Variation in Odour Profiles of Cauliflower, Curly Kale and Broccoli (Brassica oleracea L.) Cultivars Is Affected More by Genotype Rather than Herbivore Feeding
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of Plant Volatiles
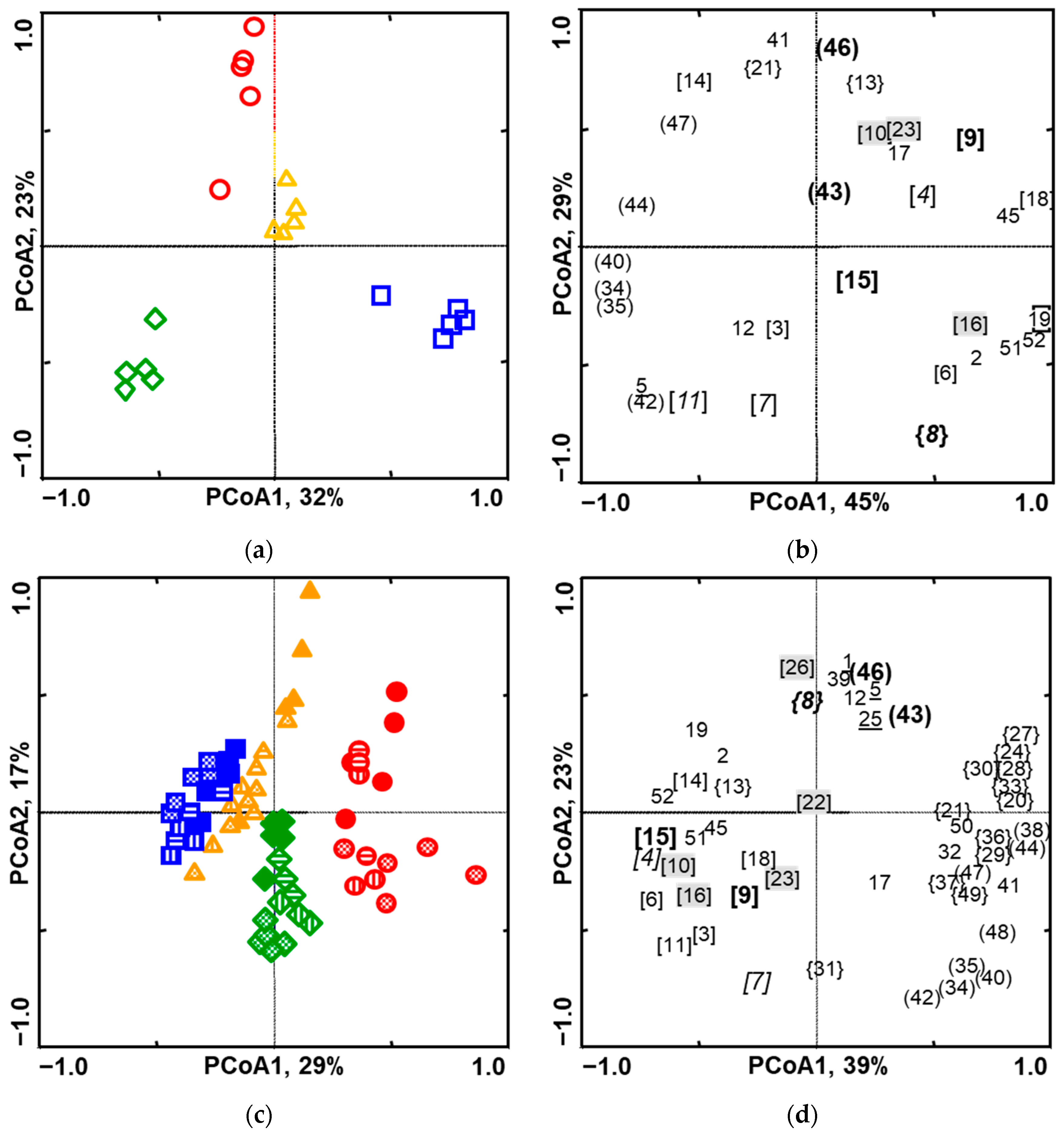
2.2. Variation in the Odour Profiles of Intact Plants
2.3. Variation in the Odour Profiles of Plant-Herbivore Systems During Caterpillar Feeding
2.4. Variation in the Odour Profiles of Damaged Plants After the Removal of Caterpillars and Faeces
2.5. Variation in the Odour Profiles of M. brassicae Versus P. rapae Infested Plants
2.6. Variation in the Odour Profiles of M. brassicae Versus P. rapae Damaged Plants After Removal of Caterpillars and Faeces
3. Discussion
| Compound Name | M. brassicae | P. rapae | C. gl | C. ru | M. me | ||
|---|---|---|---|---|---|---|---|
| OA | BA | OA | BA | OA | OA | OA | |
| Monoterpenes | |||||||
| α-Terpinene | [42] 1 | UF [42] | |||||
| γ-Terpinene | [37] | [43] | |||||
| β-Myrcene | [38,44] | ||||||
| Limonene | [38,44] | [34] | [34] | ||||
| α-Pinene | [38] | ||||||
| Oxygenated monoterpenes | |||||||
| 1.8-Cineole | [37,42] | UF [42] | [45] | ||||
| Sesquiterpenes | |||||||
| (E,E)-α-Farnesene | [37] | ||||||
| (E)-β-Farnesene | [37] | ||||||
| (Z,E)-α-Farnesene | [37] | ||||||
| Homoterpenes | |||||||
| TMTT | [37] | ||||||
| (E)-4,8-Dimethylnona-1,3,7-triene | [34] | [34] | [46] | ||||
| Ketones | |||||||
| β-Ionone | [38] | D [47] | [45] | ||||
| Esters | |||||||
| (Z)-3-Hexen-1-yl acetate | [37,42] | UF; L [42] | [38] | [34,45] | [34] | [46] | |
| (Z)-3-Hexenyl butanoate | [34,45] | [34] | |||||
| (Z)-3-Hexenyl 2-methylbutanoate | [34,45] | [34] | |||||
| (Z)-3-Hexenyl 3-methylbutanoate | [34,45] | [34] | |||||
4. Materials and Methods
4.1. Plants and Insects
4.2. Sampling of Volatiles
4.3. Analysis of Volatiles
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- De Pinho, P.G.; Valentao, P.; Gonçalves, R.F.; Sousa, C.; Seabra, R.M.; Andrade, P.B. Volatile composition of Brassica oleracea L. var. costata DC leaves using solid-phase microextraction and gas chromatography/ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
- World-Cabbage and Other Brassicas-Market Analysis, Forecast, Size, Trends and Insights, 2025-02-06 ed.; IndexBox: Luxembourg, 2025.
- Ahuja, I.; Rohloff, J.; Bones, A.M. Defence mechanisms of Brassicaceae: Implications for plant-insect interactions and potential for integrated pest management. A review. Agron. Sustain. Dev. 2010, 30, 311–348. [Google Scholar] [CrossRef]
- Dhillon, M.K.; Singh, N.; Yadava, D.K. Preventable yield losses and management of mustard aphid, Lipaphis erysimi (Kaltenbach) in different cultivars of Brassica juncea (L.) Czern & Coss. Crop Prot. 2022, 161, 106070. [Google Scholar] [CrossRef]
- Hamback, P.A.; Beckerman, A.P. Herbivory and plant resource competition: A review of two interacting interactions. Oikos 2003, 101, 26–37. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Lau, J.A.; Hamback, P.A. Community heterogeneity and the evolution of interactions between plants and insect herbivores. Q. Rev. Biol. 2006, 81, 349–376. [Google Scholar] [CrossRef]
- Haan, N.L.; Zhang, Y.; Landis, D.A. Predicting landscape configuration effects on agricultural pest suppression. Trends Ecol. Evol. 2020, 35, 175–186. [Google Scholar] [CrossRef]
- Norris, R.F.; Kogan, M. Ecology of interactions between weeds and arthropods. Annu. Rev. Entomol. 2005, 50, 479–503. [Google Scholar] [CrossRef]
- Potting, R.P.J.; Perry, J.N.; Powell, W. Insect behavioural ecology and other factors affecting the control efficacy of agro-ecosystern diversification strategies. Ecol. Model. 2005, 182, 199–216. [Google Scholar] [CrossRef]
- Underwood, N.; Hambäck, P.A.; Inouye, B.D. Pollinators, herbivores, and plant neighborhood effects. Q. Rev. Biol. 2020, 95, 37–57. [Google Scholar] [CrossRef]
- Root, R.B. Organization of a plant-arthropod association in simple and diverse habitats-fauna of collards (Brassica oleracea). Ecol. Monogr. 1973, 43, 95–120. [Google Scholar] [CrossRef]
- Rusman, Q.; Cusumano, A.; Vosteen, I. En route to resources: Foraging strategies of plant-associated insects to identify resources in complex dynamic environments. Funct. Ecol. 2024, 38, 1664–1682. [Google Scholar] [CrossRef]
- Vet, L.E.M.; Dicke, M. Ecology of infochemical use by natural enemies in a tritrophic context. Annu. Rev. Entomol. 1992, 37, 141–172. [Google Scholar] [CrossRef]
- Ali, M.Y.; Naseem, T.; Holopainen, J.K.; Liu, T.; Zhang, J.; Zhang, F. Tritrophic interactions among arthropod natural enemies, herbivores and plants considering volatile blends at different scale levels. Cells 2023, 12, 251. [Google Scholar] [CrossRef]
- Jankowska, B. The occurrence of some lepidoptera pests on different cabbage vegetables. J. Plant Prot. Res. 2006, 46, 181–190. [Google Scholar]
- Badenes-Pérez, F.R. Plant glucosinolate content and host-plant preference and suitability in the small white butterfly (Lepidoptera: Pieridae) and comparison with another specialist lepidopteran. Plants 2023, 12, 13. [Google Scholar] [CrossRef]
- Ellis, S.; White, S.; Holland, J.; Smith, B.; Collier, R.; Jukes, A. Encyclopaedia of Pests and Natural Enemies in Field Crops, 2014th ed.; Agriculture and Horticulture Development Board: Coventry, UK, 2014; pp. 1–119. [Google Scholar]
- Poelman, E.H.; Galiart, R.; Raaijmakers, C.E.; van Loon, J.J.A.; van Dam, N.M. Performance of specialist and generalist herbivores feeding on cabbage cultivars is not explained by glucosinolate profiles. Entomol. Exp. Appl. 2008, 127, 218–228. [Google Scholar] [CrossRef]
- Komino, T.; Yokoi, S.; Tsuji, H. Experiments on the daytime habitats of larvae of the cutworm moth, the armyworm moth, and the common cutworm. Jpn. J. Appl. Entomol. Zool. 1973, 17, 215–220. [Google Scholar] [CrossRef]
- Konno, K. Extremely high relative growth rate makes the cabbage white, Pieris rapae, a global pest with highly abundant and migratory nature. Sci. Rep. 2023, 13, 9697. [Google Scholar] [CrossRef]
- Hamback, P.A.; Bjorkman, M.; Ramert, B.; Hopkins, R.J. Scale-dependent responses in cabbage herbivores affect attack rates in spatially heterogeneous systems. Basic Appl. Ecol. 2009, 10, 228–236. [Google Scholar] [CrossRef]
- Omura, H.; Honda, K.; Hayashi, N. Chemical and chromatic bases for preferential visiting by the cabbage butterfly Pieris rapae to rape flowers. J. Chem. Ecol. 1999, 25, 1895–1906. [Google Scholar] [CrossRef]
- Ikeura, H.; Kobayashi, F.; Hayata, Y. How do Pieris rapae search for Brassicaceae host plants? Biochem. Syst. Ecol. 2010, 38, 1199–1203. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Stahl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1–120. [Google Scholar] [CrossRef]
- Geervliet, J.B.F.; Posthumus, M.A.; Vet, L.E.M.; Dicke, M. Comparative analysis of headspace volatiles from different caterpillar-infested or uninfested food plants of Pieris species. J. Chem. Ecol. 1997, 23, 2935–2954. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Nissinen, A.; Holopainen, J.K. Response of Plutella xylostella and its parasitoid Cotesia plutellae to volatile compounds. J. Chem. Ecol. 2005, 31, 1969–1984. [Google Scholar] [CrossRef]
- Conti, E.; Zadra, C.; Salerno, G.; Leombruni, B.; Volpe, D.; Frati, F.; Marucchini, C.; Bin, F. Changes in the volatile profile of Brassica oleracea due to feeding and oviposition by Murgantia histrionica (Heteroptera: Pentatomidae). Eur. J. Entomol. 2008, 105, 839–847. [Google Scholar] [CrossRef]
- Poelman, E.H.; Oduor, A.M.O.; Broekgaarden, C.; Hordijk, C.A.; Jansen, J.J.; Van Loon, J.J.A.; Van Dam, N.M.; Vet, L.E.M.; Dicke, M. Field parasitism rates of caterpillars on Brassica oleracea plants are reliably predicted by differential attraction of Cotesia parasitoids. Funct. Ecol. 2009, 23, 951–962. [Google Scholar] [CrossRef]
- Shiojiri, K.; Ozawa, R.; Kugimiya, S.; Uefune, M.; van Wijk, M.; Sabelis, M.W.; Takabayashi, J. Herbivore-specific, density-dependent induction of plant volatiles: Honest or “Cry Wolf” signals? PLoS ONE 2010, 5, e12161. [Google Scholar] [CrossRef]
- Fernandes, F.; Pereira, D.M.; de Pinho, P.G.; Valentao, P.; Pereira, J.A.; Bento, A.; Andrade, P.B. Headspace solid-phase microextraction and gas chromatography/ion trap-mass spectrometry applied to a living system: Pieris brassicae fed with kale. Food Chem. 2010, 119, 1681–1693. [Google Scholar] [CrossRef]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant volatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Mumm, R.; Dicke, M. Variation in natural plant products and the attraction of bodyguards involved in indirect plant defense. Can. J. Zool. 2010, 88, 628–667. [Google Scholar] [CrossRef]
- Smid, H.M.; van Loon, J.J.A.; Posthumus, M.A.; Vet, L.E.M. GC-EAG-analysis of volatiles from Brussels sprouts plants damaged by two species of Pieris caterpillars:: Olfactory receptive range of a specialist and a generalist parasitoid wasp species. Chemoecology 2002, 12, 169–176. [Google Scholar] [CrossRef]
- Husebye, H.; Chadchawan, S.; Winge, P.; Thangstad, O.P.; Bones, A.M. Guard cell- and phloem idioblast-specific expression of thioglucoside glucohydrolase 1 (myrosinase) in Arabidopsis. Plant Physiol. 2002, 128, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Bones, A.M.; Rossiter, J.T. The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry 2006, 67, 1053–1067. [Google Scholar] [CrossRef]
- Ulland, S.; Ian, E.; Stranden, M.; Borg-Karlson, A.K.; Mustaparta, H. Plant volatiles activating specific olfactory receptor neurons of the cabbage moth Mamestra brassicae L. (Lepidoptera, Noctuidae). Chem. Senses 2008, 33, 509–522. [Google Scholar] [CrossRef]
- Van Loon, J.J.A.; Frentz, W.H.; Vaneeuwijk, F.A. Electroantennogram responses to plant volatiles in 2 species of Pieris butterflies. Entomol. Exp. Appl. 1992, 62, 253–260. [Google Scholar]
- Hopkins, R.J.; van Dam, N.M.; van Loon, J.J.A. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. Entomol. 2009, 54, 57–83. [Google Scholar] [CrossRef]
- Takabayashi, J.; Dicke, M.; Posthumus, M.A. Variation in composition of predator-attracting allelochemicals emitted by herbivore-infested plants: Relative influence of plant and herbivore. Chemoecology 1991, 2, 1–6. [Google Scholar] [CrossRef]
- Turlings, T.C.; Loughrin, J.H.; McCall, P.J.; Rose, U.S.; Lewis, W.J.; Tumlinson, J.H. How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc. Natl. Acad. Sci. USA 1995, 92, 4169–4174. [Google Scholar]
- Rojas, J.C. Electrophysiological and behavioral responses of the cabbage moth to plant volatiles. J. Chem. Ecol. 1999, 25, 1867–1883. [Google Scholar] [CrossRef]
- Itoh, Y.; Okumura, Y.; Fujii, T.; Ishikawa, Y.; Omura, H. Effects of mating on host selection by female small white butterflies Pieris rapae (Lepidoptera: Pieridae). J. Comp. Physiol. A-Neuroethol. Sens. Neural Behav. Physiol. 2018, 204, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Omura, H.; Hayashi, N. Identification of floral volatiles from Ligustrum japonicum that stimulate flower visiting by cabbage butterfly, Pieris rapae. J. Chem. Ecol. 1998, 24, 2167–2180. [Google Scholar]
- Blažytė-Čereškienė, L.; Aleknavičius, D.; Apšegaitė, V.; Būda, V. Response of parasitic wasp Cotesia glomerata L. (Hymenoptera: Braconidae) to cabbage plants of two varieties: Olfactory spectra of males and females. J. Econ. Entomol. 2022, 115, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.L.; Zhang, Y.J.; Wyckhuys, K.A.G.; Wu, K.M.; Gao, X.W.; Guo, Y.Y. Electrophysiological and behavioral responses of Microplitis mediator (Hymenoptera: Braconidae) to caterpillar-induced volatiles from cotton. Environ. Entomol. 2010, 39, 600–609. [Google Scholar] [CrossRef]
- Omura, H.; Honda, K.; Hayashi, N. Floral scent of Osmanthus fragrans discourages foraging behavior of cabbage butterfly, Pieris rapae. J. Chem. Ecol. 2000, 26, 655–666. [Google Scholar]
- De Moraes, C.M.; Mescher, M.C.; Tumlinson, J.H. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 2001, 410, 577–580. [Google Scholar]
- Kessler, A.; Baldwin, I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 2001, 291, 2141–2144. [Google Scholar]
- Mumm, R.; Posthumus, M.A.; Dicke, M. Significance of terpenoids in induced indirect plant defence against herbivorous arthropods. Plant Cell Environ. 2008, 31, 575–585. [Google Scholar] [CrossRef]
- Gols, R.; Veenemans, C.; Potting, R.P.J.; Smid, H.M.; Dicke, M.; Harvey, J.A.; Bukovinszky, T. Variation in the specificity of plant volatiles and their use by a specialist and a generalist parasitoid. Anim. Behav. 2012, 83, 1231–1242. [Google Scholar] [CrossRef]
- Puente, M.; Magori, K.; Kennedy, G.G.; Gould, F. Impact of herbivore-induced plant volatiles on parasitoid foraging success: A spatial simulation of the Cotesia rubecula, Pieris rapae, and Brassica oleracea system. J. Chem. Ecol. 2008, 34, 959–970. [Google Scholar] [CrossRef]
- Pawliszyn, J. Solid-Phase Microextraction: Theory and Practice; VCH: New York, NY, USA, 1997; p. 264. [Google Scholar]
- Araniti, F.; Pantò, S.; Lupini, A.; Sunseri, F.; Abenavoli, M.R. Chemical Characterization of Volatile Organic Compounds (VOCs) Through Headspace Solid Phase Micro Extraction (SPME). In Advances in Plant Ecophysiology Techniques; Sánchez-Moreiras, A.M., Reigosa, M.J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 401–417. [Google Scholar] [CrossRef]


| No | Compound | CAS No | Group | RI | ID | Intact | M. brassicae Caterpillars Feeding | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CB | CL | CK | BR | CB | CL | CK | BR | ||||||
| 1 | Dimethyl disulphide | 624-32-0 | S | - | RC | nd | nd | nd | nd | tr | 5.5 ± 0.4 a | 5.3 ± 0.4 a | 5.8 ± 0.5 a |
| 2 | Unidentified (oxime) | IM | 908 | U | 6.4 ± 0.1 a | 5.4 ± 0.2 c | 6.3 ± 0.1 ab | 6.1 ± 0.1 b | 6.7 ± 0.3 a | 5.6 ± 0.5 b | 5.9 ± 0.0 *b | 6.3 ± 0.2 a | |
| 3 | α-Thujene | 2867-05-2 | MT | 923 | RC | 5.6 ± 0.1 a | 5.3 ± 0.4 a | 6.1 ± 0.3 a | 5.6 ± 0.1 a | 5.2 ± 0.3 a | 4.9 ± 0.4 a | 5.1 ± 0.5 a | 4.7 ± 0.5 a |
| 4 | α-Pinene | 80-56-8 | MT | 930 | RC | 4.9 ± 0.3 a | 4.1 ± 1.1 a | 4.3 ± 0.3 a | tr | 4.8 ± 0.2 b | 3.7 ± 1.0d | 5.6 ± 0.0 *a | 4.2 ± 0.3 c |
| 5 | Dimethyl trisulphide | 3658-80-8 | S | 944 | RC | nd | nd | tr | nd | nd | 5.5 ± 0.5 a | 5.2 ± 0.4 a | 5.8 ± 0.5 a |
| 6 | Sabinene | 3387-41-5 | MT | 966 | RC | 6.4 ± 0.3 ab | 6.0 ± 0.3 b | 6.6 ± 0.1 a | 6.2 ± 0.2 ab | 6.2 ± 0.1 b | 6.1 ± 0.2 b | 6.8 ± 0.1 a | 5.9 ± 0.3 b |
| 7 | β-Myrcene | 123-35-3 | MT | 985 | RC | 6.5 ± 0.5 b | 6.5 ± 0.2 b | 7.1 ± 0.1 a | 6.6 ± 0.1 b | 6.5 ± 0.2 b | 6.7 ± 0.1 b | 7.3 ± 0.1 a | 6.5 ± 0.2 b |
| 8 | (Z)-3-Hexen-1-yl acetate | 3681-71-8 | E | 991 | RC | 6.0 ± 0.2 a | nd | 6.1 ± 0.0 *a | 4.0 ± 1.1 b | 6.6 ± 0.2 c | 7.2 ± 0.2 b | 7.6 ± 0.1 a | 7.0 ± 0.1 b |
| 9 | α-Terpinene | 99-86-5 | MT | 1008 | RC | 3.8 ± 0.0 * | tr | nd | nd | tr | tr | nd | tr |
| 10 | 1,8-Cineole | 470-82-6 | OMT | 1019 | RC | 6.1 ± 0.1 a | 6.3 ± 0.3 a | 6.1 ± 0.1 a | 5.8 ± 0.1 b | 5.8 ± 0.3 ab | 6.0 ± 0.1 a | 6.1 ± 0.1 a | 5.6 ± 0.1 b |
| 11 | Limonene | 7705-14-8 | MT | 1021 | RC | 6.5 ± 0.0 *b | 6.5 ± 0.2 b | 7.2 ± 0.1 a | 6.6 ± 0.1 b | 6.6 ± 0.1 b | 6.7 ± 0.1 b | 7.2 ± 0.1 a | 6.6 ± 0.1 b |
| 12 | 2-Methylbutyl isothiocyanate | 4404-51-7 | IST | 1029 | MS | nd | nd | 1.3 ± 1.3 | nd | nd | 2.9 ± 1.2 a | tr | 3.4 ± 0.9 a |
| 13 | Methyl-2-ethyl hexanoate | 816-19-3 | E | 1033 | MS | tr | 5.0 ± 0.3 b | tr | 5.7 ± 0.1 a | tr | 4.7 ± 0.4 b | 5.1 ± 0.3 ab | 5.5 ± 0.2 a |
| 14 | (E)-β-Ocimene | 3779-61-1 | MT | 1042 | RC | nd | tr | tr | 4.4 ± 0.3 | tr | 3.9 ± 1.0 a | 4.1 ± 0.2 a | 4.2 ± 0.3 a |
| 15 | γ-Terpinene | 99-85-4 | MT | 1051 | RC | 4.7 ± 0.4 b | tr | 5.3 ± 0.4 ab | 5.3 ± 0.1 a | 3.8 ± 0.9 | 3.3 ± 0.9 b | 5.5 ± 0.1 a | 5.1 ± 0.3 a |
| 16 | (E)-Sabinene hydrate | 17699-16-0 | OMT | 1056 | RC | 5.2 ± 0.3 ab | tr | 5.5 ± 0.1 a | 4.9 ± 0.3 b | 3.9 ± 0.9 b | 5.1 ± 0.3 b | 5.7 ± 0.1 a | 4.9 ± 0.3 b |
| 17 | 1-Methoxy-1-methylethyl-benzene | 935-67-1 | AR | 1075 | MS | 4.5 ± 0.4 a | 3.5 ± 1.0 b | tr | 5.0 ± 0.3 a | tr | 4.1 ± 0.3 | tr | tr |
| 18 | p-Mentha-2,4(8)-diene | 586-63-0 | MT | 1078 | RC | 5.0 ± 0.3 a | tr | 4.9 ± 0.3 a | 4.4 ± 0.3 a | 4.6 ± 0.3 b | 3.8 ± 1.0 c | 5.4 ± 0.0 *a | 4.1 ± 0.3 bc |
| 19 | (E)-4,8-Dimethylnona-1,3,7-triene | 19945-61-0 | HT | 1107 | MSRI | 5.5 ± 0.1 | nd | nd | tr | 5.9 ± 0.2 a | 1.1 ± 1.1 c | nd | 5.2 ± 0.3 b |
| 20 | 2-Methylbutyl valerate | 55590-83-5 | E | 1129 | RC | nd | nd | nd | nd | nd | 5.1 ± 0.5 | nd | nd |
| 21 | (Z)-4-Hexen-1-yl butanoate | 69727-41-9 | E | 1131 | MS | nd | tr | nd | nd | nd | 5.2 ± 0.6 | nd | nd |
| 22 | p-Menthan-3-one | 10458-14-7 | OMT | 1132 | RC | nd | nd | nd | nd | nd | 2.2 ± 1.4 c | 4.6 ± 0.3 a | 4.0 ± 1.0 b |
| 23 | Unidentified | 1152 | MS | 5.4 ± 0.1 b | 5.6 ± 0.0 *a | tr | nd | 5.5 ± 0.1 b | 5.6 ± 0.1 ab | 5.7 ± 0.0 *a | 4.8 ± 0.4 c | ||
| 24 | (Z)-3-Hexen-1-yl butanoate | 16491-36-4 | E | 1171 | RC | nd | nd | nd | nd | nd | 6.8 ± 0.3 a | nd | 4.3 ± 1.1 b |
| 25 | Dimethyl tetrasulphide | 5756-24-1 | S | 1181 | RC | nd | nd | nd | nd | nd | 3.4 ± 1.4 b | 3.3 ± 0.8 b | 5.3 ± 0.4 a |
| 26 | β-Cyclocitral | 432-25-7 | OMT | 1194 | RC | nd | nd | nd | nd | tr | 4.8 ± 0.4 a | 4.7 ± 0.4 a | 5.4 ± 0.4 a |
| 27 | (Z)-3-Hexen-1-yl 2-methylbutanoate | 53398-85-9 | E | 1217 | RC | nd | nd | nd | nd | 5.7 ± 0.0 *c | 6.8 ± 0.3 a | 6.1 ± 0.1 b | 5.8 ± 0.2 bc |
| 28 | (Z)-3-Hexen-1-yl 3-methylbutanoate | 35154-45-1 | E | 1220 | RC | nd | nd | nd | nd | tr | 6.9 ± 0.3 a | 5.7 ± 0.1 b | 5.5 ± 0.2 b |
| 29 | Hexyl 3-methylbutanoate | 10632-13-0 | E | 1228 | RC | nd | nd | nd | nd | nd | 3.4 ± 0.9 | nd | nd |
| 30 | 3-Methyl-2-buten-1-yl hexanoate | 74298-89-8 | E | 1231 | MS | nd | nd | nd | nd | nd | 5.0 ± 0.5 | nd | tr |
| 31 | Methyl nerolate | 1862-61-9 | E | 1263 | MSRI | nd | nd | nd | nd | nd | nd | 5.5 ± 0.1 | nd |
| 32 | Unidentified | 1315 | U | nd | nd | nd | nd | nd | nd | nd | nd | ||
| 33 | (Z)-3-hexen-1-yl hexanoate | 31501-11-8 | E | 1324 | RC | nd | nd | nd | nd | nd | 6.3 ± 0.4 | nd | nd |
| 34 | (Z)-β-Elemene | 674819-48-8 | ST | 1376 | MSRI | nd | tr | 6.0 ± 0.0 * | nd | nd | 5.4 ± 0.1 b | 6.0 ± 0.1 a | nd |
| 35 | β-Elemene | 33880-83-0 | ST | 1383 | RC | nd | 6.3 ± 0.2 b | 7.3 ± 0.0 *a | nd | nd | 6.9 ± 0.2 b | 7.4 ± 0.1 a | nd |
| 36 | Unidentified (ester) | E | 1419 | U | nd | nd | nd | nd | nd | 4.5 ± 0.4 | nd | nd | |
| 37 | Octyl 3-methylbutanoate | 7786-58-5 | E | 1424 | MS | nd | nd | nd | nd | nd | 4.1 ± 0.3 | nd | nd |
| 38 | (E)-β-Farnesene | 18794-84-8 | ST | 1447 | RC | nd | nd | nd | nd | nd | 5.1 ± 0.3 | nd | nd |
| 39 | β-Ionone | 79-77-6 | KT | 1461 | RC | nd | nd | nd | nd | 3.3 ± 0.8 b | 4.9 ± 0.4 a | 4.9 ± 0.3 a | 5.4 ± 0.4 a |
| 40 | α-Acoradiene | 87173-79-3 | ST | 1463 | MSRI | nd | 4.2 ± 0.3 b | 5.8 ± 0.1 a | nd | nd | 5.1 ± 0.3 b | 5.7 ± 0.0 *a | nd |
| 41 | α-Curcumene | 644-30-4 | ST | 1468 | MSRI | nd | tr | nd | nd | nd | 5.0 ± 0.3 | nd | nd |
| 42 | β-Selinene | 21488-94-8 | ST | 1474 | MSRI | nd | nd | 5.2 ± 0.3 | nd | nd | 3.3 ± 0.9 b | 5.0 ± 0.3 a | nd |
| 43 | (Z,E)-α-Farnesene | 26560-14-5 | ST | 1482 | RC | nd | nd | nd | 5.1 ± 0.3 | tr | 5.2 ± 0.4 a | 3.3 ± 0.8 b | 5.4 ± 0.2 a |
| 44 | α-Zingiberene | 495-60-3 | ST | 1483 | MSRI | nd | tr | 5.7 ± 0.1 a | 5.4 ± 0.0 *b | nd | 5.9 ± 0.1 a | 5.5 ± 0.1 b | 5.6 ± 0.1 b |
| 45 | Unidentified | 1488 | U | 6.5 ± 0.1 a | 6.2 ± 0.0 *b | 6.3 ± 0.1 b | 5.9 ± 0.1 c | 6.1 ± 0.0 *a | 5.6 ± 0.5 ab | 6.0 ± 0.0 *a | 4.5 ± 1.1 b | ||
| 46 | (E,E)-α-Farnesene | 502-61-4 | ST | 1495 | RC | 5.9 ± 0.1 b | 6.9 ± 0.1 a | 5.4 ± 0.1 c | 7.1 ± 0.0.1 a | 6.6 ± 0.1 b | 7.1 ± 0.2 a | 5.9 ± 0.1 c | 7.2 ± 0.1 a |
| 47 | (Z)-γ-Bisabolene | 495-62-5 | ST | 1502 | MSRI | nd | tr | tr | nd | nd | 5.0 ± 0.3 a | 5.4 ± 0.4 a | tr |
| 48 | (E)-α-Bisabolene | 17627-44-0 | ST | 1530 | MSRI | nd | nd | nd | nd | nd | tr | nd | nd |
| 49 | (Z)-3-Hexen-1-yl benzoate | 25152-85-6 | E | 1542 | RC | nd | nd | nd | nd | nd | 1.9 ± 1.2 | nd | nd |
| 50 | TMTT * | 62235-06-7 | HT | 1566 | RC | nd | nd | nd | nd | nd | tr | nd | 1.1 ± 1.1 |
| 51 | Unidentified (branched hydrocarbon) | HY | 1937 | U | 4.6 ± 1.2 | nd | nd | nd | nd | nd | nd | nd | |
| 52 | Unidentified (MS similar to verticilol) | 1990 | U | 5.3 ± 1.3 | nd | nd | nd | 5.7 ± 0.2 a | nd | nd | 4.1 ± 0.3 b | ||
| No | Compound | M. brassicae caterpillars removed | P. rapae caterpillars feeding | P. rapae caterpillars removed | |||||||||
| CB | CL | CK | BR | CB | CL | CK | BR | CB | CL | CK | BR | ||
| 1 | Dimethyl disulphide | nd | nd | nd | nd | tr | 5.2 ± 0.7 a | nd | 5.3 ± 0.7 a | nd | nd | nd | nd |
| 2 | Unidentified (oxime) | 6.2 ± 0.2 a | 6.0 ± 0.2 a | 6.0 ± 0.2 a | 6.1 ± 0.2 a | 5.9 ± 0.1 b | 6.3 ± 0.1 a | 6.2 ± 0.2 a b | 6.0 ± 0.0 *b | 5.8 ± 0.1 b | 6.2 ± 0.1 a | 6.3 ± 0.2 a | 5.9 ± 0.4 ab |
| 3 | α-Thujene | 5.2 ± 0.1 b | 5.3 ± 0.4 b | 6.1 ± 0.1 a | 5.4 ± 0.4 b | 5.9 ± 0.1 a | 5.4 ± 0.1 b | 5.8 ± 0.1 a | 4.8 ± 0.5 b | 5.8 ± 0.1 a | 5.3 ± 0.2 b | 5.7 ± 0.1 a | 5.1 ± 0.1 b |
| 4 | α-Pinene | 4.8 ± 0.4 a | tr | 5.4 ± 0.2 a | 4.6 ± 0.5 a | 5.3 ± 0.3 | tr | tr | tr | 4.9 ± 0.5 | tr | tr | tr |
| 5 | Dimethyl trisulphide | nd | nd | nd | tr | nd | tr | tr | tr | nd | tr | 4.9 ± 0.6 | nd |
| 6 | Sabinene | 6.4 ± 0.2 b | 6.1 ± 0.2 b | 6.9 ± 0.1 a | 6.1 ± 0.4 b | 6.5 ± 0.2 a | 5.7 ± 0.2 b | 6.3 ± 0.0 *a | 5.8 ± 0.3 b | 6.7 ± 0.2 a | 5.5 ± 0.1 d | 6.3 ± 0.0 *b | 5.9 ± 0.0 * c |
| 7 | β-Myrcene | 6.0 ± 0.1 c | 6.8 ± 0.1 b | 7.4 ± 0.1 a | 6.7 ± 0.3 b | 6.7 ± 0.1 b | 6.7 ± 0.1 b | 7.2 ± 0.1 a | 6.5 ± 0.1 b | 6.9 ± 0.2 a | 6.8 ± 0.2 a b | 7.1 ± 0.2 a | 6.6 ± 0.0 *b |
| 8 | (Z)-3-Hexen-1-yl acetate | 6.1 ± 0.4 a | 6.3 ± 0.1 a | 5.9 ± 0.3 a | 6.1 ± 0.1 a | 6.8 ± 0.6 a | 7.3 ± 0.3 a | 7.0 ± 0.5 a | 6.7 ± 0.4 a | 6.1 ± 0.1 a | 5.2 ± 0.8 b | 6.1 ± 0.5 a b | 5.9 ± 0.0 *b |
| 9 | α-Terpinene | tr | tr | 4.7 ± 0.4 a | 2.9 ± 1.2 b | tr | tr | nd | nd | tr | tr | nd | nd |
| 10 | 1,8-Cineole | 5.8 ± 0.1 b | 6.0 ± 0.1 b | 6.3 ± 0.1 a | 5.9 ± 0.2 b | 6.3 ± 0.1 a | 5.6 ± 0.1 c | 5.9 ± 0.1 b | 5.9 ± 0.0 *b | 6.4 ± 0.1 a | 5.6 ± 0.0 * c | 6.0 ± 0.0 *b | 6.2 ± 0.3 a b |
| 11 | Limonene | 6.5 ± 0.1 b | 6.8 ± 0.2 b | 7.4 ± 0.1 a | 6.6 ± 0.3 b | 6.8 ± 0.1 a | 6.4 ± 0.0 *b | 7.0 ± 0.1 a | 6.5 ± 0.1 b | 6.9 ± 0.2 a | 6.5 ± 0.1 c | 7.0 ± 0.1 a | 6.6 ± 0.0 *b c |
| 12 | 2-Methylbutyl isothiocyanate | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 13 | Methyl-2-ethyl hexanoate | 5.2 ± 0.1 a | 4.7 ± 0.4 a | 5.1 ± 0.3 a | 5.2 ± 0.4 a | 4.7 ± 0.4 b | 5.3 ± 0.2 b | 4.8 ± 0.0.5 b | 5.7 ± 0.0 *a | 5.5 ± 0.2 b | 5.6 ± 0.1 b | 5.6 ± 0.0 *b | 6.0 ± 0.0 *a |
| 14 | (E)-β-Ocimene | tr | 4.2 ± 0.5 a | 4.1 ± 0.3 a | 4.0 ± 1.1 a | tr | tr | nd | tr | tr | tr | nd | 4.2 ± 0.3 |
| 15 | γ-Terpinene | tr | 3.7 ± 1.0 b | 5.4 ± 0.2 a | 5.1 ± 0.4 a | 5.4 ± 0.1 a | tr | 5.5 ± 0.2 a | 4.7 ± 0.5 | 5.1 ± 0.6 a b | tr | 5.3 ± 0.0 *b | 5.4 ± 0.0 *a |
| 16 | (E)-Sabinene hydrate | tr | 4.8 ± 0.4 b | 5.9 ± 0.0 *a | 4.8 ± 0.4 b | 5.8 ± 0.1 a | tr | 5.7 ± 0.0 *b | tr | 5.1 ± 0.7 a | tr | 5.3 ± 0.1 a | 5.3 ± 0.0 *a |
| 17 | 1-Methoxy-1-methylethyl-benzene | tr | tr ± | 4.4 ± 0.4 | nd | 4.2 ± 0.3 b | 5.6 ± 0.1 a | tr | nd | tr | tr | 5.7 ± 0.1 | nd |
| 18 | p-Mentha-2,4(8)-diene | nd | nd | 5.5 ± 0.1 | tr | 5.2 ± 0.1 a | 5.2 ± 0.0 *a | tr | nd | 4.4 ± 0.5 b | nd | 5.2 ± 0.0 *a | nd |
| 19 | (E)-4,8-Dimethylnona-1,3,7-triene | 6.0 ± 0.3 a | nd | nd | 5.6 ± 0.3 a | 5.7 ± 0.2 a | 4.8 ± 0.5 b | nd | tr | 2.0 ± 2.0 | nd | nd | tr |
| 20 | 2-Methylbutyl valerate | nd | 4.5 ± 1.2 | nd | nd | nd | 5.1 ± 0.1 | nd | nd | nd | 4.9 ± 0.0 * | nd | nd |
| 21 | (Z)-4-Hexen-1-yl butanoate | nd | 5.3 ± 0.1 | nd | nd | 5.5 ± 0.1 a | 5.6 ± 0.2 a | nd | nd | 1.8 ± 1.8 b | 5.2 ± 0.3 a | nd | nd |
| 22 | p-Menthan-3-one | 4.8 ± 0.4 a | 5.2 ± 0.1 a | 4.1 ± 0.2 b | tr | 5.1 ± 0.1 b | 5.3 ± 0.0 *a | 4.9 ± 0.5 a b | nd | nd | 5.0 ± 0.0 *a | 4.8 ± 0.5 a | nd |
| 23 | Unidentified | 5.2 ± 0.1 c | 5.8 ± 0.2 a | 5.4 ± 0.1 bc | 5.6 ± 0.1 ab | 5.7 ± 0.0 *b | 5.9 ± 0.1 a | 5.8 ± 0.1 a b | 5.3 ± 0.2 c | 5.5 ± 0.2 b | 5.9 ± 0.1 a | 5.9 ± 0.1 a | 5.6 ± 0.0 *b |
| 24 | (Z)-3-Hexen-1-yl butanoate | 0 | 6.1 ± 0.3 | nd | nd | nd | 6.5 ± 0.3 | nd | nd | nd | 5.4 ± 0.8 | nd | nd |
| 25 | Dimethyl tetrasulphide | 0 | nd | tr | nd | nd | nd | tr | nd | nd | nd | tr | nd |
| 26 | β-Cyclocitral | tr | 4.9 ± 0.3 a | tr | 4.2 ± 0.4 a | tr | tr | tr | tr | tr | tr | tr | tr |
| 27 | (Z)-3-Hexen-1-yl 2-methylbutanoate | 0 | 6.4 ± 0.2 a | 4.1 ± 0.2 b | 2.7 ± 1.1 b | 5.2 ± 0.7 b c | 6.6 ± 0.4 a | 5.5 ± 0.0 c * | 5.8 ± 0.1 b | nd | 6.3 ± 0.4 a | 6.4 ± 0.1 a | 2.9 ± 1.5 b |
| 28 | (Z)-3-Hexen-1-yl 3-methylbutanoate | 0 | 6.8 ± 0.2 a | 3.2 ± 1.3 c | 5.6 ± 0.2 b | 5.7 ± 0.4 b | 6.9 ± 0.2 a | 5.6 ± 0.1 b | 5.4 ± 0.8 b | 5.7 ± 0.4 a | 6.1 ± 0.6 a | 5.9 ± 0.3 a | 5.6 ± 0.0 *a |
| 29 | Hexyl 3-methylbutanoate | 0 | 5.4 ± 0.4 a | 1.5 ± 0.9 b | nd | nd | nd | nd | nd | nd | tr | nd | nd |
| 30 | 3-Methyl-2-buten-1-yl hexanoate | 0 | tr | nd | nd | nd | nd | nd | nd | nd | tr | nd | nd |
| 31 | Methyl nerolate | 0 | nd | 6.2 ± 0.1 | nd | nd | nd | 5.4 ± 0.8 | nd | nd | nd | 6.3 ± 0.0 * | nd |
| 32 | Unidentified | 0 | 4.1 ± 1.1 | nd | nd | nd | 5.7 ± 0.0 * | nd | nd | nd | nd | nd | nd |
| 33 | (Z)-3-hexen-1-yl hexanoate | 0 | 5.7 ± 0.3 | nd | nd | nd | 6.1 ± 0.4 a | 5.3 ± 0.0 *b | nd | nd | 5.1 ± 0.7 | nd | nd |
| 34 | (Z)-β-Elemene | 0 | 5.9 ± 0.1 b | 6.2 ± 0.1 a | nd | nd | 5.4 ± 0.2 b | 5.9 ± 0.2 a | nd | nd | 5.6 ± 0.2 b | 6.1 ± 0.2 a | nd |
| 35 | β-Elemene | 0 | 7.3 ± 0.1 b | 7.6 ± 0.1 a | nd | nd | 6.7 ± 0.3 b | 7.3 ± 0.1 a | nd | nd | 7.0 ± 0.2 a | 7.4 ± 0.2 a | nd |
| 36 | Unidentified (ester) | 0 | 3.7 ± 1.0 | nd | nd | nd | tr | nd | nd | nd | tr | nd | nd |
| 37 | Octyl 3-methylbutanoate | 0 | 5.3 ± 0.4 | nd | nd | nd | 4.8 ± 0.5 | nd | nd | nd | 5.6 ± 0.4 | nd | nd |
| 38 | (E)-β-Farnesene | 0 | 5.4 ± 0.4 | nd | nd | nd | 5.2 ± 0.7 | nd | nd | nd | 5.0 ± 0.7 | nd | nd |
| 39 | β-Ionone | tr | 3.8 ± 1.0 a | 4.1 ± 0.3 a | 4.3 ± 0.5 a | tr | tr | tr | tr | nd | nd | nd | tr |
| 40 | α-Acoradiene | 0 | 5.9 ± 0.2 a | 6.0 ± 0.1 a | nd | nd | 5.4 ± 0.0 *a | 5.5 ± 0.1 a | nd | nd | 5.6 ± 0.1 a | 5.6 ± 0.1 a | nd |
| 41 | α-Curcumene | 0 | 5.4 ± 0.5 | nd | nd | nd | tr | nd | nd | nd | 4.4 ± 0.5 | nd | nd |
| 42 | β-Selinene | 0 | 4.7 ± 0.5 b | 5.6 ± 0.2 a | nd | nd | tr | 5.4 ± 0.0 * | nd | nd | 4.3 ± 0.4 b | 5.6 ± 0.0 *a | nd |
| 43 | (Z,E)-α-Farnesene | tr | 5.4 ± 0.1 a | 3.0 ± 0.8 b | 5.1 ± 0.3 a | nd | 5.5 ± 0.8 | tr | 2.8 ± 1.4 | 1.8 ± 1.8 b | 5.6 ± 0.5 a | nd | 4.9 ± 0.5 a |
| 44 | α-Zingiberene | 0 | 6.3 ± 0.2 a | 5.9 ± 0.1 b | 5.2 ± 0.3 c | nd | 5.8 ± 0.3 | 5.6 ± 0.1 | tr | nd | 6.0 ± 0.2 a | 5.7 ± 0.0 *b | 4.2 ± 0.3 c |
| 45 | Unidentified | 6.0 ± 0.1 a | 5.9 ± 0.1 ab | 5.6 ± 0.1 c | 5.4 ± 0.4 bc | 6.4 ± 0.1 a | 5.6 ± 0.0 * c | 5.9 ± 0.2 b | 3.5 ± 1.6 d | 6.1 ± 0.1 a | 5.6 ± 0.1 b | 5.4 ± 0.2 b | 4.2 ± 0.4 c |
| 46 | (E,E)-α-Farnesene | 6.4 ± 0.2 b | 7.2 ± 0.1 a | 6.0 ± 0.2 b | 7.1 ± 0.1 a | 5.4 ± 0.2 c | 7.6 ± 0.5 a | tr | 6.6 ± 0.2 b | 5.9 ± 0.2 b | 7.2 ± 0.6 a | tr | 6.9 ± 0.2 a |
| 47 | (Z)-γ-Bisabolene | 0 | 5.4 ± 0.4 | tr | tr | nd | 5.6 ± 0.1 a | 5.6 ± 0.1 a | tr | nd | 4.5 ± 0.6 a | 5.0 ± 0.6 a | 4.2 ± 0.3 a |
| 48 | (E)-α-Bisabolene | 0 | 5.1 ± 0.3 a | 3.4 ± 0.9 b | nd | nd | 4.7 ± 0.4 | nd | nd | nd | 4.7 ± 0.5 | nd | nd |
| 49 | (Z)-3-Hexen-1-yl benzoate | 0 | 3.2 ± 1.4 | tr | nd | nd | tr | nd | nd | nd | tr | nd | nd |
| 50 | TMTT * | 0 | 3.8 ± 1.0 | nd | nd | nd | tr | nd | nd | nd | tr | nd | nd |
| 51 | Unidentified (hydrocarbon) | 3.8 ± 1.0 | nd | nd | nd | 5.5 ± 0.1 | nd | nd | nd | 5.5 ± 0.2 | nd | nd | nd |
| 52 | Unidentified (MS similar to verticilol) | 5.6 ± 0.1 a | nd | nd | 4.8 ± 0.4 b | 6.4 ± 0.1 | nd | nd | tr | 6.4 ± 0.3 a | nd | nd | 5.8 ± 0.1 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mozūraitis, R.; Hambäck, P.; Borg-Karlson, A.-K.; Hopkins, R.J. Variation in Odour Profiles of Cauliflower, Curly Kale and Broccoli (Brassica oleracea L.) Cultivars Is Affected More by Genotype Rather than Herbivore Feeding. Plants 2025, 14, 1014. https://doi.org/10.3390/plants14071014
Mozūraitis R, Hambäck P, Borg-Karlson A-K, Hopkins RJ. Variation in Odour Profiles of Cauliflower, Curly Kale and Broccoli (Brassica oleracea L.) Cultivars Is Affected More by Genotype Rather than Herbivore Feeding. Plants. 2025; 14(7):1014. https://doi.org/10.3390/plants14071014
Chicago/Turabian StyleMozūraitis, Raimondas, Peter Hambäck, Anna-Karin Borg-Karlson, and Richard James Hopkins. 2025. "Variation in Odour Profiles of Cauliflower, Curly Kale and Broccoli (Brassica oleracea L.) Cultivars Is Affected More by Genotype Rather than Herbivore Feeding" Plants 14, no. 7: 1014. https://doi.org/10.3390/plants14071014
APA StyleMozūraitis, R., Hambäck, P., Borg-Karlson, A.-K., & Hopkins, R. J. (2025). Variation in Odour Profiles of Cauliflower, Curly Kale and Broccoli (Brassica oleracea L.) Cultivars Is Affected More by Genotype Rather than Herbivore Feeding. Plants, 14(7), 1014. https://doi.org/10.3390/plants14071014








