Comparative Transcriptome Analysis of Gene Responses of Salt-Tolerant and Salt-Sensitive Watermelon Cultivars’ Roots to Salt Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experiment Design
2.2. Measurement of Physiological Parameters
2.3. RNA Sequencing and Data Analysis
2.4. Fluorescent Quantitative Real-Time PCR (qRT-PCR) Verification
2.5. Yeast Constructs
2.6. Tolerance Assay and Growth Curve
2.7. Data Processing and Visualization
3. Results
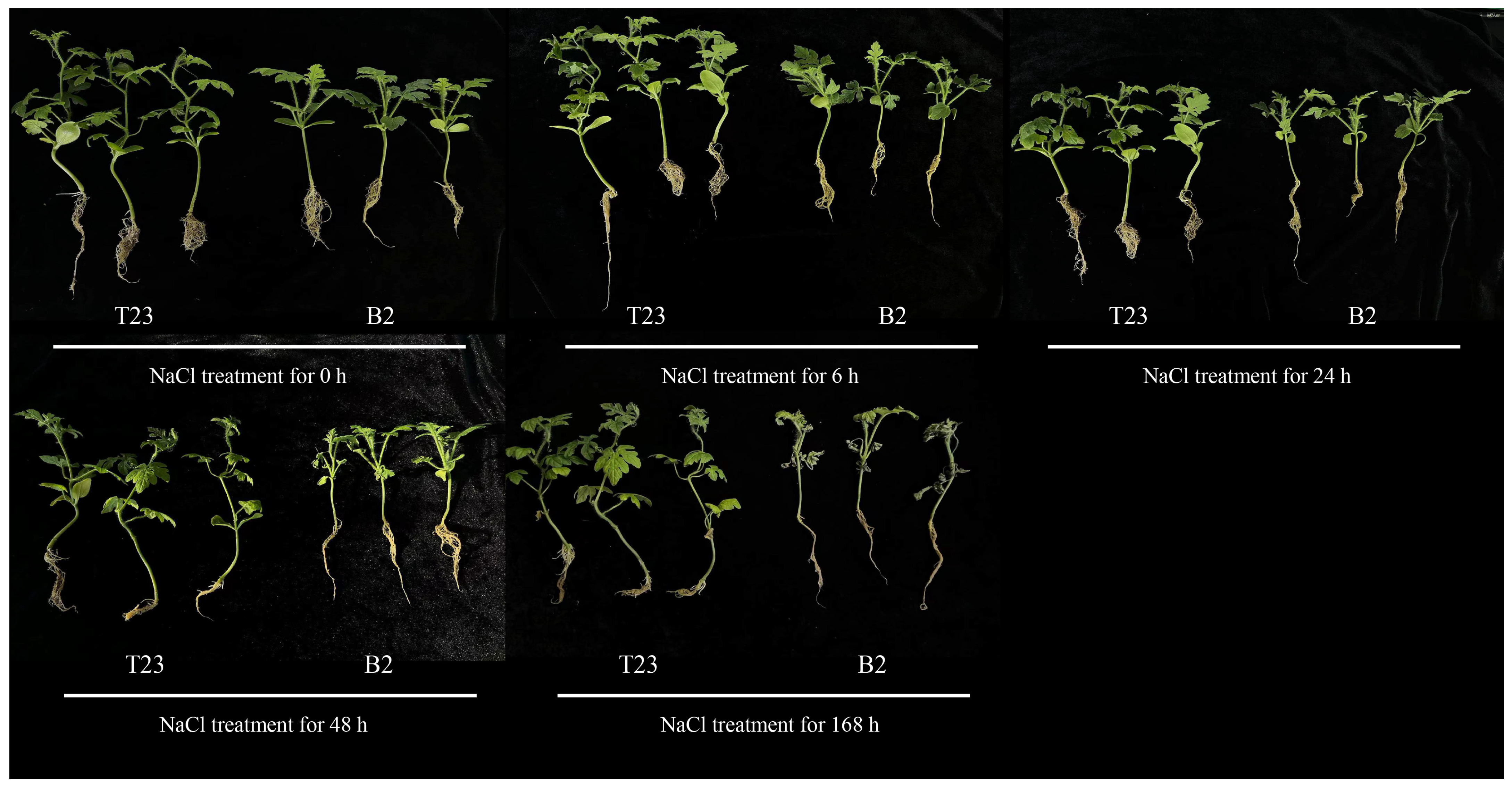
3.1. Phenotypic Changes in Watermelon Seedlings of Two Varieties Under Salt Stress
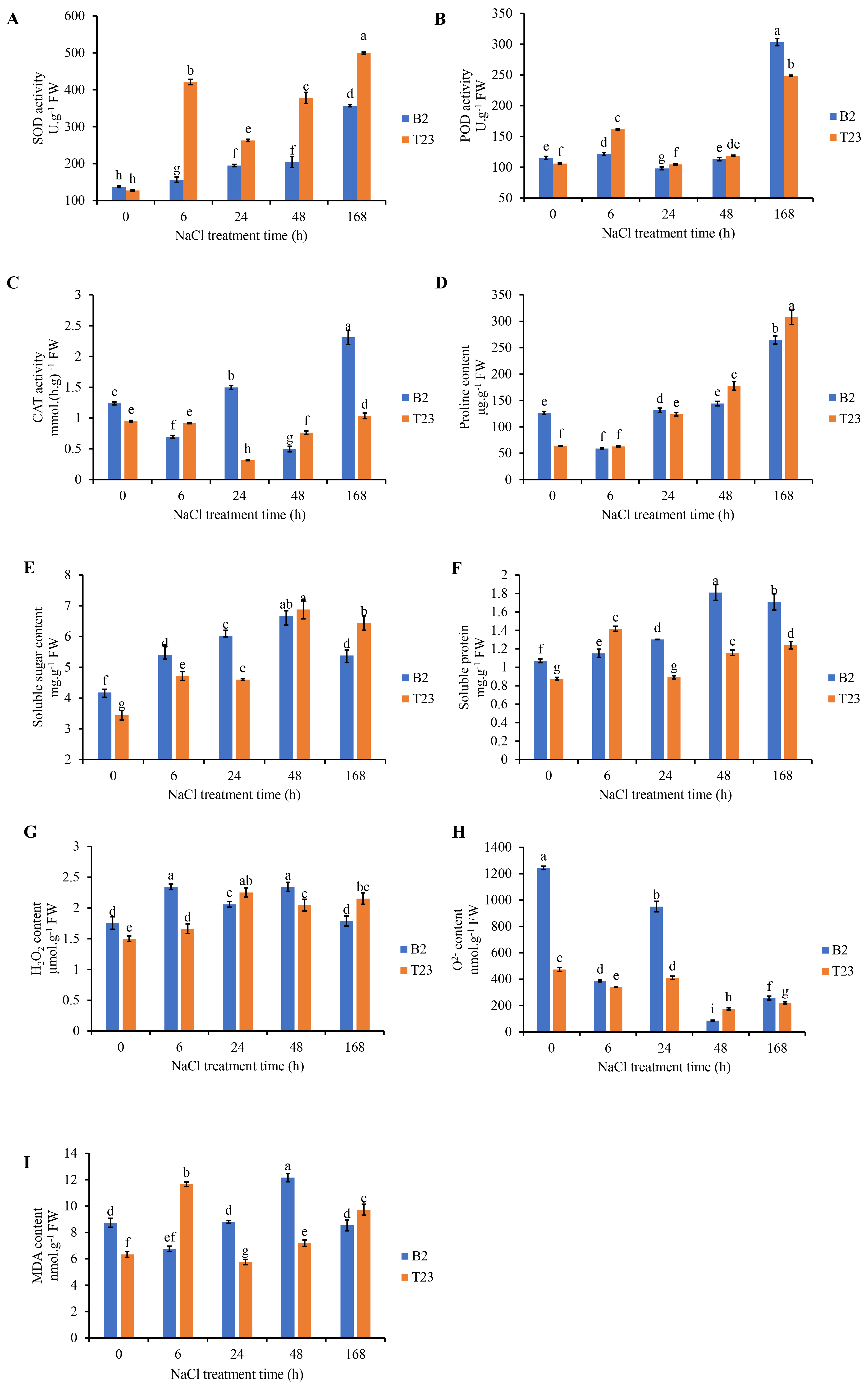
3.2. Analysis of Physiological Parameters Under Salt Stress
3.3. Evaluation of RNA-Seq Data Quality
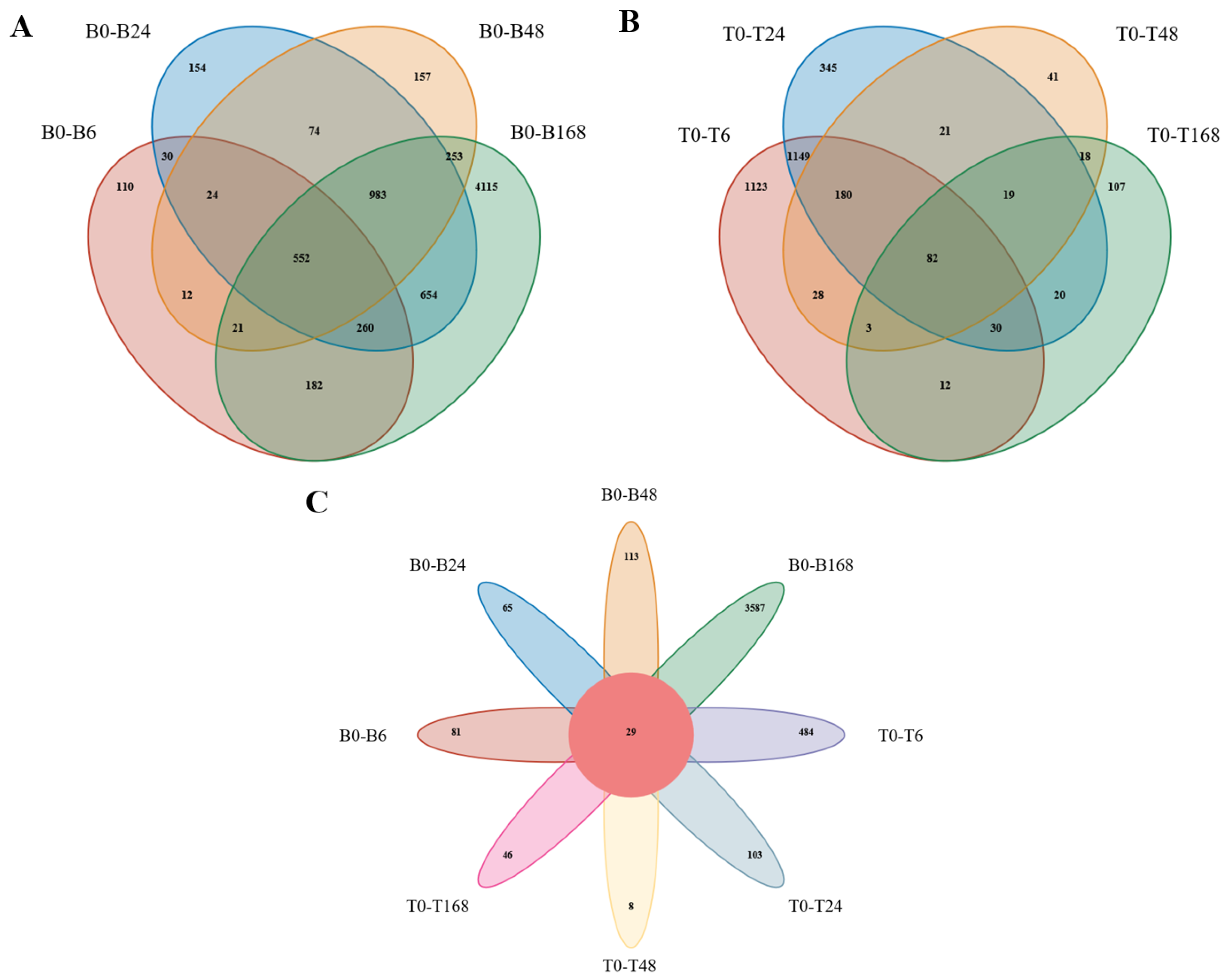
3.4. Differential Expression Gene Analysis
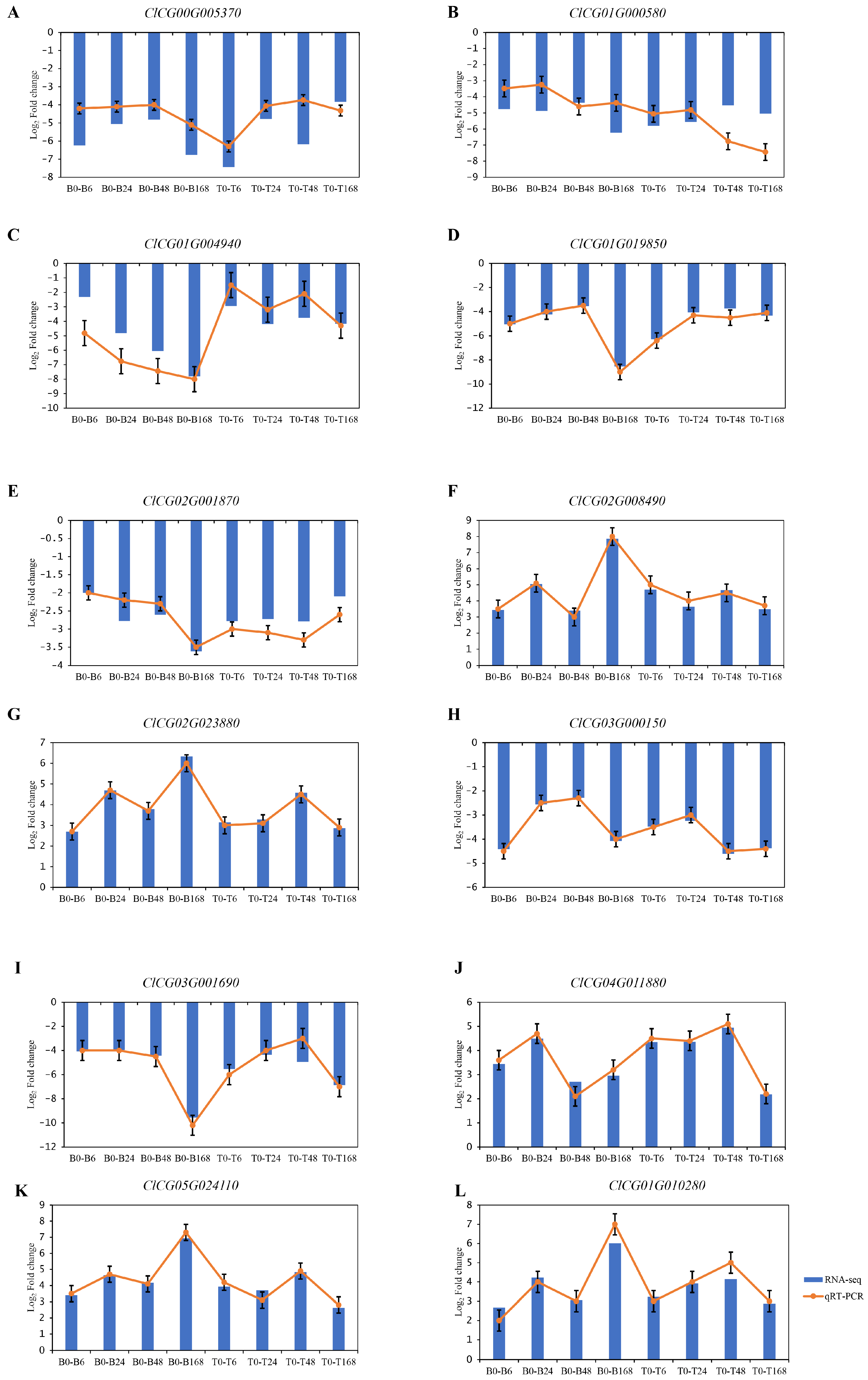
3.5. Transcriptome Data Verified by qRT-PCR
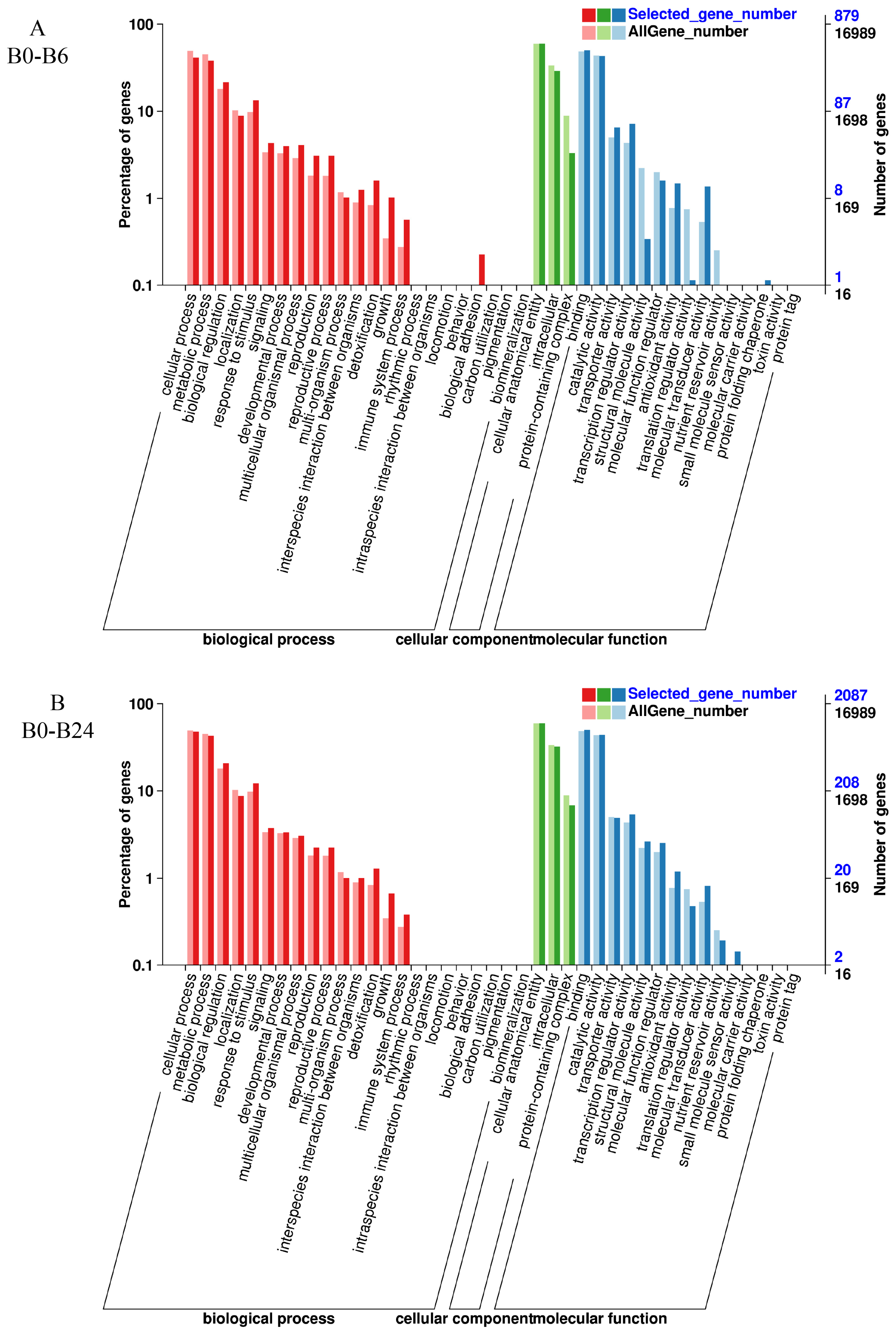
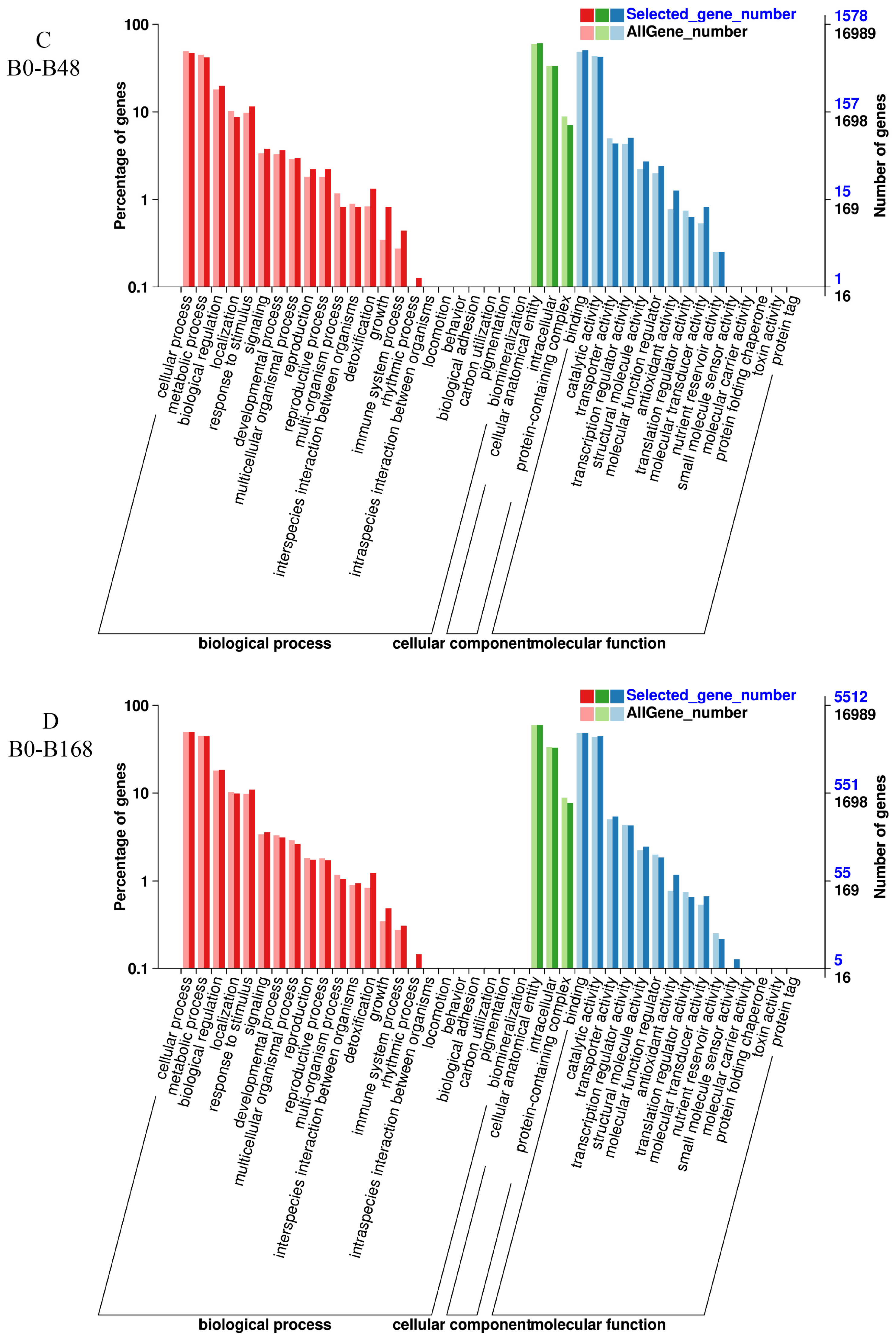
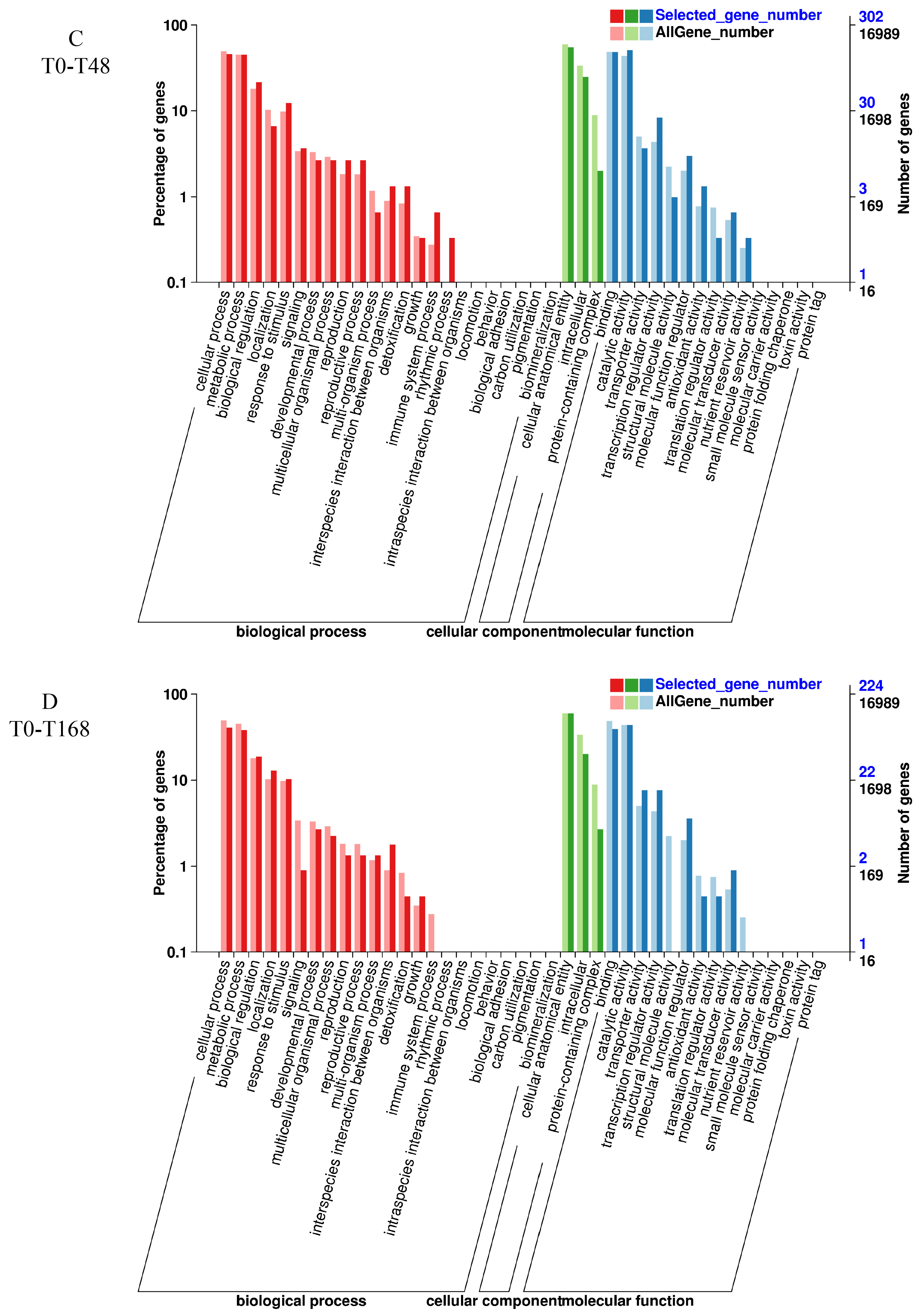
3.6. GO Enrichment Analysis
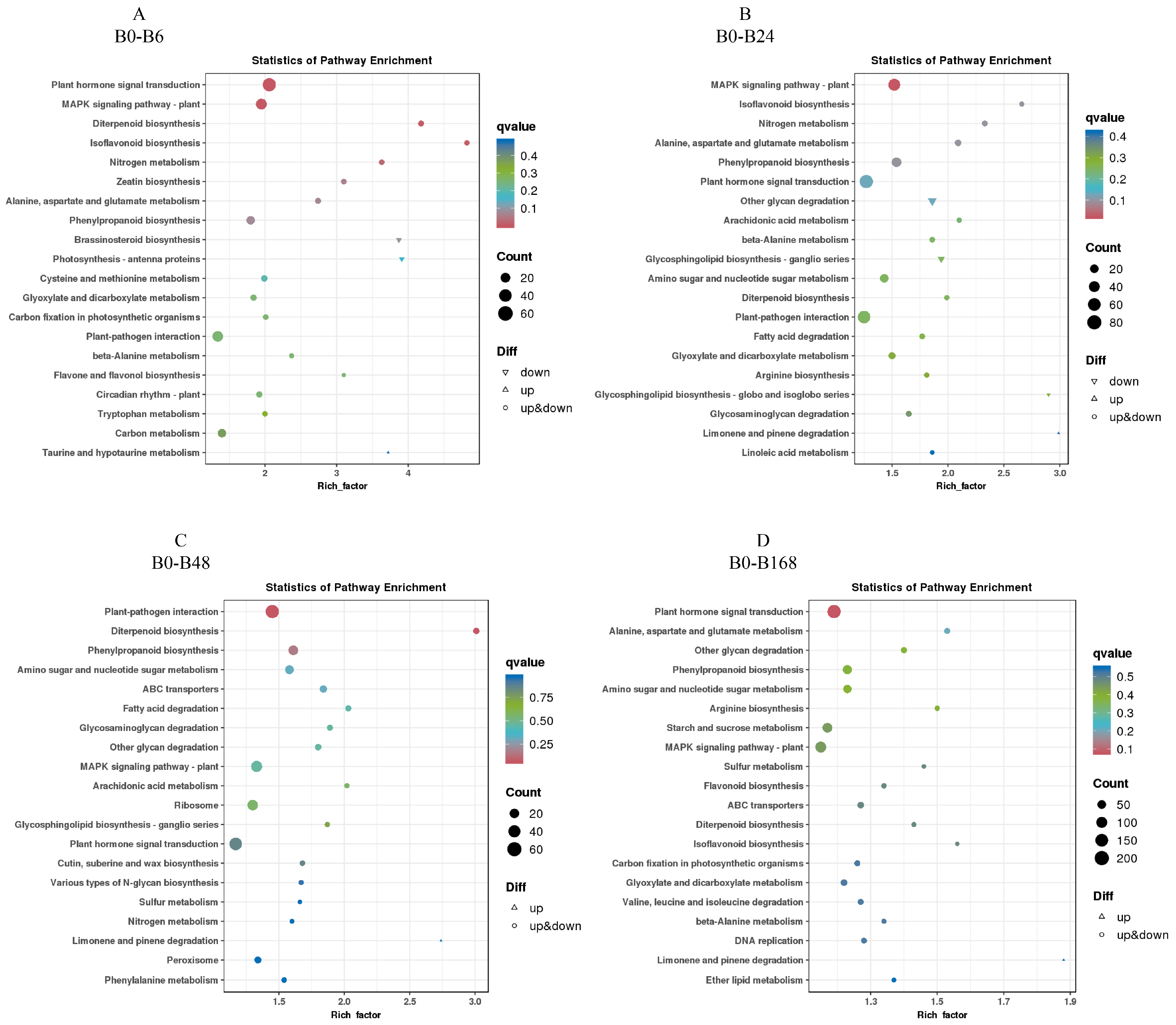
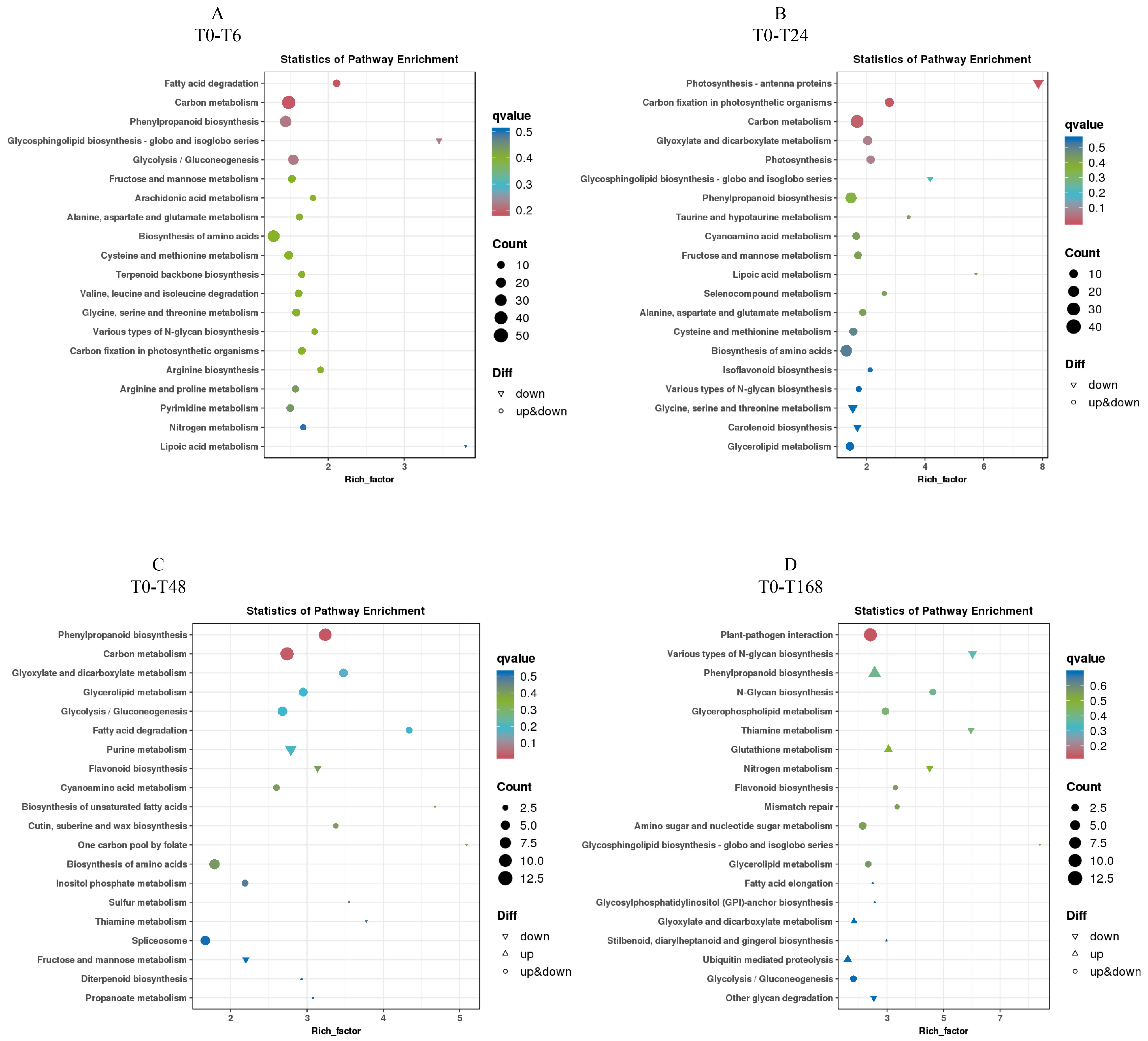
3.7. KEGG Enrichment Analysis of Differentially Expressed Genes
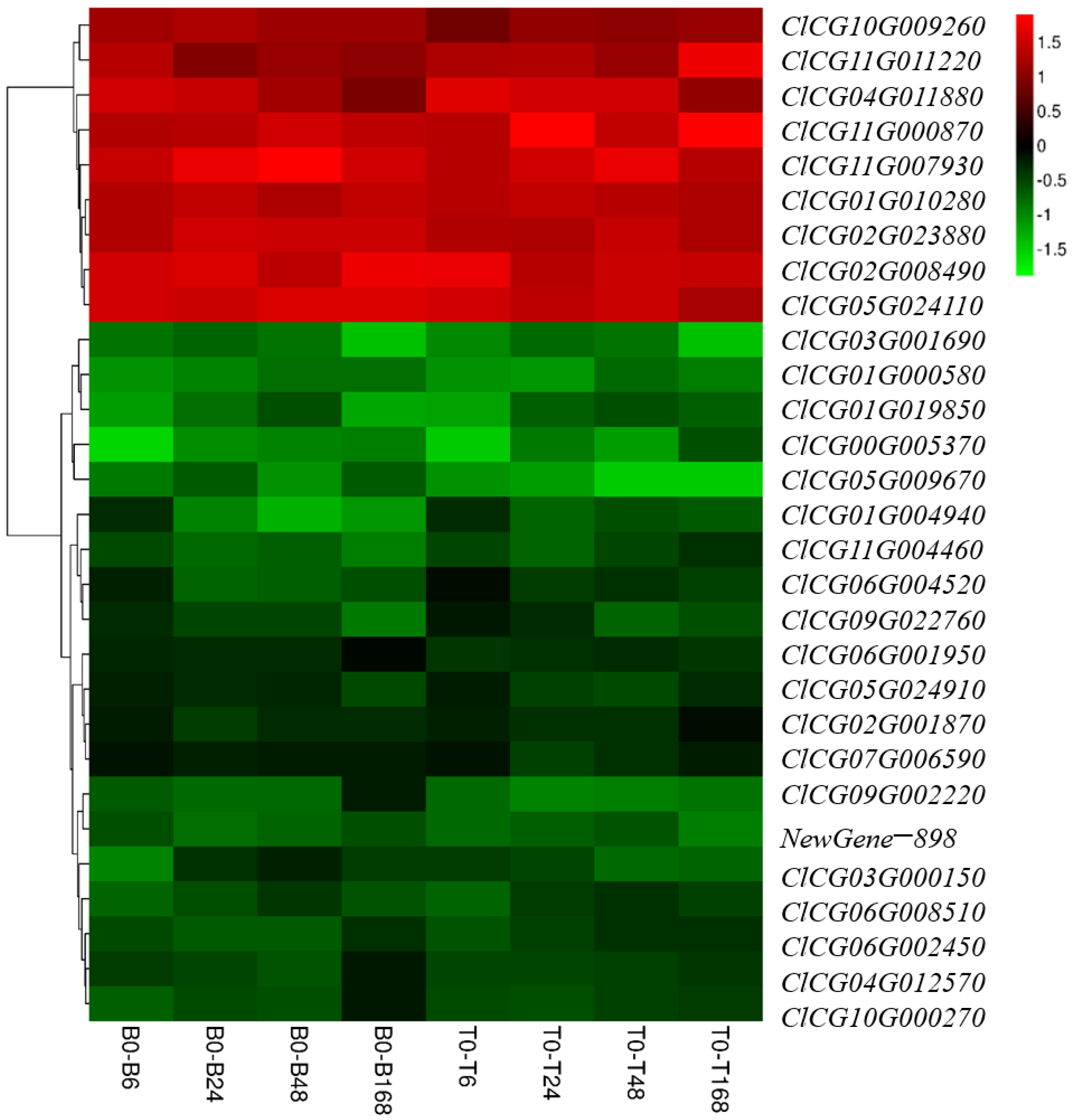
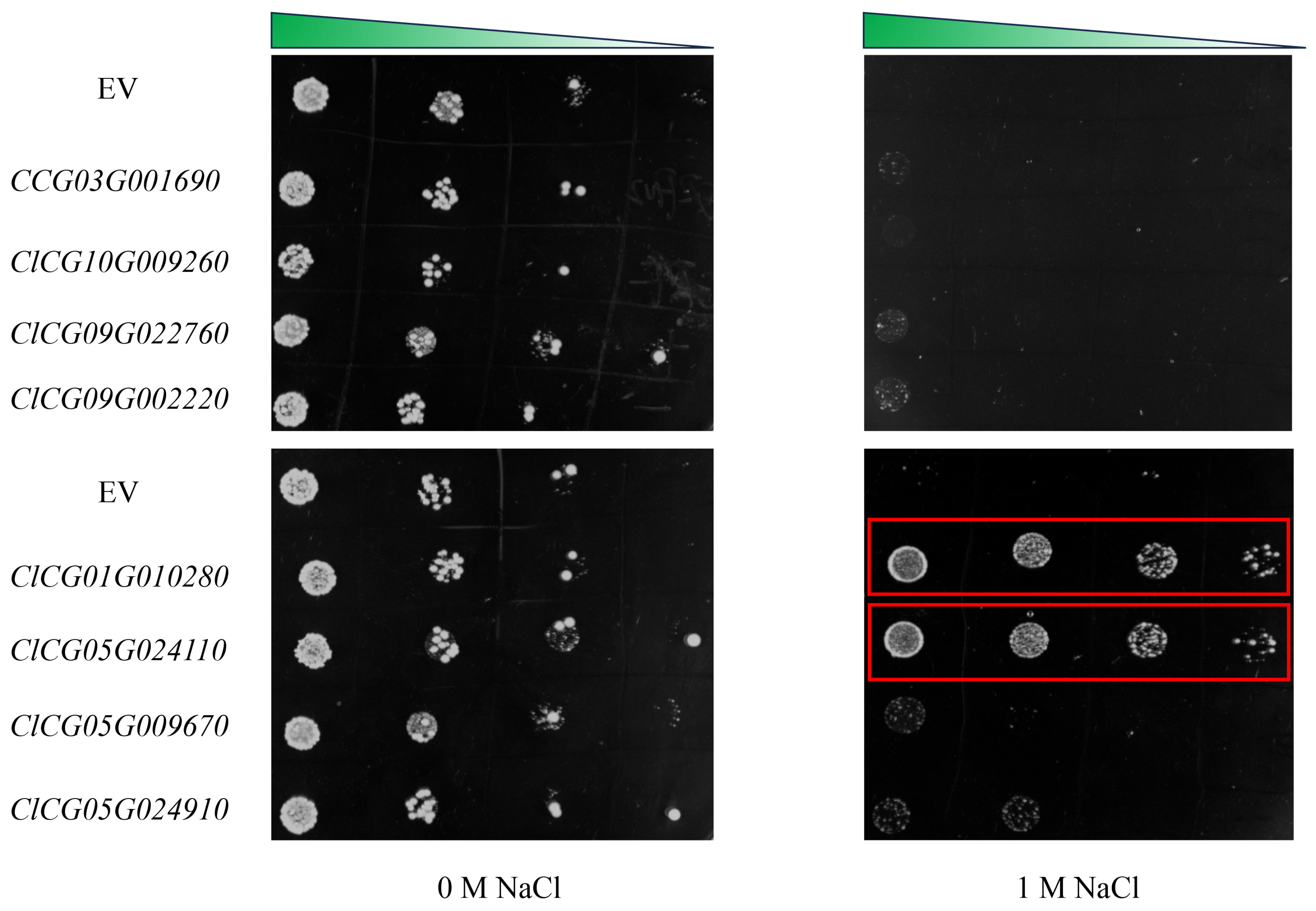
3.8. Exploration and Preliminary Verification of Salt-Tolerant Candidate Genes
4. Discussion
4.1. Physiological Response to Salt Stress by Watermelon Seedlings
4.2. Change in Gene Transcription of Watermelon Seedlings Under Salt Stress
4.3. Salt-Tolerant Candidate Genes ClCG01G010280 and ClCG05G024110 in Watermelon
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, H.J.; Zhao, S.J.; Lu, X.Q.; He, N.; Gao, L.; Dou, J.L.; Bie, Z.L.; Liu, W.G. Genome duplication improves the resistance of watermelon root to salt stress. Plant Physiol. Biochem. 2018, 133, 11–21. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Lao, M.T. The effects of salt stress on ornamental plants and integrative cultivation practices. Sci. Hortic. 2018, 240, 430–439. [Google Scholar] [CrossRef]
- Bantis, F.; Koukounaras, A. Ascophyllum nodosum and Silicon-Based Biostimulants Differentially Affect the Physiology and Growth of Watermelon Transplants under Abiotic Stress Factors: The Case of Salinity. Plants 2023, 12, 433. [Google Scholar] [CrossRef] [PubMed]
- da Silva, F.J.L.; de Lima, G.S.; da Silva, S.S.; Soares, L.A.D.; Torres, R.A.F.; Gheyi, H.R.; Paiva, F.J.D.; Mendonca, A.J.T.; Ferreira, J.T.A.; da Silva, A.A.R.; et al. Effect of hydrogen peroxide application on physiology, production, and post-harvest quality of mini watermelon under salt stress. Arid Land Res. Manag. 2024, 39, 262–288. [Google Scholar] [CrossRef]
- de Sousa, A.B.O.; Duarte, S.N.; Neto, O.N.D.; Souza, A.C.M.; Sampaio, P.R.F.; Dias, C. Production and quality of mini watermelon cv. Smile irrigated with saline water. Rev. Bras. Eng. Agric. E Ambient. 2016, 20, 897–902. [Google Scholar] [CrossRef]
- Gomes do O, L.M.; Cova, A.M.W.; Neto, A.D.D.; da Silva, N.D.; Santos, A.L.; Silva, P.C.C.; Gheyi, H.R.; da Silva, L.L. Osmotic adjustment, production, and post-harvest quality of mini watermelon genotypes differing in salt tolerance. Sci. Hortic. 2022, 306, 111463. [Google Scholar] [CrossRef]
- Chen, Y.H.; Zhang, S.Y.; Du, S.F.; Jiang, J.; Wang, G.Y. Transcriptome and Metabonomic Analysis of Tamarix ramosissima Potassium (K+) Channels and Transporters in Response to NaCl Stress. Genes 2022, 13, 1313. [Google Scholar] [CrossRef]
- Hu, J.; Hu, X.K.; Zhang, H.W.; Yu, Q.S. Moderate NaCl alleviates osmotic stress in Lycium ruthenicum. Plant Growth Regul. 2022, 96, 25–35. [Google Scholar] [CrossRef]
- Kim, Y.H.; Wang, M.Q.; Bai, Y.; Zeng, Z.H.; Guo, F.; Han, N.; Bian, H.W.; Wang, J.H.; Pan, J.W.; Zhu, M.Y. Bcl-2 suppresses activation of VPEs by inhibiting cytosolic Ca2+ level with elevated K+ efflux in NaCl-induced PCD in rice. Plant Physiol. Biochem. 2014, 80, 168–175. [Google Scholar] [CrossRef]
- Fu, H.Q.; Yang, Y.Q. How Plants Tolerate Salt Stress. Curr. Issues Mol. Biol. 2023, 45, 5914–5934. [Google Scholar] [CrossRef]
- Zhao, S.S.; Zhang, Q.K.; Liu, M.Y.; Zhou, H.P.; Ma, C.L.; Wang, P.P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.P.; Shi, H.F.; Yang, Y.Q.; Feng, X.X.; Chen, X.; Xiao, F.; Lin, H.H.; Guo, Y. Insights into plant salt stress signaling and tolerance. J. Genet. Genom. 2024, 51, 16–34. [Google Scholar] [CrossRef]
- Farooq, M.A.; Ul Haq, M.; Zhang, L.P.; Wu, S.H.; Mushtaq, N.; Tahir, H.; Wang, Z.W. Transcriptomic Insights into Salt Stress Response in Two Pepper Species: The Role of MAPK and Plant Hormone Signaling Pathways. Int. J. Mol. Sci. 2024, 25, 9355. [Google Scholar] [CrossRef]
- Xu, J.X.; Wang, T.T.; Wang, X.Y.; Yan, H.H.; Liu, P.; Hou, X.; Gao, Y.; Yang, L.; Zhang, L. Exogenous Eugenol Alleviates Salt Stress in Tobacco Seedlings by Regulating the Antioxidant System and Hormone Signaling. Int. J. Mol. Sci. 2024, 25, 6771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Shen, Y.X.; Han, M.; Su, Y.; Feng, X.L.; Gao, T.; Zhou, X.J.; Wu, Q.; Sun, G.L.; Wang, Y.L. Potential Response Patterns of Endogenous Hormones in Cliff Species Opisthopappus taihangensis and Opisthopappus longilobus under Salt Stress. Plants 2024, 13, 557. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.Q.; Wang, L.; Li, C.Q.; Zhao, Y.H.; Zhang, N.; Yan, L.; Li, C.M.; Niu, Y.S. Integrated Transcriptomic, Proteomic, and Metabolomic Analyses Revealed Molecular Mechanism for Salt Resistance in Soybean (Glycine max L.) Seedlings. Int. J. Mol. Sci. 2024, 25, 3559. [Google Scholar] [CrossRef]
- Valifard, M.; Mohsenzadeh, S.; Kholdebarin, B.; Rowshan, V.; Niazi, A.; Moghadam, A. Effect of salt stress on terpenoid biosynthesis in Salvia mirzayanii: From gene to metabolite. J. Hortic. Sci. Biotechnol. 2019, 94, 389–399. [Google Scholar] [CrossRef]
- Dudhate, A.; Shinde, H.; Yu, P.; Tsugama, D.; Gupta, S.K.; Liu, S.K.; Takano, T. Comprehensive analysis of NAC transcription factor family uncovers drought and salinity stress response in pearl millet (Pennisetum glaucum). BMC Genom. 2021, 22, 70. [Google Scholar] [CrossRef]
- Hussain, Q.; Asim, M.; Zhang, R.; Khan, R.; Farooq, S.; Wu, J.S. Transcription Factors Interact with ABA through Gene Expression and Signaling Pathways to Mitigate Drought and Salinity Stress. Biomolecules 2021, 11, 1159. [Google Scholar] [CrossRef]
- Khoso, M.A.; Hussain, A.; Ritonga, F.N.; Ali, Q.; Channa, M.M.; Alshegaihi, R.M.; Meng, Q.L.; Ali, M.; Zaman, W.; Brohi, R.D.; et al. WRKY transcription factors (TFs): Molecular switches to regulate drought, temperature, and salinity stresses in plants. Front. Plant Sci. 2022, 13, 1039329. [Google Scholar] [CrossRef]
- Wang, Z.J.; Zhu, J.; Yuan, W.Y.; Wang, Y.; Hu, P.P.; Jiao, C.Y.; Xia, H.M.; Wang, D.D.; Cai, Q.W.; Li, J.; et al. Genome-wide characterization of bZIP transcription factors and their expression patterns in response to drought and salinity stress in Jatropha curcas. Int. J. Biol. Macromol. 2021, 181, 1207–1223. [Google Scholar] [CrossRef] [PubMed]
- Zang, D.D.; Wang, J.X.; Zhang, X.; Liu, Z.J.; Wang, Y.C. Arabidopsis heat shock transcription factor HSFA7b positively mediates salt stress tolerance by binding to an E-box-like motif to regulate gene expression. J. Exp. Bot. 2019, 70, 5355–5374. [Google Scholar] [CrossRef]
- Zhou, X.G.; Sun, Z.F.; Huang, Y.Z.; He, D.; Lu, L.; Wei, M.T.; Lin, S.M.; Luo, W.X.; Liao, X.Z.; Jin, S.S.; et al. WRKY45 positively regulates salinity and osmotic stress responses in Arabidopsis. Plant Physiol. Biochem. 2025, 219, 109408. [Google Scholar] [CrossRef]
- Li, X.Y.; Gao, M.L.; Guo, Y.; Zhang, Z.W.; Zhang, Z.M.; Chi, L.; Qu, Z.C.; Wang, L.; Huang, R.Y. 6-Benzyladenine alleviates NaCl stress in watermelon (Citrullus lanatus) seedlings by improving photosynthesis and upregulating antioxidant defences. Funct. Plant Biol. 2023, 50, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.Y.; Wang, S.S.; Wei, M.; Gong, B.; Shi, Q.H. Effect of Different Rootstocks on the Salt Stress Tolerance in Watermelon Seedlings. Hortic. Plant J. 2018, 4, 239–249. [Google Scholar] [CrossRef]
- Borsai, O.; Al Hassan, M.; Negrusier, C.; Raigón, M.D.; Boscaiu, M.; Sestras, R.E.; Vicente, O. Responses to Salt Stress in Portulaca: Insight into Its Tolerance Mechanisms. Plants 2020, 9, 1660. [Google Scholar] [CrossRef]
- Dien, D.C.; Mochizuki, T.; Yamakawa, T. Effect of various drought stresses and subsequent recovery on proline, total soluble sugar and starch metabolisms in Rice (Oryza sativa L.) varieties. Plant Prod. Sci. 2019, 22, 530–545. [Google Scholar] [CrossRef]
- Rady, M.M.; Taha, R.S.; Mandi, A.H.A. Proline enhances growth, productivity and anatomy of two varieties of Lupinus termis L. grown under salt stress. S. Afr. J. Bot. 2016, 102, 221–227. [Google Scholar] [CrossRef]
- Ul Mushtaq, N.; Saleem, S.; Tahir, I.; Seth, C.S.; Ul Rehman, R. Crosstalk in proline biosynthesis regulates proline augmentation and resilience to salt stress in Panicum miliaceum L. Environ. Exp. Bot. 2024, 224, 105810. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Nafees, M.; Moosa, A.; Ferrante, A.; Darras, A. Melatonin induces proline, secondary metabolites, sugars and antioxidants activity to regulate oxidative stress and ROS scavenging in salt stressed sword lily. Heliyon 2024, 10, e32569. [Google Scholar] [CrossRef]
- Zhang, P.F.; Liu, L.; Wang, X.; Wang, Z.Y.; Zhang, H.; Chen, J.T.; Liu, X.Y.; Wang, Y.B.; Li, C.F. Beneficial Effects of Exogenous Melatonin on Overcoming Salt Stress in Sugar Beets (Beta vulgaris L.). Plants 2021, 10, 886. [Google Scholar] [CrossRef]
- Li, W.J.; Meng, R.; Liu, Y.; Chen, S.M.; Jiang, J.F.; Wang, L.K.; Zhao, S.; Wang, Z.X.; Fang, W.M.; Chen, F.D.; et al. Heterografted chrysanthemums enhance salt stress tolerance by integrating reactive oxygen species, soluble sugar, and proline. Hortic. Res. 2022, 9, uhac073. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.H.; Elango, D.; Liu, H.X.; Jin, D.D.; Wang, X.Y.; Wu, Y. Metabolome and transcriptome analysis reveals molecular mechanisms of watermelon under salt stress. Environ. Exp. Bot. 2023, 206, 105200. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Yuan, G.P.; Gao, B.W.; An, G.L.; Li, W.H.; Si, W.J.; Sun, D.X.; Liu, J.P. Comparative Transcriptome Profiling Provides Insights into Plant Salt Tolerance in Watermelon (Citrullus lanatus). Life 2022, 12, 1033. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.S.; Joshi, M.; Joshi, V. Transcriptomic Analysis of Short-Term Salt Stress Response in Watermelon Seedlings. Int. J. Mol. Sci. 2020, 21, 6036. [Google Scholar] [CrossRef]
- Fang, X.; Mo, J.J.; Zhou, H.K.; Shen, X.F.; Xie, Y.L.; Xu, J.H.; Yang, S. Comparative transcriptome analysis of gene responses of salt-tolerant and salt-sensitive rice cultivars to salt stress. Sci. Rep. 2023, 13, 19065. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.R.; Wang, Z.C.; Zhao, L.; Liu, J.; Meng, J.; Luan, Y.S. Secreted Peptide SpPIP1 Modulates Disease Resistance and Salt Tolerance in Tomato. J. Agric. Food Chem. 2023, 71, 12264–12279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, D.D.; Sa, R.; Wang, L.; Sheng, Y.Y. Cloning and Function Analysis of the CsTAU1 in Response to Salt-Alkali Stress. Genes 2024, 15, 613. [Google Scholar] [CrossRef]
- Liu, X.; Yang, X.X.; Zhang, B. Transcriptome analysis and functional identification of GmMYB46 in soybean seedlings under salt stress. Peerj 2021, 9, e12492. [Google Scholar] [CrossRef]
- Li, X.H.; Ye, G.; Shen, Z.Y.; Li, J.J.; Hao, D.L.; Kong, W.Y.; Wang, H.R.; Zhang, L.; Chen, J.B.; Guo, H.L. Na plus and K plus homeostasis in different organs of contrasting Zoysia japonica accessions under salt stress. Environ. Exp. Bot. 2023, 214, 105455. [Google Scholar] [CrossRef]
- Liu, P.; Li, Q.; Gao, Y.A.; Wang, H.; Chai, L.; Yu, H.J.; Jiang, W.J. A New Perspective on the Effect of UV-B on L-Ascorbic Acid Metabolism in Cucumber Seedlings. J. Agric. Food Chem. 2019, 67, 4444–4452. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Yang, C.H.; Li, J.R.; Wu, Q.X.; Yang, X.X.; Huang, A.Y.; Zhang, J.; Ye, A.Y.; Dou, Y.M.; Yan, L.L.; Zhou, W.Z.; et al. AutismKB 2.0: A knowledgebase for the genetic evidence of autism spectrum disorder. Database-J. Biol. Databases Curation 2018, 2018, bay106. [Google Scholar] [CrossRef]
- Harshitha, R.; Arunraj, D.R. Real-time quantitative PCR: A tool for absolute and relative quantification. Biochem. Mol. Biol. Educ. 2021, 49, 800–812. [Google Scholar] [CrossRef] [PubMed]
- He, L.L.; Jing, Y.; Shen, J.L.; Li, X.N.; Liu, H.P.; Geng, Z.L.; Wang, M.; Li, Y.Q.; Chen, D.H.; Gao, J.W.; et al. Mitochondrial Pyruvate Carriers Prevent Cadmium Toxicity by Sustaining the TCA Cycle and Glutathione Synthesis. Plant Physiol. 2019, 180, 198–211. [Google Scholar] [CrossRef]
- Jing, Y.; Shi, L.; Li, X.; Zheng, H.; Gao, J.W.; Wang, M.; He, L.L.; Zhang, W. OXS2 is Required for Salt Tolerance Mainly through Associating with Salt Inducible Genes, CA1 and Araport11, in Arabidopsis. Sci. Rep. 2019, 9, 20341. [Google Scholar] [CrossRef]
- Amjad, M.; Akhtar, J.; Murtaza, B.; Abbas, G.; Jawad, H. Differential accumulation of potassium results in varied salt-tolerance response in tomato (Solanum lycopersicum L.) cultivars. Hortic. Environ. Biotechnol. 2016, 57, 248–258. [Google Scholar] [CrossRef]
- Beyaz, R.; Kir, H. Physio-biochemical analyses in seedlings of sorghum-sudangrass hybrids that are grown under salt stress under in vitro conditions. Turk. J. Biochem.-Turk Biyokim. Derg. 2020, 45, 177–184. [Google Scholar] [CrossRef]
- Mehmood, S.; Siddiqi, E.H.; Iqbal, M.S.; Malik, M.F.; Hussain, K.; Nawaz, K.; Akbar, M.; Fatima, S.; Bilal, M.; Mukhtar, E. Salt-induced variation of inorganic nutrients, antioxidant enzymes, leaf proline and malondialdehyde (MDA) content in canola (brassica napus L.). Appl. Ecol. Environ. Res. 2018, 16, 4299–4309. [Google Scholar] [CrossRef]
- Amjad, M.; Akhtar, J.; Anwar-Ul-Haq, M.; Riaz, M.A.; Saqib, Z.A.; Murtaza, B.; Naeem, M.A. Effectiveness of potassium in mitigating the salt-induced oxidative stress in contrasting tomato genotypes. J. Plant Nutr. 2016, 39, 1926–1935. [Google Scholar] [CrossRef]
- Kordrostami, M.; Rabiei, B.; Kumleh, H.H. Different physiobiochemical and transcriptomic reactions of rice (Oryza sativa L.) cultivars differing in terms of salt sensitivity under salinity stress. Environ. Sci. Pollut. Res. 2017, 24, 7184–7196. [Google Scholar] [CrossRef]
- Swarcewicz, B.; Sawikowska, A.; Marczak, L.; Luczak, M.; Ciesiolka, D.; Krystkowiak, K.; Kuczynska, A.; Pislewska-Bednarek, M.; Krajewski, P.; Stobiecki, M. Effect of drought stress on metabolite contents in barley recombinant inbred line population revealed by untargeted GC-MS profiling. Acta Physiol. Plant. 2017, 39, 158. [Google Scholar] [CrossRef]
- Wang, N.N.; Qi, F.; Wang, F.; Lin, Y.J.; Xiaoyang, C.; Peng, Z.W.; Zhang, B.; Qi, X.; Deyholos, M.K.; Zhang, J. Evaluation of Differentially Expressed Genes in Leaves vs. Roots Subjected to Drought Stress in Flax (Linum usitatissimum L.). Int. J. Mol. Sci. 2023, 24, 12019. [Google Scholar] [CrossRef]
- Chen, G.; Fan, P.S.; Feng, W.M.; Guan, A.Q.; Lu, Y.Y.; Wan, Y.L. Effects of 5-aminolevulinic acid on nitrogen metabolism and ion distribution of watermelon seedlings under salt stress. Russ. J. Plant Physiol. 2017, 64, 116–123. [Google Scholar] [CrossRef]
- do O, L.M.G.; Cova, A.M.W.; Neto, A.D.D.; da Silva, N.D.; Silva, P.C.C.; Ribas, R.F.; Santos, A.L.; Gheyi, H.R. Physiological and biochemical responses of mini watermelon irrigated with brackish water under two types of irrigation system. Semin.-Cienc. Agrar. 2022, 43, 1497–1515. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.Q.; Wen, W.X.; Sun, J.; Shu, S.; Guo, S.R. Transcriptome and Proteome Analysis Identifies Salt Stress Response Genes in Bottle Gourd Rootstock-Grafted Watermelon Seedlings. Agronomy 2023, 13, 618. [Google Scholar] [CrossRef]
- Du, X.L.; Wang, G.; Ji, J.; Shi, L.P.; Guan, C.F.; Jin, C. Comparative transcriptome analysis of transcription factors in different maize varieties under salt stress conditions. Plant Growth Regul. 2017, 81, 183–195. [Google Scholar] [CrossRef]
- Wang, M.Q.; Wang, Y.F.; Zhang, Y.F.; Li, C.X.; Gong, S.C.; Yan, S.Q.; Li, G.L.; Hu, G.H.; Ren, H.L.; Yang, J.F.; et al. Comparative transcriptome analysis of salt-sensitive and salt-tolerant maize reveals potential mechanisms to enhance salt resistance. Genes Genom. 2019, 41, 781–801. [Google Scholar] [CrossRef]
- Chen, L.F.; Meng, Y.; Bai, Y.; Yu, H.H.; Qian, Y.; Zhang, D.Y.; Zhou, Y.W. Starch and Sucrose Metabolism and Plant Hormone Signaling Pathways Play Crucial Roles in Aquilegia Salt Stress Adaption. Int. J. Mol. Sci. 2023, 24, 3948. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Wang, Q.Y.; Gao, Z.W.; Wang, Y.; Liu, Y.J.; Ma, Z.P.; Chen, Y.W.; Zhang, Y.C.; Yan, F.; Li, J.W. Analysis of Phytohormone Signal Transduction in Sophora alopecuroides under Salt Stress. Int. J. Mol. Sci. 2021, 22, 7313. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.M.; Liu, J.N.; Yan, L.P.; Liang, Q.; Fang, H.C.; Wang, C.X.; Dong, Y.H.; Chai, Z.J.; Zhou, R.; Bao, Y.; et al. Comparative Transcriptome Analysis Unravels Defense Pathways of Fraxinus velutina Torr Against Salt Stress. Front. Plant Sci. 2022, 13, 842726. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Liu, J.G.; Zhao, G.Y.; Geng, Z.; Qi, H.; Dou, H.K.; Zhang, H.S. Dynamic transcriptome and co-expression network analysis of the cotton (Gossypium hirsutum) root response to salinity stress at the seedling stage. Acta Physiol. Plant. 2020, 42, 143. [Google Scholar] [CrossRef]
- Cheng, Y.; Cheng, X.Q.; Wei, K.; Wang, Y. Comparative Transcriptome Analysis of Salt-Tolerant and -Sensitive Soybean Cultivars under Salt Stress. Int. J. Mol. Sci. 2024, 25, 9818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, B.; Zhang, P.; Han, Q.H.; Zhao, G.W.; Zhao, F.C. Comparative Transcriptome Analysis Reveals the Underlying Response Mechanism to Salt Stress in Maize Seedling Roots. Metabolites 2023, 13, 1155. [Google Scholar] [CrossRef]
- Shah, S.H.A.; Wang, H.B.; Xu, H.H.; Yu, Z.H.; Hou, X.L.; Li, Y. Comparative Transcriptome Analysis Reveals the Protective Role of Melatonin during Salt Stress by Regulating the Photosynthesis and Ascorbic Acid Metabolism Pathways in Brassica campestris. Int. J. Mol. Sci. 2024, 25, 5092. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.H.; Ren, G.Z.; Qiao, H.Y.; Xiang, X.D.; Huang, L.S.; Chang, J.H. Comparative Transcriptome Analysis of Seedling Stage of Two Sorghum Cultivars Under Salt Stress. J. Plant Growth Regul. 2018, 37, 986–998. [Google Scholar] [CrossRef]
- Doireau, R.; Jaslan, J.; Cubero-Font, P.; Demes-Causse, E.; Bertaux, K.; Cassan, C.; Pétriarcq, P.; De Angeli, A. AtALMT5 mediates vacuolar fumarate import and regulates the malate/fumarate balance in Arabidopsis. New Phytol. 2024, 244, 811–824. [Google Scholar] [CrossRef]
- Frei, B.; Eisenach, C.; Martinoia, E.; Hussein, S.; Chen, X.Z.; Arrivault, S.; Neuhaus, H.E. Purification and functional characterization of the vacuolar malate transporter tDT from Arabidopsis. J. Biol. Chem. 2018, 293, 4180–4190. [Google Scholar] [CrossRef]
- Medeiros, D.B.; Barros, K.A.; Barros, J.A.S.; Omena-Garcia, R.P.; Arrivault, S.; Sanglard, L.; Detmann, K.C.; Silva, W.B.; Daloso, D.M.; DaMatta, F.M.; et al. Impaired Malate and Fumarate Accumulation Due to the Mutation of the Tonoplast Dicarboxylate Transporter Has Little Effects on Stomatal Behavior. Plant Physiol. 2017, 175, 1068–1081. [Google Scholar] [CrossRef]
- Wei, X.Y.; Xu, L.; Dong, S.S.; He, N.N.; Xi, Q.Q.; Yao, D.; Wang, Q.Q.; Zuo, Y.; Ling, C.; Qi, M.T.; et al. SaTDT enhanced plant tolerance to NaCl stress by modulating the levels of malic acid and citric acid in cells. Plant Mol. Biol. 2025, 115, 4. [Google Scholar] [CrossRef]
- Fan, W.; Xu, J.M.; Lou, H.Q.; Xiao, C.; Chen, W.W.; Yang, J.L. Physiological and Molecular Analysis of Aluminium-Induced Organic Acid Anion Secretion from Grain Amaranth (Amaranthus hypochondriacus L.) Roots. Int. J. Mol. Sci. 2016, 17, 608. [Google Scholar] [CrossRef]
- Yu, S.; Wu, M.X.; Wang, X.Q.; Li, M.K.; Gao, X.H.; Xu, X.R.; Zhang, Y.T.; Liu, X.R.; Yu, L.H.; Zhang, Y.F. Common Bean (Phaseolus vulgaris L.) NAC Transcriptional Factor PvNAC52 Enhances Transgenic Arabidopsis Resistance to Salt, Alkali, Osmotic, and ABA Stress by Upregulating Stress-Responsive Genes. Int. J. Mol. Sci. 2024, 25, 5818. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Y.; Gongbuzhaxi; Wang, C.Y.; Xue, F.; Zhang, H.; Ji, W.Q. Wheat NAC transcription factor TaNAC29 is involved in response to salt stress. Plant Physiol. Biochem. 2015, 96, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Mijiti, M.; Wang, Y.C.; Wang, L.Q.; Habuding, X. Tamarix hispida NAC Transcription Factor ThNAC4 Confers Salt and Drought Stress Tolerance to Transgenic Tamarix and Arabidopsis. Plants 2022, 11, 2647. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.F.; Lv, B.; Li, J.; Luo, L.Q.; Lu, S.C.; Zhang, X.; Ma, H.; Ming, F. The NAC Family Transcription Factor OsNAP Confers Abiotic Stress Response Through the ABA Pathway. Plant Cell Physiol. 2014, 55, 604–619. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Gao, C.; Li, S.; Wang, X.; Dong, Y.; Wang, C.; Jiao, Z.; Sun, J. Comparative Transcriptome Analysis of Gene Responses of Salt-Tolerant and Salt-Sensitive Watermelon Cultivars’ Roots to Salt Stress. Plants 2025, 14, 1013. https://doi.org/10.3390/plants14071013
Liu P, Gao C, Li S, Wang X, Dong Y, Wang C, Jiao Z, Sun J. Comparative Transcriptome Analysis of Gene Responses of Salt-Tolerant and Salt-Sensitive Watermelon Cultivars’ Roots to Salt Stress. Plants. 2025; 14(7):1013. https://doi.org/10.3390/plants14071013
Chicago/Turabian StyleLiu, Peng, Chao Gao, Shuai Li, Xudong Wang, Yumei Dong, Chongqi Wang, Zigao Jiao, and Jianlei Sun. 2025. "Comparative Transcriptome Analysis of Gene Responses of Salt-Tolerant and Salt-Sensitive Watermelon Cultivars’ Roots to Salt Stress" Plants 14, no. 7: 1013. https://doi.org/10.3390/plants14071013
APA StyleLiu, P., Gao, C., Li, S., Wang, X., Dong, Y., Wang, C., Jiao, Z., & Sun, J. (2025). Comparative Transcriptome Analysis of Gene Responses of Salt-Tolerant and Salt-Sensitive Watermelon Cultivars’ Roots to Salt Stress. Plants, 14(7), 1013. https://doi.org/10.3390/plants14071013




