Preliminary Review of the Diploid Taxa in Hieracium s.s.
Abstract
1. Introduction
2. Phylogeny and Evolutionary Scenarios
3. Considerations on Diploid Taxa
4. Ploidy Analyses/Data
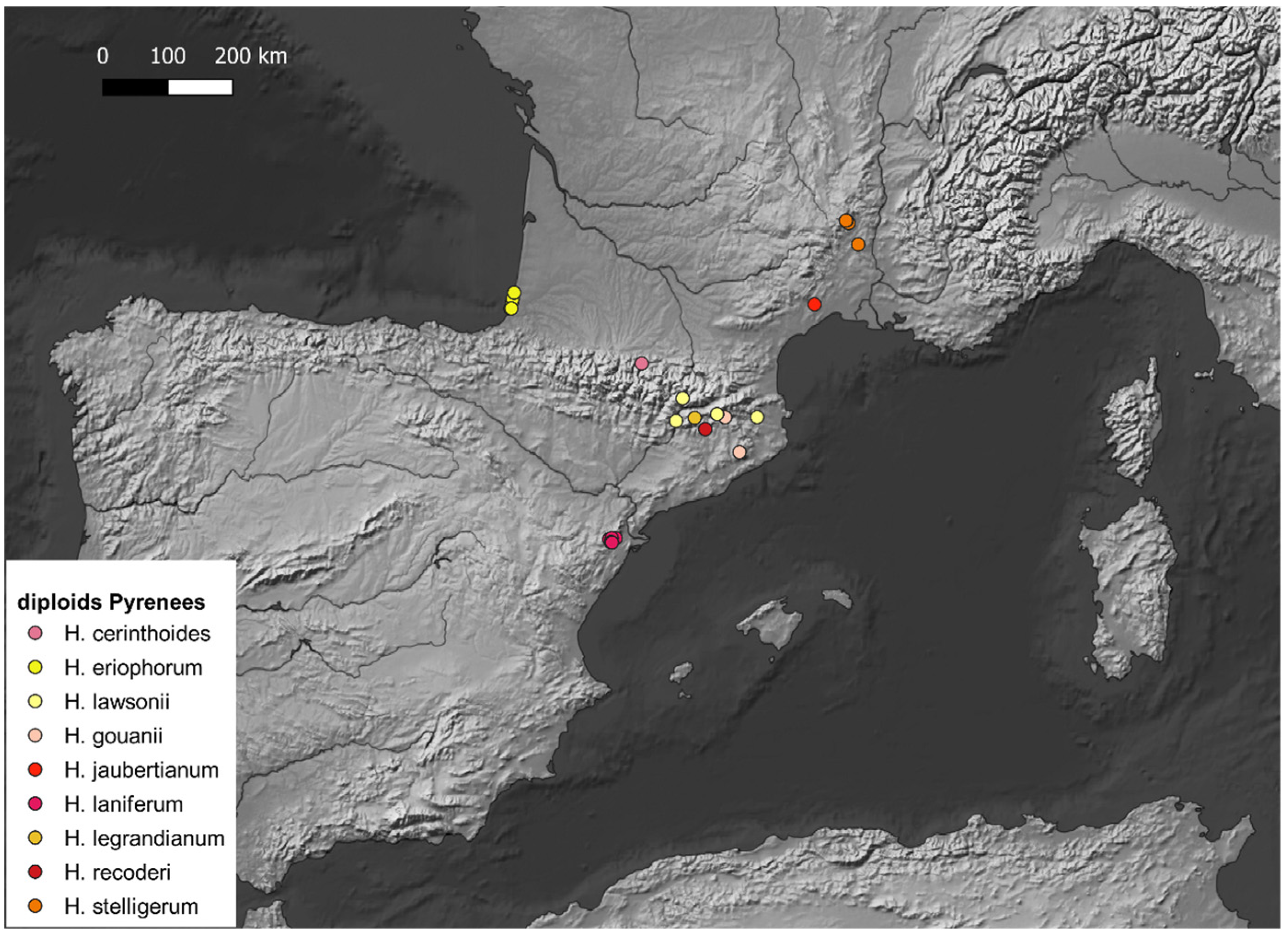
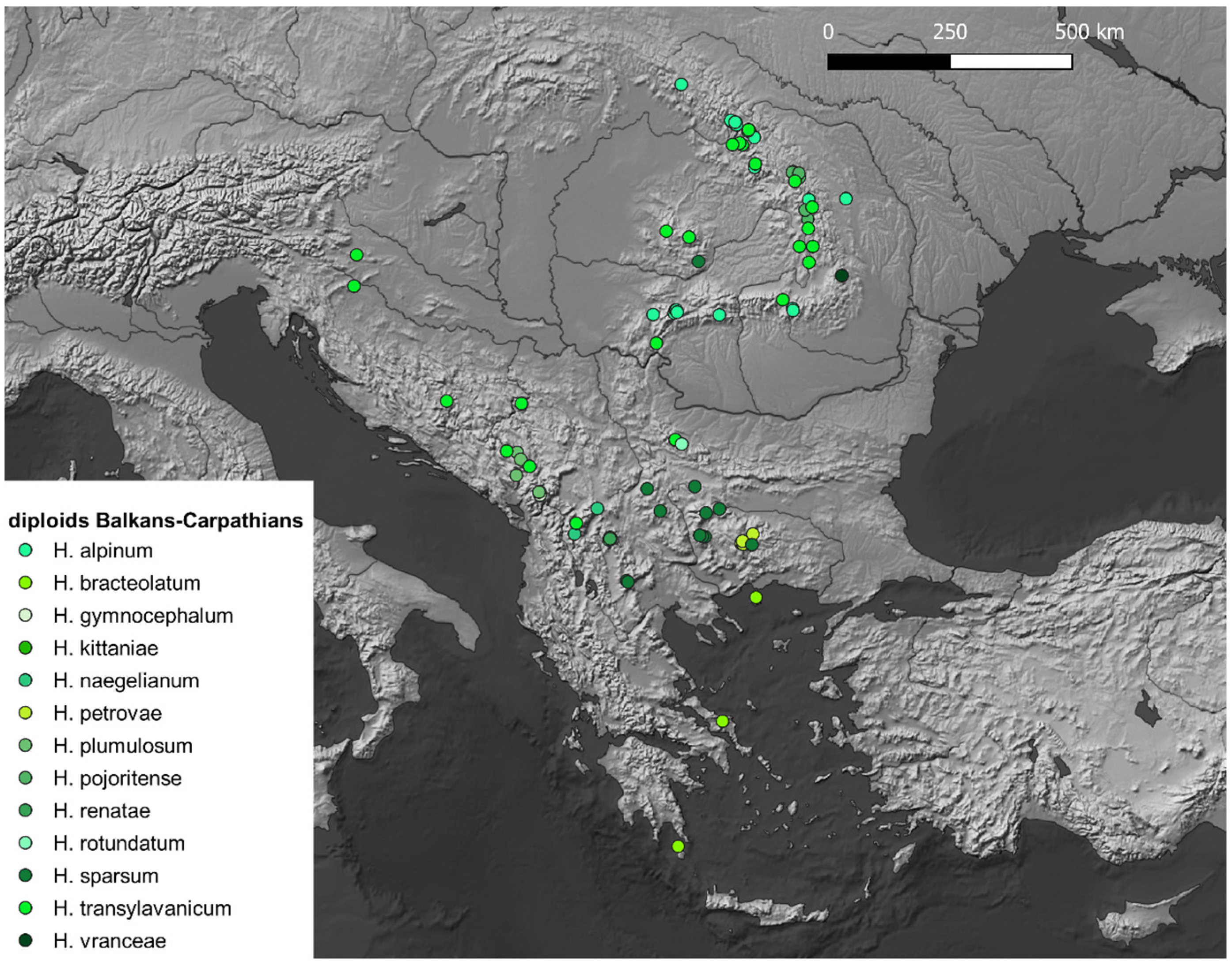
5. Species List
- H. alpinum L.
- H. bifidum Kit. ex Hornem. s.l.
- H. bracteolatum Sm.
- H. cerinthoides L. s.l.
- H. dollineri Sch.Bip. ex Neilr.
- H. eriophorum St.-Amans
- H. gouanii Arv.-Touv.
- H. gymnocephalum Griseb. ex Pant.
- H. intybaceum All.
- H. jaubertianum Timb.-Lagr. & Loret (≡H. glaucinum Jord. subsp. jaubertianum (Timb.-Lagr. & Loret) O.Bolòs & Vigo)
- H. kittanae Vladimir.
- H. laniferum Cav.
- H. lawsonii Vill. s.l. (incl. H. rupicaprinum Arv.-Touv. & Gaut., H. flocciferum Arv.-Touv.)
- H. legrandianum Arv.-Touv. (incl. H. amplexicaule L. sensu auct.)
- H. lucidum Guss.
- H. naegelianum Pančić
- H. petrovae Vladimir. & Szeląg
- H. plumulosum A.Kern. (≡H. waldsteinii subsp. plumulosum (A.Kern.) Freyn)
- H. pojoritense Woł.
- H. porrifolium L.
- H. prenanthoides Vill. s.s.
- H. racemosum Waldst & Kit. ex Willd. s.l.
- H. recoderi de Retz
- H. sparsum Friv. s.s.
- H. stelligerum Froel.
- H. tomentosum L. s.s.
- H. transylvanicum Heuff.
- H. umbellatum L.
- H. valdepilosum Vill. subsp. subsinuatum (Nägeli & Peter) Zahn
- H. virgaurea Coss. (≡H. racemosum subsp. virgaurea (Coss.) Zahn)
- H. vranceae Mráz
6. Hieracium Hotspots
7. Final Considerations/Remarks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schuhwerk, F. Some thoughts on the taxonomy of Hieracium. Ber. Bayer. Bot. Ges. 2002, 72, 193–198. [Google Scholar]
- Tison, J.-M. L’étude du genre Hieracium L. (Asteraceae): Possibilités et impossibilités actuelles du système zahnien, adaptation à la Flore pratique de la région méditerranéenne française. Bull. Soc. Éch. Pl. Vasc. Eur. Bass. Medit. 2004, 29, 27–103. [Google Scholar]
- Haveman, R. Freakish patterns: Species and species concepts in apomicts. Nordic J. Bot. 2013, 31, 257–269. [Google Scholar] [CrossRef]
- Majeský, Ľ.; Krahulec, F.; Vašut, R.J. How apomictic taxa are treated in current taxonomy: A review. Taxon 2017, 66, 1017–1040. [Google Scholar] [CrossRef]
- Sell, P.D. An introduction to the study of the British Hieracia, 1. History and classification. Watsonia 1987, 16, 365–371. [Google Scholar]
- Tyler, T. Patterns of morphometric variation and a new supraspecific classification of apomictic taxa of Hieracium (Asteraceae) from Denmark and southern Sweden. Pl. Syst. Evol. 2006, 261, 39–88. [Google Scholar] [CrossRef]
- Zahn, K.H. Hieracium. In Das Pflanzenreich; Engler, A., Ed.; Wilhelm Engelmann: Leipzig, Germany, 1921; Volumes 75 and 77, pp. 1–864. [Google Scholar]
- Zahn, K.H. Hieracium. In Das Pflanzenreich; Engler, A., Ed.; Wilhelm Engelmann: Leipzig, Germany, 1922; Volumes 79 and 80, pp. 865–1146. [Google Scholar]
- Zahn, K.H. Hieracium. In Das Pflanzenreich; Engler, A., Ed.; Wilhelm Engelmann: Leipzig, Germany, 1923; Volume 82 (IV/280), pp. 1147–1705. [Google Scholar]
- Stace, C.A. Sectional names in the genus Hieracium (Asteraceae) sensu stricto. Edinburgh J. Bot. 1998, 55, 417–441. [Google Scholar] [CrossRef]
- Asker, S.E.; Jerling, L. Apomixis in Plants; CRC Press: Boca Raton, FL, USA, 1992; pp. 1–314. [Google Scholar]
- Mráz, P.; Chrtek, J.; Fehrer, J.; Plačková, I. Rare recent natural hybridization in the genus Hieracium s. str. evidence from morphology, allozymes and chloroplast DNA. Pl. Syst. Evol. 2005, 255, 177–192. [Google Scholar] [CrossRef]
- Mráz, P.; Chrtek, J.; Fehrer, J. Interspecific hybridization in the genus Hieracium s. str. evidence for bidirectional gene flow and spontaneous allopolyploidization. Pl. Syst. Evol. 2011, 293, 237–245. [Google Scholar] [CrossRef]
- Mráz, P.; Paule, J. Experimental hybridization in the genus Hieracium s. str.: Crosses between diploid taxa. Preslia 2006, 78, 1–26. [Google Scholar]
- Fehrer, J.; Krak, K.; Chrtek, J. Intra-individual polymorphism in diploid and apomictic polyploid hawkweeds (Hieracium, Lactuceae, Asteraceae): Disentangling phylogenetic signal, reticulation, and noise. BMC Evol. Biol. 2009, 9, 239. [Google Scholar] [CrossRef] [PubMed]
- Mráz, P.; Chrtek, J.; Kirschner, J. Genetic variation in the Hieracium rohacsense group (Hieracium sect. Alpina). Phyton 2001, 41, 269–276. [Google Scholar]
- Chrtek, J.J.; Mráz, P.; Severa, M. Chromosome numbers in selected species of Hieracium s. str. (Hieracium subgen. Hieracium) in the Western Carpathians. Preslia 2004, 76, 119–140. [Google Scholar]
- Chrtek, J.J.; Mráz, P.; Zahradníček, J.; Mateo, G.; Szeląg, Z. Chromosome numbers and DNA ploidy levels of selected species of Hieracium s.str. (Asteraceae). Folia Geobot. 2007, 42, 411–430. [Google Scholar] [CrossRef]
- Mráz, P.; Zdvořák, P.; Hartmann, M.; Štefánek, M.; Chrtek, J. Can obligate apomixis and more stable reproductive assurance explain the distributional successes of asexual triploids in Hieracium alpinum (Asteraceae)? Pl. Biol. 2019, 21, 227–236. [Google Scholar] [CrossRef]
- Tyler, T.; Jönsson, J. Ploidy level analysis of apomictic Hieracium (Asteraceae) reveal unexpected patterns and variation. Nordic J. Bot. 2009, 27, 490–502. [Google Scholar] [CrossRef]
- Juel, H.O. Vergleichende Untersuchungen uber typische und parthenogenetische Fortpflanzung bei der Gattung Antennaria. Kungl. Svenska Vetenskapsakad. Handl. 1900, 33, 1–59. [Google Scholar]
- Hand, M.L.; Vít, P.; Krahulcová, A.; Johnson, S.D.; Oelkers, K.; Siddons, H.; Chrtek, J.; Fehrer, J.; Koltunow, A.M.G. Evolution of apomixis loci in Pilosella and Hieracium (Asteraceae) inferred from the conservation of apomixis-linked markers in natural and experimental populations. Heredity 2015, 114, 17–26. [Google Scholar] [CrossRef]
- Mráz, P.; Zdvořák, P. Reproductive pathways in Hieracium s.s. (Asteraceae): Strict sexuality in diploids and apomixis in polyploids. Ann. Bot. 2019, 123, 391–403. [Google Scholar] [CrossRef]
- Bergman, B. Studies on the Embryo Sac Mother Cell and its Development in Hieracium Subg. Archieracium. Svensk Bot. Tidskr. 1941, 35, 1. [Google Scholar]
- Skawińska, R. Apomixis in Hieracium alpinum. Acta Biol. Cracov. Ser. Bot. 1963, 5, 7–14. [Google Scholar]
- Rosenberg, O. Die semiheterotypisehe Teilung und ihre Bedeutung ftir die Entstehung verdoppelter Chromosomenzahlen. Hereditas 1927, 8, 305–338. [Google Scholar]
- Gentscheff, G.; Gustafsson, A. The balance system of meiosis in Hieracium. Hereditas 1940, 26, 209–249. [Google Scholar]
- Mráz, P. Mentor effects in the genus Hieracium s. str. (Compositae, Lactuceae). Folia Geobot. 2003, 38, 345–350. [Google Scholar] [CrossRef][Green Version]
- Slade, K.; Rich, T.C.G. Pollen studies in British Hieracium sect. Alpina (Asteraceae). Watsonia 2007, 26, 443–450. [Google Scholar]
- Mráz, P.; Chrtek, J.; Šingliarová, B. Geographical parthenogenesis, genome size variation and pollen production in the arctic-alpine species Hieracium alpinum. Bot. Helv. 2009, 119, 41–51. [Google Scholar] [CrossRef][Green Version]
- Kilian, N.; Gemeinholzer, B.; Lack, H.W. Cichorieae. In Systematics, Evolution, and Biogeography of Compositae; Funk, V.A., Susanna, A., Stuessy, T.F., Bayer, R.J., Eds.; International Association for Plant Taxonomy: Vienna, Austria, 2009; pp. 343–383. [Google Scholar]
- Fehrer, J.; Gemeinholzer, B.; Chrtek, J.; Bräutigam, S. Incongruent plastid and nuclear DNA phylogenies reveal ancient intergeneric hybridization in Pilosella hawkweeds (Hieracium, Cichorieae, Asteraceae). Mol. Phylogenet. Evol. 2007, 42, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Fehrer, J.; Slavíková, R.; Paštová, L.; Josefiová, J.; Mráz, P.; Chrtek, J.; Bertrand, Y.J. Molecular evolution and organization of ribosomal DNA in the hawkweed tribe Hieraciinae (Cichorieae, Asteraceae). Frontiers Pl. Sci. 2021, 12, 647375. [Google Scholar] [CrossRef]
- Krak, K.; Caklová, P.; Chrtek, J.; Fehrer, J. Reconstruction of phylogenetic relationships in a highly reticulate group with deep coalescence and recent speciation (Hieracium, Asteraceae). Heredity 2013, 110, 138–151. [Google Scholar] [CrossRef]
- Mráz, P.; Filipaş, L.; Bărbos, M.I.; Kadlecová, J.; Paštová, L.; Belyayev, A.; Fehrer, J. An unexpected new diploid Hieracium from Europe: Integrative taxonomic approach with a phylogeny of diploid Hieracium taxa. Taxon 2019, 68, 1258–1277. [Google Scholar] [CrossRef]
- Chrtek, J.; Mráz, P.; Belyayev, A.; Paštová, L.; Mrázová, V.; Caklová, P.; Josefiová, J.; Zagorski, D.; Hartmann, M.; Jandová, M.; et al. Evolutionary history and genetic diversity of apomictic allopolyploids in Hieracium s.str.: Morphological versus genomic features. Amer. J. Bot. 2020, 107, 66–90. [Google Scholar] [CrossRef]
- Chrtek, J.; Zahradníček, J.; Krak, K.; Fehrer, J. Genome size in Hieracium subgenus Hieracium (Asteraceae) is strongly correlated with major phylogenetic groups. Ann. Bot. 2009, 104, 161–178. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Belyayev, A.; Paštová, L.; Fehrer, J.; Josefiová, J.; Chrtek, J.; Mráz, P. Mapping of Hieracium (Asteraceae) chromosomes with genus-specific satDNA elements derived from next-generation sequencing data. Pl. Syst. Evol. 2018, 304, 387–396. [Google Scholar] [CrossRef]
- Chrtek, J.; Mráz, P.; Sennikov, A.N. Hieracium × grofae—A rediscovered diploid hybrid from the Ukrainian Carpathians. Biologia 2006, 61, 365–373. [Google Scholar] [CrossRef][Green Version]
- Hörandl, E. Geographical parthenogenesis: Opportunities for asexuality. In Lost Sex: The Evolutionary Biology of Parthenogenesis; Schön, I., Martens, K., Dijk, P., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 161–186. [Google Scholar]
- Stebbins, G.L. Polyploidy and the distribution of the arctic-alpine flora: New evidence and a new approach. Bot. Helv. 1984, 94, 1–13. [Google Scholar]
- Mráz, P.; Mrázová, V. Greater reproductive assurance of asexual plant compared with sexual relative in a low-density sympatric population: Experimental evidence for pollen limitation. J. Evol. Biol. 2021, 34, 1503–1509. [Google Scholar] [CrossRef]
- Merxmüller, H. Diploide Hieracien. Anales Inst. Bot. Cavanilles 1975, 32, 89–196. [Google Scholar]
- Chrtek, J. Chromosome numbers in selected species of Hieracium (Compositae) in the Sudeten Mts. and the Western and Ukrainian Eastern Carpathians. Fragm. Florist. Geobot. 1996, 41, 783–790. [Google Scholar]
- Vladimirov, V. A new diploid Hieracium (Asteraceae: Lactuceae) from Bulgaria. Bot. J. Linn. Soc. 2003, 143, 213–218. [Google Scholar] [CrossRef][Green Version]
- Vladimirov, V.; Szeląg, Z. A new diploid species of Hieracium sect. Pannosa (Asteraceae) from Bulgaria. Bot. J. Linn. Soc. 2006, 150, 261–265. [Google Scholar] [CrossRef]
- Castro, M.; Mateo, G.; Rossello, J.A. Chromosome numbers in Hieracium and Pilosella species (Asteraceae) from the Iberian Peninsula and the Balearic Islands. Bot. J. Linn. Soc. 2007, 153, 311–320. [Google Scholar] [CrossRef][Green Version]
- Szeląg, Z.; Ilnicki, T.; Niketić, M.; Tomović, G. Diploid chromosome numbers in five Hieracium species from Serbia and Montenegro. Acta Biol. Cracov., Set. Bot. 2007, 49, 119–121. [Google Scholar]
- Szeląg, Z. Hieracia balcanica V. A new diploid species in Hieracium sect. Naegeliana (Asteraceae) from Macedonia. Ann. Bot. Fenn. 2010, 47, 315–319. [Google Scholar] [CrossRef]
- Ilnicki, T.; Szeląg, Z. Chromosome numbers in Hieracium and Pilosella (Asteraceae) from Central and Southeastern Europe. Acta Biol. Cracov., Ser. Bot. 2011, 53, 102–110. [Google Scholar] [CrossRef]
- Musiał, K.; Szeląg, Z. Chromosome numbers in Hieracium (Asteraceae) from Central and Southeastern Europe V. Acta Biol. Cracov. Ser. Bot. 2019, 61, 63–68. [Google Scholar] [CrossRef]
- Juel, H.O. Die Tetradenteilungen bei Taraxacum und anderen Cichorieen. Kungl. Svenska Vetenskapsakad. Handl. 1905, 39, 1–21. [Google Scholar]
- Yurukova-Grancharova, P.; Robeva-Davidova, P.; Vladimirov, V. On the embryology and mode of reproduction of selected diploid species of Hieracium s.l. (Asteraceae) from Bulgaria. Flora 2006, 201, 668–675. [Google Scholar] [CrossRef]
- Chrtek, J. Taxonomy of the Hieracium alpinum group in the Sudeten Mts., the West and the Ukrainian East Carpathians. Folia Geobot. Phytotax. 1997, 32, 69–97. [Google Scholar] [CrossRef]
- Mráz, P.; Tomčíková, D. Experimental hybridization in the genus Hieracium s. str.—Crosses between diploid H. umbellatum and triploid H. sabaudum. Thaiszia-J. Bot. 2004, 14 (Suppl. S1), 15–16. [Google Scholar]
- Chrtek, J.; Hartmann, M.; Mrázova, V.; Zdvořák, P.; Štefánek, M.; Mráz, P. Seed traits, terminal velocity and germination in sexual diploid and apomictic triploid Hieracium alpinum (Asteraceae): Are apomicts better dispersers? Flora 2018, 240, 76–81. [Google Scholar] [CrossRef]
- Grabowska-Joachimiak, A.; Kwolek, D.; Pięta, E.; Szeląg, Z.; Joachimiak, A.J. rDNA-FISH pattern in selected Hieracium species representing different ploidy levels. Acta Soc. Bot. Poloniae 2023, 92, 1–10. [Google Scholar] [CrossRef]
- Grabowska-Joachimiak, A.; Żytkowicz, M.; Kwolek, D.; Szeląg, Z. Chromosome complex of the relict diploid species Hieracium bracteolatum. Acta Biol. Cracov. Ser. Bot. 2021, 63, 29–34. [Google Scholar] [CrossRef]
- Zagorski, D.; Hartmann, M.; Bertrand, Y.J.; Paštová, L.; Slavíková, R.; Josefiová, J.; Fehrer, J. Characterization and dynamics of repeatomes in closely related species of Hieracium (Asteraceae) and their synthetic and apomictic hybrids. Frontiers Pl. Sci. 2020, 11, 591053. [Google Scholar] [CrossRef] [PubMed]
- Ilnicki, T.; Hasterok, R.; Szeląg, Z. Cytogenetic analysis of Hieracium transylvanicum (Asteraceae). Caryologia 2010, 63, 192–196. [Google Scholar]
- Selvi, F.; Fiorini, G. Karyology of Hieracium L. subg. Hieracium (Asteraceae) from Mount Amiata (Central Italy). Caryologia 1996, 49, 287–299. [Google Scholar] [CrossRef][Green Version]
- Hörandl, E. The classification of asexual organisms: Old myths, new facts, and a novel pluralistic approach. Taxon 2018, 67, 1066–1081. [Google Scholar] [CrossRef]
- Schuhwerk, F. Published Chromosome Counts in Hieracium. (Updated to 1996). Available online: http://www.botanischestaatssammlung.de/projects/chrzlit.html (accessed on 15 October 2024).
- Paule, J.; Gregor, T.; Schmidt, M.; Gerstner, E.-M.; Dersch, G.; Dressler, S.; Wesche, K.; Zizka, G. Chromosome Numbers of the Flora of Germany. Available online: https://chromosomes.senckenberg.de (accessed on 25 October 2024).
- Leitch, I.J.; Johnston, E.; Pellicer, J.; Hidalgo, O.; Bennett, M.D. Plant DNA C-Values Database. Available online: https://cvalues.science.kew.org/ (accessed on 30 October 2024).
- Goldblatt, P.; Johnson, D.E. IPCN Chromosome Reports. Available online: http://legacy.tropicos.org/Project/IPCN (accessed on 4 November 2024).
- Rice, A.; Glick, L.; Abadi, S.; Einhorn, M.; Kopelman, N.M.; Salman-Minkov, A.; Mayzel, J.; Chay, O.; Mayrose, I. The Chromosome Counts Database (CCDB)—A Community Resource of Plant Chromosome Numbers. Available online: https://taux.evolseq.net (accessed on 10 November 2024).
- Sell, P.D.; West, C. Hieracium. In Flora Europaea; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1976; Volume 4, pp. 358–410. [Google Scholar]
- Gottschlich, G. Hieracium. In Flora d’Italia, 2nd ed.; Pignatti, S., Ed.; Edagricole: Milan, Italy, 2018; Volume 3, pp. 1138–1196. [Google Scholar]
- Mráz, P. Chromosome numbers in selected species of Hieracium sect. Alpina from Central and Eastern Europe. Folia Geobot. 2001, 36, 321–332. [Google Scholar]
- Mráz, P.; Szeląg, Z. Chromosome numbers and reproductive systems in selected species of the genera Hieracium L. and Pilosella Hill (Asteraceae) from Romania. Ann. Bot. Fenn. 2004, 41, 405–414. [Google Scholar]
- Musiał, K.; Vladimirov, V.; Szeląg, Z. Chromosome numbers in Hieracium (Asteraceae) from Central and Southeastern Europe VI. Acta Biol. Cracov. Ser. Bot. 2020, 62, 43–50. [Google Scholar] [CrossRef]
- Mateo, G.; Talavera, S.; Egido, F. Hieracium. In Flora Iberica; Castroviejo, S., Talavera, S., Buira, A., Quintanar, A., Gacìa, M.A., Talavera, M., Fernàndez Piedra, P., Eds.; Real Jardín Botánico CSIC: Madrid, Spain, 2017; Volume XVI, Part II, pp. 1170–1258. [Google Scholar]
- Tison, J.-M.; Foucault, B. Hieracium. In Flora Gallica; Flore de France; Biotope edition: Mèze, France, 2014; pp. 434–463. [Google Scholar]
- Delay, J. Endémiques Pyrénéennes (Composées). Inf. Ann. Caryosyst. Cytogenet. 1969, 3, 24. [Google Scholar]
- Goñalons, L.; Mateo, G. The genus Hieracium (Asteraceae) in Catalonia (Northeastern Iberian Peninsula, Spain). Fl. Montiber. 2016, 65, 88–121. [Google Scholar]
- Ferrer Gallego, P.P.; Greuter, W.; Del Egido, F.; Mateo, G. Typification of the Linnaean name Hieracium cerinthoides (Compositae). Willdenowia 2015, 45, 385–389. [Google Scholar] [CrossRef]
- Schuhwerk, F. Chromosomenzahlen von Hieracium (Compositae, Cichorieae)–Teil 5. Ber. Bayer. Bot. Ges. 2010, 80, 141–160. [Google Scholar]
- Schuhwerk, F.; Lippert, W. Chromosomenzahlen von Hieracium (Compositae, Lactuceae) Teil 3. Sendtnera 1999, 6, 197–214. [Google Scholar]
- Frey, D.J.; Haag, C.R.; Kozlowski, G.; Tison, J.M.; Mráz, P. High genetic and morphological diversity despite range contraction in the diploid Hieracium eriophorum (Asteraceae) endemic to the coastal sand dunes of south-west France. Bot. J. Linn. Soc. 2012, 169, 365–377. [Google Scholar] [CrossRef]
- Bolòs, O.; Vigo, J. Hieracium. In Flora dels Països Catalans; Bolòs, O., Vigo, J., Eds.; Barcino: Barcelona, Spain, 1995; Volume 3, pp. 1035–1155. [Google Scholar]
- Buttler, K.P. Hieracium. In Mountain Flora of Greece; Strid, A., Tan, K., Eds.; Edinburgh University Press: Edinburgh, UK, 1991; Volume 2, pp. 595–642. [Google Scholar]
- Szeląg, Z.; Ilnicki, T. Diploid chromosome numbers in Hieracium and Pilosella (Asteraceae) from Macedonia and Montenegro. Acta Biol. Cracov. Ser. Bot. 2011, 53, 124–126. [Google Scholar] [CrossRef]
- Niketić, M. The Hieracium gymnocephalum complex: A taxonomic revision. In Proceedings of the 6th Hieracium Workshop, Hirschegg/Kleinwalsertal Allgäuer Alpen, Wien, Austria, 17–23 July 2002. [Google Scholar]
- Niketić, M.; Bareka, P.; Kamari, G. Karyosystematic study of selected Hieracium taxa (Compositae) from Mt Durmitor (Montenegro). Chron. Bot. 2003, 16, 23–45. [Google Scholar]
- Zahradníček, J.; Chrtek, J. Cytotype distribution and phylogeography of Hieracium intybaceum (Asteraceae). Bot. J. Linn. Soc. 2015, 179, 487–498. [Google Scholar] [CrossRef][Green Version]
- Dobeš, C.; Hahn, B.; Morawetz, W. Chromosomenzahlen zur Gefäßpflanzen-Flora Österreichs. Linzer Biol. Beitr. 1997, 29, 5–43. [Google Scholar]
- Favarger, C. Notes de caryologie Alpine VI. Bull. Soc. Neuchâteloise Sci. Nat. 1997, 120, 19–33. [Google Scholar]
- Greuter, W.; von Raab-Straube, E. Med-Checklist. In A Critical Inventory of Vascular Plants of the Circum-Editerrean Countries. 2. Dicotyledones (Compositae); OPTIMA: Geneva, Switzerland, 2008. [Google Scholar]
- Natarajan, G. Étude caryosystematique de quelques dicotylédones de la Garrigue Languedocienne. Naturalia Monspel. Sér. Bot. 1988, 52, 85–123. [Google Scholar]
- Natarajan, G. Chromosome Number Reports LXXII; Löve, A., Ed. Taxon 1981, 30, 698–699. [Google Scholar]
- Ferrer Gallego, P.P.; Mateo, G. Tipificación del nombre cavanillesiano Hieracium laniferum (Compositae). Fl. Montiber. 2016, 63, 13–17. [Google Scholar] [CrossRef]
- Schuhwerk, F.; Lippert, W. Chromosomenzahlen von Hieracium (Compositae, Lactuceae) Teil 2. Sendtnera 1998, 5, 269–286. [Google Scholar]
- Tison, J.M.; Greuter, W. Euro+Med-Checklist Notulae, 1 [Notulae ad floram euro-mediterraneam pertinentes 30]; von Raab-Straube, E.; Raus, T., Eds. Willdenowia 2013, 43, 154–155. [Google Scholar] [CrossRef]
- Mateo, G. Sobre las especies iberiopirenaicas del género Hieracium L. distribuidas en la Hieraciotheca de Arvet-Touvet y Gautier. Fl. Montib. 2016, 62, 100–143. [Google Scholar]
- Di Gristina, E.; Raimondo, F.M.; Mazzola, P. Diversity in the genus Hieracium Linnaeus s. str. (Asteraceae) in Sicily. Biodivers. J. 2015, 6, 205–214. [Google Scholar]
- Di Gristina, E.; Raimondo, F.M.; Domina, G. Conservation status assessment of the endemic Hieracium s. str. (Asteraceae) occurring in Sicily. Ann. Bot. 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Pasta, S.; Gristina, A.S.; Marcenò, C.; De Simone, L.; Garfì, G.; Giacalone, G.; Ilardi, V.; Kozlowski, G.; Scuderi, L.; Guarino, R. Discovering hidden treasures: Unveiling a new population of the narrow endemic Hieracium lucidum Guss. (Asteraceae) on the Mounts of Palermo (NW Sicily, Italy). Hacquetia 2024, 23, 213–219. [Google Scholar] [CrossRef]
- Brullo, S.; Pavone, P. Numeri cromosomici per la Flora Italiana. Inform. Bot. Ital. 1978, 10, 248–265. [Google Scholar]
- Brullo, S.; Campo, G.; Romano, S. Indagini citotassonomiche sul genere Hieracium (Asteraceae) in Sicilia. Inform. Bot. Ital. 2004, 36, 481–485. [Google Scholar]
- Geraci, A.; Di Gristina, E.; Schicchi, R. Reports (1640–1644). In Mediterranean Chromosome Number Reports–17; Kamari, G., Blanché, C., Garbari, F., Eds. Fl. Medit. 2007, 17, 314–319. [Google Scholar]
- Gianguzzi, L.; La Mantia, A. Hieracium lucidum. The IUCN Red List of Threatened Species 2006: E.T61624A12525812. Available online: https://www.iucnredlist.org/species/61624/12525812 (accessed on 16 February 2025).
- Szeląg, Z. A Synopsis of Hieracium sect. Cernua (Asteraceae). Polish Bot. J. 2003, 48, 87–95. [Google Scholar]
- Vladimirov, V. Diploid species of the genus Hieracium s.l. in Bulgaria. Abh. Ber. Naturkundemus. Görlitz 2000, 72, S16. [Google Scholar]
- Szeląg, Z. Hieracia balcanica XIII. Typification of the Hieracium (Asteraceae) names described by Josif Pančić from Montenegro. Polish Bot. J. 2016, 61, 59–64. [Google Scholar]
- Nyárády, E.I. Hieracium. In Flora Republicii Populare Romîne; Săvulescu, T., Ed.; Editura Academiei Republicii Populare Romîne: București, Romania, 1965; Volume X, pp. 370–713. [Google Scholar]
- Ştefureac, T.I.; Tăcină, A. Cytotaxonomical and chorological investigations of the endemic taxon Hieracium pojoritense Woł. Rev. Roumaine Biol. 1979, 24, 109–120. [Google Scholar]
- Favarger, C. Notes de caryologie Alpine IV. Bull. Soc. Neuchâteloise Sci. Nat. 1965, 88, 5–60. [Google Scholar]
- Marcucci, R.; Tornadore, N. Reports (1089-1098). In Mediterranean chromosome number reports—9; Kamari, G., Felber, F., Garbari, F., Eds. Fl. Medit. 1999, 9, 372–378. [Google Scholar]
- Ferrer Gallego, P.P. Typification of fourteen Linnaean names in the genus Hieracium (Compositae). Taxon 2021, 70, 880–896. [Google Scholar] [CrossRef]
- Van Es, J.; Tison, J.-M. Notices Descriptives de Hieracium des Alpes Françaises; Conservatoire Botanique National Alpin: Gap, France, 2018; pp. 1–255. [Google Scholar]
- Favarger, C. Notes de caryologie Alpine V. Bull. Soc. Neuchâteloise Sci. Nat. 1969, 92, 13–30. [Google Scholar]
- De Retz, B. Contributions à la connaissance de la flore hiéraciologique de la France et de l’Espagne. 5. Taxons nouveaux pour le genre Hieracium dans les Pyrénées françaises et en Espagne. Bull. Soc. Bot. France 1978, 125, 209–217. [Google Scholar] [CrossRef][Green Version]
- Christoff, M. Die genetische grundlage der apomiktischen fortpflanzung bei Hieracium aurantiacum L. Z. Indukt. Abstammungs-Vererbungsl. 1942, 80, 103–125. [Google Scholar]
- Vladimirov, V.; Szeląg, Z. Reports (1271–1277). In Mediterranean chromosome number reports—11; Kamari, G., Blanché, C., Garbari, F., Eds. Fl. Medit. 2001, 11, 478–483. [Google Scholar]
- Pashuk, T. Khromozomnye chisla vidov subal’piyskogo poyasa Chernogory (Ukrainskie Karpaty) (Chromosome numbers of species of the subalpine belt of the Chornohora Mts. (Ukrainian Carpathians). Bot. Zhurn. 1987, 72, 1069–1074. [Google Scholar]
- Polya, L. Magyarorszàgi növényfajok kromoszómaszámai. II. (Chromosome numbers of Hungarian plants. II.). Ann. Biol. Univ. Debrecen 1950, 1, 46–56. [Google Scholar]
- Wulff, H.D. Chromosomenstudien an der schleswig-holsteinischen Angiospermen-Flora. V. Ber. Deutsch. Bot. Ges. 1950, 63, 65–71. [Google Scholar]
- Lövkvist, B. Chromosome and differentiation studies in flowering plants of Skåne, south Sweden. I. General aspects. Type species with coastal differentiation. Bot. Not. 1962, 115, 261–287. [Google Scholar]
- Sorsa, V. Chromosomenzahlen finnischer Kormophyten I. Ann. Acad. Sci. Fenn. Ser. A IV Biol. 1962, 58, 1–14. [Google Scholar]
- Sorsa, V. Cytological observations in four species of Compositae in Finland. Arch. Soc. Zool. Bot. Fenn. Vanamo 1963, 18, 65–67. [Google Scholar]
- Gadella, T.W.J.; Kliphuis, E. Chromosome numbers of flowering plants in the Netherlands. Ill. Proc. Roy. Neth. Acad. Sci. Ser. C 1967, 69, 541–556. [Google Scholar]
- Gadella, T.W.J.; Kliphuis, E. Chromosome numbers of flowering plants in the Netherlands. IV. Proc. Roy. Neth. Acad. Sci. Ser. C 1968, 71, 168–183. [Google Scholar]
- Laane, M.M. Further chromosome studies in Norwegian vascular plants. Blyttia 1969, 27, 5–17. [Google Scholar]
- Májovský, J. Index of chromosome numbers of Slovakian flora (part 1). Acta Fac. Rerum Nat. Univ. Comen. Bot. 1970, 16, 1–26. [Google Scholar]
- Skalińska, M.; Jankun, H.; Wcisło, H. Studies in chromosome numbers of Polish Angiosperms. Eigth contribution. Acta Biol. Cracov., Ser. Bot. 1971, 14, 55–102. [Google Scholar]
- Gadella, T.W.; Kliphuis, E. Chromosome numbers of flowering plants in the Netherlands. VI. Meded. Bot. Mus. Herb. Rijks Univ. Utrecht 1973, 392, 303–311. [Google Scholar]
- Morton, J.K. Chromosome numbers of British plants. Watsonia 1974, 10, 169. [Google Scholar]
- Uhríková, A.; Feráková, V. Chromosome number reports LVI; Löve, A., Ed. Taxon 1977, 26, 263. [Google Scholar] [CrossRef]
- Strid, A.; Franzén, R. Chromosome number reports LXIX; Löve, A., Ed. Taxon 1980, 29, 709–710. [Google Scholar] [CrossRef]
- Kockx van Roon, M.; Wieffering, J.H. IOBP chromosome number reports LXXV; Löve, A., Ed. Taxon 1982, 31, 342–368. [Google Scholar]
- Jalas, J.; Pellinen, K. Chromosome counts on Erigeron, Hieracium, Pilosella and Sonchus (Compositae), mainly from Finland. Ann. Bot. Fenn. 1985, 22, 45–47. [Google Scholar]
- Hrušovská-Osuská, L. Karyological study of some taxa of the flora of the northern part of Považský Inovec. Part I. Acta Fac. Rerum Nat. Univ. Comen. Bot. 1988, 35, 69–79. [Google Scholar]
- Szeląg, Z.; Vladimirov, V. Chromosome numbers of Polish Hieracia (Asteraceae). Polish Bot. J. 2005, 50, 139–143. [Google Scholar]
- Hayirlioğlu-Ayaz, S.; Inceer, H. Chromosome numbers of some species of the genus Hieracium s.str. (Asteraceae) from Turkey. Folia Geobot. 2004, 39, 319–325. [Google Scholar] [CrossRef]
- Sennikov, A.N. A new typification of Hieracium umbellatum (Asteraceae). Nordic J. Bot. 2007, 25, 99–103. [Google Scholar] [CrossRef]
- Loret, H.; Barrandon, A. Flore de Montpellier, 2nd ed.; G. Masson: Paris, France, 1886; pp. 623–624. [Google Scholar]
- Mills, J.N.; Stace, C.A. Chromosome Numbers of British plants. 2. Watsonia 1974, 10, 167–168. [Google Scholar]
- Tomb, A.S.; Chambers, K.L.; Kyhos, D.W.; Powell, A.M.; Raven, P.H. Chromosome numbers in the Compositae. XIV. Lactuceae. Amer. J. Bot. 1978, 65, 717–721. [Google Scholar] [CrossRef]
- Moore, D.M. Flora Europaea Check-List and Chromosome Index; Cambridge University Press: Cambridge, UK, 1982; pp. 167–169. [Google Scholar]
- Ostenfeld, C.H. Some experiments on the origin of new forms in the genus Hieracium sub-genus Archieracium. J. Genet. 1921, 11, 117–122. [Google Scholar]
- Tupitsyna, N.N. Hieracium. In Flora Sibiri; Krasnoborov, I.M., Ed.; Nauka: Novosibirsk, Russia, 1997; Volume 13, pp. 308–336. [Google Scholar]
- Rostovtseva, T.S. Chisla khromosom nekotorykh vidov semeystva Asteraceae II. (Chromosome numbers of some species of the family Asteraceae II.). Bot. Zurn. 1983, 68, 660–664. [Google Scholar]
- Krogulevich, R.E. Kariologicheskii analiz vidov flory Vostochnogo Saiana (Karyologycal analysis of the species of the flora of Eastern Sayan). In Flora Pribaikal’ya (Flora of the Pre-Baikal); Malyshev, L.I., Peshlova, G.A., Eds.; Nauka: Novosibirsk, Russia, 1978; pp. 19–48. [Google Scholar]
- Pulkina, S.V.; Tupitsyna, N.N. Poliploidnye kompleksy v rode Hieracium (Asteraceae). (Polyploid complexes in Hieracium (Asteraceae). Turczaninowia 2000, 3, 79–81. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldesi, G.; Tison, J.-M.; Orsenigo, S. Preliminary Review of the Diploid Taxa in Hieracium s.s. Plants 2025, 14, 1057. https://doi.org/10.3390/plants14071057
Baldesi G, Tison J-M, Orsenigo S. Preliminary Review of the Diploid Taxa in Hieracium s.s. Plants. 2025; 14(7):1057. https://doi.org/10.3390/plants14071057
Chicago/Turabian StyleBaldesi, Giacomo, Jean-Marc Tison, and Simone Orsenigo. 2025. "Preliminary Review of the Diploid Taxa in Hieracium s.s." Plants 14, no. 7: 1057. https://doi.org/10.3390/plants14071057
APA StyleBaldesi, G., Tison, J.-M., & Orsenigo, S. (2025). Preliminary Review of the Diploid Taxa in Hieracium s.s. Plants, 14(7), 1057. https://doi.org/10.3390/plants14071057




