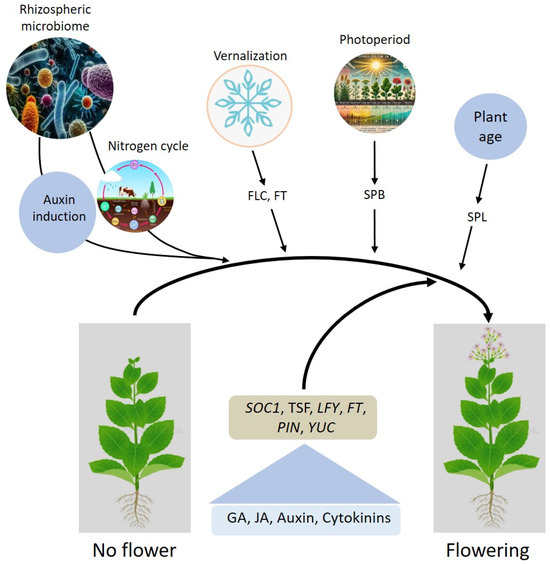
Figure 4.
Factors regulating the transition from vegetative to flowering stages in plants. Various environmental and developmental signals, including the rhizospheric microbiome, nitrogen cycle, auxin induction, vernalization, photoperiod, and plant age, converge to regulate flowering. Key pathways and genes involved include FLC (FLOWERING LOCUS C) and FT (FLOWERING LOCUS T) for vernalization, SPB (SUPPRESSOR OF PHYTOCHROME B) for photoperiod, and SPL (SQUAMOSA PROMOTER BINDING PROTEIN-LIKE) for plant age. These signals modulate the expression of central flowering regulators such as SOC1 (SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1), TSF (TWIN SISTER OF FT), LFY (LEAFY), FT, PIN (PIN-FORMED), and YUC (YUCCA). Hormones like gibberellic acid (GA), jasmonic acid (JA), auxin, and cytokinins further facilitate the transition from vegetative growth to flowering.
Author Contributions
M.A., L., S.S.H. and M.A.K. wrote the original draft; R.J., S.B. and A.A.-H. collected all the data, drew the figures, and revised the original draft; K.-M.K. and S.A. supervised and arranged resources. All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable.
Data Availability Statement
The data are available in the manuscript.
Acknowledgments
This research was funded by the “Cooperative Research Program for Agri-culture Science and Technology Development (Project No. RS-2025-00512751)”, Rural Develop-ment Administration, Republic of Korea.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Volckaert, E.; Gobin, B.; Verheye, W. Ornamental plants and floriculture. In Soils, Plant Growth and Crop Production; Verheye, W.H., Ed.; EOLSS Publications: Paris, France, 2010; Volume 3. [Google Scholar]
- Yahia, E.M. Classification of horticultural commodities. In Postharvest Technology of Perishable Horticultural Commodities; Elsevier: Amsterdam, The Netherlands, 2019; pp. 71–97. [Google Scholar]
- Gabellini, S.; Scaramuzzi, S. Evolving consumption trends, marketing strategies, and governance settings in ornamental horticulture: A grey literature review. Horticulturae 2022, 8, 234. [Google Scholar] [CrossRef]
- Hendricks, J.; Briercliffe, T.; Oosterom, B.; Treer, A.; Kok, G.; Edwards, T.; Kong, H. Ornamental Horticulture, A Growing Industry. In International Vision Project Reports; AIPH Horticulture House: Chilton Didcot, Oxfordshire, UK, 2019. [Google Scholar]
- Löbke, A. Record Sales for the Flower and Plant Market; Messe Essen GmbH: Essen, Germany, 2022. [Google Scholar]
- Bugallo, V.; Facciuto, G. Selection process in ornamental plant breeding. Ornam. Hortic. 2023, 29, 68–75. [Google Scholar] [CrossRef]
- Arslan, M.; Yanmaz, R. Use of ornamental vegetables, medicinal and aromatic plants in urban landscape design. In Proceedings of the II International Conference on Landscape and Urban Horticulture 881, Bologna, Italy, 9–13 June 2009; pp. 207–211. [Google Scholar]
- Coyago-Cruz, E.; Moya, M.; Méndez, G.; Villacís, M.; Rojas-Silva, P.; Corell, M.; Mapelli-Brahm, P.; Vicario, I.M.; Meléndez-Martínez, A.J. Exploring Plants with Flowers: From Therapeutic Nutritional Benefits to Innovative Sustainable Uses. Foods 2023, 12, 4066. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.J.A.J. Allusions to Floral Emblem of G20 Nations. Int. J. Multidiscip. Res. 2023, 5. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. An overview on the market of edible flowers. Food Rev. Int. 2020, 36, 258–275. [Google Scholar] [CrossRef]
- Urakami, J.; Huss, E.; Nagamine, M.; Czamanski-Cohen, J.; Zaccai, M. The Emotional Experience of Flowers: Zoomed In, Zoomed Out and Painted. Horticulturae 2022, 8, 668. [Google Scholar] [CrossRef]
- Sood, Y.; Lal, M.; Kalia, A.; Verma, S. Edible Flowers: Super Foods with Potential Health Benefits. Int. J. Plant Soil Sci. 2024, 36, 213–221. [Google Scholar] [CrossRef]
- Marchioni, I.; Gabriele, M.; Carmassi, G.; Ruffoni, B.; Pistelli, L.; Pistelli, L.; Najar, B. Phytochemical, Nutritional and Mineral Content of Four Edible Flowers. Foods 2024, 13, 939. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Badwaik, L.S.; Annapure, U.; Casanova, F.; Alaskar, K. A review on the journey of edible flowers from farm to consumer’s plate. Appl. Food Res. 2023, 3, 100312. [Google Scholar] [CrossRef]
- Pires, E.D.O., Jr.; Di Gioia, F.; Rouphael, Y.; García-Caparrós, P.; Tzortzakis, N.; Ferreira, I.C.; Barros, L.; Petropoulos, S.A.; Caleja, C. Edible flowers as an emerging horticultural product: A review on sensorial properties, mineral and aroma profile. Trends Food Sci. Technol. 2023, 137, 31–54. [Google Scholar]
- Chávez-Hernández, E.C.; Quiroz, S.; García-Ponce, B.; Álvarez-Buylla, E.R. The flowering transition pathways converge into a complex gene regulatory network that underlies the phase changes of the shoot apical meristem in Arabidopsis thaliana. Front. Plant Sci. 2022, 13, 852047. [Google Scholar] [CrossRef]
- Sasaki, E.; Frommlet, F.; Nordborg, M. The genetic architecture of the network underlying flowering time variation in Arabidopsis thaliana. bioRxiv 2017. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, E.; Liu, Q.A.; Wang, J. High CO2 adaptation mechanisms revealed in the miR156-regulated flowering time pathway. PLoS Comput. Biol. 2023, 19, e1011738. [Google Scholar]
- He, T.; Hill, C.B.; Angessa, T.T.; Zhang, X.-Q.; Chen, K.; Moody, D.; Telfer, P.; Westcott, S.; Li, C. Gene-set association and epistatic analyses reveal complex gene interaction networks affecting flowering time in a worldwide barley collection. J. Exp. Bot. 2019, 70, 5603–5616. [Google Scholar] [PubMed]
- Tran, L.M.H. The effect of phytohormones on the flowering of plants. Plant Sci. Today 2023, 10, 138–142. [Google Scholar]
- Wang, X.; Zhao, F.; Wu, Q.; Xing, S.; Yu, Y.; Qi, S. Physiological and transcriptome analyses to infer regulatory networks in flowering transition of Rosa rugosa. Ornam. Plant Res. 2023, 3, 4. [Google Scholar]
- Goh, C.; Yang, A. Effects of growth regulators and decapitation on flowering of Dendrobium orchid hybrids. Plant Sci. Lett. 1978, 12, 287–292. [Google Scholar] [CrossRef]
- Bhalerao, R.P.; Bennett, M.J. The case for morphogens in plants. Nat. Cell Biol. 2003, 5, 939–943. [Google Scholar]
- Zoulias, N.; Duttke, S.H.; Garcês, H.; Spencer, V.; Kim, M. The role of auxin in the pattern formation of the Asteraceae flower head (capitulum). Plant Physiol. 2019, 179, 391–401. [Google Scholar]
- Blanchard, M.G.; Runkle, E.S. Benzyladenine promotes flowering in Doritaenopsis and Phalaenopsis orchids. J. Plant Growth Regul. 2008, 27, 141–150. [Google Scholar]
- Su, W.-R.; Chen, W.-S.; Koshioka, M.; Mander, L.N.; Hung, L.-S.; Chen, W.-H.; Fu, Y.-M.; Huang, K.-L. Changes in gibberellin levels in the flowering shoot of Phalaenopsis hybrida under high temperature conditionswhen flower development is blocked. Plant Physiol. Biochem. 2001, 39, 45–50. [Google Scholar]
- Hew, C.; Clifford, P. Plant growth regulators and the orchid cut-flower industry. Plant Growth Regul. 1993, 13, 231–239. [Google Scholar] [CrossRef]
- Li, J.; Jiang, J.; Qian, Q.; Xu, Y.; Zhang, C.; Xiao, J.; Du, C.; Luo, W.; Zou, G.; Chen, M. Mutation of rice BC12/GDD1, which encodes a kinesin-like protein that binds to a GA biosynthesis gene promoter, leads to dwarfism with impaired cell elongation. Plant Cell 2011, 23, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Ye, T.; Lu, Y.; Chen, X.; Wu, Y. The inhibitory effect of ABA on floral transition is mediated by ABI5 in Arabidopsis. J. Exp. Bot. 2013, 64, 675–684. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.; Matusova, R.; Zhongkui, S.; Beale, M.H. Secondary metabolite signalling in host–parasitic plant interactions. Curr. Opin. Plant Biol. 2003, 6, 358–364. [Google Scholar] [CrossRef]
- Ruttink, T.; Arend, M.; Morreel, K.; Storme, V.; Rombauts, S.; Fromm, J.; Bhalerao, R.P.; Boerjan, W.; Rohde, A. A molecular timetable for apical bud formation and dormancy induction in poplar. Plant Cell 2007, 19, 2370–2390. [Google Scholar] [CrossRef]
- Tylewicz, S.; Petterle, A.; Marttila, S.; Miskolczi, P.; Azeez, A.; Singh, R.K.; Immanen, J.; Mähler, N.; Hvidsten, T.R.; Eklund, D.M. Photoperiodic control of seasonal growth is mediated by ABA acting on cell-cell communication. Science 2018, 360, 212–215. [Google Scholar] [PubMed]
- Ahmad, S.; Lu, C.; Gao, J.; Ren, R.; Wei, Y.; Wu, J.; Jin, J.; Zheng, C.; Zhu, G.; Yang, F. Genetic insights into the regulatory pathways for continuous flowering in a unique orchid Arundina graminifolia. BMC Plant Biol. 2021, 21, 587. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Chen, W.-S.; Chen, W.-H.; Hung, L.-S.; Chang, P.-S. Influence of abscisic acid on flowering in Phalaenopsis hybrida. Plant Physiol. Biochem. 2002, 40, 97–100. [Google Scholar] [CrossRef]
- Burgess, E.C.; Schaeffer, R.N. The floral microbiome and its management in agroecosystems: A perspective. J. Agric. Food Chem. 2022, 70, 9819–9825. [Google Scholar] [CrossRef]
- Brysch-Herzberg, M. Ecology of yeasts in plant–bumblebee mutualism in Central Europe. FEMS Microbiol. Ecol. 2004, 50, 87–100. [Google Scholar] [CrossRef]
- Chappell, C.R.; Fukami, T. Nectar yeasts: A natural microcosm for ecology. Yeast 2018, 35, 417–423. [Google Scholar] [CrossRef]
- Figueroa, L.L.; Blinder, M.; Grincavitch, C.; Jelinek, A.; Mann, E.K.; Merva, L.A.; Metz, L.E.; Zhao, A.Y.; Irwin, R.E.; McArt, S.H. Bee pathogen transmission dynamics: Deposition, persistence and acquisition on flowers. Proc. R. Soc. B 2019, 286, 20190603. [Google Scholar] [PubMed]
- Lindow, S.E.; Suslow, T.V. Temporal dynamics of the biocontrol agent Pseudomonas fluorescens strain A506 in flowers in inoculated pear trees. Phytopathology 2003, 93, 727–737. [Google Scholar] [CrossRef] [PubMed]
- von Arx, M.; Moore, A.; Davidowitz, G.; Arnold, A.E. Diversity and distribution of microbial communities in floral nectar of two night-blooming plants of the Sonoran Desert. PLoS ONE 2019, 14, e0225309. [Google Scholar] [CrossRef]
- Junker, R.R.; Loewel, C.; Gross, R.; Dötterl, S.; Keller, A.; Blüthgen, N. Composition of epiphytic bacterial communities differs on petals and leaves. Plant Biol. 2011, 13, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Hinton, D.; Bacon, C. The distribution and ultrastructure of the endophyte of toxic tall fescue. Can. J. Bot. 1985, 63, 36–42. [Google Scholar]
- Hodgson, S.; de Cates, C.; Hodgson, J.; Morley, N.J.; Sutton, B.C.; Gange, A.C. Vertical transmission of fungal endophytes is widespread in forbs. Ecol. Evol. 2014, 4, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Peay, K.G.; Belisle, M.; Fukami, T. Phylogenetic relatedness predicts priority effects in nectar yeast communities. Proc. R. Soc. B Biol. Sci. 2012, 279, 749–758. [Google Scholar]
- Morris, M.M.; Frixione, N.J.; Burkert, A.C.; Dinsdale, E.A.; Vannette, R.L. Microbial abundance, composition, and function in nectar are shaped by flower visitor identity. FEMS Microbiol. Ecol. 2020, 96, fiaa003. [Google Scholar] [CrossRef] [PubMed]
- Boutroux, L. Sur la conservation des ferments alcooliques dans la nature. Ann. Des Sci. Nat. Série IV Bot. 1884, 17, 145–209. [Google Scholar]
- Belisle, M.; Peay, K.G.; Fukami, T. Flowers as islands: Spatial distribution of nectar-inhabiting microfungi among plants of Mimulus aurantiacus, a hummingbird-pollinated shrub. Microb. Ecol. 2012, 63, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Lachance, M.-A.; Starmer, W.T.; Rosa, C.A.; Bowles, J.M.; Barker, J.S.F.; Janzen, D.H. Biogeography of the yeasts of ephemeral flowers and their insects. FEMS Yeast Res. 2001, 1, 1–8. [Google Scholar] [PubMed]
- Samuni-Blank, M.; Izhaki, I.; Laviad, S.; Bar-Massada, A.; Gerchman, Y.; Halpern, M. The role of abiotic environmental conditions and herbivory in shaping bacterial community composition in floral nectar. PLoS ONE 2014, 9, e99107. [Google Scholar]
- Chanbusarakum, L.; Ullman, D. Characterization of bacterial symbionts in Frankliniella occidentalis (Pergande), Western flower thrips. J. Invertebr. Pathol. 2008, 99, 318–325. [Google Scholar] [CrossRef]
- Ambika Manirajan, B.; Ratering, S.; Rusch, V.; Schwiertz, A.; Geissler-Plaum, R.; Cardinale, M.; Schnell, S. Bacterial microbiota associated with flower pollen is influenced by pollination type, and shows a high degree of diversity and species-specificity. Environ. Microbiol. 2016, 18, 5161–5174. [Google Scholar]
- Russell, A.L.; Rebolleda-Gómez, M.; Shaible, T.M.; Ashman, T.L. Movers and shakers: Bumble bee foraging behavior shapes the dispersal of microbes among and within flowers. Ecosphere 2019, 10, e02714. [Google Scholar]
- Lu, T.; Ke, M.; Lavoie, M.; Jin, Y.; Fan, X.; Zhang, Z.; Fu, Z.; Sun, L.; Gillings, M.; Peñuelas, J. Rhizosphere microorganisms can influence the timing of plant flowering. Microbiome 2018, 6, 231. [Google Scholar]
- Putterill, J.; Laurie, R.; Macknight, R. It’s time to flower: The genetic control of flowering time. Bioessays 2004, 26, 363–373. [Google Scholar]
- Amasino, R.M.; Michaels, S.D. The timing of flowering. Plant Physiol. 2010, 154, 516–520. [Google Scholar] [PubMed]
- Colleoni, P.E.; van Es, S.W.; Winkelmolen, T.; Immink, R.G.; van Esse, G.W. Flowering time genes branching out. J. Exp. Bot. 2024, 75, 4195–4209. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, J.; Yin, H.; Li, X.; Liu, W.; Fan, Z. Function of FT in Flowering Induction in Two Camellia Species. Plants 2024, 13, 784. [Google Scholar] [CrossRef]
- Liu, B.; Woods, D.P.; Li, W.; Amasino, R.M. INDETERMINATE1-mediated expression of FT family genes is required for proper timing of flowering in Brachypodium distachyon. Proc. Natl. Acad. Sci. USA 2023, 120, e2312052120. [Google Scholar] [CrossRef]
- Jun, S.E.; Manzoor, M.A.; Kim, M.-J.; Youn, Y.; Nam, J.; Hyung, N.-I.; Kim, G.-T. Molecular cloning and functional characterization of CmSOC1 gene and its promoter region from Chrysanthemum morifolium. Sci. Hortic. 2024, 329, 112991. [Google Scholar]
- Poulet, A.; Zhao, M.; Peng, Y.; Tham, F.; Jaudal, M.; Zhang, L.; van Wolfswinkel, J.C.; Putterill, J. Gene-edited Mtsoc1 triple mutant Medicago plants do not flower. Front. Plant Sci. 2024, 15, 1357924. [Google Scholar] [CrossRef]
- Li, X.; Ping, A.; Qi, X.; Li, M.; Hou, L. Cloning, expression and functional analysis of the SOC1 homologous gene in pak choi (Brassica rapa ssp. Chinensis makino). Biotechnol. Biotechnol. Equip. 2022, 36, 848–857. [Google Scholar] [CrossRef]
- Jue, D.; Li, Z.; Zhang, W.; Tang, J.; Xie, T.; Sang, X.; Guo, Q. Identification and functional analysis of the LEAFY gene in longan flower induction. BMC Genom. 2024, 25, 308. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Li, Y.; Yang, Y.; Zhou, Y.; Zhao, K.; Zhang, Q. Isolation, functional characterization and evolutionary study of LFY1 gene in Prunus mume. Plant Cell Tissue Organ Cult. 2019, 136, 523–536. [Google Scholar] [CrossRef]
- Zhang, T.; Chao, Y.; Kang, J.; Ding, W.; Yang, Q. Molecular cloning and characterization of a gene regulating flowering time from Alfalfa (Medicago sativa L.). Mol. Biol. Rep. 2013, 40, 4597–4603. [Google Scholar] [CrossRef]
- Mahima; Chatterjee, S.; Singh, S.; Sarkar, A.K. LDL1 and LDL2 histone demethylases interact with FVE to regulate flowering in Arabidopsis. bioRxiv 2022. [Google Scholar] [CrossRef]
- Rédei, G.P. Supervital mutants of Arabidopsis. Genetics 1962, 47, 443. [Google Scholar] [PubMed]
- Harmer, S.L.; Hogenesch, J.B.; Straume, M.; Chang, H.-S.; Han, B.; Zhu, T.; Wang, X.; Kreps, J.A.; Kay, S.A. Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 2000, 290, 2110–2113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Y.; Tobin, E.M. Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell 1998, 93, 1207–1217. [Google Scholar] [PubMed]
- Alabadí, D.; Oyama, T.; Yanovsky, M.J.; Harmon, F.G.; Más, P.; Kay, S.A. Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 2001, 293, 880–883. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Wheatley, K.; Hanzawa, Y.; Wright, L.; Mizoguchi, M.; Song, H.-R.; Carré, I.A.; Coupland, G. LHY and CCA1 are partially redundant genes required to maintain circadian rhythms in Arabidopsis. Dev. Cell 2002, 2, 629–641. [Google Scholar] [CrossRef]
- Hicks, K.A.; Albertson, T.M.; Wagner, D.R. EARLY FLOWERING3 encodes a novel protein that regulates circadian clock function and flowering in Arabidopsis. Plant Cell 2001, 13, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Peng, D.; Zhou, Y.; Zhao, K. The genetic and hormonal inducers of continuous flowering in orchids: An emerging view. Cells 2022, 11, 657. [Google Scholar] [CrossRef] [PubMed]
- Amasino, R.M. Vernalization and flowering time. Curr. Opin. Biotechnol. 2005, 16, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H. Current understanding of flowering pathways in plants: Focusing on the vernalization pathway in Arabidopsis and several vegetable crop plants. Hortic. Environ. Biotechnol. 2020, 61, 209–227. [Google Scholar] [CrossRef]
- Choi, K.; Kim, J.; Hwang, H.-J.; Kim, S.; Park, C.; Kim, S.Y.; Lee, I. The FRIGIDA complex activates transcription of FLC, a strong flowering repressor in Arabidopsis, by recruiting chromatin modification factors. Plant Cell 2011, 23, 289–303. [Google Scholar] [CrossRef]
- Henderson, I.R.; Shindo, C.; Dean, C. The need for winter in the switch to flowering. Annu. Rev. Genet. 2003, 37, 371–392. [Google Scholar] [CrossRef] [PubMed]
- Bastow, R.; Mylne, J.S.; Lister, C.; Lippman, Z.; Martienssen, R.A.; Dean, C. Vernalization requires epigenetic silencing of FLC by histone methylation. Nature 2004, 427, 164–167. [Google Scholar] [CrossRef]
- Molitor, A.; Shen, W.-H. The polycomb complex PRC1: Composition and function in plants. J. Genet. Genom. 2013, 40, 231–238. [Google Scholar] [CrossRef]
- Wood, C.C.; Robertson, M.; Tanner, G.; Peacock, W.J.; Dennis, E.S.; Helliwell, C.A. The Arabidopsis thaliana vernalization response requires a polycomb-like protein complex that also includes VERNALIZATION INSENSITIVE 3. Proc. Natl. Acad. Sci. USA 2006, 103, 14631–14636. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.B.; Sung, S. Vernalization-mediated epigenetic silencing by a long intronic noncoding RNA. Science 2011, 331, 76–79. [Google Scholar] [CrossRef]
- Li, M.; Wen, Z.; Meng, J.; Cheng, T.; Zhang, Q.; Sun, L. The genomics of ornamental plants: Current status and opportunities. Ornam. Plant Res. 2022, 2, 6. [Google Scholar] [CrossRef]
- Shahri, W.; Gul, F.; Tahir, I. Ornamental Plants: Some Molecular Aspects. In The Global Floriculture Industry; Apple Academic Press: Palm Bay, FL, USA, 2020; pp. 59–81. [Google Scholar]
- Mekapogu, M.; Song, H.-Y.; Lim, S.-H.; Jung, J.-A. Genetic Engineering and Genome Editing Advances to Enhance Floral Attributes in Ornamental Plants: An Update. Plants 2023, 12, 3983. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Zhang, F.; Guan, J.; Ma, Y. Genetic and epigenetic regulation of disease resistance in horticultural plants. Front. Genet. 2023, 14, 1277571. [Google Scholar] [CrossRef] [PubMed]
- Meijón, M.; Jesús Cañal, M.; Valledor, L.; Rodríguez, R.; Feito, I. Epigenetic and physiological effects of gibberellin inhibitors and chemical pruners on the floral transition of azalea. Physiol. Plant. 2011, 141, 276–288. [Google Scholar] [CrossRef]
- Gallego-Giraldo, L.; García-Martínez, J.L.; Moritz, T.; López-Díaz, I. Flowering in tobacco needs gibberellins but is not promoted by the levels of active GA1 and GA4 in the apical shoot. Plant Cell Physiol. 2007, 48, 615–625. [Google Scholar]
- Sandoval-Oliveros, R.; Guevara-Olvera, L.; Beltrán, J.; Gómez-Mena, C.; Acosta-García, G. Developmental landmarks during floral ontogeny of jalapeño chili pepper (Capsicum annuum L.) and the effect of gibberellin on ovary growth. Plant Reprod. 2017, 30, 119–129. [Google Scholar]
- Zhang, D.; Ren, L.; Yue, J.-H.; Wang, L.; Zhuo, L.-H.; Shen, X.-H. GA4 and IAA were involved in the morphogenesis and development of flowers in Agapanthus praecox ssp. orientalis. J. Plant Physiol. 2014, 171, 966–976. [Google Scholar] [PubMed]
- Cheng, Y.; Zhao, Y. A role for auxin in flower development. J. Integr. Plant Biol. 2007, 49, 99–104. [Google Scholar]
- Yamaguchi, N.; Jeong, C.W.; Nole-Wilson, S.; Krizek, B.A.; Wagner, D. AINTEGUMENTA and AINTEGUMENTA-LIKE6/PLETHORA3 induce LEAFY expression in response to auxin to promote the onset of flower formation in Arabidopsis. Plant Physiol. 2016, 170, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Enomoto, K.; Fukunaga, Y.; Kuo, C. Regeneration of tepals, stamens and ovules in explants from perianth of Hyacinthus orientalis L. importance of explant age and exogenous hormones. Planta 1988, 175, 478–484. [Google Scholar] [PubMed]
- Shu, K.; Chen, Q.; Wu, Y.; Liu, R.; Zhang, H.; Wang, S.; Tang, S.; Yang, W.; Xie, Q. ABSCISIC ACID-INSENSITIVE 4 negatively regulates flowering through directly promoting Arabidopsis FLOWERING LOCUS C transcription. J. Exp. Bot. 2016, 67, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Renau-Morata, B.; Nebauer, S.G.; García-Carpintero, V.; Canizares, J.; Minguet, E.G.; De los Mozos, M.; Molina, R.V. Flower induction and development in saffron: Timing and hormone signalling pathways. Ind. Crops Prod. 2021, 164, 113370. [Google Scholar]
- Mutasa-Göttgens, E.; Hedden, P. Gibberellin as a factor in floral regulatory networks. J. Exp. Bot. 2009, 60, 1979–1989. [Google Scholar] [PubMed]
- Griffiths, J.; Murase, K.; Rieu, I.; Zentella, R.; Zhang, Z.-L.; Powers, S.J.; Gong, F.; Phillips, A.L.; Hedden, P.; Sun, T.-P. Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 2006, 18, 3399–3414. [Google Scholar]
- Rieu, I.; Ruiz-Rivero, O.; Fernandez-Garcia, N.; Griffiths, J.; Powers, S.J.; Gong, F.; Linhartova, T.; Eriksson, S.; Nilsson, O.; Thomas, S.G. The gibberellin biosynthetic genes AtGA20ox1 and AtGA20ox2 act, partially redundantly, to promote growth and development throughout the Arabidopsis life cycle. Plant J. 2008, 53, 488–504. [Google Scholar] [PubMed]
- Nester, J.E.; Zeevaart, J.A. Flower development in normal tomato and a gibberellin-deficient (ga-2) mutant. Am. J. Bot. 1988, 75, 45–55. [Google Scholar]
- Plack, A. Effect of gibberellic acid on corolla size. Nature 1958, 182, 610. [Google Scholar] [CrossRef]
- Hu, J.; Mitchum, M.G.; Barnaby, N.; Ayele, B.T.; Ogawa, M.; Nam, E.; Lai, W.-C.; Hanada, A.; Alonso, J.M.; Ecker, J.R. Potential sites of bioactive gibberellin production during reproductive growth in Arabidopsis. Plant Cell 2008, 20, 320–336. [Google Scholar]
- Cheng, H.; Qin, L.; Lee, S.; Fu, X.; Richards, D.E.; Cao, D.; Luo, D.; Harberd, N.P.; Peng, J. Gibberellin regulates Arabidopsis floral development via suppression of DELLA protein function. Development 2004, 131, 1055–1064. [Google Scholar] [PubMed]
- Song, S.; Qi, T.; Huang, H.; Xie, D. Regulation of stamen development by coordinated actions of jasmonate, auxin, and gibberellin in Arabidopsis. Mol. Plant 2013, 6, 1065–1073. [Google Scholar]
- Yari, V.; Roein, Z.; Sabouri, A. Exogenous 5-azaCitidine accelerates flowering and external GA3 increases ornamental value in Iranian Anemone accessions. Sci. Rep. 2021, 11, 7478. [Google Scholar]
- Brooking, I.; Jamieson, P. Temperature and photoperiod response of vernalization in near-isogenic lines of wheat. Field Crops Res. 2002, 79, 21–38. [Google Scholar]
- Izhaki, A.; Borochov, A.; Zamski, E.; Weiss, D. Gibberellin regulates post-microsporogenesis processes in petunia anthers. Physiol. Plant. 2002, 115, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Chhun, T.; Aya, K.; Asano, K.; Yamamoto, E.; Morinaka, Y.; Watanabe, M.; Kitano, H.; Ashikari, M.; Matsuoka, M.; Ueguchi-Tanaka, M. Gibberellin regulates pollen viability and pollen tube growth in rice. Plant Cell 2007, 19, 3876–3888. [Google Scholar] [CrossRef] [PubMed]
- Barendse, G.; Rodrigues Pereira, A.; Berkers, P.; Driessen, F.; van Eyden-Emons, A.; Linskens, H. Growth hormones in pollen, styles and ovaries of Petunia hybrida and of Lilium species. Acta Bot. Neerl. 1970, 19, 175–186. [Google Scholar] [CrossRef]
- Fuchs, E.; Atsmon, D.; Halevy, A.H. Adventitious staminate flower formation in gibberellin treated gynoecious cucumber plants. Plant Cell Physiol. 1977, 18, 1193–1201. [Google Scholar] [CrossRef]
- Galun, E. Effects of gibberellic acid and naphthalene-acetic acid on sex expression and some morphological characters in the cucumber plant. Phyton 1959, 13, 1–8. [Google Scholar]
- Banarjee, S.; Basu, P. Hormonal regulators of flowering and fruit development: Effect of GA and ethereal on fruit setting and development of Momordica charinata L. Biol. Plant 1992, 34, 63–70. [Google Scholar]
- Lee, Z.; Kim, S.; Choi, S.J.; Joung, E.; Kwon, M.; Park, H.J.; Shim, J.S. Regulation of flowering time by environmental factors in plants. Plants 2023, 12, 3680. [Google Scholar] [CrossRef]
- Dong, X.; Li, Y.; Guan, Y.; Wang, S.; Luo, H.; Li, X.; Li, H.; Zhang, Z. Auxin-induced AUXIN RESPONSE FACTOR4 activates APETALA1 and FRUITFULL to promote flowering in woodland strawberry. Hortic. Res. 2021, 8, 115. [Google Scholar] [CrossRef]
- Liang, Y.; Jiang, C.; Liu, Y.; Gao, Y.; Lu, J.; Aiwaili, P.; Fei, Z.; Jiang, C.-Z.; Hong, B.; Ma, C. Auxin regulates sucrose transport to repress petal abscission in rose (Rosa hybrida). Plant Cell 2020, 32, 3485–3499. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Shan, N.; Liu, H.; Yao, X.; Wang, Z.; Bai, R.; Guo, Y.; Duan, Y.; Wang, C.; Sui, X. Transcriptional control of local auxin distribution by the CsDFB1-CsPHB module regulates floral organogenesis in cucumber. Proc. Natl. Acad. Sci. USA 2021, 118, e2023942118. [Google Scholar] [CrossRef]
- Liu, X.; Dinh, T.T.; Li, D.; Shi, B.; Li, Y.; Cao, X.; Guo, L.; Pan, Y.; Jiao, Y.; Chen, X. AUXIN RESPONSE FACTOR 3 integrates the functions of AGAMOUS and APETALA 2 in floral meristem determinacy. Plant J. 2014, 80, 629–641. [Google Scholar] [CrossRef]
- D’Aloia, M.; Bonhomme, D.; Bouché, F.; Tamseddak, K.; Ormenese, S.; Torti, S.; Coupland, G.; Périlleux, C. Cytokinin promotes flowering of Arabidopsis via transcriptional activation of the FT paralogue TSF. Plant J. 2011, 65, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Zieslin, N.; Mor, Y.; Khayat, E.; Levy, M. The use of cytokinins for promotion of flower production in roses. In Proceedings of the II Symposium on Growth Regulators in Floriculture 167, Skierniewice, Poland, 30 July–4 August 1984; pp. 433–434. [Google Scholar]
- Eshghi, S.; Tafazoli, E. Possible role of cytokinins in flower induction in strawberry. Am. J. Plant Physiol. 2007, 2, 167–174. [Google Scholar] [CrossRef]
- Qiu, Y.; Guan, S.C.; Wen, C.; Li, P.; Gao, Z.; Chen, X. Auxin and cytokinin coordinate the dormancy and outgrowth of axillary bud in strawberry runner. BMC Plant Biol. 2019, 19, 528. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Sharma, S.; Jose-Santhi, J.; Kalia, D.; Singh, R.K. Hormones regulate the flowering process in saffron differently depending on the developmental stage. Front. Plant Sci. 2023, 14, 1107172. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, C.; Yang, H.; Jiao, Y. Cytokinin pathway mediates APETALA1 function in the establishment of determinate floral meristems in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 6840–6845. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Suh, S.S.; Lee, H.; Choi, K.R.; Hong, C.B.; Paek, N.C.; Kim, S.G.; Lee, I. The SOC1 MADS-box gene integrates vernalization and gibberellin signals for flowering in Arabidopsis. Plant J. 2003, 35, 613–623. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Y.; Tang, X.; Rao, H.; Ren, C.; Chen, J.; Wu, Q.; Jiang, Y.; Geng, F.; Pei, J. Transcriptome profiling of the flowering transition in saffron (Crocus sativus L.). Sci. Rep. 2020, 10, 9680. [Google Scholar]
- Martignago, D.; Siemiatkowska, B.; Lombardi, A.; Conti, L. Abscisic acid and flowering regulation: Many targets, different places. Int. J. Mol. Sci. 2020, 21, 9700. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, J.; Ohashi, Y.; Takahashi, R.; Nakai, K.; Takahashi, Y. DELLA degradation by gibberellin promotes flowering via GAF1-TPR-dependent repression of floral repressors in Arabidopsis. Plant Cell 2021, 33, 2258–2272. [Google Scholar]
- Zhang, S.; Gottschalk, C.; van Nocker, S. Genetic mechanisms in the repression of flowering by gibberellins in apple (Malus x domestica Borkh.). BMC Genom. 2019, 20, 747. [Google Scholar]
- King, R.W.; Hisamatsu, T.; Goldschmidt, E.E.; Blundell, C. The nature of floral signals in Arabidopsis. I. Photosynthesis and a far-red photoresponse independently regulate flowering by increasing expression of FLOWERING LOCUS T (FT). J. Exp. Bot. 2008, 59, 3811–3820. [Google Scholar]
- Lawson, R.H. Economic importance and trends in ornamental horticulture. In Proceedings of the IX International Symposium on Virus Diseases of Ornamental Plants 432, Herzliya, Israel, 17–22 March 1996; pp. 226–237. [Google Scholar]
- Noman, A.; Aqeel, M.; Deng, J.; Khalid, N.; Sanaullah, T.; Shuilin, H. Biotechnological advancements for improving floral attributes in ornamental plants. Front. Plant Sci. 2017, 8, 530. [Google Scholar]
- Magray, M.M. Plant growth regulators and their role in horticultural crop production and development. Res. Manag. Hortic. Crops 2021, 1, 31–51. [Google Scholar]
- Nambara, E.; Yan, D.; Wen, J.; Sharma, A.; Nguyen, F.; Yan, A.; Uruma, K.; Yano, K. Plant Hormones: Gene Family Organization and Homolog Interactions of Genes for Gibberellin Metabolism and Signaling in Allotetraploid Brassica napus. In Plant Omics: Advances in Big Data Biology; CABI GB: Wallingford, UK, 2022; pp. 151–171. [Google Scholar]
- Gaspar, T.; Kevers, C.; Penel, C.; Greppin, H.; Reid, D.M.; Thorpe, T.A. Plant hormones and plant growth regulators in plant tissue culture. Vitr. Cell Dev. Biol.-Plant 1996, 32, 272–289. [Google Scholar] [CrossRef]
- Seaton, K.; Bettin, A.; Grüneberg, H. New ornamental plants for horticulture. In Horticulture: Plants for People and Places, Volume 1: Production Horticulture; Springer: Berlin/Heidelberg, Germany, 2014; pp. 435–463. [Google Scholar]
- Serek, M.; Woltering, E.; Sisler, E.; Frello, S.; Sriskandarajah, S. Controlling ethylene responses in flowers at the receptor level. Biotechnol. Adv. 2006, 24, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Ryu, H.; Cho, Y.-G. Plant hormones in salt stress tolerance. J. Plant Biol. 2015, 58, 147–155. [Google Scholar] [CrossRef]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. Res. 2018, 25, 33103–33118. [Google Scholar] [CrossRef] [PubMed]
- Mehbub, H.; Akter, A.; Akter, M.A.; Mandal, M.S.H.; Hoque, M.A.; Tuleja, M.; Mehraj, H. Tissue culture in ornamentals: Cultivation factors, propagation techniques, and its application. Plants 2022, 11, 3208. [Google Scholar] [CrossRef]
- Kuligowska, K.; Lütken, H.; Müller, R. Towards development of new ornamental plants: Status and progress in wide hybridization. Planta 2016, 244, 1–17. [Google Scholar] [CrossRef]
- Alsoufi, A.S.; Ahmed, Z.S.; Salim, A.M. The efficiency of interaction between cytokines and Auxins in Micropropagation of Chrysanthemum plant (Chrysanthemum indicum L.). In IOP Conference Series: Earth and Environmental Science; IOP Publishing Ltd.: Bristol, UK, 2021; p. 012048. [Google Scholar]
- Reid, M.S.; Wu, M.-J. Ethylene in flower development and senescence. In The Plant Hormone Ethylene; CRC Press: Boca Raton, FL, USA, 2018; pp. 215–234. [Google Scholar]
- Castro-Camba, R.; Sánchez, C.; Vidal, N.; Vielba, J.M. Plant development and crop yield: The role of gibberellins. Plants 2022, 11, 2650. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Peñuelas, J. Gardening and urban landscaping: Significant players in global change. Trends Plant Sci. 2008, 13, 60–65. [Google Scholar] [CrossRef]
- El Malahi, S.; Sbah, N.; Zim, J.; Ennami, M.; Zakri, B.; Mokhtari, W.; Taimourya, H.; Mokhtari, M.; Hassani, L.M.I. Enhancing rooting efficiency and nutrient uptake in Rosa damascena Mill. cuttings: Insights into auxin and cutting type optimization. Plant Sci. Today 2024, 11, 119–131. [Google Scholar] [CrossRef]
- Anjum, S.; Mirza, U.; Shafi, N.; Parray, J.A. Plant–microbe interactions: Perspectives in promoting plant health. In Microbiome Drivers of Ecosystem Function; Elsevier: Amsterdam, The Netherlands, 2024; pp. 79–90. [Google Scholar]
- Finkel, O.M.; Castrillo, G.; Paredes, S.H.; González, I.S.; Dangl, J.L. Understanding and exploiting plant beneficial microbes. Curr. Opin. Plant Biol. 2017, 38, 155–163. [Google Scholar] [PubMed]
- Singh, M.; Singh, D.; Gupta, A.; Pandey, K.D.; Singh, P.; Kumar, A. Plant growth promoting rhizobacteria: Application in biofertilizers and biocontrol of phytopathogens. In PGPR Amelioration in Sustainable Agriculture; Elsevier: Amsterdam, The Netherlands, 2019; pp. 41–66. [Google Scholar]
- Turner, T.R.; James, E.K.; Poole, P.S. The plant microbiome. Genome Biol. 2013, 14, 209. [Google Scholar]
- Poupin, M.J.; Timmermann, T.; Vega, A.; Zuñiga, A.; González, B. Effects of the plant growth-promoting bacterium Burkholderia phytofirmans PsJN throughout the life cycle of Arabidopsis thaliana. PLoS ONE 2013, 8, e69435. [Google Scholar]
- Flores, A.C.; Luna, A.A.E.; Portugal, V.O. Yield and quality enhancement of marigold flowers by inoculation with Bacillus subtilis and Glomus fasciculatum. J. Sustain. Agric. 2007, 31, 21–31. [Google Scholar]
- de los Santos-Villalobos, S.; de Folter, S.; Délano-Frier, J.P.; Gómez-Lim, M.A.; Guzmán-Ortiz, D.A.; Pena-Cabriales, J.J. Growth promotion and flowering induction in mango (Mangifera indica L. cv “Ataulfo”) trees by Burkholderia and Rhizobium Inoculation: Morphometric, biochemical, and molecular events. J. Plant Growth Regul. 2013, 32, 615–627. [Google Scholar]
- Lyons, R.; Rusu, A.; Stiller, J.; Powell, J.; Manners, J.M.; Kazan, K. Investigating the association between flowering time and defense in the Arabidopsis thaliana-Fusarium oxysporum interaction. PLoS ONE 2015, 10, e0127699. [Google Scholar]
- Das, A.; Kamal, S.; Shakil, N.A.; Sherameti, I.; Oelmüller, R.; Dua, M.; Tuteja, N.; Johri, A.K.; Varma, A. The root endophyte fungus Piriformospora indica leads to early flowering, higher biomass and altered secondary metabolites of the medicinal plant, Coleus forskohlii. Plant Signal. Behav. 2012, 7, 103–112. [Google Scholar] [PubMed]
- Halifu, S.; Deng, X.; Song, X.; Song, R. Effects of two Trichoderma strains on plant growth, rhizosphere soil nutrients, and fungal community of Pinus sylvestris var. mongolica annual seedlings. Forests 2019, 10, 758. [Google Scholar] [CrossRef]
- Xing, L.-J.; Wei, L.I.; Zhai, Y.-L.; Hu, X.-Y.; Guo, S.-X. Arbuscular mycorrhizal fungi promote early flowering and prolong flowering in Antirrhinum majus L. by regulating endogenous hormone balance under field-planting conditions. Not. Bot. Horti Agrobot. 2022, 50, 12503. [Google Scholar]
- Toffoli, L.M.; Martínez-Zamora, M.G.; Medrano, N.N.; Fontana, C.A.; Lovaisa, N.C.; Delaporte-Quintana, P.; Elias, J.M.; Salazar, S.M.; Pedraza, R.O. Natural occurrence of Azospirillum brasilense in petunia with capacity to improve plant growth and flowering. J. Basic Microbiol. 2021, 61, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Zulueta-Rodriguez, R.; Cordoba-Matson, M.V.; Hernandez-Montiel, L.G.; Murillo-Amador, B.; Rueda-Puente, E.; Lara, L. Effect of Pseudomonas putida on growth and anthocyanin pigment in two poinsettia (Euphorbia pulcherrima) cultivars. Sci. World J. 2014, 2014, 810192. [Google Scholar] [CrossRef] [PubMed]
- Vaingankar, J.D.; Rodrigues, B. Effect of arbuscular mycorrhizal (AM) inoculation on growth and flowering in Crossandra infundibuliformis (L.) Nees. J. Plant Nutr. 2015, 38, 1478–1488. [Google Scholar] [CrossRef]
- Yanti, Y.; Warnita, W.; Reflin, R.; Nasution, C.R. Characterizations of endophytic Bacillus strains from tomato roots as growth promoter and biocontrol of Ralstonia solanacearum. Biodiversitas J. Biol. Divers. 2018, 19, 906–911. [Google Scholar] [CrossRef]
- Goicoechea; Aguirreolea; Cenoz, G.-M. Gas exchange and flowering in Verticillium-wilted pepper plants. J. Phytopathol. 2001, 149, 281–286. [Google Scholar] [CrossRef]
- Nordstedt, N.P.; Chapin, L.J.; Taylor, C.G.; Jones, M.L. Identification of Pseudomonas spp. that increase ornamental crop quality during abiotic stress. Front. Plant Sci. 2020, 10, 1754. [Google Scholar] [CrossRef]
- Sharaf-Eldin, M.; Elkholy, S.; Fernández, J.-A.; Junge, H.; Cheetham, R.; Guardiola, J.; Weathers, P. Bacillus subtilis FZB24® affects flower quantity and quality of saffron (Crocus sativus). Planta Med. 2008, 74, 1316–1320. [Google Scholar] [CrossRef]
- Ousley, M.; Lynch, J.; Whipps, J. The effects of addition of Trichoderma inocula on flowering and shoot growth of bedding plants. Sci. Hortic. 1994, 59, 147–155. [Google Scholar] [CrossRef]
- Cui, Z.; Huntley, R.B.; Zeng, Q.; Steven, B. Temporal and spatial dynamics in the apple flower microbiome in the presence of the phytopathogen Erwinia amylovora. ISME J. 2021, 15, 318–329. [Google Scholar] [CrossRef]
- Christensen, S.M.; Munkres, I.; Vannette, R.L. Nectar bacteria stimulate pollen germination and bursting to enhance microbial fitness. Curr. Biol. 2021, 31, 4373–4380.e6. [Google Scholar] [CrossRef]
- Gätjens-Boniche, O.; Jiménez-Madrigal, J.P.; Whetten, R.W.; Valenzuela-Diaz, S.; Alemán-Gutiérrez, A.; Hanson, P.E.; Pinto-Tomás, A.A. Microbiome and plant cell transformation trigger insect gall induction in cassava. Front. Plant Sci. 2023, 14, 1237966. [Google Scholar]
- Crowley-Gall, A.; Rering, C.C.; Rudolph, A.B.; Vannette, R.L.; Beck, J.J. Volatile microbial semiochemicals and insect perception at flowers. Curr. Opin. Insect Sci. 2021, 44, 23–34. [Google Scholar] [PubMed]
- Dombrowski, N.; Schlaeppi, K.; Agler, M.T.; Hacquard, S.; Kemen, E.; Garrido-Oter, R.; Wunder, J.; Coupland, G.; Schulze-Lefert, P. Root microbiota dynamics of perennial Arabis alpina are dependent on soil residence time but independent of flowering time. ISME J. 2017, 11, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.R.; Lundberg, D.S.; Coleman-Derr, D.; Tringe, S.G.; Dangl, J.L.; Mitchell-Olds, T. Natural soil microbes alter flowering phenology and the intensity of selection on flowering time in a wild Arabidopsis relative. Ecol. Lett. 2014, 17, 717–726. [Google Scholar]
- Yoo, S.K.; Chung, K.S.; Kim, J.; Lee, J.H.; Hong, S.M.; Yoo, S.J.; Yoo, S.Y.; Lee, J.S.; Ahn, J.H. Constans activates suppressor of overexpression of constans 1 through Flowering Locus T to promote flowering in Arabidopsis. Plant Physiol. 2005, 139, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Turck, F.; Fornara, F.; Coupland, G. Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu. Rev. Plant Biol. 2008, 59, 573–594. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Ying, H.; Helliwell, C.A.; Taylor, J.M.; Peacock, W.J.; Dennis, E.S. FLOWERING LOCUS C (FLC) regulates development pathways throughout the life cycle of Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 6680–6685. [Google Scholar] [CrossRef]
- Peer, L.A.; Bhat, M.Y.; Ahmad, N.; Mir, B.A. Floral induction pathways: Decision making and determination in plants to flower-A comprehensive review. J. Appl. Biol. Biotechnol 2021, 9, 7–17. [Google Scholar]
- Izawa, T. What is going on with the hormonal control of flowering in plants? Plant J. 2021, 105, 431–445. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Liu, Z.; Li, X.; Wang, Y.; Liu, W.; Li, X.; Hu, J.; Zhu, W.; Wang, C. Reciprocal regulation of flower induction by ELF3α and ELF3β generated via alternative promoter usage. Plant Cell 2023, 35, 2095–2113. [Google Scholar] [CrossRef]
- Eichmann, R.; Richards, L.; Schäfer, P. Hormones as go-betweens in plant microbiome assembly. Plant J. 2021, 105, 518–541. [Google Scholar] [CrossRef]
- Shah, A.; Tyagi, S.; Saratale, G.D.; Guzik, U.; Hu, A.; Sreevathsa, R.; Reddy, V.D.; Rai, V.; Mulla, S.I. A comprehensive review on the influence of light on signaling cross-talk and molecular communication against phyto-microbiome interactions. Crit. Rev. Biotechnol. 2021, 41, 370–393. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Datta, S.; Ramamurthy, P.C.; Singh, J. Molecular mechanism and signaling pathways interplay between plant hormones during plant-microbe crosstalk. In Microbial Management of Plant Stresses; Elsevier: Amsterdam, The Netherlands, 2021; pp. 93–105. [Google Scholar]
- Darras, A.I. Implementation of sustainable practices to ornamental plant cultivation worldwide: A critical review. Agronomy 2020, 10, 1570. [Google Scholar] [CrossRef]
- Atal, H.L.; Srilakshmi, D.; Debbarma, K.; Jena, L.; Ichancha, M. A Review on Breeding in Ornamental Crops for Abiotic Stress Tolerance. Int. J. Plant Soil Sci. 2022, 34, 134–138. [Google Scholar] [CrossRef]
- Chandler, S.F.; Tribe, D. Modern techniques for plant breeding in ornamentals. In Floriculture and Ornamental Plants; Springer: Berlin/Heidelberg, Germany, 2022; pp. 523–555. [Google Scholar]
- Roberts, W.R.; Roalson, E.H. Co-expression clustering across flower development identifies modules for diverse floral forms in Achimenes (Gesneriaceae). PeerJ 2020, 8, e8778. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Song, A.; Zhang, X.; Li, S.; Su, J.; Xia, W.; Zhao, K.; Zhao, W.; Guan, Y.; Fang, W. The core regulatory networks and hub genes regulating flower development in Chrysanthemum morifolium. Plant Mol. Biol. 2020, 103, 669–688. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Kang, X.; Wang, M.; Haider, W.; Price, W.B.; Hajek, B.; Hanzawa, Y. Transcriptome-enabled network inference revealed the GmCOL1 feed-forward loop and its roles in photoperiodic flowering of soybean. Front. Plant Sci. 2019, 10, 1221. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).