Metabolic Regulation and Saline–Alkali Stress Response in Novel Symbionts of Epichloë bromicola-Bromus inermis
Abstract
1. Introduction
2. Results
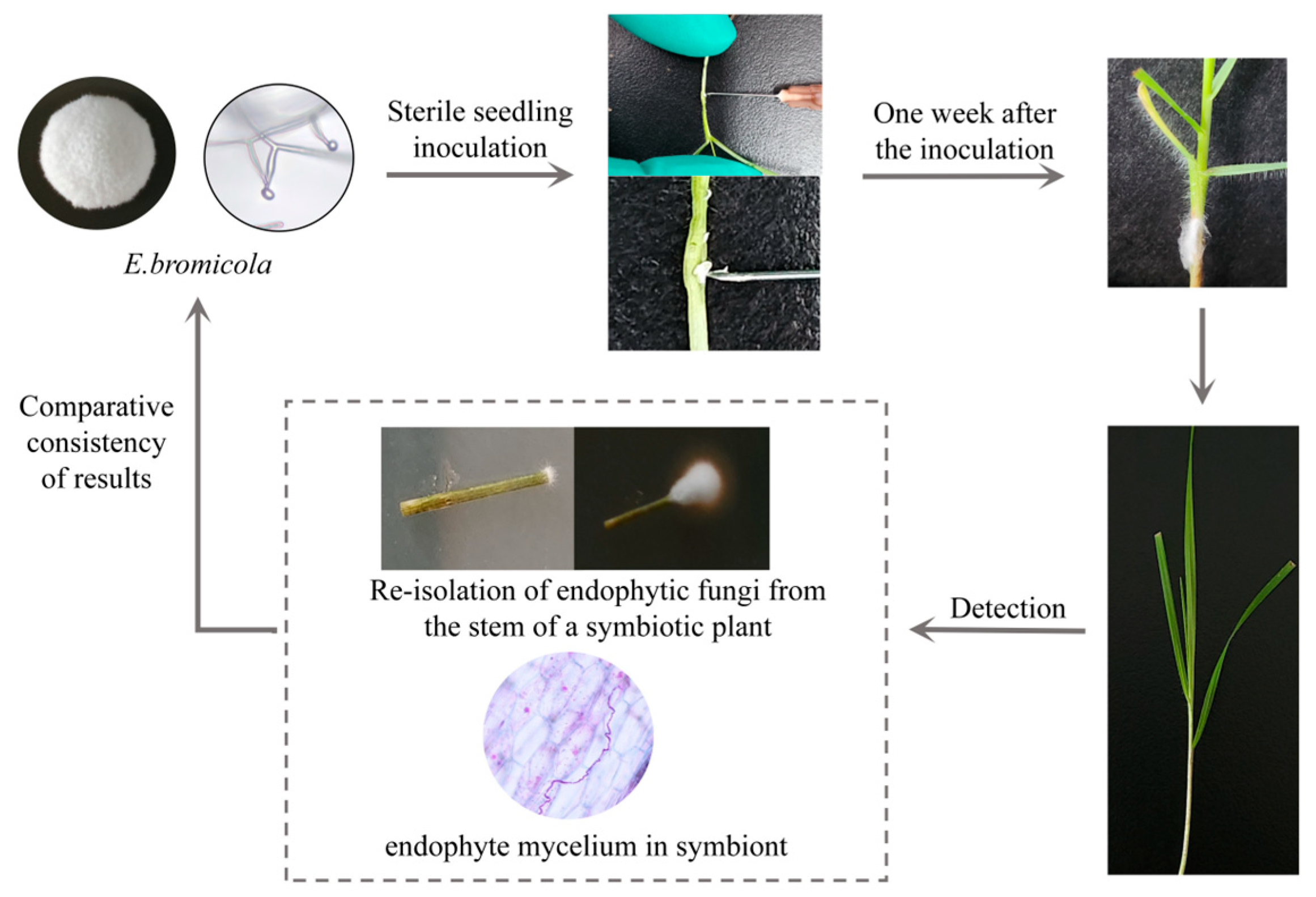
2.1. Artificial Inoculation Result
2.2. Effects of E. bromicola on Metabolism of B. inermis in a New Host
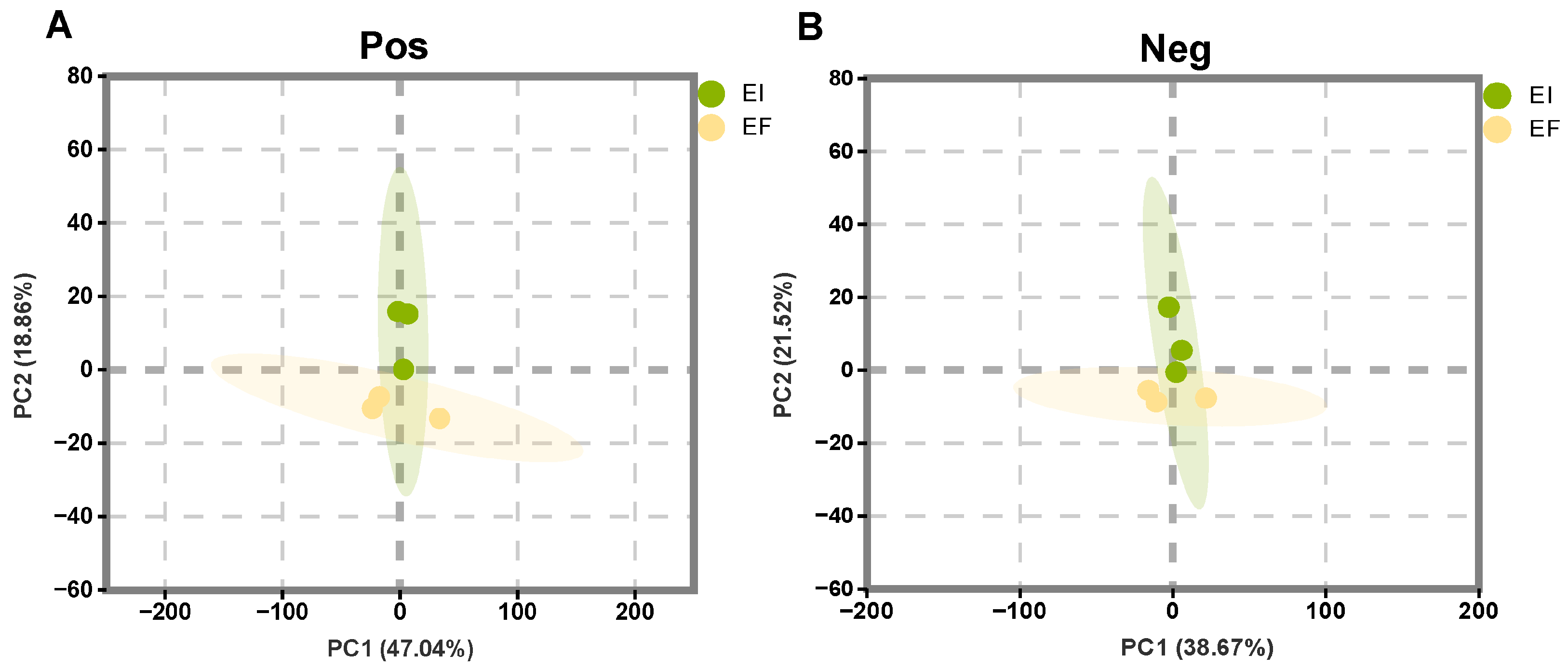
2.2.1. Principal Component Analysis
2.2.2. Screening of Differential Metabolites
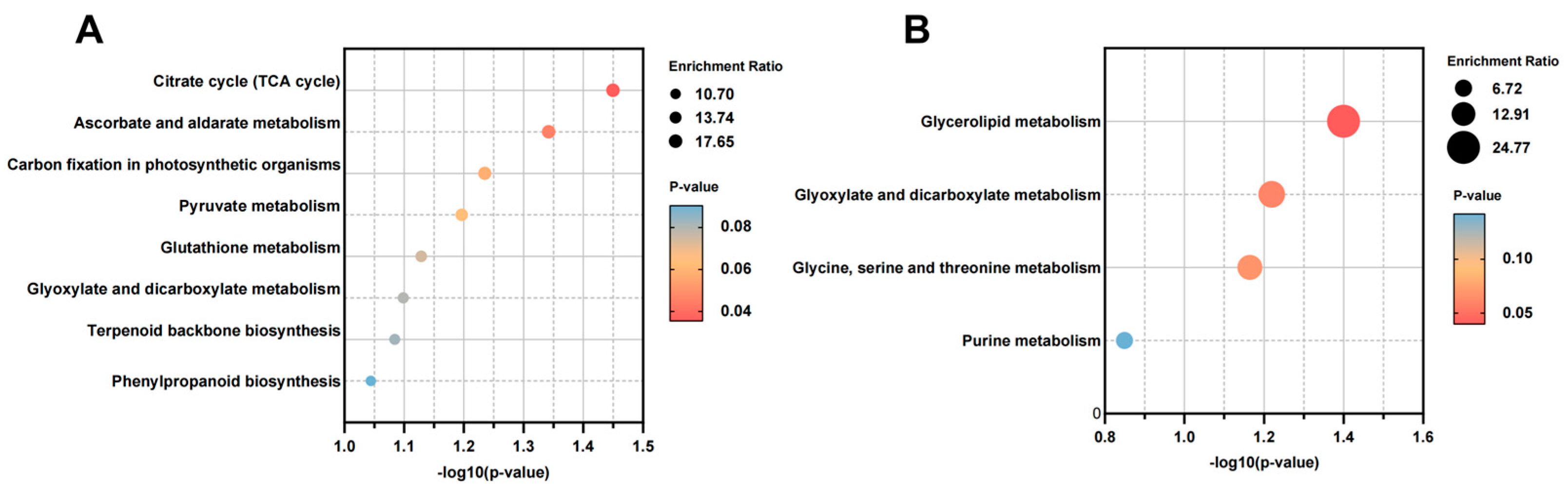
2.2.3. KEGG Pathway Enrichment Analysis of Differential Metabolites
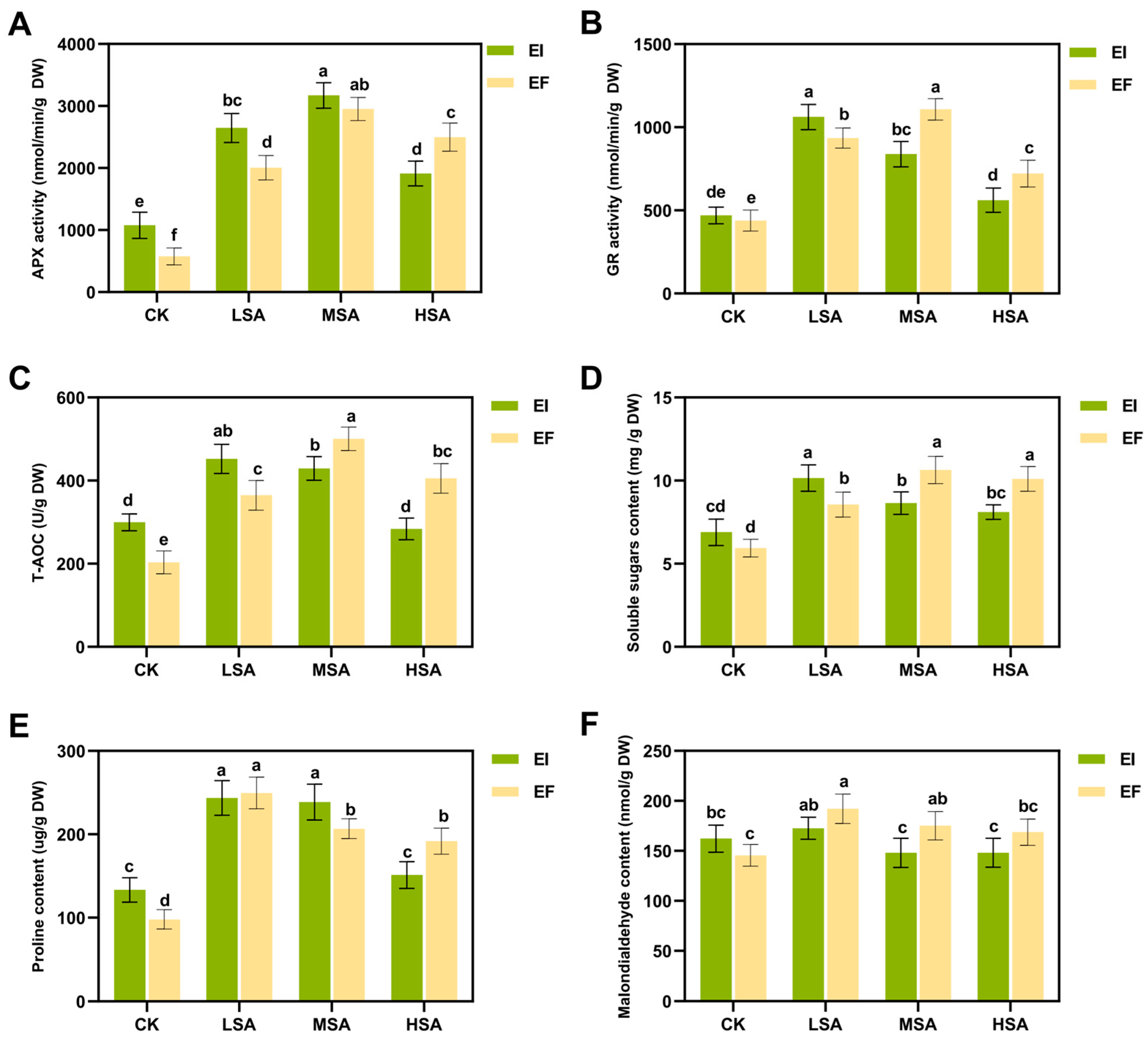
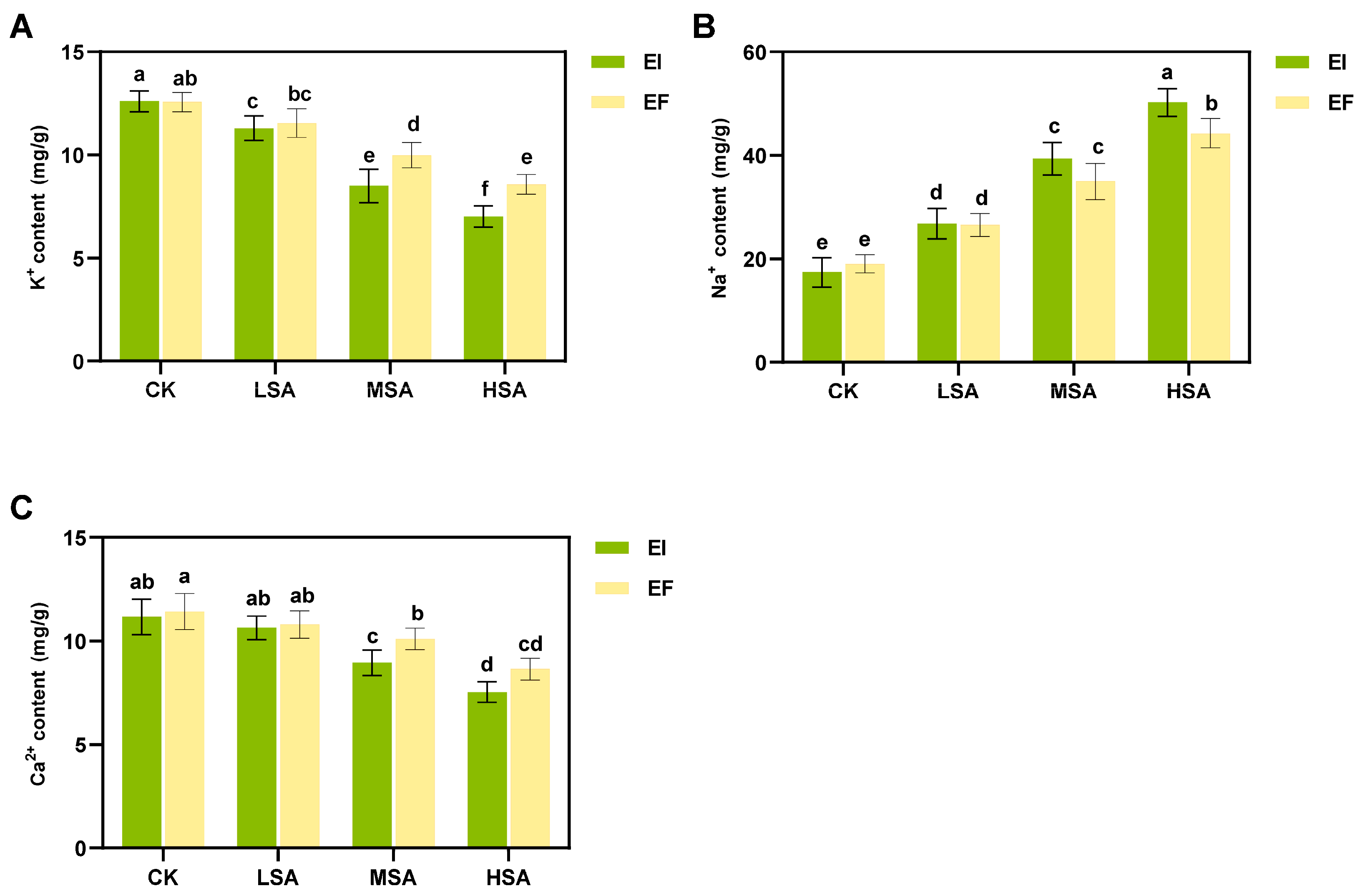
2.3. Physiological Response of Epichloë bromicola-B. inermis to Saline–Alkali Stress
3. Discussion
3.1. Research on Artificial Inoculation Methods
3.2. Effects of E. bromicola on Metabolism of B. inermis in a New Host
3.3. Physiological Response of Symbiont to Saline–Alkali Stress
4. Materials and Methods
4.1. Strain and Plant Origin
4.2. Artificial Inoculation Method
4.3. Detection of Fungal Infection Rate in B. inermis
4.4. LC-MS Detection of Symbiont and Non-Symbiont
4.5. Salt and Alkali Stress Test of Symbiont
4.6. Data Visualization and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saikkonen, K.; Young, C.A.; Helander, M.; Schardl, C.L. Endophytic Epichloë species and their grass hosts: From evolution to applications. Plant Mol. Biol. 2016, 90, 6. [Google Scholar] [CrossRef]
- Lee, K.; Missaoui, A.; Mahmud, K.; Presley, H.; Lonnee, M. Interaction between Grasses and Epichloë Endophytes and Its Significance to Biotic and Abiotic Stress Tolerance and the Rhizosphere. Microorganisms 2021, 9, 2186. [Google Scholar] [CrossRef]
- Bastías, D.A.; Bustos, L.B.; Jáuregui, R.; Barrera, A.; Acuña-Rodríguez, I.S.; Molina-Montenegro, M.A.; Gundel, P.E. Epichloë Fungal Endophytes Influence Seed-Associated Bacterial Communities. Front. Microbiol. 2022, 12, 795354. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L. Epichloë species: Fungal symbionts of grasses. Annu. Rev. Phytopathol. 1996, 34, 109–130. [Google Scholar] [CrossRef] [PubMed]
- Scott, B. Epichloë endophytes: Fungal symbionts of grasses. Curr. Opin. Microbiol. 2001, 4, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Bacon, C.W.; Porter, J.K.; Robbins, J.D.; Luttrell, E.S. Epichloë typhina from toxic tall fescue grasses. Appl. Environ. Microbiol. 1977, 34, 576–581. [Google Scholar] [CrossRef]
- Becker, Y.; Green, K.A.; Scott, B.; Becker, A.M. Artificial Inoculation of Epichloë festucae into Lolium perenne, and Visualisation of Endophytic and Epiphyllous Fungal Growth. Bio. Protoc. 2018, 8, e2990. [Google Scholar] [CrossRef]
- Latch, G.C.M.; Christensen, M.J. Artificial infection of grasses with endophytes. Ann. Appl. Biol. 1985, 107, 17–24. [Google Scholar] [CrossRef]
- Wang, Y.Y. Studies on Endophytic Fungi of Festuca spp. (F. ovina, F. stapfii). Master’s Dissertation, Beijing Forestry University, Beijing, China, 2009. [Google Scholar]
- Wille, P.; Boller, T.; Kaltz, O. Mixed inoculation alters infection success of strains of the endophyte Epichloë bromicola on its grass host Bromus erectus. Proc. Biol. Sci. 2002, 269, 397–402. [Google Scholar] [CrossRef]
- Eady, C. The Impact of Alkaloid-Producing Epichloë Endophyte on Forage Ryegrass Breeding: A New Zealand Perspective. Toxins 2021, 13, 158. [Google Scholar] [CrossRef]
- Gunter, S.A.; Beck, P.A. Novel endophyte-infected tall fescue for growing beef cattle. J. Anim. Sci. 2004, 82, E75–E82. [Google Scholar] [CrossRef] [PubMed]
- Bluett, S.J.; Thom, E.R.; Clark, D.A.; Macdonald, K.A.; Minneé, E.M.K. Effects of perennial ryegrass infected with either AR1 or wild endophyte on dairy production in the Waikato. New Zeal. J. Agric. Res. 2005, 48, 197–212. [Google Scholar] [CrossRef]
- Forte, F.P.; Schmid, J.; Dijkwel, P.P.; Nagy, I.; Hume, D.E.; Johnson, R.D.; Simpson, W.R.; Monk, S.M.; Zhang, N.; Sehrish, T.; et al. Fungal Endophyte Colonization Patterns Alter Over Time in the Novel Association Between Lolium perenne and Epichloë Endophyte AR37. Front. Plant Sci. 2020, 11, 570026. [Google Scholar] [CrossRef]
- Li, C.J.; Wang, Z.F.; Chen, T.X.; Nan, Z. Creation of novel barley germplasm using an Epichloë endophyte. Chin. Sci. Bull. 2021, 66, 2608–2617. [Google Scholar]
- Li, R.Q.; Wang, Y.X.; Sun, Y.L.; Zhang, L.; Chen, A.P. Effects of salt stress on the growth, physiology, and biochemistry of five Bromus inermis varieties. Acta Prataculturae Sin. 2023, 32, 99. [Google Scholar] [CrossRef]
- Song, W.; Gao, X.; Li, H.; Li, S.; Wang, J.; Wang, X.; Wang, T.; Ye, Y.; Hu, P.; Li, X.; et al. Transcriptome analysis and physiological changes in the leaves of two Bromus inermis L. genotypes in response to salt stress. Front. Plant Sci. 2023, 14, 1313113. [Google Scholar] [CrossRef]
- Mu, Q.E.; Zhang, M.; Li, Y.; Feng, F.; Yu, X.; Nie, J. Metabolomic analysis reveals the effect of insecticide chlorpyrifos on rice plant metabolism. Metabolites 2022, 12, 1289. [Google Scholar] [CrossRef]
- Chu, Y.; Kwon, T.; Nam, J. Enzymatic and metabolic engineering for efficient production of syringin, sinapyl alcohol 4-O-glucoside, in Arabidopsis thaliana. Phytochemistry 2014, 102, 55–63. [Google Scholar] [CrossRef]
- Miller, R.N.G.; Costa Alves, G.S.; Van Sluys, M.A. Plant immunity: Unravelling the complexity of plant responses to biotic stresses. Ann. Bot. 2017, 119, 681–687. [Google Scholar] [CrossRef]
- Wang, C.; Ahsan, T.; Ding, A.; Han, D.; Gao, J.; Liang, C.H.; Du, S.T.; Wei, Y.; Huang, Y.Q.; Zhang, S.H. Correction: Colonization of Serendipita indica enhances resistance against Phoma arachidicola in Arachis hypogaea L. World J. Microbiol. Biotechnol. 2025, 41, 46. [Google Scholar] [CrossRef]
- Lavell, A.A.; Benning, C. Cellular Organization and Regulation of Plant Glycerolipid Metabolism. Plant Cell Physiol. 2019, 60, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhu, C.; Li, B.; Ning, W.; Yin, J. Combining Physiology and Transcriptome to Reveal Mechanisms of Hosta ‘Golden Cadet’ in Response to Alkali Stress. Plants 2025, 14, 593. [Google Scholar] [CrossRef]
- Kearney, J.F.; Parrott, W.A.; Hill, N.S. Infection of somatic embroys of Tall Fescue with Acremonium coenophialum. Crop Sci. 1991, 31, 979–984. [Google Scholar] [CrossRef]
- Johnson, M.C.; Bush, L.P.; Siegel, M.R. Infection of tall fescue with Acremonium coenophialum by means of callus culture. Plant Dis. 1986, 70, 380–382. [Google Scholar] [CrossRef]
- Chen, L. Molecular Detection, Genotypes and Chemotypes of Epichloë Endophytes in Achnatherum Inebrians. Master’s Dissertation, Lanzhou University, Lanzhou, China, 2015. [Google Scholar]
- Shi, C.; An, S.; Yao, Z.; Young, C.A.; Panaccione, D.G.; Lee, S.T.; Schardl, C.L.; Li, C. Toxin-producing Epichloë bromicola strains symbiotic with the forage grass Elymus dahuricus in China. Mycologia 2017, 109, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yu, X.; Yin, J.; Chen, L.; Zhao, N.; Gao, Y.; Ren, A. Epichloë Endophyte Enhanced Insect Resistance of Host Grass Leymus Chinensis by Affecting Volatile Organic Compound Emissions. J. Chem. Ecol. 2024, 50, 1067–1076. [Google Scholar] [CrossRef]
- Foyer, C.H.; Kyndt, T.; Hancock, R.D. Vitamin C in Plants: Novel Concepts, New Perspectives, and Outstanding Issues. Antioxid. Redox Signal. 2020, 32, 463–485. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- De Gara, L.; de Pinto, M.C.; Tommasi, F. The antioxidant systems vis-à-vis reactive oxygen species during plant–pathogen interaction. Plant Physiol. Biochem. 2003, 41, 863–870. [Google Scholar] [CrossRef]
- Sahu, P.K.; Jayalakshmi, K.; Tilgam, J.; Gupta, A.; Nagaraju, Y.; Kumar, A.; Hamid, S.; Singh, H.V.; Minkina, T.; Rajput, V.D.; et al. ROS generated from biotic stress: Effects on plants and alleviation by endophytic microbes. Front. Plant Sci. 2022, 13, 1042936. [Google Scholar] [CrossRef]
- Heinemann, B.; Hildebrandt, T.M. The role of amino acid metabolism in signaling and metabolic adaptation to stress-induced energy deficiency in plants. J. Exp. Bot. 2021, 72, 4634–4645. [Google Scholar] [CrossRef]
- Zhang, Y.; Fernie, A.R. On the role of the tricarboxylic acid cycle in plant productivity. J. Integr. Plant Biol. 2018, 60, 1199–1216. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Wang, J.; Christensen, M.J.; Liu, J.; Zhang, Y.; Liu, Y.; Cheng, C. Metabolomics insights into the mechanism by which Epichloë gansuensis endophyte increased Achnatherum inebrians tolerance to low nitrogen stress. Plant Soil 2021, 463, 487–508. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, J.; Shao, S.; Zhou, H.; Kang, J.; Yu, X.; Huang, M.; Qiu, G.; Shen, L. Combining metabolomics and transcriptomics to analyze key response metabolites and molecular mechanisms of Aspergillus fumigatus under cadmium stress. Environ. Pollut. 2024, 356, 124344. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, C.E.; Gundel, P.E.; Helander, M.; Saikkonen, K. Endophytic mediation of reactive oxygen species and antioxidant activity in plants: A review. Fungal Divers. 2012, 54, 1–10. [Google Scholar] [CrossRef]
- Lu, H.; Wei, T.; Lou, H.; Shu, X.; Chen, Q. A Critical Review on Communication Mechanism within Plant-Endophytic Fungi Interactions to Cope with Biotic and Abiotic Stresses. J. Fungi 2021, 7, 719. [Google Scholar] [CrossRef]
- Li, S.; Meng, H.; Yang, Y.; Zhao, J.; Xia, Y.; Wang, S.; Wang, F.; Zheng, G.; Li, J. Overexpression of AtruLEA1 from Acer truncatum Bunge Enhanced Arabidopsis Drought and Salt Tolerance by Improving ROS-Scavenging Capability. Plants 2025, 14, 117. [Google Scholar] [CrossRef]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Saifullah; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring control: The evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef]
- Chen, T.; Johnson, R.; Chen, S.; Lv, H.; Zhou, J.; Li, C. Infection by the fungal endophyte Epichloë bromicola enhances the tolerance of wild barley (Hordeum brevisubulatum) to salt and alkali stresses. Plant Soil 2018, 428, 353–370. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; White, J. Effects of Epichloë endophyte infection on growth, physiological properties and seed germination of wild barley under saline conditions. J. Agron. Crop Sci. 2020, 206, 43–51. [Google Scholar] [CrossRef]
- Song, M.; Chai, Q.; Li, X.; Yao, X.; Li, C.; Christensen, M.J.; Nan, Z. An asexual Epichloë endophyte modifies the nutrient stoichiometry of wild barley (Hordeum brevisubulatum) under salt stress. Plant Soil 2015, 387, 153–165. [Google Scholar] [CrossRef]
- Wang, C.; Shi, C.; Huang, W.; Zhang, M.; He, J. The Impact of Aboveground Epichloë Endophytic Fungi on the Rhizosphere Microbial Functions of the Host Melica transsilvanica. Microorganisms 2024, 12, 956. [Google Scholar] [CrossRef] [PubMed]


| Method | Fungi | Number of Inoculated Seedlings | Seedling Survival Rate (%) | Fungal Infection Rate (%) |
|---|---|---|---|---|
| sterile seedling slit inoculation method | E. bromicola | 1455 | 78.1 | 2.1 |
| CK | 427 | 80.6 | 0 |
| Material | Method | |
|---|---|---|
| Sterile seedling | Cut | Cut the sterile seedling 2 cm above ground with sterile scissors and cover the wound with inoculated mycelium. |
| Slit | A 2–3 mm incision was made at the meristem of a sterile seedling with a sterile scalpel, and hypha was inserted into the wound. | |
| Injection | The spore suspension was injected into the meristem of sterile seedlings. | |
| Seeds | Soaking | The disinfected seeds were placed in a petri dish with bacterial suspension and soaked for 24 h. |
| Piercing and soaking | The sterilized seeds were pierced near the embryo and then sealed and soaked in bacterial suspension for 24 h. | |
| Slit | The sterilized seeds are cut into small holes near the embryo and the mycelium is inserted into the wound. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Shi, C.; Wang, C.; Yao, Y.; He, J. Metabolic Regulation and Saline–Alkali Stress Response in Novel Symbionts of Epichloë bromicola-Bromus inermis. Plants 2025, 14, 1089. https://doi.org/10.3390/plants14071089
Zhang M, Shi C, Wang C, Yao Y, He J. Metabolic Regulation and Saline–Alkali Stress Response in Novel Symbionts of Epichloë bromicola-Bromus inermis. Plants. 2025; 14(7):1089. https://doi.org/10.3390/plants14071089
Chicago/Turabian StyleZhang, Mengmeng, Chong Shi, Chuanzhe Wang, Yuehan Yao, and Jiakun He. 2025. "Metabolic Regulation and Saline–Alkali Stress Response in Novel Symbionts of Epichloë bromicola-Bromus inermis" Plants 14, no. 7: 1089. https://doi.org/10.3390/plants14071089
APA StyleZhang, M., Shi, C., Wang, C., Yao, Y., & He, J. (2025). Metabolic Regulation and Saline–Alkali Stress Response in Novel Symbionts of Epichloë bromicola-Bromus inermis. Plants, 14(7), 1089. https://doi.org/10.3390/plants14071089







