Jasmonic Acid Enhances Rice Cadmium Tolerance by Suppressing Cadmium Uptake and Translocation
Abstract
1. Introduction
2. Results
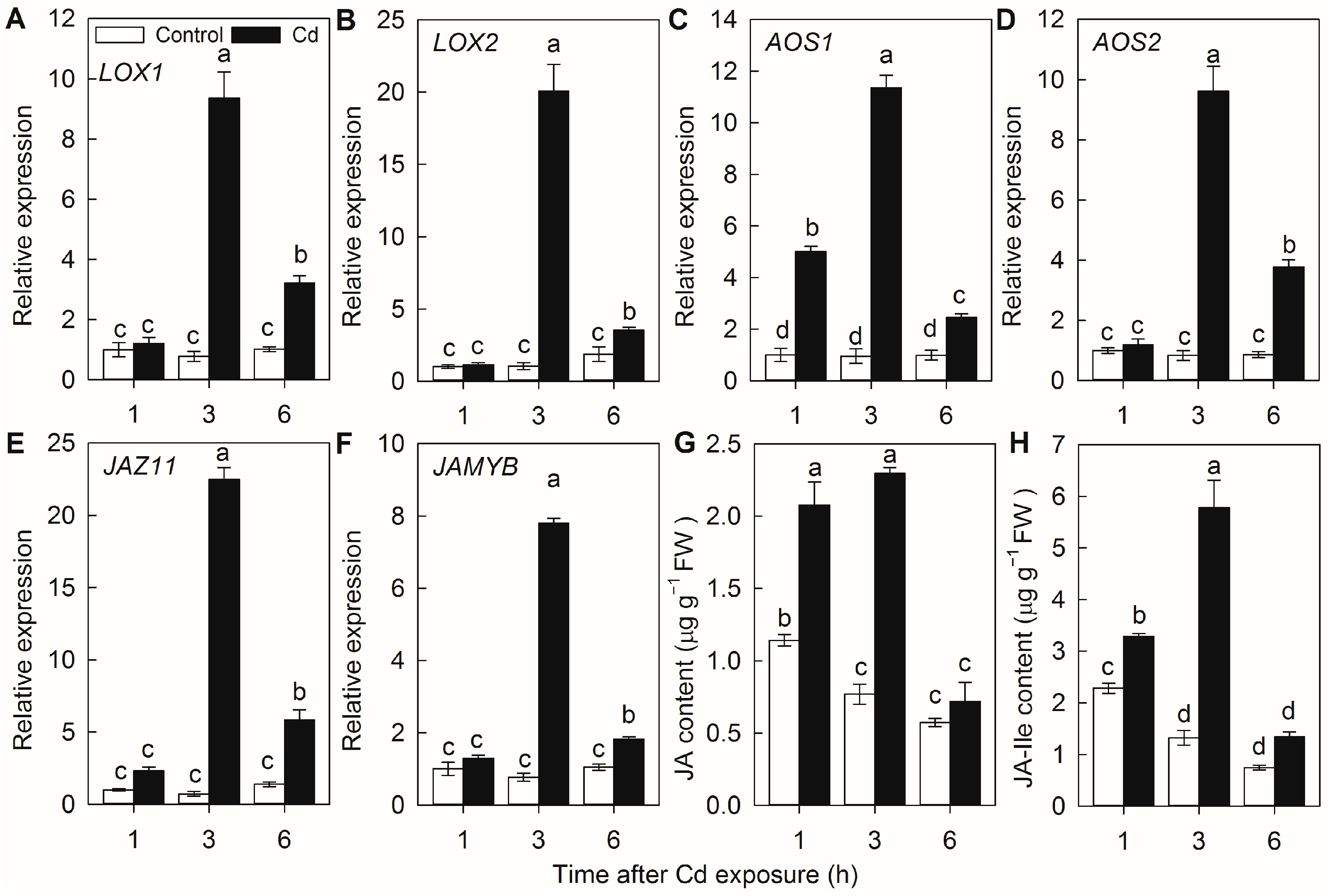
2.1. JA Synthesis Is Enhanced by Cd Exposure
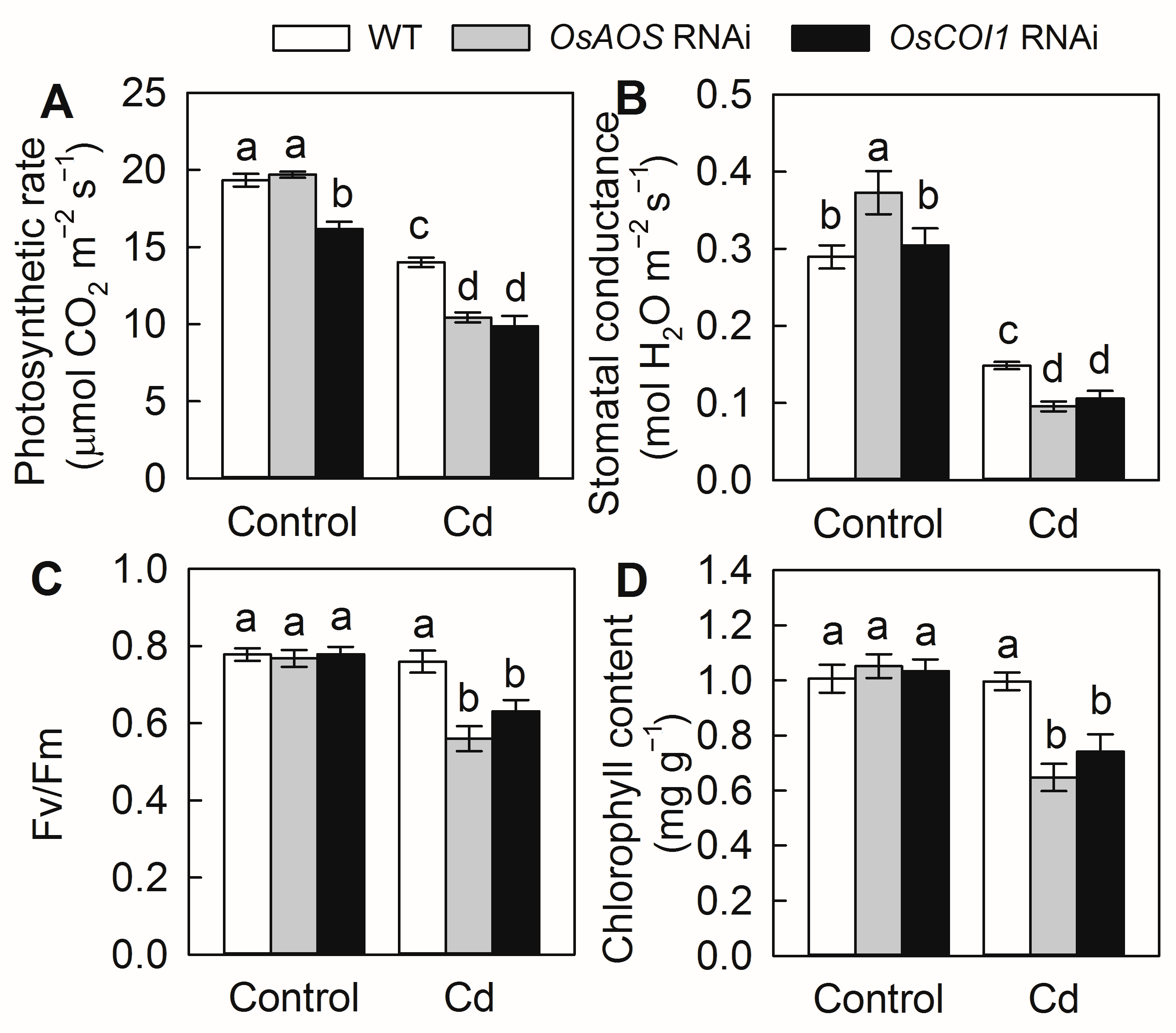
2.2. Silencing of OsAOS and OsCOI1 Aggravates Cd Toxicity in Rice
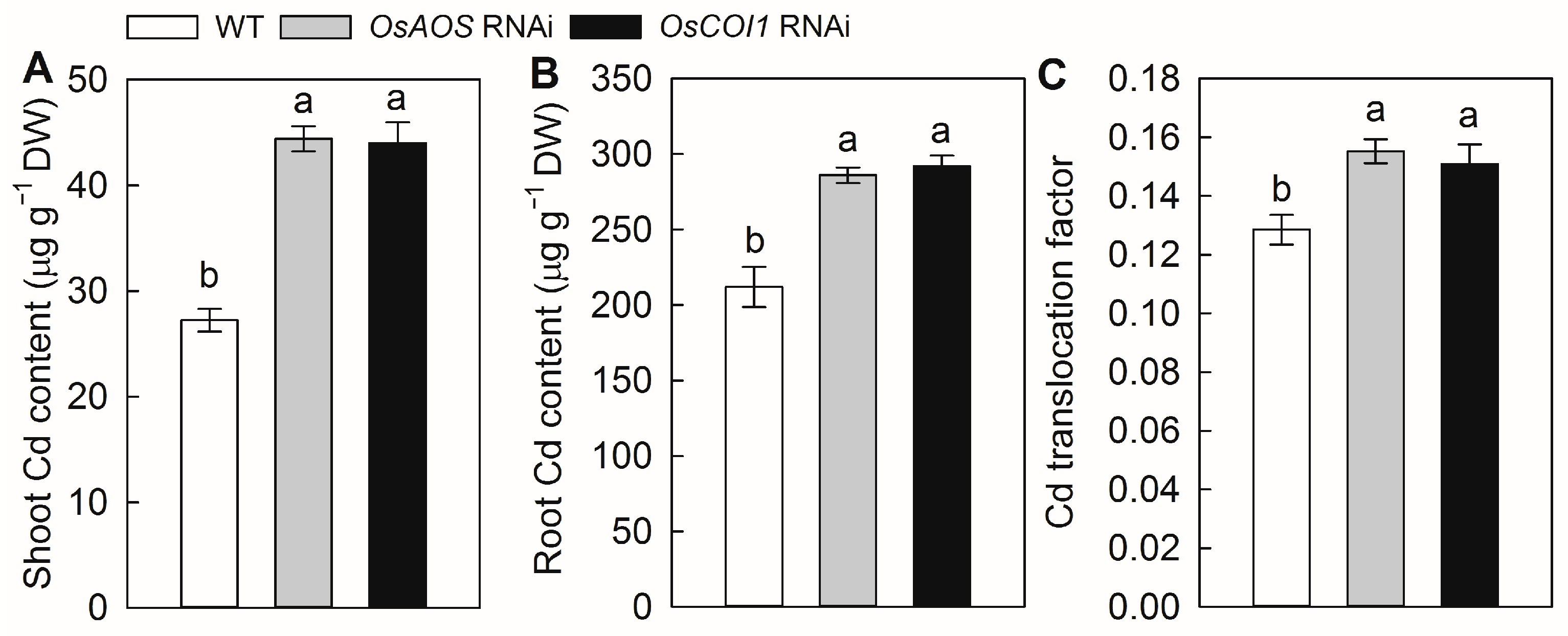
2.3. Silencing of OsAOS and OsCOI1 Increases Cd Accumulation
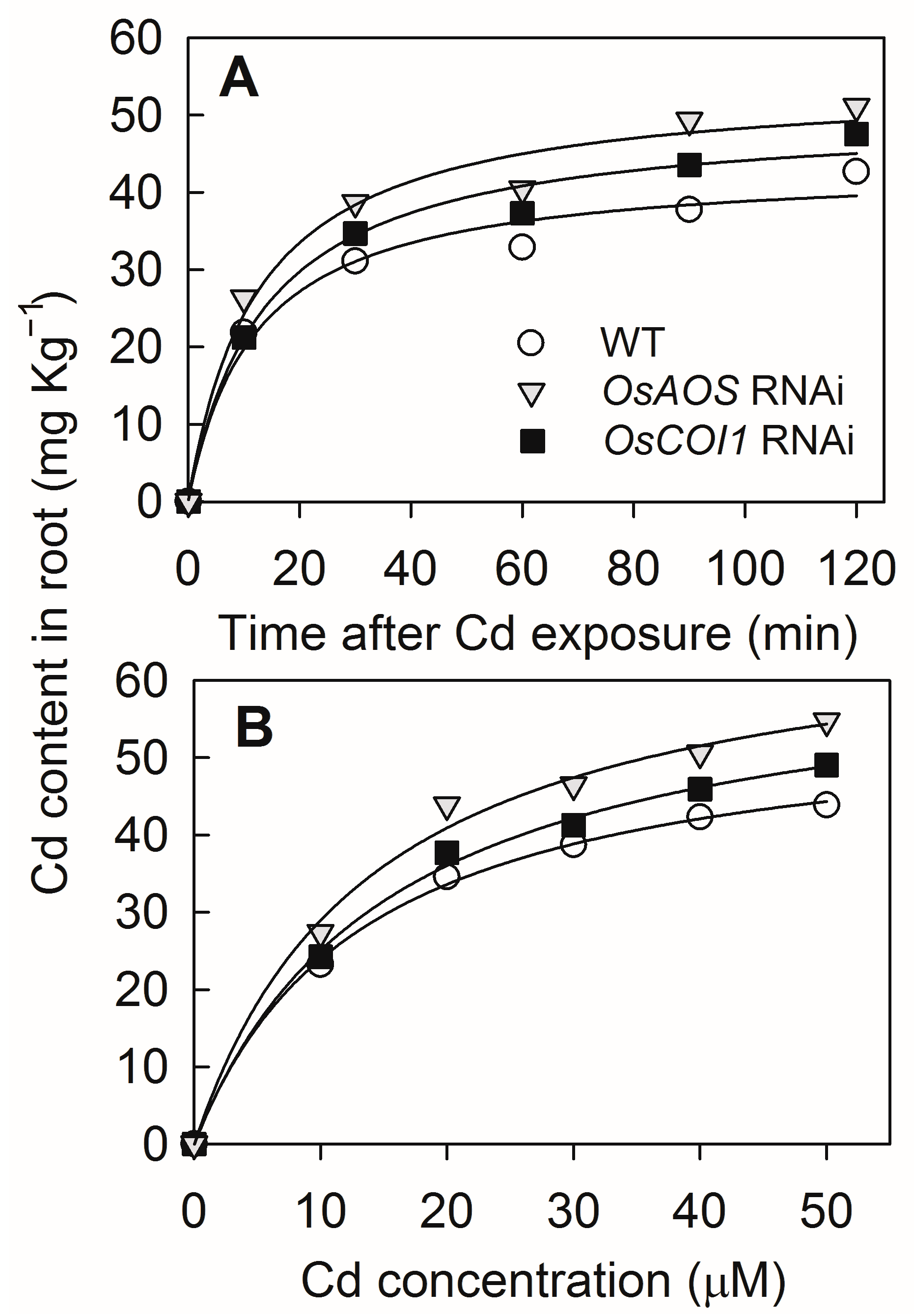
2.4. Silencing of OsAOS and OsCOI1 Enhances Root Cd Uptake
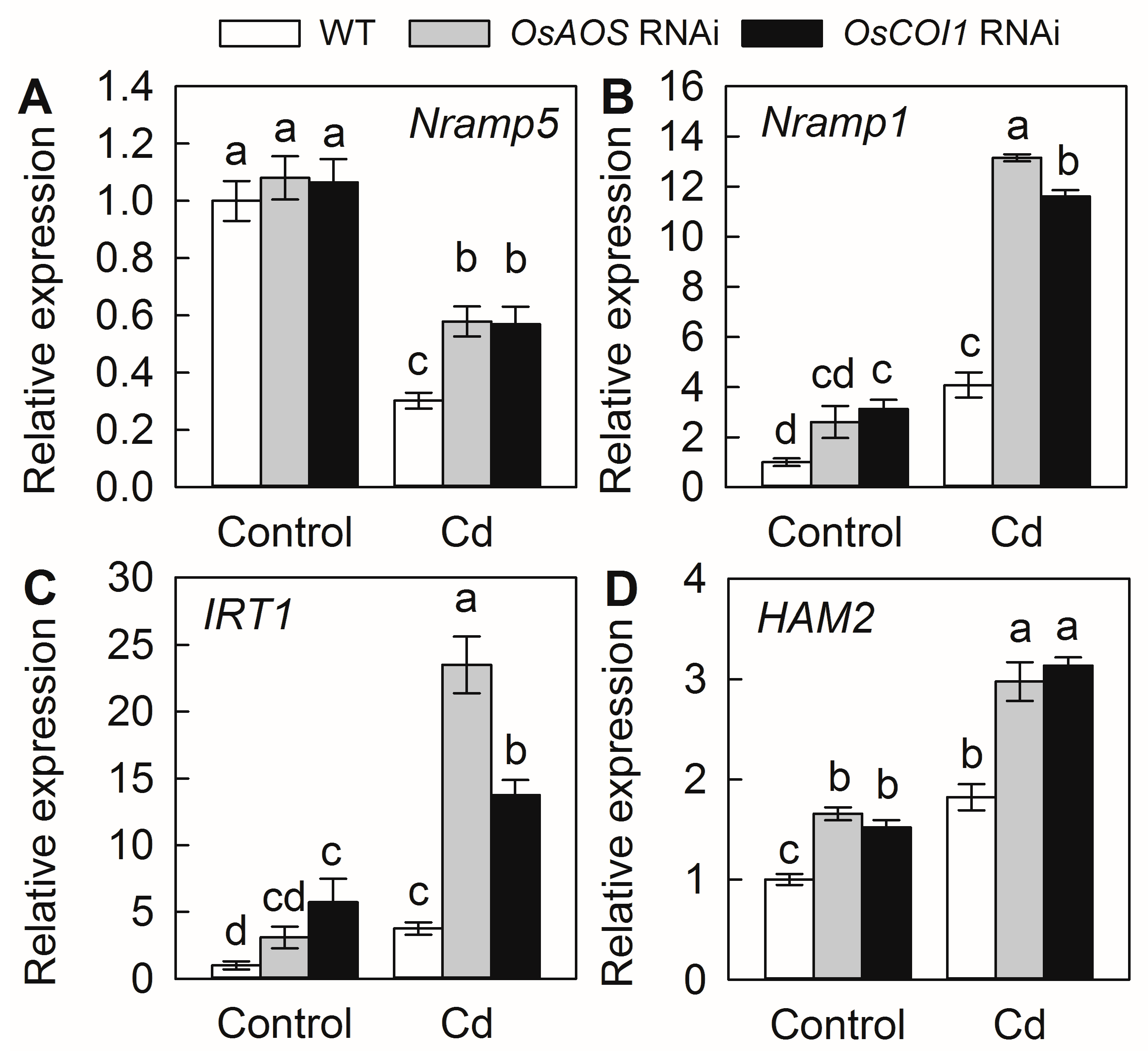
2.5. Silencing of OsAOS and OsCOI1 Promotes Expression of Genes for Cd Uptake and Translocation
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Leaf Gas Exchange Analysis
4.3. Chlorophyll Fluorescence Analysis
4.4. JA and JA-Ile Analysis
4.5. Cd Content Measurement
4.6. Short-Term Cd Uptake Determination
4.7. Gene Expression Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Cd | Cadmium |
| JA | Jasmonic acid |
| RNAi | RNA interference |
| WT | Wild type |
| AOS | Allene oxide synthase |
| COI | CORONATINE INSENSITIVE |
| LOX | Lipoxygenase |
| IRT | iron-regulated transporter |
| Nramp | Natural resistance-associated macrophage protein |
| HMA | P-type heavy metal ATPase |
| RT-qPCR | Reverse transcription–quantitative PCR |
References
- Clemens, S.; Aarts, M.G.; Thomine, S.; Verbruggen, N. Plant Science: The Key to Preventing Slow Cadmium Poisoning. Trends Plant Sci. 2013, 18, 92–99. [Google Scholar] [PubMed]
- Zhao, D.; Wang, P.; Zhao, F.-J. Toxic Metals and Metalloids in Food: Current Status, Health Risks, and Mitigation Strategies. Curr. Environ. Health Rep. 2024, 11, 468–483. [Google Scholar] [PubMed]
- Han, R.; Wang, Z.; Wang, S.; Sun, G.; Xiao, Z.; Hao, Y.; Nriagu, J.; Teng, H.H.; Li, G. A Combined Strategy to Mitigate the Accumulation of Arsenic and Cadmium in Rice (Oryza sativa L.). Sci. Total Environ. 2023, 896, 165226. [Google Scholar] [PubMed]
- Zhang, H.; Reynolds, M. Cadmium Exposure in Living Organisms: A Short Review. Sci. Total Environ. 2019, 678, 761–767. [Google Scholar]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium Toxicity in Plants: Impacts and Remediation Strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar]
- Zou, M.; Zhou, S.; Zhou, Y.; Jia, Z.; Guo, T.; Wang, J. Cadmium Pollution of Soil-Rice Ecosystems in Rice Cultivation Dominated Regions in China: A Review. Environ. Pollut. 2021, 280, 116965. [Google Scholar]
- Zhao, F.-J.; Ma, Y.; Zhu, Y.-G.; Tang, Z.; McGrath, S.P. Soil Contamination in China: Current Status and Mitigation Strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar]
- Chen, D.; Chen, D.; Xue, R.; Long, J.; Lin, X.; Lin, Y.; Jia, L.; Zeng, R.; Song, Y. Effects of Boron, Silicon and Their Interactions on Cadmium Accumulation and Toxicity in Rice Plants. J. Hazard. Mater. 2019, 367, 447–455. [Google Scholar]
- Zhao, F.-J.; Wang, P. Arsenic and Cadmium Accumulation in Rice and Mitigation Strategies. Plant Soil 2020, 446, 1–21. [Google Scholar]
- Huang, S.; Yamaji, N.; Ma, J.F. Metal Transport Systems in Plants. Annu. Rev. Plant Biol. 2024, 75, 1–25. [Google Scholar]
- Feng, J.; Shen, R.F.; Shao, J.F. Transport of Cadmium from Soil to Grain in Cereal Crops: A Review. Pedosphere 2021, 31, 3–10. [Google Scholar]
- Song, Y.; Jin, L.; Wang, X. Cadmium Absorption and Transportation Pathways in Plants. Int. J. Phytorem. 2017, 19, 133–141. [Google Scholar]
- Sterckeman, T.; Thomine, S. Mechanisms of Cadmium Accumulation in Plants. Crit. Rev. Plant Sci. 2020, 39, 322–359. [Google Scholar]
- Sasaki, A.; Yamaji, N.; Yokosho, K.; Ma, J.F. Nramp5 Is a Major Transporter Responsible for Manganese and Cadmium Uptake in Rice. Plant Cell 2012, 24, 2155–2167. [Google Scholar]
- Yan, H.; Xu, W.; Xie, J.; Gao, Y.; Wu, L.; Sun, L.; Feng, L.; Chen, X.; Zhang, T.; Dai, C.; et al. Variation of a Major Facilitator Superfamily Gene Contributes to Differential Cadmium Accumulation between Rice Subspecies. Nat. Commun. 2019, 10, 2562. [Google Scholar]
- Chang, J.-D.; Huang, S.; Yamaji, N.; Zhang, W.; Ma, J.F.; Zhao, F.-J. OsNRAMP1 Transporter Contributes to Cadmium and Manganese Uptake in Rice. Plant Cell Environ. 2020, 43, 2476–2491. [Google Scholar]
- Hu, J.; Chen, G.; Xu, K.; Wang, J. Cadmium in Cereal Crops: Uptake and Transport Mechanisms and Minimizing Strategies. J. Agric. Food Chem. 2022, 70, 5961–5974. [Google Scholar]
- Satoh-Nagasawa, N.; Mori, M.; Nakazawa, N.; Kawamoto, T.; Nagato, Y.; Sakurai, K.; Takahashi, H.; Watanabe, A.; Akagi, H. Mutations in Rice (Oryza sativa) Heavy Metal ATPase 2 (OsHMA2) Restrict the Translocation of Zinc and Cadmium. Plant Cell Physiol. 2012, 53, 213–224. [Google Scholar]
- Takahashi, R.; Ishimaru, Y.; Shimo, H.; Ogo, Y.; Senoura, T.; Nishizawa, N.K.; Nakanishi, H. The OsHMA2 Transporter Is Involved in Root-to-Shoot Translocation of Zn and Cd in Rice. Plant Cell Environ. 2012, 35, 1948–1957. [Google Scholar]
- Zhu, X.F.; Jiang, T.; Wang, Z.W.; Lei, G.J.; Shi, Y.Z.; Li, G.X.; Zheng, S.J. Gibberellic Acid Alleviates Cadmium Toxicity by Reducing Nitric Oxide Accumulation and Expression of IRT1 in Arabidopsis thaliana. J. Hazard. Mater. 2012, 239, 302–307. [Google Scholar]
- Zhu, X.F.; Wang, Z.W.; Dong, F.; Lei, G.J.; Shi, Y.Z.; Li, G.X.; Zheng, S.J. Exogenous Auxin Alleviates Cadmium Toxicity in Arabidopsis thaliana by Stimulating Synthesis of Hemicellulose 1 and Increasing the Cadmium Fixation Capacity of Root Cell Walls. J. Hazard. Mater. 2013, 263, 398–403. [Google Scholar] [PubMed]
- Rodríguez-Serrano, M.; Romero-Puertas, M.C.; Pazmino, D.M.; Testillano, P.S.; Risueño, M.C.; Del Río, L.A.; Sandalio, L.M. Cellular Response of Pea Plants to Cadmium Toxicity: Cross Talk between Reactive Oxygen Species, Nitric Oxide, and Calcium. Plant Physiol. 2009, 150, 229–243. [Google Scholar] [PubMed]
- Lei, G.J.; Sun, L.; Sun, Y.; Zhu, X.F.; Li, G.X.; Zheng, S.J. Jasmonic Acid Alleviates Cadmium Toxicity in Arabidopsis via Suppression of Cadmium Uptake and Translocation. J. Integr. Plant Biol. 2020, 62, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, G.; Cao, C.; Liu, P. Metabolism, Signaling, and Transport of Jasmonates. Plant Commun. 2021, 2, 100231. [Google Scholar]
- Howe, G.A.; Major, I.T.; Koo, A.J. Modularity in Jasmonate Signaling for Multistress Resilience. Annu. Rev. Plant Biol. 2018, 69, 387–415. [Google Scholar]
- Maksymiec, W.; Wianowska, D.; Dawidowicz, A.L.; Radkiewicz, S.; Mardarowicz, M.; Krupa, Z. The Level of Jasmonic Acid in Arabidopsis thaliana and Phaseolus coccineus Plants under Heavy Metal Stress. J. Plant Physiol. 2005, 162, 1338–1346. [Google Scholar]
- Yan, Z.; Chen, J.; Li, X. Methyl Jasmonate as Modulator of Cd Toxicity in Capsicum frutescens Var. Fasciculatum Seedlings. Ecotoxicol. Environ. Saf. 2013, 98, 203–209. [Google Scholar]
- Yan, Z.; Zhang, W.; Chen, J.; Li, X. Methyl Jasmonate Alleviates Cadmium Toxicity in Solanum Nigrum by Regulating Metal Uptake and Antioxidative Capacity. Biol. Plant. 2015, 59, 373–381. [Google Scholar]
- Alikhani, O.; Abbaspour, H.; Afshar, A.S.; Motavalizadehkakhki, A. Studying the Effects of Methyl Jasmonate on Growth Parameters and Anti-Oxidative Capacity in Wheat (Triticum aestivum L.) under Cadmium Stress. Environ. Sci. 2017. Available online: https://api.semanticscholar.org/CorpusID:212614059 (accessed on 9 January 2025).
- Yang, J.B.; Wang, H.Y.; Huang, J.; Shan, C.J.; Yan, J.; Zhong, C.W.; Hu, D.; Zhang, Q.; Shen, R.F.; Zhu, X.F.; et al. Jasmonic Acid Improves Cadmium Tolerance in Rice (Oryza sativa) by Reducing the Production of Nitric Oxide. Ecotoxicol. Environ. Saf. 2025, 290, 117722. [Google Scholar]
- Wei, T.; Li, H.; Wang, Y.; Chi, M.; Guo, J.; Jia, H.; Zhang, C. Alleviation of Cadmium Toxicity and Minimizing Its Accumulation in Rice Plants by Methyl Jasmonate: Performance and Mechanisms. J. Biotechnol. 2025, 398, 133–145. [Google Scholar]
- Li, Y.; Zhang, S.; Bao, Q.; Chu, Y.; Sun, H.; Huang, Y. Jasmonic Acid Alleviates Cadmium Toxicity through Regulating the Antioxidant Response and Enhancing the Chelation of Cadmium in Rice (Oryza sativa L.). Environ. Pollut. 2022, 304, 119178. [Google Scholar] [PubMed]
- Ye, M.; Song, Y.; Long, J.; Wang, R.; Baerson, S.R.; Pan, Z.; Zhu-Salzman, K.; Xie, J.; Cai, K.; Luo, S.; et al. Priming of Jasmonate-Mediated Antiherbivore Defense Responses in Rice by Silicon. Proc. Natl. Acad. Sci. USA 2013, 110, E3631–E3639. [Google Scholar] [PubMed]
- Kanu, A.S.; Ashraf, U.; Mansaray, L.R.; Abbas, F.; Fiaz, S.; Amanullah, S.; Charley, C.S.; Tang, X. Exogenous Methyl Jasmonate Application Improved Physio-Biochemical Attributes, Yield, Quality, and Cadmium Tolerance in Fragrant Rice. Front. Plant Sci. 2022, 13, 849477. [Google Scholar]
- Zhao, S.; Ma, Q.; Xu, X.; Li, G.; Hao, L. Tomato Jasmonic Acid-Deficient Mutant Spr2 Seedling Response to Cadmium Stress. J. Plant Growth Regul. 2016, 35, 603–610. [Google Scholar]
- Cui, Y.; Chen, C.-L.; Cui, M.; Zhou, W.-J.; Wu, H.-L.; Ling, H.-Q. Four IVa bHLH Transcription Factors Are Novel Interactors of FIT and Mediate JA Inhibition of Iron Uptake in Arabidopsis. Mol. Plant 2018, 11, 1166–1183. [Google Scholar]
- Chen, D.; Cao, B.; Qi, L.; Yin, L.; Wang, S.; Deng, X. Silicon-Moderated K-Deficiency-Induced Leaf Chlorosis by Decreasing Putrescine Accumulation in Sorghum. Ann. Bot. 2016, 118, 305–315. [Google Scholar]
- Song, J.; Bian, J.; Xue, N.; Xu, Y.; Wu, J. Inter-species mRNA Transfer Among Green Peach Aphids, Dodder Parasites, and Cucumber Host Plants. Plant Divers. 2021, 44, 1–10. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Liu, Z.; Li, X.; Liu, X.; Fang, L.; Zeng, R.; Wang, Q.; Song, Y.; Chen, D. Jasmonic Acid Enhances Rice Cadmium Tolerance by Suppressing Cadmium Uptake and Translocation. Plants 2025, 14, 1068. https://doi.org/10.3390/plants14071068
Zhang H, Liu Z, Li X, Liu X, Fang L, Zeng R, Wang Q, Song Y, Chen D. Jasmonic Acid Enhances Rice Cadmium Tolerance by Suppressing Cadmium Uptake and Translocation. Plants. 2025; 14(7):1068. https://doi.org/10.3390/plants14071068
Chicago/Turabian StyleZhang, Hao, Zhengkai Liu, Xinyu Li, Xiaodong Liu, Linzhi Fang, Rensen Zeng, Qiongli Wang, Yuanyuan Song, and Daoqian Chen. 2025. "Jasmonic Acid Enhances Rice Cadmium Tolerance by Suppressing Cadmium Uptake and Translocation" Plants 14, no. 7: 1068. https://doi.org/10.3390/plants14071068
APA StyleZhang, H., Liu, Z., Li, X., Liu, X., Fang, L., Zeng, R., Wang, Q., Song, Y., & Chen, D. (2025). Jasmonic Acid Enhances Rice Cadmium Tolerance by Suppressing Cadmium Uptake and Translocation. Plants, 14(7), 1068. https://doi.org/10.3390/plants14071068







