Green Extraction Strategies and Bioactivity of Rheum cordatum Losinsk: Antioxidant, Antimicrobial, and Molecular Docking Insights
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material
2.2. Extraction Methods
2.2.1. Ultrasound-Assisted Extraction (UAE)
2.2.2. Subcritical Ethanol Extraction (sbcEtOH-E)
2.2.3. Supercritical Carbon Dioxide Extraction (ScCO2-E)
2.3. LC-MSMS Analysis
2.4. Antioxidant Activity
2.4.1. DPPH Radical Scavenging Activity Method
2.4.2. ABTS Radical Scavenging Activity Method
2.4.3. Cu2+-Cu+ Reducing Activity
2.4.4. Total Phenolic Content Determination
2.5. Antimicrobial Activity
2.5.1. Test Organism and Growth Conditions
2.5.2. Determination of Minimum Inhibitory Concentration (MIC)
2.6. Molecular Docking Studies
2.7. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Activity
3.2. In Vitro Antimicrobial Activity
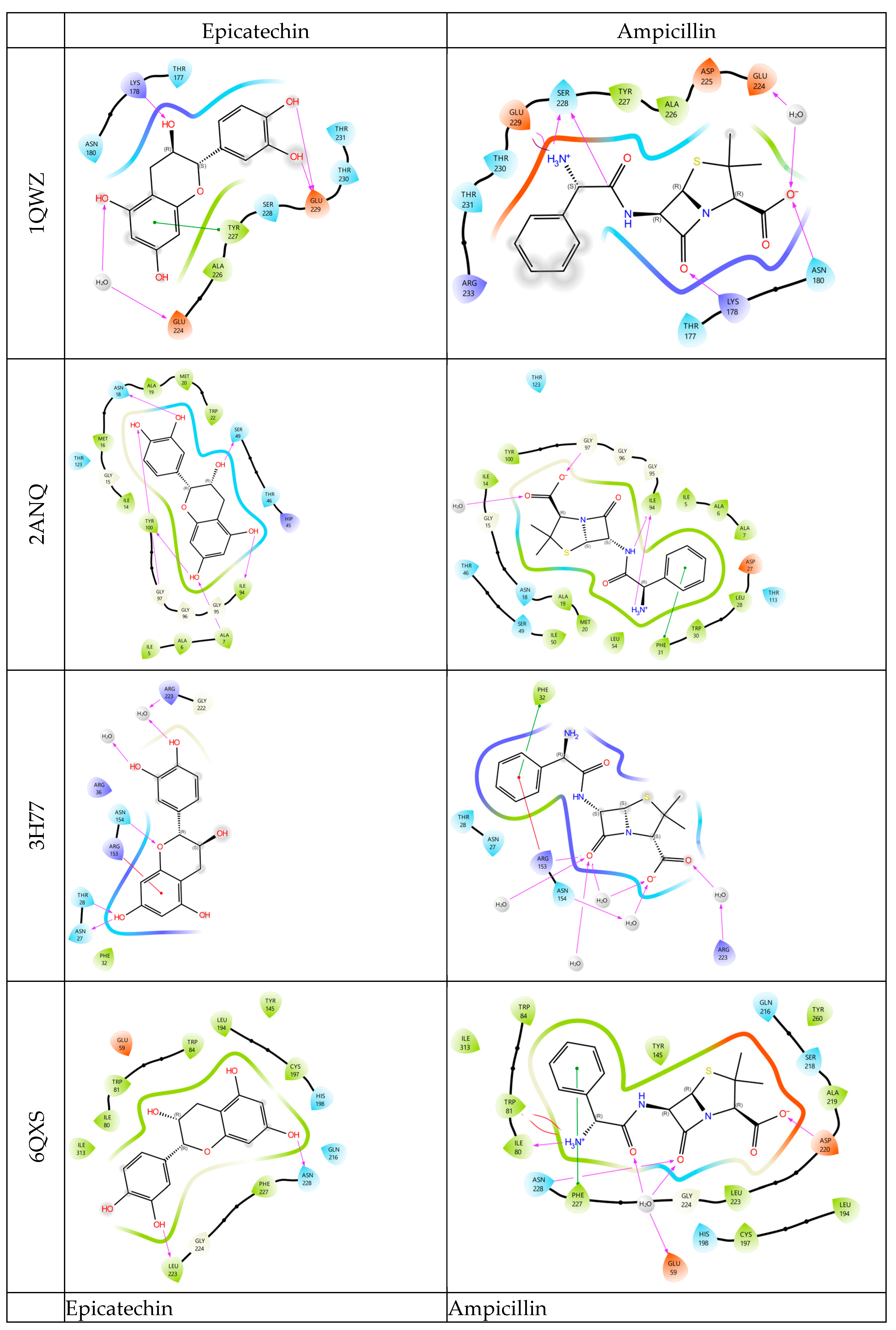
3.3. Molecular Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sitarek, P.; Kowalczyk, T.; Wieczfinska, J.; Merecz-Sadowska, A.; Górski, K.; Śliwiński, T.; Skała, E. Plant extracts as a natural source of bioactive compounds and potential remedy for the treatment of certain skin diseases. Curr. Pharm. Des. 2020, 26, 2859–2875. [Google Scholar]
- Packer, J.; Turpin, G.; Ens, E.; Venkataya, B.; Community, M.; Rangers, Y.; Hunter, J. Building partnerships for linking biomedical science with traditional knowledge of customary medicines: A case study with two Australian Indigenous communities. J. Ethnobiol. Ethnomed. 2019, 15, 69. [Google Scholar]
- Amangeldinova, M.; Ersatır, M.; Necip, A.; Yilmaz, M.A.; Cimentepe, M.; Kudrina, N.; Terletskaya, N.V.; Ozturk Cimentepe, O.; Yildirim, M. Simultaneous quantitative screening of 53 phytochemicals from Rheum tataricum L. roots: A comparative study of supercritical CO2, subcritical ethanol, and ultrasound-assisted extraction for enhanced antioxidant, antibacterial activities, and molecular docking study. Front. Plant Sci. 2024, 15, 1513875. [Google Scholar] [CrossRef]
- Xiang, H.; Zuo, J.; Guo, F.; Dong, D. What we already know about rhubarb: A comprehensive review. Chin. Med. 2020, 15, 88. [Google Scholar]
- Sun, Y.; Wang, A.; Wan, D.; Wang, Q.; Liu, J. Rapid radiation of Rheum (Polygonaceae) and parallel evolution of morphological traits. Mol. Phylogenet. Evol. 2012, 63, 150–158. [Google Scholar] [PubMed]
- Barceloux, D.G. Rhubarb and oxalosis (Rheum species). Dis.-a-Mon. 2009, 6, 403–411. [Google Scholar]
- Hevor, E.A. Preservation of Fresh-Cut Apples with Selected Natural Compounds. M.Sc. Thesis, Czech University of Life Sciences Prague, Prague, Czech Republic, 2023. [Google Scholar]
- Yildirim, M.; Degirmenci, U.; Akkapulu, M.; Comelekoglu, U.; Balli, E.; Metin Ozcan, T.; Berköz, M.; Yalin, A.E.; Yalin, S. The effect of Rheum ribes L. on oxidative stress in diabetic rats. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 20200058. [Google Scholar]
- Park, S.K.; Lee, Y.K. Antioxidant activity in rheum emodi wall (Himalayan Rhubarb). Molecules 2021, 26, 2555. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Kim, C.Y. Lipid peroxidation and antioxidant activities of the aqueous rhizome extract of Rheum officinale Baillon. J. Food Qual. 2018, 2018, 5258276. [Google Scholar]
- Zhumashova, G.; Kukula-Koch, W.; Koch, W.; Baj, T.; Sayakova, G.; Shukirbekova, A.; Głowniak, K.; Sakipova, Z. Phytochemical and antioxidant studies on a rare Rheum cordatum Losinsk. Species from Kazakhstan. Oxidative Med. Cell. Longev. 2019, 2019, 5465463. [Google Scholar]
- Aygün, A.; Gülbağça, F.; Nas, M.S.; Alma, M.H.; Çalımlı, M.H.; Ustaoglu, B.; Altunoglu, Y.C.; Baloğlu, M.C.; Cellat, K.; Şen, F. Biological synthesis of silver nanoparticles using Rheum ribes and evaluation of their anticarcinogenic and antimicrobial potential: A novel approach in phytonanotechnology. J. Pharm. Biomed. Anal. 2020, 179, 113012. [Google Scholar] [CrossRef]
- Yang, W.-T.; Ke, C.-Y.; Wu, W.-T.; Tseng, Y.-H.; Lee, R.-P. Antimicrobial and anti-inflammatory potential of Angelica dahurica and Rheum officinale extract accelerates wound healing in Staphylococcus aureus-infected wounds. Sci. Rep. 2020, 10, 5596. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.-X.; Li, J.-C.; Miao, X.-L.; Guo, X.; Shang, X.-F.; Wang, W.-W.; Li, B.; Wang, Y.; Pan, H.; Zhang, J.-Y. Ultrasound-assisted extraction of five anthraquinones from Rheum palmatum water extract residues and the antimicrobial activities. Ind. Crops Prod. 2021, 162, 113288. [Google Scholar]
- Liu, J.; Leng, L.; Gao, H.; Yang, W.; Liu, A. A large-scale detection, identification and quantification of target metabolites using dMRM-MS combined with transcriptome of two rheum species focused on anthraquinone and flavonids biosynthesis. Res. Sq. 2019. [Google Scholar] [CrossRef]
- Liu, J.; Leng, L.; Liu, Y.; Gao, H.; Yang, W.; Chen, S.; Liu, A. Identification and quantification of target metabolites combined with transcriptome of two rheum species focused on anthraquinone and flavonoids biosynthesis. Sci. Rep. 2020, 10, 20241. [Google Scholar]
- Khattak, A.K.; Syeda, M.; Shahzad, S. General overview of phytochemistry and pharmacological potential of Rheum palmatum (Chinese rhubarb). Innovare J. Ayurvedic Sci. 2020, 8, 1–5. [Google Scholar]
- Liu, Q.; Shen, J.; Li, P.; Li, Y.; He, C.; Xiao, P. Stilbenoids isolated from the roots of Rheum lhasaense under the guidance of the acetylcholinesterase inhibition activity. J. Nat. Med. 2021, 75, 372–380. [Google Scholar]
- Riyanto, S.; Sukari, M.A.; Rahmani, M.; Ee, G.C.; Yap, Y.; Aimi, N.; Kitajima, M. Alkaloids from Aegle marmelos (Rutaceae). Malays. J. Anal. Sci. 2001, 7, 463–465. [Google Scholar]
- WHO. Traditional Medicine. Available online: https://www.who.int/news-room/questions-and-answers/item/traditional-medicine (accessed on 9 August 2023).
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar]
- Kubátová, A.; Lagadec, A.J.; Miller, D.J.; Hawthorne, S.B. Selective extraction of oxygenates from savory and peppermint using subcritical water. Flavour Fragr. J. 2001, 16, 64–73. [Google Scholar] [CrossRef]
- Li, H.; Pordesimo, L.; Weiss, J. High intensity ultrasound-assisted extraction of oil from soybeans. Food Res. Int. 2004, 37, 731–738. [Google Scholar] [CrossRef]
- Demirkol, O.; Erşatır, M.; Giray, E.S.; Kırıcı, S. Comparison of the effects of green and sustainable extraction methods on the extraction yield and chemical composition of Ruta chalepensis roots. Sustain. Chem. Pharm. 2022, 29, 100750. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Yıldırım, M.; Erşatır, M.; Poyraz, S.; Amangeldinova, M.; Kudrina, N.O.; Terletskaya, N.V. Green Extraction of Plant Materials Using Supercritical CO2: Insights into Methods, Analysis, and Bioactivity. Plants 2024, 13, 2295. [Google Scholar] [CrossRef] [PubMed]
- Rawson, A.; Tiwari, B.; Brunton, N.; Brennan, C.; Cullen, P.; O’donnell, C. Application of supercritical carbon dioxide to fruit and vegetables: Extraction, processing, and preservation. Food Rev. Int. 2012, 28, 253–276. [Google Scholar] [CrossRef]
- Eisenbach, W.O. Supercritical carbon dioxide as an extraction agent. In Carbon Dioxide as a Source of Carbon: Biochemical and Chemical Uses; Springer: Berlin, Germany, 1987; pp. 371–388. [Google Scholar]
- Villacís-Chiriboga, J.; Voorspoels, S.; Uyttebroek, M.; Ruales, J.; Van Camp, J.; Vera, E.; Elst, K. Supercritical CO2 extraction of bioactive compounds from mango (Mangifera indica L.) peel and pulp. Foods 2021, 10, 2201. [Google Scholar] [CrossRef]
- Costa, F.M.d.; Ortega, T.L.C.; Arvelos, S.; Traczynski, M.R.; Silva, E.A.d.; Cardozo-Filho, L.; Eponina, H.C.; Watanabe, E.O. Evaluation of supercritical carbon dioxide extraction to obtain bioactive compounds from Vernonia amygdalina delile leaves. Chem. Ind. Chem. Eng. Q. 2020, 26, 113–124. [Google Scholar] [CrossRef]
- Fu, X.; Wang, D.; Belwal, T.; Xie, J.; Xu, Y.; Li, L.; Zou, L.; Zhang, L.; Luo, Z. Natural deep eutectic solvent enhanced pulse-ultrasonication assisted extraction as a multi-stability protective and efficient green strategy to extract anthocyanin from blueberry pomace. LWT 2021, 144, 111220. [Google Scholar] [CrossRef]
- Qin, L.; Yu, J.; Zhu, J.; Kong, B.; Chen, Q. Ultrasonic-assisted extraction of polyphenol from the seeds of Allium senescens L. and its antioxidative role in Harbin dry sausage. Meat Sci. 2021, 172, 108351. [Google Scholar] [CrossRef]
- Saleh, I.A.; Vinatoru, M.; Mason, T.J.; Abdel-Azim, N.; Aboutabl, E.; Hammouda, F. A possible general mechanism for ultrasound-assisted extraction (UAE) suggested from the results of UAE of chlorogenic acid from Cynara scolymus L.(artichoke) leaves. Ultrason. Sonochem. 2016, 31, 330–336. [Google Scholar] [CrossRef]
- Nolasco-González, Y.; Chacón-López, M.A.; Ortiz-Basurto, R.I.; Aguilera-Aguirre, S.; González-Aguilar, G.A.; Rodríguez-Aguayo, C.; Navarro-Cortez, M.C.; García-Galindo, H.S.; García-Magaña, M.d.L.; Meza-Espinoza, L. Annona muricata leaves as a source of bioactive compounds: Extraction and quantification using ultrasound. Horticulturae 2022, 8, 560. [Google Scholar] [CrossRef]
- Turrini, F.; Donno, D.; Beccaro, G.L.; Zunin, P.; Pittaluga, A.; Boggia, R. Pulsed ultrasound-assisted extraction as an alternative method to conventional maceration for the extraction of the polyphenolic fraction of Ribes nigrum buds: A new category of food supplements proposed by the Finnover project. Foods 2019, 8, 466. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, I.M.; Taher, Z.M.; Rahmat, Z.; Chua, L.S. A review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef]
- Rahaman, A.; Zeng, X.-A.; Kumari, A.; Rafiq, M.; Siddeeg, A.; Manzoor, M.F.; Baloch, Z.; Ahmed, Z. Influence of ultrasound-assisted osmotic dehydration on texture, bioactive compounds and metabolites analysis of plum. Ultrason. Sonochem. 2019, 58, 104643. [Google Scholar] [PubMed]
- Hadidi, M.; Ibarz, A.; Pagan, J. Optimisation and kinetic study of the ultrasonic-assisted extraction of total saponins from alfalfa (Medicago sativa) and its bioaccessibility using the response surface methodology. Food Chem. 2020, 309, 125786. [Google Scholar]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar]
- Munir, M.; Kheirkhah, H.; Baroutian, S.; Quek, S.Y.; Young, B.R. Subcritical water extraction of bioactive compounds from waste onion skin. J. Clean. Prod. 2018, 183, 487–494. [Google Scholar] [CrossRef]
- Gbashi, S.; Adebo, O.A.; Piater, L.; Madala, N.E.; Njobeh, P.B. Subcritical water extraction of biological materials. Sep. Purif. Rev. 2017, 46, 21–34. [Google Scholar]
- Yilmaz, M.A. Simultaneous quantitative screening of 53 phytochemicals in 33 species of medicinal and aromatic plants: A detailed, robust and comprehensive LC–MS/MS method validation. Ind. Crops Prod. 2020, 149, 112347. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar]
- Necip, A.; Işık, M. Bioactivities of Hypericum perforatum L. and Equisetum arvense L. fractions obtained with different solvents. Int. J. Life Sci. Biotechnol. 2019, 2, 221–230. [Google Scholar] [CrossRef]
- Necip, A.; Işık, M.; Güzel, A.; Takım, K.; Kaygısız, F. Nicotiana rustica L’nin Bazı Önemli Metabolik Enzimleri Üzerindeki İnhibisyon Etkisi, LC-MS/MS Analizi, Antioksidan Özellikleri. Ksu Tarim Ve Doga Derg.-Ksu J. Agric. Nat. 2021, 24, 930–938. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [PubMed]
- Necip, A.; Demirtas, I.; Tayhan, S.E.; Işık, M.; Bilgin, S.; Turan, İ.F.; İpek, Y.; Beydemir, Ş. Isolation of phenolic compounds from eco-friendly white bee propolis: Antioxidant, wound-healing, and anti—Alzheimer effects. Food Sci. Nutr. 2024, 12, 1928–1939. [Google Scholar] [CrossRef]
- Necip, A.; Durgun, M. Antioxidant properties, total phenolic content and LC-MS/MS analysis of Mentha pulegium, Lepidium draba and Centaurea solstitialis. J. Inst. Sci. Technol. 2022, 12, 2375–2385. [Google Scholar]
- Carbonell, S.A.; de Aquino Neto, F.R.; Cardoso, J.N.; dos Santos Pereira, A.; Amaral, A.C.F.; Barnes, R.A. Rapid screening of natural products by high-resolution high-temperature gas chromatography. J. Chromatogr. Sci. 2000, 38, 234–240. [Google Scholar]
- Yildirim, M.; Yasar, E.; Necip, A.; Cimentepe, M.; Demirbağ, B.; Kilic, A. Facile synthesis and spectral analysis of the bioactive spiroborate compounds as a novel therapeutic agent for computational insights, biological evaluation, and applications. J. Organomet. Chem. 2025, 1027, 123510. [Google Scholar] [CrossRef]
- Yildirim, M.; Kilic, A.; Cimentepe, M.; Necip, A.; Turedi, S. Synthesis of bioactive quercetin-boronate esters as a novel biological agent: Enzyme inhibition, anti-microbial properties, computational insights and anti-cancer activity. J. Mol. Struct. 2025, 1321, 140216. [Google Scholar] [CrossRef]
- Yildirim, M.; Cimentepe, M.; Dogan, K.; Necip, A.; Karakoc, V. Advancing drug delivery and antimicrobial activity: Development and molecular docking analysis of quercetin-loaded pHEMA cryogel membranes. J. Mol. Struct. 2025, 1319, 139271. [Google Scholar] [CrossRef]
- Ntamo, Y.; Jack, B.; Ziqubu, K.; Mazibuko-Mbeje, S.E.; Nkambule, B.B.; Nyambuya, T.M.; Mabhida, S.E.; Hanser, S.; Orlando, P.; Tiano, L.; et al. Epigallocatechin gallate as a nutraceutical to potentially target the metabolic syndrome: Novel insights into therapeutic effects beyond its antioxidant and anti-inflammatory properties. Crit. Rev. Food Sci. Nutr. 2024, 64, 87–109. [Google Scholar]
- Couto, A.G.; Kassuya, C.A.; Calixto, J.B.; Petrovick, P.R. Anti-inflammatory, antiallodynic effects and quantitative analysis of gallic acid in spray dried powders from Phyllanthus niruri leaves, stems, roots and whole plant. Rev. Bras. Farmacogn. 2013, 23, 124–131. [Google Scholar] [CrossRef]
- Xu, N.; Liu, S.; Zhang, Y.; Chen, Y.; Zuo, Y.; Tan, X.; Liao, B.; Li, P.; Feng, J. Oxidative stress signaling in the pathogenesis of diabetic cardiomyopathy and the potential therapeutic role of antioxidant naringenin. Redox Rep. 2023, 28, 2246720. [Google Scholar]
- Choi, S.S.; Park, H.R.; Lee, K.A. A comparative study of rutin and rutin glycoside: Antioxidant activity, anti-inflammatory effect, effect on platelet aggregation and blood coagulation. Antioxidants 2021, 10, 1696. [Google Scholar] [CrossRef] [PubMed]
- Shariati, S.; Mohtadi, S.; Khodayar, M.J.; Salehcheh, M.; Azadnasab, R.; Mansouri, E.; Moosavi, M. Quinic acid alleviates liver toxicity induced by acetaminophen in mice via anti-oxidative and anti-inflammatory effects. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 1–12. [Google Scholar] [CrossRef]
- Benali, T.; Bakrim, S.; Ghchime, R.; Benkhaira, N.; El Omari, N.; Balahbib, A.; Taha, D.; Zengin, G.; Hasan, M.M.; Bibi, S.; et al. Pharmacological insights into the multifaceted biological properties of quinic acid. Biotechnol. Genet. Eng. Rev. 2024, 40, 3408–3437. [Google Scholar] [PubMed]
- Mohammadinejad, A.; Mohajeri, T.; Aleyaghoob, G.; Heidarian, F.; Kazemi Oskuee, R. Ellagic acid as a potent anticancer drug: A comprehensive review on in vitro, in vivo, in silico, and drug delivery studies. Biotechnol. Appl. Biochem. 2022, 69, 2323–2356. [Google Scholar]
- Moradi, M.; Farbood, Y.; Mard, S.A.; Dianat, M.; Goudarzi, G.; Khorsandi, L.; Seyedian, S.S. p-Coumaric acid has pure anti-inflammatory characteristics against hepatopathy caused by ischemia-reperfusion in the liver and dust exposure. Iran. J. Basic Med. Sci. 2023, 26, 164. [Google Scholar]
- Raudsepp, P.; Helmja, K.; Raal, A.; Vaher, M.; Püssa, T. Comparative study of antioxidant capacity of Rheum rhaponticum root polyphenols. In Proceedings of the XXIVth International Conference on Polyphenols (ICP 2008), Salamanca, Spain, 7–11 July 2008. [Google Scholar]
- Amirkhosravi, A.; Farhadi, S.; Vasei, S.; Raeiszadeh, M.; Mehrabani, M. The influence of harvest time on total phenolic and flavonoid contents, antioxidant, antibacterial and cytotoxicity of Rheum khorasanicum root extract. Ann. Pharm. Françaises 2023, 81, 475–483. [Google Scholar]
- Malik, M.A.; Bhat, S.A.; Rehman, M.U.; Sidique, S.; Akhoon, Z.A.; Shrivastava, P.; Sheikh, B.A. Phytochemical analysis and antimicrobial activity of Rheum emodi (Rhubarb) rhizomes. Pharma Innov. 2018, 7, 17. [Google Scholar]
- Rolta, R.; Kumar, V.; Sourirajan, A.; Upadhyay, N.K.; Dev, K. Bioassay guided fractionation of rhizome extract of Rheum emodi wall as bio-availability enhancer of antibiotics against bacterial and fungal pathogens. J. Ethnopharmacol. 2020, 257, 112867. [Google Scholar]
- Canli, K.; Yetgin, A.; Akata, I.; Altuner, E.M. In vitro antimicrobial activity screening of Rheum rhabarbarum roots. Int. J. Pharm. Sci. Invent. 2016, 5, 1–4. [Google Scholar]
- Önem, E.; Sarısu, H.C.; Ibrahım, B. The effect of Rheum ribes L. extracts on bacterial communication and antibacterial activity. Süleyman Demirel Üniversitesi Sağlık Bilim. Derg. 2020, 11, 436–442. [Google Scholar]

| No. | Analytes | ScCO2-100 | ScCO2-400 | SbcEtOH-60 | SbcEtOH-80_ | UAE-EtOH-1h | UAE-EtOH-4h | UAE-MeOH-1h | UAE-MeOH-4h |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Quinic acid | 0.421 | 0.297 | 1.616 | 2.357 | 0.373 | 0.352 | 0.644 | 0.503 |
| 2 | Fumaric aid | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 3 | Aconitic acid | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 4 | Gallic acid | 76.967 | 42.596 | 54.526 | 72.364 | 49.735 | 44.883 | 46.59 | 48.396 |
| 5 | Epigallocatechin | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 6 | Protocatechuic acid | 3.29 | 1.613 | 1.602 | 2.836 | 0.847 | 0.682 | 0.9 | 0.968 |
| 7 | Catechin | 3.806 | N.D. | 5.112 | 6.132 | 6.989 | 4.621 | 6.208 | 6.37 |
| 8 | Gentisic acid | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 9 | Chlorogenic acid | N.D. | N.D. | 0.133 | 0.681 | N.D. | N.D. | N.D. | N.D. |
| 10 | Protocatechuic aldehyde | 0.263 | 0.584 | 0.369 | 0.502 | N.D. | 0.035 | 0.068 | 0.064 |
| 11 | Tannic acid | 3.661 | 1.738 | 2.895 | 5.97 | 1.263 | 1.693 | 2.573 | 3.592 |
| 12 | Epigallocatechin gallate | 18.167 | 2.729 | 21.469 | 14.835 | 30.557 | 26.634 | 29.218 | 28.09 |
| 13 | Cynarin | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 14 | 4-OH Benzoic acid | 1.693 | 1.37 | N.D. | 0.463 | N.D. | N.D. | N.D. | N.D. |
| 15 | Epicatechin | 8.143 | N.D. | 20.751 | 20.337 | 24.413 | 15.652 | 20.086 | 19.913 |
| 16 | Vanilic acid | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 17 | Caffeic acid | 0.543 | 0.165 | 0.074 | 0.145 | 0.052 | 0.039 | 0.043 | 0.038 |
| 18 | Syringic acid | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 19 | Vanillin | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 20 | Syringic aldehyde | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 21 | Daidzin | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 22 | Epicatechin gallate | 77.341 | 9.998 | 61.431 | 50.102 | 83.53 | 73.078 | 80.27 | 79.744 |
| 23 | Piceid | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 24 | p-Coumaric acid | 0.855 | 0.491 | 0.093 | 0.147 | 0.08 | 0.086 | 0.12 | 0.065 |
| 25 | Ferulic acid-D3-ISh | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
| 26 | Ferulic acid | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 27 | Sinapic acid | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 28 | Coumarin | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 29 | Salicylic acid | 0.027 | 0.035 | 0.018 | N.D. | N.D. | N.D. | N.D. | N.D. |
| 30 | Cyranoside | N.D. | N.D. | 0.05 | 0.041 | 0.048 | 0.043 | 0.054 | 0.046 |
| 31 | Miquelianin | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 32 | Rutin-D3-IS | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
| 33 | Rutin | 4.778 | 1.383 | 0.24 | 0.214 | 0.32 | 0.241 | 0.247 | 0.211 |
| 34 | isoquercitrin | 1.865 | 0.209 | 0.346 | 0.318 | 0.452 | 0.398 | 0.407 | 0.368 |
| 35 | Hesperidin | 3.791 | 1.029 | 0.197 | 0.716 | 0.284 | 0.182 | 0.254 | 0.212 |
| 36 | o-Coumaric acid | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 37 | Genistin | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 38 | Rosmarinic acid | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 39 | Ellagic acid | 1.671 | 0.545 | 0.842 | 0.513 | 3.425 | 2.501 | 3.254 | 2.507 |
| 40 | Cosmosiin | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 41 | Quercitrin | 0.204 | N.D. | 0.058 | 0.037 | 0.073 | 0.069 | 0.065 | 0.058 |
| 42 | Astragalin | 0.166 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 43 | Nicotiflorin | 1.069 | 0.221 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 44 | Fisetin | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 45 | Daidzein | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 46 | Quercetin-D3-IS | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
| 47 | Quercetin | 0.837 | 0.09 | 0.093 | 0.074 | 0.075 | 0.08 | 0.115 | 0.056 |
| 48 | Naringenin | 0.114 | 0.053 | 0.013 | 0.018 | 0.015 | 0.013 | 0.013 | 0.016 |
| 49 | Hesperetin | 0.058 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 50 | Luteolin | 0.016 | 0.004 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.004 |
| 51 | Genistein | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 52 | Kaempferol | 0.116 | 0.035 | 0.009 | 0.012 | 0.015 | N.D. | 0.012 | 0.01 |
| 53 | Apigenin | 0.016 | 0.011 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 54 | Amentoflavone | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 55 | Chrysin | 0.09 | 0.075 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 56 | Acacetin | 0.012 | 0.023 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| DPPH (IC50 mg/mL) | R2 | ABTS (IC50 mg/mL) | R2 | CUPRAC (mg TE/mL) | Toplam Fenolik (mg GAE/g) | |
|---|---|---|---|---|---|---|
| BHA | 0.0023 ± 0.0002 | 0.994 | 0.0021 ± 0.0002 | 0.981 | ||
| BHT | 0.0038 | 0.961 | 0.0034 | 0.993 | ||
| Trolox | 0.0076 | 0.991 | 0.0041 | 0.998 | ||
| UAE-MeOH-1h | 0.0606 ± 0.003 a | 0.964 | 0.0056 ± 0.0003 a | 0.994 | 0.0236 ± 0.0012 | 195.355 ± 9.75 |
| UAE-EtOH-1h | 0.0645 ± 0.003 a | 0.965 | 0.0055 ± 0.0003 | 0.995 | 0.0228 ± 0.0012 | 208.066 ± 10.4 |
| UAE-MeOH-4h | 0.0654 ± 0.003 a | 0.968 | 0.0058 ± 0.0003 a | 0.994 | 0.0195 ± 0.001 b,c | 204.644 ± 11 |
| UAE-EtOH-4h | 0.0659 ± 0.004 a | 0.969 | 0.0057 ± 0.0003 a | 0.995 | 0.0197 ± 0.001 b,c,d | 207.088 ± 11 c |
| sbcEtOH-E 140-60 | 0.1122 ± 0.005 a,b,c,d,e | 0.964 | 0.0080 ± 0.0004 a,b,c,d,e | 0.964 | 0.0113 ± 0.001 b,c,d,e | 176.289 ± 9 b |
| sbcEtOH-E 140-80 | 0.1297 ± 0.006 a,d,e,f | 0.966 | 0.0078 ± 0.0003 a,c,d,e | 0.965 | 0.0105 ± 0.001 b,c,d,e | 189.002 ± 10 c,e |
| ScCO2 100-60 | 0.0352 ± 0.002 a,b,c,d,e | 0.997 | 0.0273 ± 0.0013 | 0.995 | 0.0139 ± 0.001 b,c,d,e,g | 178.245 ± 9 b,e,f |
| ScCO2 400-60 | 0.0348 ± 0.002 a,b,c,d,e | 0.992 | 0.0256 ± 0.0012 | 0.992 | 0.0081 ± 0.0006 b,c,d,e,f,h | 188.022 ± 10 c,e |
| S. aureus | E. faecalis | P. aeruginosa | E. coli | |||||
|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| UAE-EtOH-1h | 250 | 250 | 125 | 125 | 250 | 500 | 500 | 500 |
| UAE-EtOH-4h | 500 | 1000 | 1000 | 2000 | 500 | 1000 | 500 | 1000 |
| UAE-MeOH-1h | 250 | 250 | 125 | 125 | 250 | 500 | 500 | 500 |
| UAE-MeOH-4h | 250 | 250 | 125 | 125 | 250 | 500 | 500 | 500 |
| sbcEtOH-E 140-60 | 250 | 250 | 62.5 | 125 | 500 | 500 | 250 | 500 |
| sbcEtOH-E 140-80 | 125 | 250 | 62.5 | 125 | 250 | 500 | 250 | 500 |
| ScCO2 100-60 | 250 | 500 | 31.25 | 250 | 250 | 250 | 250 | 500 |
| ScCO2 400-60 | 500 | 1000 | 62.5 | 500 | 500 | 500 | 500 | 1000 |
| Ampicillin | * | * | 31.25 | 3.9 | ||||
| 1QWZ | 2ANQ | 3H77 | 6QXS | |||||
|---|---|---|---|---|---|---|---|---|
| Docking Score | Glide Emodel | Docking Score | Glide Emodel | Docking Score | Glide Emodel | Docking Score | Glide Emodel | |
| Epicatechin | −6.127 | −49.236 | −9.479 | −66.637 | −5.836 | −39.315 | −7.067 | −63.494 |
| Epicatechin gallate | −3.410 | −39.416 | −6.769 | −76.817 | −4.955 | −40.342 | −7.616 | −78.489 |
| Epigallocatechin gallate | −3.915 | −43.334 | −9.385 | −52.025 | −5.548 | −48.280 | −7.009 | −72.014 |
| Gallic acid | −4.861 | −24.020 | −7.830 | −72.009 | −5.777 | −37.897 | −6.431 | −54.483 |
| Ampicillin | −5.733 | −63.137 | −9.041 | −86.410 | −6.299 | −56.984 | −8.234 | −88.989 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amangeldinova, M.; Ersatır, M.; Necip, A.; Cimentepe, M.; Kudrina, N.; Terletskaya, N.; Cimentepe, O.O.; Cakır, O.; Yilmaz, M.A.; Yildirim, M. Green Extraction Strategies and Bioactivity of Rheum cordatum Losinsk: Antioxidant, Antimicrobial, and Molecular Docking Insights. Plants 2025, 14, 1071. https://doi.org/10.3390/plants14071071
Amangeldinova M, Ersatır M, Necip A, Cimentepe M, Kudrina N, Terletskaya N, Cimentepe OO, Cakır O, Yilmaz MA, Yildirim M. Green Extraction Strategies and Bioactivity of Rheum cordatum Losinsk: Antioxidant, Antimicrobial, and Molecular Docking Insights. Plants. 2025; 14(7):1071. https://doi.org/10.3390/plants14071071
Chicago/Turabian StyleAmangeldinova, Madina, Mehmet Ersatır, Adem Necip, Mehmet Cimentepe, Nataliya Kudrina, Nina Terletskaya, Ozge Oztürk Cimentepe, Oguz Cakır, Mustafa Abdullah Yilmaz, and Metin Yildirim. 2025. "Green Extraction Strategies and Bioactivity of Rheum cordatum Losinsk: Antioxidant, Antimicrobial, and Molecular Docking Insights" Plants 14, no. 7: 1071. https://doi.org/10.3390/plants14071071
APA StyleAmangeldinova, M., Ersatır, M., Necip, A., Cimentepe, M., Kudrina, N., Terletskaya, N., Cimentepe, O. O., Cakır, O., Yilmaz, M. A., & Yildirim, M. (2025). Green Extraction Strategies and Bioactivity of Rheum cordatum Losinsk: Antioxidant, Antimicrobial, and Molecular Docking Insights. Plants, 14(7), 1071. https://doi.org/10.3390/plants14071071








